Simple Summary
Surgery is the most effective treatment for early-stage lung cancer, but it poses a heavy physical burden. Accordingly, understanding the perioperative daily life conditions of patients is important to maintain their health status and to provide appropriate treatment. We performed a prospective study to examine the socioclinical factors associated with the physical quality of life of patients who underwent surgery for lung cancer at Shonan Kamakura General Hospital, Kanagawa, Japan. In the preoperative setting, living alone and lower performance status were independently associated with worse physical quality of life. In the postoperative setting at 6 months, later smoking cessation, lower performance status, living alone, and higher comorbid burden were independently associated with worse physical quality of life. In order to maintain quality of life and provide enough treatment, perioperative management should include taking care of the patient’s physical condition, lifestyle, smoking, and comorbid status.
Abstract
Surgery is the most effective treatment for early-stage lung cancer; however, it poses a higher physical burden than other treatment options. Therefore, understanding the perioperative course of patients is important. Using the Short Form Health Survey 36, we prospectively measured the physical quality of life of patients who underwent anatomical pulmonary resection for non-small cell lung cancer at Shonan Kamakura General Hospital, Kanagawa, Japan (n = 87). In the preoperative setting, patients who had lower performance status and lived alone had significantly worse physical quality of life scores on multivariate analysis (regression coefficient (95% confidence interval), −9.37 (−13.43–−5.32) and −10.22 (−13.74–−7.40), respectively, p < 0.0001 for both). At 6 months postoperatively, patients who stopped smoking within 1 year preoperatively (stopped smoking within 1 year vs. remote or never smokers, 41.0 ± 10.5 vs. 48.6 ± 7.2, p = 0.002), had lower performance status (0 vs. 1–2, 49.3 ± 6.6 vs. 38.6 ± 9.6, p < 0.0001), lived alone (living alone vs. living with somebody, 41.6 ± 9.7 vs. 48.1 ± 7.9, p = 0.021), and had higher comorbid burden (Charlson comorbidity index <3 vs. ≥3, 48.2 ± 6.9 vs. 39.1 ± 14.7, p = 0.003) had significantly worse physical quality of life scores on univariate analysis. More recent smoking (regression coefficient (95% confidence interval), −4.90 (−8.78–1.0), p = 0.014), lower performance status (8.90 (5.10–12.70), p < 0.0001), living alone (5.76 (1.39–10.13), p = 0.01), and higher comorbid burden (−6.94 (−11.78–−2.10), p = 0.006) were significant independent predictors of worse postoperative physical quality of life on multivariate analysis. Therefore, patients with these conditions might need additional support to maintain their physical condition after anatomical lung cancer surgery.
1. Introduction
Surgery is considered the most effective and best curative option for patients with early-stage lung cancer, but it poses a higher physical burden than other treatment modalities for lung cancer. Lung cancer is a disease of the elderly [1] and the leading cause of cancer-related mortality worldwide [2]. In fact, septuagenarians comprise the most common surgical population in Japan [3]. As aging of the population progresses, evaluation of the influence of surgery on patients has become more meaningful, because compared with younger patients, elderly patients with lung cancer have more comorbidities and are more vulnerable [4].
In some countries, there has been a shift from big families to nuclear families in recent decades [5,6,7]. This change in family structure has increased the number of elderly people living alone. Under these circumstances, detailed understanding of the perioperative course, especially the daily life, of patients with lung cancer has become more important.
Evaluation of health-related quality of life (HR-QOL) has been reported to be a method for the assessment of the postoperative condition of these patients. Several reports have confirmed that the postoperative decline in QOL is associated with survival after lung cancer surgery and the course of treatment [8,9]. We previously reported that the perioperative mental QOL of patients with lung cancer was significantly associated with smoking status, living alone, and the Charlson comorbidity index (CCI) [10]. This study aimed to investigate the socioclinical factors associated with the perioperative physical QOL (P-QOL) using the Short Form Health Survey 36 (SF-36) to understand the influence of surgery and adjuvant therapies on the P-QOL of patients, especially in daily life.
2. Patients and Study Methods
Two thoracic surgeons (R.F. and T.N.) performed the surgeries of this study. These surgeons worked as full-time employees at Shonan Kamakura General Hospital (SKGH), and part-time employees at Shonan Fujisawa Tokushukai Hospital (SFTH), which is about 10 km from SKGH due to the lack of a thoracic surgeon in SFTH. Of the 108 patients who underwent surgery for non-small cell lung cancer (NSCLC) at our institution between April 2015 and November 2017, 100 patients provided written informed consent to participate in this study during the preoperative visit at the time of consenting for surgery. The participants were asked to fill out the questionnaire within 1 month of surgery at preoperative visit and at 1, 3, 6, and 12 months postoperatively as an outpatient at SKGH or SFTH by thoracic surgeons. Patients who underwent partial resection (n = 8), repeat lobectomy (n = 1), lobectomy with infiltrated rib resection (n = 1), and exploratory thoracoscopic surgery (n = 1) were excluded because the surgical stress of these procedures is fundamentally different from that of initial lobectomy or segmentectomy. Patients facing difficulty in completing their questionnaires postoperatively because of major complications (n = 2, cerebral infarction and severe brain edema, respectively) were also excluded. Finally, 87 patients who underwent anatomical pulmonary resection for NSCLC were analyzed; 74 patients (85%) underwent surgery thoracoscopically and 13 (15%) by thoracotomy. The patient’s data, including postoperative adjuvant therapies, were collected through the medical record review.
2.1. Quality of Life Assessment
QOL was assessed using the Japanese version of SF-36 [11]. The SF-36, a self-rated questionnaire that comprises 36 items grouped into eight scales, including physical and mental health, is used to assess eight QOL dimensions [12]. As the main focus of the study was to understand the effect of surgery and adjuvant therapies on the P-QOL of the patients, especially in daily life, four domains from the eight SF-36 subscales were selected as elements of physical health. In this study, we evaluated only physical health, which comprises the following four subscales: physical function (PF), role-physical (RP), bodily pain (BP), and general health (GH) (Appendix A).
The raw scores were standardized and ranged from 0 to 100, where 0 represented the worst stage of health and 100 represented the best possible. The national standard level (NSL) with a confidence interval of 95% was used to consider the physical health status. The reliability and validity of the SF-36 questionnaire have been confirmed in international cancer studies [13,14,15,16].
2.2. Smoking Status
During the first visit, the surgeons instructed all patients to stop smoking; thereafter, we checked with the patients and their family whether they had stopped smoking completely at a follow-up visit. We did not use any tools or support for stopping smoking. If the patient could not stop smoking, we refused their request for surgery. Smoking status was classified into two groups, including stopped smoking within 1 year and remote or never smokers (i.e., never smoked or stopped smoking more than 1 year preoperatively). All study participants stopped smoking at least 2 weeks preoperatively.
2.3. Performance Status
To evaluate the preoperative physical activity of the patients, we used the Eastern Cooperative Oncology Group (ECOG) performance status (PS), which includes the following scores: 0, fully active; 1, restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature; 2, ambulatory and capable of self-care but unable to carry out any work activities; 3, capable of only limited self-care; 4, completely disabled; and 5, dead [17]. We confirmed the preoperative PS of each patient using the patients’ medical records.
2.4. Living Conditions
We classified the living conditions of patients into two groups, living alone and with somebody.
2.5. Charlson Comorbidity Index
We evaluated the CCI of each patient based on the preoperative comorbid status. Patients were considered to have a comorbidity if a listed disease was confirmed on the medical records or if the patient received treatment for it. We used the modified CCI, as proposed by Birim et al. [18].
We prospectively investigated the progress of perioperative P-QOL as well as the relationship between preoperative and 6-month postoperative P-QOL and patient characteristics, including age, sex, smoking status, ECOG PS, living conditions, and CCI. The scores at 6 months postoperatively were chosen as the postoperative P-QOL because most of the postoperative scores of the four P-QOL subscales plateaued at 3–6 months postoperatively, which is similar to other studies [19,20,21]. Patient data were extracted from the medical records. This study was approved by our institutional review board.
2.6. Statistical Analysis
In accordance with the SF-36 procedures, the scores were converted to a linear scale that ranged from 0 to 100 for each patient. For each of the four subscales, lower scores represented greater symptom burden. For all patients, the mean score of the four subscales was calculated and used to evaluate the relationship between socioclinical factors and P-QOL. All statistical analyses were performed using EZR version 1.55 (free statistical software), which is a modified version of the R commander and was designed to add statistical functions that are frequently used in biostatistics [22].
The paired t-test was used to compare the preoperative and postoperative mean P-QOL scores on all four subscales. The Student’s t-test was used to compare preoperative and postoperative P-QOL scores between two groups, which were divided based on each clinical factor (age, sex, smoking status, PS, living conditions, and CCI). Multivariate analysis was used for the factors with significant differences on univariate analysis. Multiple regression analysis was used for multivariate analysis.
3. Results
Overall, 87 patients participated in this study and the survey completion percentage (the number of omitted responses) to all items of the QOL questionnaire was 100% (0) preoperatively and 91% (21), 96% (12), 89% (36), and 89% (36) at 1, 3, 6, and 12 months postoperatively, respectively. The response rate of the surveys was 100% preoperatively and 94%, 97%, 90%, and 90%, at 1, 3, 6, and 12 months postoperatively, respectively. Of the 87 patients, 64 (74%) underwent surgery alone, while 23 (26%) underwent adjuvant therapy within 1 year postoperatively, including platinum-based chemotherapy (n = 11, 48%), oral uracil-tegafur (n = 10, 44%), radiotherapy (n = 1, 4%), and afatinib (n = 1, 4%). Of the 11 patients (13%) who had lung cancer recurrence within 1 year postoperatively, only 1 (1%) died because of lung cancer. The sociodemographic and clinical characteristics of the patients are presented in Table 1.

Table 1.
Sociodemographic and clinical characteristics of patients (N = 87).
3.1. Baseline P-QOL Subscales
All baseline (preoperative) P-QOL scores were equal to the Japanese NSL (50%) in the four subscales (Figure 1). On univariate analyses, the mean value of the four P-QOL subscales significantly differed according to age (p = 0.022), smoking status (p = 0.025), living conditions (p < 0.001), and ECOG PS (p < 0.0001) (Table 2), but not significantly by sex and CCI. We conducted multivariate analysis for these four factors showing significant differences on univariate analysis. Living alone and lower PS (≥1) were found to be significantly associated with worse preoperative P-QOL scores on multivariate analysis (Table 3). Specifically, the mean value of P-QOL scores on all four subscales of the patients living alone with lower PS (≥1) was 33.4, and that of those living with somebody with good PS (0) was 54.0.
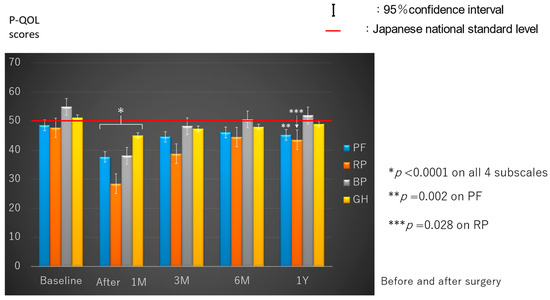
Figure 1.
Perioperative progress on the four subscales of physical quality of life. PF, physical function; RP, role-physical; BP, bodily pain; GH, general health.

Table 2.
Association between clinical factors and preoperative physical quality of life on univariate analysis.

Table 3.
Association between clinical factors and preoperative physical quality of life on multivariate analysis.
3.2. Postoperative P-QOL Evaluation
The P-QOL scores on all four subscales significantly decreased at 1 month postoperatively compared with the preoperative scores (p < 0.0001). Further, they almost plateaued 6 months postoperatively. The P-QOL scores on BP recovered to baseline at 3 months postoperatively, but the PF (p = 0.002) and RP (p = 0.028) scores significantly decreased even at 1 year postoperatively compared with the preoperative scores (Figure 1).
3.3. Predictors of Postoperative P-QOL
We compared the patient characteristics and postoperative P-QOL scores, which had plateaued at 6 months postoperatively. On univariate analysis (Table 4), the mean P-QOL on the four subscales at 6 months postoperatively significantly differed according to smoking status (stopped smoking within 1 year vs. remote or never smokers, 41.0 ± 10.5 vs. 48.6 ± 7.2, p = 0.002), PS (0 vs. 1–2, 49.3 ± 6.6 vs. 38.6 ± 9.6, p < 0.0001), living conditions (living alone vs. living with somebody, 41.6 ± 9.7 vs. 48.1 ± 7.9, p = 0.02), and CCI (<3 vs. ≥3, 48.2 ± 6.9 vs. 39.1 ± 14.7, p = 0.003). Even on multivariate analysis, all four factors significantly affected the P-QOL at 6 months postoperatively (Table 5).

Table 4.
Association between clinical factors and postoperative physical quality of life scores on univariate analysis.

Table 5.
Association between clinical factors and postoperative physical quality of life on multivariate analysis.
4. Discussion
Surgery is considered the best treatment for patients with early-stage NSCLC, but its invasiveness had a negative impact on patient HR-QOL. This study revealed that the P-QOL scores on all four subscales significantly decreased at 1 month postoperatively compared with baseline, and they plateaued at 6 months postoperatively. The P-QOL scores on PF and RP did not recover even at 1 year postoperatively, even though majority (85%) of the surgeries were performed thoracoscopically. Preoperatively, the patients who lived alone and with lower PS (≥1) had significantly lower P-QOL scores in multivariate analysis. Moreover, postoperatively, multivariate analysis revealed that later smoking cessation (i.e., within 1 year preoperatively), lower PS (≥1), living alone, and more comorbid status (CCI ≥ 3) were independently associated with significantly lower P-QOL at 6 months postoperatively.
PS is an important factor in determining the QOL, choice of treatment, and prognosis of patients with cancer. A better PS suggests a better prognosis [23]. In patients with lung cancer, poor PS is a known negative prognostic factor [24,25]. Conversely, only a few articles have reported the association between PS and perioperative P-QOL in patients with lung cancer. Our results revealed that lower PS (≥1) was independently associated with significantly lower preoperative and postoperative P-QOL. In a randomized controlled trial, Liu Z et al. reported that a short-term (2 weeks) multimodal rehabilitation strategy in the perioperative period improved the functional capacity of patients who underwent thoracoscopic lobectomy for lung cancer [26]. For patients with lung cancer and PS ≥ 1, we may consider perioperative rehabilitation in order to maintain their QOL and provide curative treatment.
Living alone affects the HR-QOL of patients with multiple comorbidities [27], and several authors have found that living conditions may affect the survival of patients with cancer [28,29,30]. In this study, we found that the patients who lived alone had significantly worse preoperative and postoperative P-QOL scores. We think that living alone, especially for the elderly, limits support from family members, which then makes remaining motivated to overcome their disease and receive necessary medical treatment more challenging. Moreover, Cheng et al. reported that older adults who lived alone were at a high risk of developing sarcopenia and probable sarcopenia [31]. Sarcopenia is defined as a progressive loss of muscle mass and reduced muscle strength and functional ability, which can lead to worse P-QOL. The factor of “living alone” should be considered as one of the risks to P-QOL. Living conditions of patients during perioperative period should be accounted for to avoid QOL deteriorating by cooperating with nurses and social workers.
Smoking has certain negative impacts on human QOL [32,33] and is a risk factor for the development of various cancers, including lung cancer [34,35]. Accordingly, our results revealed that the preoperative and postoperative P-QOL scores were relatively worse in patients who stopped smoking within 1 year. The findings might not be generalized, as not all centers/countries deny surgery to patients who continue to smoke; thus, smoking cessation in the postoperative period is a confounding variable. Preservation of QOL is important for providing necessary treatment and improving the treatment outcomes or prognosis of patients. Goldenberg et al. demonstrated that smoking cessation significantly improved QOL and that compared with nonsmokers, smokers consistently reported lower physical domain scores [32]. In addition, Hays et al. noted that a longer period of smoking cessation produced better HR-QOL [36]. They also found that compared with placebo, the nonnicotine medications varenicline and SR bupropion for the treatment of tobacco use and dependence led to improved HR-QOL. These results suggested that smoking cessation treatment should be considered for patients who find it difficult to stop smoking preoperatively and to prevent postoperative smoking relapse. Recent quitters (i.e., stopped smoking within 4 weeks of surgery) appeared to have an increased incidence of pulmonary complications [37], although no pulmonary complications were observed in six patients who had stopped smoking within 4 weeks of surgery.
The CCI, which was developed by Charlson et al. in 1987 [38], had been strongly associated with a relatively high risk of surgery in patients with NSCLC. Moreover, in two large-scale phase III trials of the National Cancer Institute of Canada, an unfavorable CCI score was associated with a poor prognosis [39]. In patients with prostate cancer, CCI was reported to be useful, mainly for predicting long-term QOL and PF scores [40]. In this study on patients with NSCLC, a CCI of ≥3 was found to lead to worse postoperative P-QOL. However, many clinical trials frequently exclude patients with the common comorbidities and do not perfectly reflect the characteristics of patients who are routinely treated for NSCLC. Therefore, we considered that compared with PS assessment by physicians, the SF-36 patient self-assessment of QOL score would more accurately reflect the real health status of the patients [41]. Compared with the conventional PS analysis, both the CCI and QOL score may be more accurate indicators of the health status of patients with NSCLC and might help in the decision on the best and individualized therapeutic option.
Our study had some limitations. First, it was conducted at a single institution on a small scale. Second, we could not obtain complete responses to the self-rated questionnaire from the patients. Third, we did not consider the influence of postoperative adjuvant therapy on P-QOL, although the majority of the patients underwent only surgery. Of the 23 patients who underwent adjuvant therapies, 10 patients (44%) underwent oral uracil-tegafur. It has been reported that overall HR-QOL did not deteriorate during adjuvant chemotherapy with oral uracil-tegafur plus leucovorin in patients with colorectal cancer [42]. Among ten patients, only one patient experienced Grade 3 pancytopenia at 10 months after the beginning of uracil-tegafur, while nine patients had only Grade 1 toxicities. These findings suggest that oral uracil-tegafur has less negative impact than platinum-based systemin chemotherapy, which has been reported to have severe side effects such as nephrotoxicity, hematological toxicity, gastrointestinal toxicity, and neurotoxicity [43]. Therefore, we believe that the total negative impact of adjuvant therapies on the P-QOL of the patients was relatively mild. Fourth, we evaluated only four physical domains of the eight subscales of SF-36. During SF-36 validity testing, PF and RP had the most pure physical health interpretation [44]. Moreover, during SF-36 reliability testing, all eight subscales showed high intrinsic consistency [12]. From these results, we believe the method to evaluate P-QOL used in this study is appropriate.
Despite these limitations, our results are clinically meaningful, because the evaluation of postoperative status was performed by the patients themselves, and the identified independent predictors of worse postoperative P-QOL can be confirmed preoperatively. Therefore, preoperative use of the SF-36 can potentially identify patient factors that increase the likelihood of lower postoperative P-QOL.
5. Conclusions
Living alone and lower PS (≥1) were independently associated with significantly worse P-QOL in preoperative settings, while later smoking cessation, PS ≥1, living alone, and higher comorbid burden (CCI ≥ 3) were independently associated with significantly worse P-QOL at 6 months postoperatively. Even after 1 year and with the use of less invasive procedures in the majority of cases, patients with lung cancer experienced a reduced P-QOL in the postoperative period, which might be due to the effects of surgery and other adjuvant therapies. Further studies are warranted to differentiate the effect of surgery from that of adjuvant therapies to P-QOL. To maintain the P-QOL and provide more fruitful medical treatment of this population, attention should be paid to their physical condition, lifestyle, smoking, and comorbid status and necessary measures should be taken in accordance with the situation during perioperative surgical management.
Author Contributions
R.F. conceived and performed the study, and wrote the manuscript; H.S., M.H. (Makoto Hibino), S.H. and T.K. examined the patients; R.F. and T.N. performed the surgeries; S.T. performed pathological diagnoses; K.A. and E.S. collected the questionnaires and inputted the data; M.H. (Masahiro Hirata) performed statistical analysis; Y.S. (Yuichi Saito) confirmed the data; and Y.S. (Yukinori Sakao) supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Shonan Kamakura General Hospital Institutional Review Board (SKEC-17-A1, 19 March 2015).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
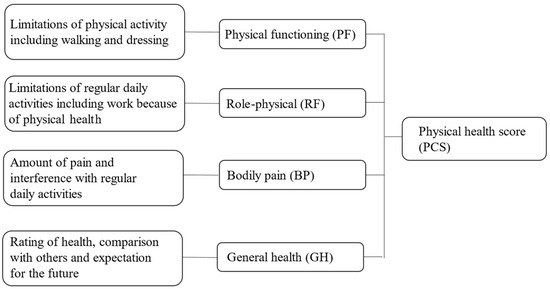
Figure A1.
Short Form 36 Health Survey (SF-36) instrument in elements of physical health care.
References
- Gajra, A.; Jatoi, A. Non-small-cell lung cancer in elderly patients: A discussion of treatment options. J. Clin. Oncol. 2014, 32, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Bade, B.C.; Dela Cruz, C.S.D. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery; Minatoya, K.; Sato, Y.; Toh, Y.; Abe, T.; Endo, S.; Hirata, Y.; Ishida, M.; Iwata, H.; Kamei, T.; et al. Thoracic and cardiovascular surgeries in Japan during 2019: Annual report by the Japanese Association for Thoracic Surgery. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 595–628. [Google Scholar] [PubMed]
- Booton, R.; Jones, M.; Thatcher, N. Lung cancer 7: Management of lung cancer in elderly patients. Thorax 2003, 58, 711–720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, N.; Andrew, N.E.; Cadilhac, D.A.; Yu, X.; Li, Z.; Wang, J.; Liang, Y. Health-related quality of life among elderly individuals living alone in an urban area of Shaanxi Province, China: A cross-sectional study. J. Int. Med. Res. 2020, 48, 300060520913146. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, K. Going nuclear? Family structure and young women’s health in India, 1992–2006. Demography 2013, 50, 853–880. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, E.C.; Kim, S.J.; Lee, S.G. Quality of life of family members living with cancer patients. Asian Pac. J. Cancer Prev. 2015, 16, 6913–6917. [Google Scholar] [CrossRef] [PubMed]
- Poghosyan, H.; Sheldon, L.K.; Leveille, S.G.; Cooley, M.E. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: A systematic review. Lung Cancer 2013, 81, 11–26. [Google Scholar] [CrossRef]
- Movsas, B.; Moughan, J.; Sarna, L.; Langer, C.; Werner-Wasik, M.; Nicolaou, N.; Komaki, R.; Machtay, M.; Wasserman, T.; Bruner, D.W. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: An analysis of RTOG 9801. J. Clin. Oncol. 2009, 27, 5816–5822. [Google Scholar] [CrossRef] [PubMed]
- Fukai, R.; Nishida, T.; Igarashi, Y.; Murata, T.; Sunoh, Y.; Miyake, K.; Isogai, N.; Shimoyama, R.; Kawachi, J.; Kashiwagi, H. Perioperative progress and predictor of the postoperative mental quality of life for lung cancer patients. Br. J. Cancer Res. 2021, 4, 485–490. [Google Scholar]
- Fukuhara, S.; Bito, S.; Green, J.; Hsiao, A.; Kurokawa, K. Translation, adaptation, and validation of the SF-36 health survey for use in Japan. J. Clin. Epidemiol. 1998, 51, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- McHorney, C.A.; Ware, J.E., Jr.; Lu, J.F.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care 1994, 32, 40–66. [Google Scholar] [CrossRef] [PubMed]
- Möller, A.; Sartipy, U. Predictors of postoperative quality of life after surgery for lung cancer. J. Thorac. Oncol. 2012, 7, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Myrdal, G.; Valtysdottir, S.; Lambe, M.; Ståhle, E. Quality of life following lung cancer surgery. Thorax 2003, 58, 194–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarna, L.; Cooley, M.E.; Brown, J.K.; Chernecky, C.; Padilla, G.; Danao, L.; Chakravarty, D.; Elashoff, D. Women with lung cancer: Quality of life after thoracotomy: A 6-month prospective study. Cancer Nurs. 2010, 33, 85–92. [Google Scholar] [CrossRef]
- Ostroff, J.S.; Krebs, P.; Coups, E.J.; Burkhalter, J.E.; Feinstein, M.B.; Steingart, R.M.; Logue, A.E.; Park, B.J. Health-related quality of life among early-stage, non-small cell, lung cancer survivors. Lung Cancer 2011, 71, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Birim, O.; Maat, A.P.W.M.; Kappetein, A.P.; van Meerbeeck, J.P.; Damhuis, R.A.M.; Bogers, A.J.J.C. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2003, 23, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Win, T.; Sharples, L.; Wells, F.C.; Ritchie, A.J.; Munday, H.; Laroche, C.M. Effect of lung cancer surgery on quality of life. Thorax 2005, 60, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Balduyck, B.; Hendriks, J.; Lauwers, P.; Sardari Nia, P.; Van Schil, P. Quality of life evolution after lung cancer surgery in septuagenarians: A prospective study. Eur. J. Cardiothorac. Surg. 2009, 35, 1070–1075, discussion 1075. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Socci, L.; Refai, M.; Salati, M.; Xiumé, F.; Sabbatini, A. Quality of life before and after major lung resection for lung cancer: A prospective follow-up analysis. Ann. Thorac. Surg. 2007, 84, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Sok, M.; Zavrl, M.; Greif, B.; Srpčič, M. Objective assessment of WHO/ECOG performance status. Support. Care Cancer 2019, 27, 3793–3798. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Hanaoka, J.; Oshio, Y.; Hashimoto, M.; Igarashi, T.; Kataoka, Y.; Kaku, R.; Namura, Y.; Akazawa, A. Decrease in performance status after lobectomy mean poor prognosis in elderly lung cancer patients. J. Thorac. Dis. 2017, 9, 1525–1530. [Google Scholar] [CrossRef]
- Friedlaender, A.; Liu, S.V.; Passaro, A.; Metro, G.; Banna, G.; Addeo, A. The role of performance status in small-cell lung cancer in the era of immune checkpoint inhibitors. Clin. Lung Cancer 2020, 21, e539–e543. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qiu, T.; Pei, L.; Zhang, Y.; Xu, L.; Cui, Y.; Liang, N.; Li, S.; Chen, W.; Huang, Y. Two-Week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: A randomized controlled trial. Anesth. Analg. 2020, 131, 840–849. [Google Scholar] [CrossRef]
- Ahn, Y.E.; Koh, C.K. Effects of living alone and sedentary behavior on quality of life in patients with multimorbidities: A secondary analysis of cross-sectional survey data obtained from the national community database. J. Nurs. Res. 2021, 29, e173. [Google Scholar] [CrossRef]
- Lee, S.; Ma, C.; Zhang, S.; Ou, F.S.; Bainter, T.M.; Niedzwiecki, D.; Saltz, L.B.; Mayer, R.J.; Whittom, R.; Hantel, A.; et al. Marital status, living arrangement, and cancer recurrence and survival in patients with stage Ⅲ colon cancer: Findings from CALBG 89803 (alliance). Oncologist 2022, 27, e494–e505. [Google Scholar] [CrossRef]
- Cavalli-Björkman, N.; Qvortrup, C.; Sebjørnsen, S.; Pfeiffer, P.; Wentzel-Larsen, T.; Glimelius, B.; Sorbye, H. Lower treatment intensity and poorer survival in metastatic colorectal cancer patients who live alone. Br. J. Cancer 2012, 107, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Dalton, S.O.; Schüz, J.; Engholm, G.; Johansen, C.; Kjaer, S.K.; Steding-Jessen, M.; Storm, H.H.; Olsen, J.H. Social inequality in incidence of and survival from cancer in a population-based study in Denmark, 1994–2003: Summary of findings. Eur. J. Cancer 2008, 44, 2074–2085. [Google Scholar] [CrossRef]
- Cheng, L.; Sit, J.W.H.; Chan, H.Y.L.; Choi, K.C.; Cheung, R.K.Y.; Wong, M.M.H.; Li, F.Y.K.; Lee, T.Y.; Fung, E.S.M.; Tai, K.M.; et al. Sarcopenia risk and associated factors among Chinese community-dwelling older adults living alone. Sci. Rep. 2021, 11, 22219. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, M.; Danovitch, I.; IsHak, W.W. Quality of life and smoking. Am. J. Addict. 2014, 23, 540–562. [Google Scholar] [CrossRef] [PubMed]
- Efendi, V.; Özalevli, S.; Naz, İ.; Kilinç, O. The effects of smoking on body composition, pulmonary function, physical activity and health-related quality of life among healthy women. Tuberk. Toraks 2018, 66, 101–108. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.; Munden, R.F. Lung cancer epidemiology, risk factors, and prevention. Radiol. Clin. N. Am. 2012, 50, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Loeb, L.A.; Ernster, V.L.; Warner, K.E.; Abbotts, J.; Laszlo, J. Smoking and lung cancer: An overview. Cancer Res. 1984, 44, 5940–5958. [Google Scholar] [PubMed]
- Hays, J.T.; Croghan, I.T.; Baker, C.L.; Cappelleri, J.C.; Bushmakin, A.G. Changes in health-related quality of life with smoking cessation treatment. Eur. J. Public Health 2012, 22, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.J.P.; Rasburn, N.J.; Abdelnour-Berchtold, E.; Brunelli, A.; Cerfolio, R.J.; Gonzalez, M.; Ljungqvist, O.; Petersen, R.H.; Popescu, W.M.; Slinger, P.D.; et al. Guidelines for enhanced recovery after lung surgery: Recommendations of the enhanced recovery after surgery (ERAS®) society and the European society of thoracic surgeons (ESTS). Eur. J. Cardiothorac. Surg. 2019, 55, 91–115. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chron. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Asmis, T.R.; Ding, K.; Seymour, L.; Shepherd, F.A.; Leighl, N.B.; Winton, T.L.; Whitehead, M.; Spaans, J.N.; Graham, B.C.; Goss, G.D. Age and comorbidity as independent prognostic factors in the treatment of non-small cell lung cancer: A review of National Cancer Institute of Canada Clinical Trials Group trials. J. Clin. Oncol. 2008, 26, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, T.; Levitt, S.; Kowalski, J.; Nilsson, S.; Brandberg, Y. Use of the Charlson combined comorbidity index to predict postradiotherapy quality of life for prostate cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Jacot, W.; Colinet, B.; Bertrand, D.; Lacombe, S.; Bozonnat, M.C.; Daurès, J.P.; Pujol, J.L.; OncoLR Health Network. Quality of life and comorbidity score as prognostic determinants in non-small-cell lung cancer patients. Ann. Oncol. 2008, 19, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, A.; Nakao, K.; Watanabe, M.; Matsui, N.; Tsunoda, Y. Health-related quality of life in patients with colorectal cancer who receive oral uracil and tegafur plus leucovorin. Jpn. J. Clin. Oncol. 2010, 40, 412–419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- McHorney, C.A.; Ware, J.E., Jr.; Raczek, A.E. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 1993, 31, 247–263. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).