Efficacy and Safety of Ripretinib in Advanced Gastrointestinal Stromal Tumors within an Expanded Access Program: A Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Participants
2.2. Variables
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Selection, Baseline Characteristics and Disposition
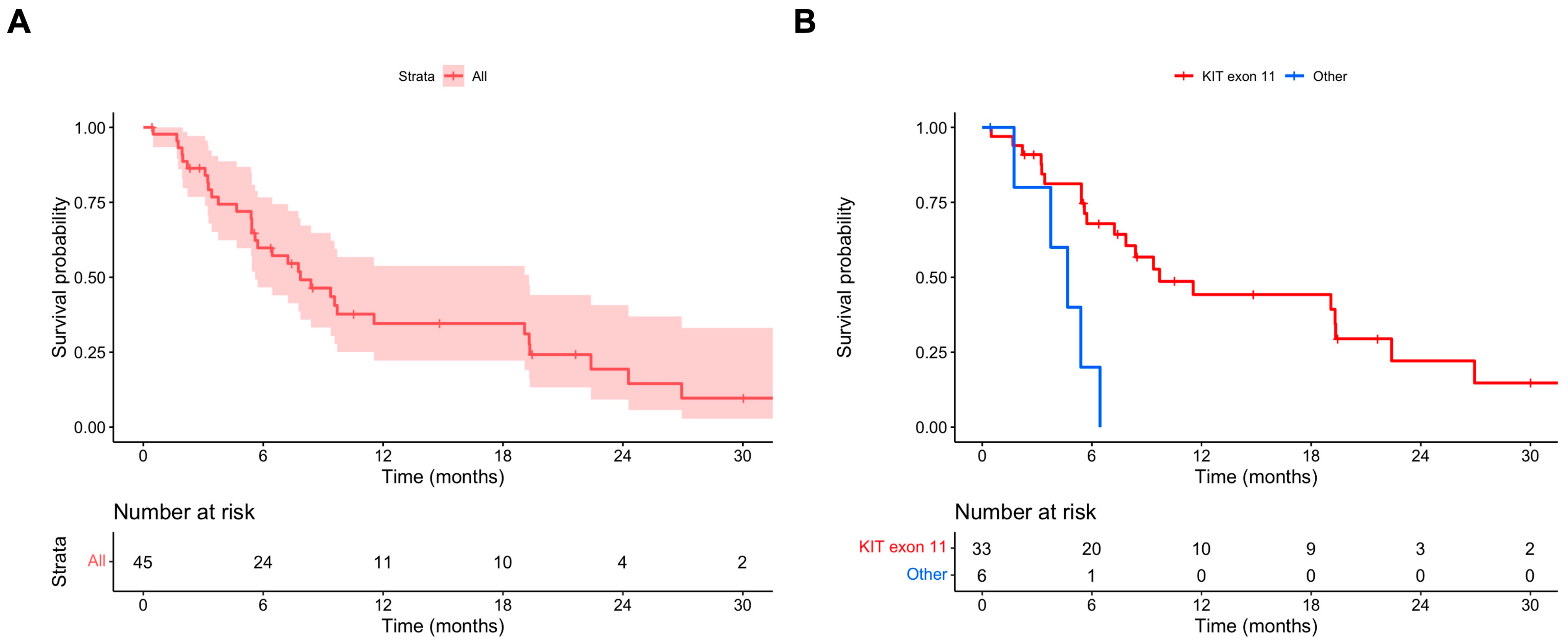
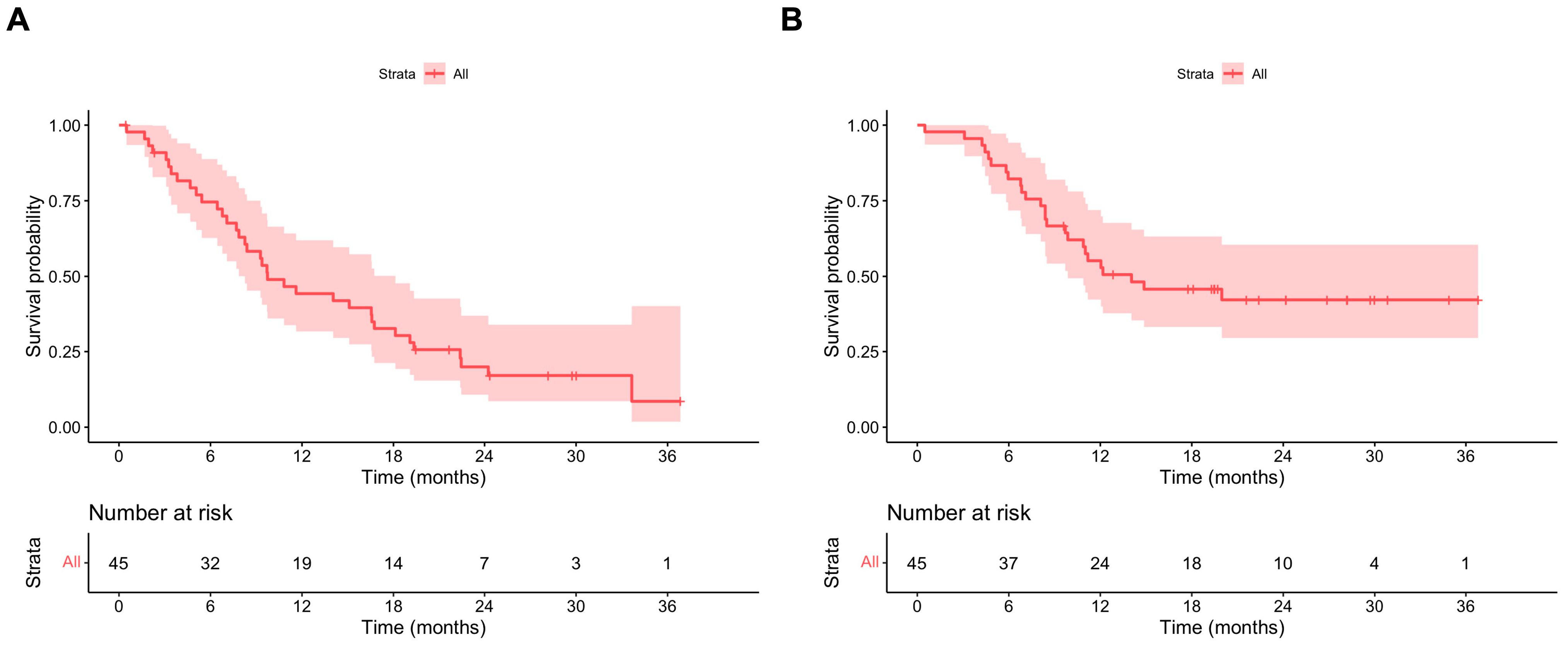
3.2. Outcomes: Ripretinib OD
3.3. Outcomes: Ripretinib BD
3.4. Outcomes: Ripretinib ITT and OS
3.5. Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blay, J.Y.; Kang, Y.K.; Nishida, T.; von Mehren, M. Gastrointestinal stromal tumours. Nat. Rev. Dis. Primers 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Zalcberg, J.; Le Cesne, A.; Reichardt, P.; Blay, J.Y.; Lindner, L.H.; Judson, I.R.; Schoffski, P.; Leyvraz, S.; Italiano, A.; et al. Ten-Year Progression-Free and Overall Survival in Patients with Unresectable or Metastatic GI Stromal Tumors: Long-Term Analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup Phase III Randomized Trial on Imatinib at Two Dose Levels. J. Clin. Oncol. 2017, 35, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Blay, J.Y.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Goggin, C.; Stansfeld, A.; Mahalingam, P.; Thway, K.; Smith, M.J.; Huang, P.; Jones, R.L.; Napolitano, A. Ripretinib in advanced gastrointestinal stromal tumors: An overview of current evidence and drug approval. Future Oncol. 2022, 18, 2967–2978. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schoffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Jones, R.L.; Blay, J.Y.; Gelderblom, H.; George, S.; Schoffski, P.; von Mehren, M.; Zalcberg, J.R.; Kang, Y.K.; Razak, A.A.; et al. Ripretinib Versus Sunitinib in Patients with Advanced Gastrointestinal Stromal Tumor after Treatment with Imatinib (INTRIGUE): A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2022, 40, 3918–3928. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. FDA Approves Ripretinib for Advanced Gastrointestinal Stromal Tumor. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ripretinib-advanced-gastrointestinal-stromal-tumor (accessed on 8 January 2023).
- European Medicines Agency. Market Authorisation Qinlock; European Medicines Agency: Amsterdam, The Netherlands, 2021.
- R Development Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021.
- Serrano, C.; Rothschild, S.; Villacampa, G.; Heinrich, M.C.; George, S.; Blay, J.Y.; Sicklick, J.K.; Schwartz, G.K.; Rastogi, S.; Jones, R.L.; et al. Rethinking placebos: Embracing synthetic control arms in clinical trials for rare tumors. Nat. Med. 2023, 29, 2689–2692. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Ryu, M.H.; Yoo, C.; Ryoo, B.Y.; Kim, H.J.; Lee, J.J.; Nam, B.H.; Ramaiya, N.; Jagannathan, J.; Demetri, G.D. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): A randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, B.; Nannini, M.; Badalamenti, G.; Grignani, G.; Fumagalli, E.; Gasperoni, S.; D’Ambrosio, L.; Incorvaia, L.; Stellato, M.; Spalato Ceruso, M.; et al. Imatinib rechallenge in patients with advanced gastrointestinal stromal tumors following progression with imatinib, sunitinib and regorafenib. Ther. Adv. Med. Oncol. 2018, 10, 1758835918794623. [Google Scholar] [CrossRef]
- George, S.; Chi, P.; Heinrich, M.C.; von Mehren, M.; Jones, R.L.; Ganjoo, K.; Trent, J.; Gelderblom, H.; Razak, A.A.; Gordon, M.S.; et al. Ripretinib intrapatient dose escalation after disease progression provides clinically meaningful outcomes in advanced gastrointestinal stromal tumour. Eur. J. Cancer 2021, 155, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Zalcberg, J.R.; Heinrich, M.C.; George, S.; Bauer, S.; Schoffski, P.; Serrano, C.; Gelderblom, H.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Clinical Benefit of Ripretinib Dose Escalation after Disease Progression in Advanced Gastrointestinal Stromal Tumor: An Analysis of the INVICTUS Study. Oncologist 2021, 26, e2053–e2060. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.S.; Choi, H.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Charnsangavej, C. We should desist using RECIST, at least in GIST. J. Clin. Oncol. 2007, 25, 1760–1764. [Google Scholar] [CrossRef]
- Bauer, S.; Heinrich, M.C.; George, S.; Zalcberg, J.R.; Serrano, C.; Gelderblom, H.; Jones, R.L.; Attia, S.; D’Amato, G.; Chi, P.; et al. Clinical Activity of Ripretinib in Patients with Advanced Gastrointestinal Stromal Tumor Harboring Heterogeneous KIT/PDGFRA Mutations in the Phase III INVICTUS Study. Clin. Cancer Res. 2021, 27, 6333–6342. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Jones, R.L.; George, S.; Gelderblom, H.; Schöffski, P.; Mehren, M.V.; Zalcberg, J.R.; Kang, Y.-K.; Razak, A.R.A.; Trent, J.C.; et al. Ripretinib versus sunitinib in gastrointestinal stromal tumor: ctDNA biomarker analysis of the phase 3 INTRIGUE trial. Nat. Med. 2024, 30, 498–506. [Google Scholar] [CrossRef]
- Schoffski, P.; George, S.; Heinrich, M.C.; Zalcberg, J.R.; Bauer, S.; Gelderblom, H.; Serrano, C.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Patient-reported outcomes in individuals with advanced gastrointestinal stromal tumor treated with ripretinib in the fourth-line setting: Analysis from the phase 3 INVICTUS trial. BMC Cancer 2022, 22, 1302. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Jones, R.L.; Blay, J.Y.; George, S.; von Mehren, M.; Zalcberg, J.R.; Kang, Y.K.; Razak, A.A.; Trent, J.; Attia, S.; et al. Patient-reported outcomes and tolerability in patients receiving ripretinib versus sunitinib after treatment with imatinib in INTRIGUE, a phase 3, open-label study. Eur. J. Cancer 2023, 192, 113245. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Huang, W.K.; Yen, C.C.; Yang, C.Y.; Sung, M.T.; Wong, N.S.; Chua, D.T.T.; Lee, S.W.M.; Chen, J.S.; Yeh, C.N. Compassionate Use of Ripretinib for Patients with Metastatic Gastrointestinal Stromal Tumors: Taiwan and Hong Kong Experience. Front. Oncol. 2022, 12, 883399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, P.; Qiu, H.; Fang, Y.; Liu, H.; Zhou, Y.; Xu, H.; Yu, J.; Zhang, J.; Wang, M.; et al. Large-Scale, Multicenter, Prospective Registry Study of Ripretinib in Advanced GIST: A Real-World Study from China. Adv. Ther. 2023, 40, 3817–3829. [Google Scholar] [CrossRef] [PubMed]
| Total Number of Patients | 45 | 100% |
|---|---|---|
| Sex | ||
| Female | 19 | 42.2% |
| Male | 26 | 57.8% |
| Age | ||
| Median, Q1–Q3 | 62 | 57–72 |
| Primary mutational status | ||
| KIT exon 11 | 33 | 73.3% |
| KIT exon 9 | 3 | 6.7% |
| PDGFRA | 3 | 6.7% |
| N/A | 6 | 13.3% |
| Primary tumor site | ||
| Stomach | 13 | 28.9% |
| Small bowel | 23 | 51.1% |
| Other | 9 | 20.0% |
| Stage | ||
| Locally advanced | 1 | 2.2% |
| Metastatic | 44 | 97.8% |
| 1 site | 15 | 34.1% |
| 2 sites | 18 | 40.9% |
| 3 or more sites | 11 | 25.0% |
| ECOG | ||
| 0 | 1 | 2.2% |
| 1 | 42 | 93.3% |
| 2 | 2 | 4.4% |
| Number of previous lines | ||
| 2 | 19 | 42.2% |
| 3 or more | 26 | 57.8% |
| Previous lines | ||
| Imatinib | 45 | 100% |
| Sunitinib | 43 | 95.6% |
| Regorafenib | 24 | 53.3% |
| Avapritinib | 13 | 28.9% |
| Variable | Univariate HR (95% CI) | p Value | Multivariate HR (95% CI) | p Value |
|---|---|---|---|---|
| Sex (male vs. female) | 1.54 (0.75–3.15) | 0.238 | NI 1 | |
| Age | 1.00 (0.97–1.03) | 0.923 | NI | |
| Primary mutation (others vs. KIT exon 11) | 4.98 (1.65–15.03) | 0.004 * | 4.67 (1.53–14.29) | 0.007 * |
| Primary tumor site | NI | |||
| Small bowel vs. gastric | 0.96 (0.41–2.22) | 0.918 | ||
| Others vs. gastric | 0.98 (0.36–2.65) | 0.862 | ||
| Number of metastatic sites | ||||
| 2 vs. 0/1 | 1.79 (0.76–4.23) | 0.185 | 1.59 (0.61–4.14) | 0.342 |
| 3 or more vs. 0/1 | 2.28 (0.86–6.02) | 0.098 | 2.19 (0.72–6.71) | 0.170 |
| Number of previous lines (3 or more vs. 2) | 0.88 (0.43–1.77) | 0.711 | NI | |
| Previous regorafenib | 1.09 (0.53–2.21) | 0.919 | NI | |
| Previous avapritinib | 1.27 (0.59–2.77) | 0.578 | NI |
| Variable | Univariate HR (95% CI) | p Value | Multivariate HR (95% CI) | p Value |
|---|---|---|---|---|
| Sex (male vs. female) | 1.46 (0.75–2.84) | 0.263 | NI 1 | |
| Age | 1.01 (0.98–1.04) | 0.397 | NI | |
| Primary mutation (others vs. KIT exon 11) | 2.75 (1.00–7.58) | 0.051 | 3.06 (1.08–8.67) | 0.036 * |
| Primary tumor site | NI | |||
| Small bowel vs. gastric | 1.20 (0.55–2.62) | 0.641 | ||
| Others vs. gastric | 0.78 (0.29–2.08) | 0.616 | ||
| Number of metastatic sites | ||||
| 2 vs. 0/1 | 2.01 (0.88–4.57) | 0.096 | 1.94 (0.78–4.78) | 0.153 |
| 3 or more vs. 0/1 | 2.41 (0.97–6.02) | 0.059 | 3.00 (1.05–8.57) | 0.040 * |
| Number of previous lines (3 or more vs. 2) | 0.85 (0.44–1.67) | 0.645 | NI | |
| Previous regorafenib | 0.85 (0.44–1.64) | 0.623 | NI | |
| Previous avapritinib | 1.15 (0.54–2.47) | 0.714 | NI |
| Ripretinib OD (N = 45) | Ripretinib BD (N = 23) | |||
|---|---|---|---|---|
| Adverse Event | Any G (N, %) | G3+ (N, %) | Any G (N, %) | G3+ (N, %) |
| Fatigue | 35 (77.8%) | 0 (0.0%) | 18 (78.3%) | 0 (0.0%) |
| Alopecia | 21 (46.7%) | 0 (0.0%) | 9 (39.1%) | 0 (0.0%) |
| Muscle cramp | 20 (44.4%) | 0 (0.0%) | 4 (17.4%) | 0 (0.0%) |
| PPE | 17 (37.8%) | 1 (2.2%) | 9 (39.1%) | 0 (0.0%) |
| Constipation | 17 (37.8%) | 1 (2.2%) | 8 (34.8%) | 0 (0.0%) |
| Arthralgia/myalgia | 15 (33.3%) | 1 (2.2%) | 6 (26.1%) | 0 (0.0%) |
| Weight loss | 14 (31.1%) | 0 (0.0%) | 13 (56.5%) | 0 (0.0%) |
| Diarrhea | 13 (28.9%) | 2 (4.4%) | 15 (65.2%) | 1 (4.4%) |
| Anorexia | 10 (22.2%) | 0 (0.0%) | 8 (34.8%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.Y.; Ferro-López, L.; Barquin, E.; Lindsay, D.; Thway, K.; Smith, M.J.; Benson, C.; Jones, R.L.; Napolitano, A. Efficacy and Safety of Ripretinib in Advanced Gastrointestinal Stromal Tumors within an Expanded Access Program: A Cohort Study. Cancers 2024, 16, 985. https://doi.org/10.3390/cancers16050985
Lim SY, Ferro-López L, Barquin E, Lindsay D, Thway K, Smith MJ, Benson C, Jones RL, Napolitano A. Efficacy and Safety of Ripretinib in Advanced Gastrointestinal Stromal Tumors within an Expanded Access Program: A Cohort Study. Cancers. 2024; 16(5):985. https://doi.org/10.3390/cancers16050985
Chicago/Turabian StyleLim, Su Yin, Laura Ferro-López, Elizabeth Barquin, Daniel Lindsay, Khin Thway, Myles J. Smith, Charlotte Benson, Robin L. Jones, and Andrea Napolitano. 2024. "Efficacy and Safety of Ripretinib in Advanced Gastrointestinal Stromal Tumors within an Expanded Access Program: A Cohort Study" Cancers 16, no. 5: 985. https://doi.org/10.3390/cancers16050985
APA StyleLim, S. Y., Ferro-López, L., Barquin, E., Lindsay, D., Thway, K., Smith, M. J., Benson, C., Jones, R. L., & Napolitano, A. (2024). Efficacy and Safety of Ripretinib in Advanced Gastrointestinal Stromal Tumors within an Expanded Access Program: A Cohort Study. Cancers, 16(5), 985. https://doi.org/10.3390/cancers16050985








