Simple Summary
This study examined the frequency and impact of body weight loss during combination therapy with immune checkpoint inhibitors (ICIs) and chemotherapy for advanced non-small cell lung cancer (NSCLC). Among 370 patients treated at 13 institutions, 38.1% experienced weight loss exceeding 5% (WL group) during therapy. A 2-month landmark analysis revealed that the WL group had significantly worse overall survival (OS) and progression-free survival (PFS) compared to those without substantial weight loss (OS: 14.0 vs. 31.1 months, p < 0.001; PFS: 6.8 vs. 10.9 months, p = 0.002). This negative impact of weight loss was observed even in patients with normal or high body weight at baseline. The findings indicate that weight loss > 5% during ICI and chemotherapy adversely affects treatment outcomes, underscoring the importance of monitoring and managing weight changes in patients with cancer undergoing this therapy.
Abstract
Background: Limited data are available on the frequency and significance of body weight loss during cancer therapy. This study investigated the frequency of patients who experienced body weight loss during immune checkpoint inhibitor (ICI) plus chemotherapy for advanced non-small cell lung cancer (NSCLC) and the impact of weight loss on treatment outcomes. Methods: Using the clinical data of 370 patients with NSCLC who received a combination of ICI and chemotherapy at 13 institutions, this study investigated the frequency of body weight loss > 5% during treatment and determined the impact of body weight loss on patient outcomes. Results: Of the 370 included patients, 141 (38.1%) lost more than 5% of their body weight during ICI plus chemotherapy (WL group). The 2-month landmark analysis showed that patients who experienced body weight loss of >5% during treatment had worse overall survival (OS) and progression-free survival (PFS) than those who did not (OS 14.0 and 31.1 months in the WL non-WL groups, respectively, p < 0.001; PFS 6.8 and 10.9 months in the WL non-WL groups, respectively, p = 0.002). Furthermore, a negative impact of body weight loss on survival was observed even in those who had obesity (body mass index [BMI] ≥ 25.0) at the start of therapy (OS 12.8 and 25.4 months in the WL non-WL groups, respectively, p < 0.001; PFS 5.7 and 10.7 months in the WL non-WL groups, respectively, p = 0.038). Conclusions: In conclusion, weight loss of >5% during ICI plus chemotherapy negatively influenced patient outcomes. Further and broader studies should investigate the role of nutritional status, specifically weight change and nutritional support, in responsiveness to ICI plus chemotherapy.
1. Introduction
The development of immune checkpoint inhibitors (ICIs) has revolutionized cancer treatment, offering improved survival outcomes for various malignancies, including non-small cell lung cancer (NSCLC) [1]. Anti-programmed death receptor-1 (PD-1) and anti-programmed death ligand-1 (PD-L1) inhibitors, used alone or in combination with chemotherapy, are now the standard treatments for advanced NSCLC [2,3,4,5,6]. Despite these advancements, some patients do not benefit from ICIs owing to host factors. Cancer cachexia and sarcopenia, characterized by significant weight and muscle loss, are among the negative factors affecting the efficacy of ICI therapy and remain major factors that negatively influence patient outcomes, likely owing to their immunosuppressive effects. For example, many studies have shown that patients with NSCLC who experience weight loss prior to ICI therapy are at a higher risk of tumor progression and have worse overall survival (OS) than those without weight loss [7,8]. The effects of weight loss on cancer treatment are a critical clinical concern because they reflect the underlying metabolic dysregulation and can lead to reduced treatment efficacy. A recent study reported that weight loss at the initiation of pembrolizumab treatment in patients with advanced NSCLC was associated with increased catabolic activity, higher pembrolizumab clearance, and significantly shorter OS [7]. These findings highlighted the complex interplay between metabolic changes and drug metabolism during ICI therapy. Moreover, cancer-related cachexia and sarcopenia are closely linked to systemic inflammation and immune suppression, both of which may interfere with the efficacy of ICIs and chemotherapy [8,9,10].
Considerable attention has been paid to the prognostic impact of weight loss before treatment as a negative indicator of the efficacy of ICI therapy. However, there is limited understanding of the frequency and clinical significance of weight loss during ICI therapy [11]. Studies have demonstrated that weight loss during cancer treatment is common and can significantly worsen treatment outcomes [12]. However, most of these studies were conducted after the era of ICI therapy and focused on conventional chemotherapy or single-agent ICIs, leaving a gap in knowledge regarding body weight loss during ICI combination therapies.
This study addressed these gaps by investigating the frequency and clinical significance of body weight loss during combination therapy with ICIs and chemotherapy in patients with advanced NSCLC. By focusing on weight loss during treatment, this study aimed to provide insight into its potential as a prognostic indicator and its relationship with treatment outcomes, including OS and progression-free survival (PFS). The findings of this study may contribute to improving patient management strategies by highlighting the importance of monitoring and mitigating weight loss during therapy. Furthermore, this study adds to the growing body of evidence emphasizing the metabolic and immunological complexities associated with advanced cancer and its treatment.
2. Materials and Methods
2.1. Patients
This was a branch study analyzing data from the Okayama Lung Cancer Study Group-Immune Checkpoint Database, which contains the clinical data of consecutive patients with NSCLC who started first-line systemic therapy (except for molecular-targeted therapy) for advanced NSCLC at 13 institutions in Japan from December 2018 to December 2020 [13,14].
2.2. Outcome and Exposure
The main outcome measures in the analysis were OS and PFS. OS was defined as the period from the initiation of ICI therapy to death, whereas PFS was defined as the period from the initiation of ICI therapy to disease progression or death. A landmark analysis was performed to avoid immortal time bias, and the landmark time was set at 2 months because, in practice, the evaluation of the treatment effect is performed at 2 months. In the landmark analysis, patients with worsening or death events in the first 2 months were excluded. Significant weight loss was defined as >5%, based on the definition of cachexia [15]. Patients were defined as having weight loss if they lost ≥5% of their body weight according to their weight at the initiation of therapy.
2.3. Statistical Analysis
The Cox proportional hazards model was used to analyze possible factors affecting patient survival. The model variables included >5% weight loss during therapy, baseline body mass index (BMI), age, sex, performance status (PS), histology, and PD-L1 expression. In the main analysis, the overall population was divided into two groups: patients with >5% weight loss compared to body weight at the initiation of therapy during ICI plus chemotherapy (WL group) and those without >5% weight loss (non-WL group). Parametric data were compared between the two groups using the Student’s t-test, and Fisher’s exact test was used to compare nonparametric data. The significance level was set at p < 0.05. Analyses were conducted using Stata statistical software (version 18; Stata Corp LLC, College Station, TX, USA).
3. Results
3.1. More Than One-Third of the Patients Significantly Lost Weight During Immunochemotherapy
In total, 370 patients with available body weight data during ICI treatment plus chemotherapy were included in this study. Of the 370 patients, 141 (38.1%) lost >5% of their weight during ICI plus chemotherapy, excluding body weight loss due to tumors.
Progression: this study also investigated the proportion of patients who lost weight only among those with the best response to therapy: complete response (CR) or partial response (PR). Overall, 212 patients achieved CR or PR in response to ICI plus chemotherapy. Among the 212 patients responsive to ICI plus chemotherapy, 83 (39.2%) lost >5% of their weight during treatment. The proportion of patients with weight loss was similar for all patients and those who achieved CR or PR with ICI plus chemotherapy.
The characteristics of the WL and non-WL groups are presented in Table 1. Pre-treatment BMI was not significantly different between the groups (median BMI 22.8 kg/m2 in the WL group and 22.1 kg/m2 in the non-WL group, p = 0.168). The median age was significantly higher in the WL group (71 years) than in the non-WL group (69 years) (p = 0.014). No other differences were observed between the groups.

Table 1.
Patient characteristics of the WL group and non-WL group.
3.2. Body Weight Loss During Treatment Negatively Affected Outcomes of ICI Plus Chemotherapy
The effects of body weight loss during treatment on patient outcomes were then determined. There were no significant differences in responses between the groups (WL vs. non-WL: disease control rate: 90.7% vs. 90.8%, p = 1.000; objective response rate: 58.7% vs. 56.3%, p = 0.666) (Table 2). The effect of body weight loss on survival outcomes was also assessed (Figure 1). Patients in the WL group had significantly worse PFS and OS than those in the non-WL group. The OS was 14.0 months in the WL group (95% confidence interval [CI]: 11.7–23.1) and 31.1 months in the non-WL group (95% CI: 22.8–not reached; hazard ratio [HR]: 2.18, 95% CI: 1.53–3.10, p < 0.001) (Figure 1A). The PFS was 6.8 months in the WL group (95% CI: 5.7–8.8) and 10.9 months in the non-WL group (95% CI: 9.0–12.8; HR: 1.56, 95% CI: 1.17–2.09, p = 0.003) (Figure 1B). The multivariable Cox regression analysis, including weight loss of >5% during the therapy, pre-treatment BMI, age, sex, PS, histology, and PD-L1 expression, showed that weight loss of >5% during the therapy was an independent poor factor for OS (HR 2.24, 95% CI: 1.51–3.36, p < 0.001) and PFS (HR 1.69, 95% CI: 1.20–2.36, p = 0.002) (Table 3).

Table 2.
Response outcomes to ICI plus chemotherapy in the WL and non-WL groups.
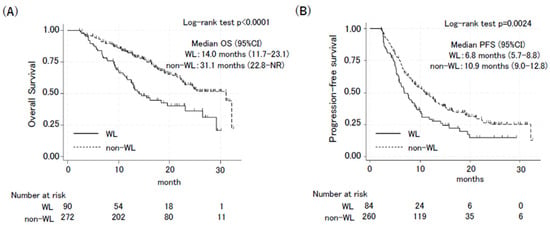
Figure 1.
Kaplan–Meier curves of overall survival (A) and progression-free survival (B) in the WL group and non-WL group. OS and PFS between the WL group and non-WL group were compared using a 2-month landmark analysis. Confidence interval (CI).

Table 3.
Multivariate analysis of the factor associated with progression-free survival (PFS) and overall survival (OS).
3.3. Poor Prognosis Due to Weight Loss Was Also Observed in Patients with Standard Weight and Obesity
Finally, this study investigated whether the impact of body weight loss during therapy differed according to pre-treatment body weight status. The patients were then classified into low, standard, and high BMI groups using the indicators in Japan (low BMI: <18.5; standard BMI: ≥18.5 and <25; high BMI: ≥25.0) [15]. Among the patients with a low BMI, there was no difference in OS or PFS between the WL and non-WL groups. The OS was 15.5 months in the WL group [(95% CI: 6.7–not reached (NR)] and 17.1 months in the non-WL group (95% CI: 10.7–NR; HR: 0.68, 95% CI: 0.51–2.84, p = 0.679) (Figure 2A). The PFS was 7.6 months in the WL group (95% CI: 4.7–NR) and 5.9 months in the non-WL group (95% CI: 4.7–8.4; HR: 0.33, 95% CI: 0.32–1.47, p = 0.326) (Figure 2B).
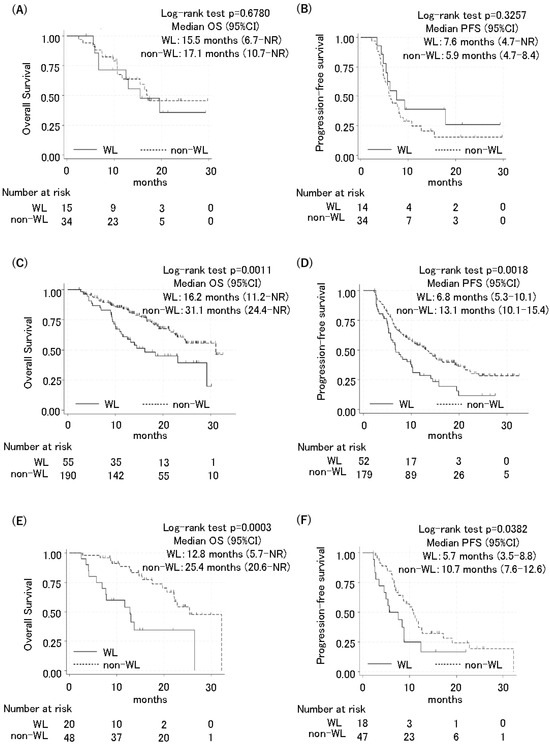
Figure 2.
Kaplan–Meier curves of progression-free survival and overall survival for patients with low BMI, standard BMI, and high BMI. Overall survival and progression-free survival between the WL group and non-WL group were compared using a 2-month landmark analysis in patients with low BMI (<18.5) (A,B), standard BMI (18.5–24.9) (C,D), and high BMI (≥25) (E,F).
Among the patients with a standard BMI, those in the WL group had a significantly shorter OS and PFS compared to those in the non-WL group (OS 16.2 months in the WL group [95% CI: 11.2–NR] and 31.1 months in the non-WL group (95% CI: 24.4–NR; HR: 2.09, 95% CI: 1.33–3.29, p = 0.001); and PFS 6.8 months in the WL group (95% CI: 5.3–10.1) and 13.1 months in the non-WL group (95% CI: 10.1–15.4; HR: 1.38, 95% CI: 1.23–2.56, p = 0.002) (Figure 2C,D). Similarly, among the patients with a high BMI, the WL group also had significantly shorter OS and PFS compared to the non-WL group. The OS was 12.8 months in the WL group (95% CI: 5.7–NR) and 25.4 months in the non-WL group (95% CI: 20.6–NR; HR: 3.74, 95% CI: 1.75–8.01, p < 0.001). The PFS 5.7 months in the WL group (95% CI: 3.5–8.8) and 10.7 months in the non-WL group (95% CI: 7.6–12.6; HR: 1.94, 95% CI: 1.02–3.66, p = 0.038) (Figure 2E,F).
3.4. Frequency of Most Immune-Related Adverse Events Was Not Different Between Groups
Furthermore, this study investigated the frequency of immune-related adverse events and compared the WL and non-WL groups (Table 4). There was no significant difference in the frequency.

Table 4.
irAEs in the WL and non-WL groups treated with ICI plus chemotherapy.
4. Discussion
This study investigated the frequency of patients with advanced NSCLC who experienced body weight loss during ICI plus chemotherapy and examined the association between weight loss during therapy and survival outcomes. More than one-third of the patients lost more than 5% of their body weight during ICI plus chemotherapy, and poor OS and PFS were associated with weight loss.
Pre-treatment with low body weight or cachexia has been repeatedly reported to be associated with poor outcomes in patients with cancer [16,17,18]. Cachexia is a multifactorial syndrome frequently associated with cancer that includes loss of skeletal muscle, fatigue, functional impairment, decreased quality of life (QoL), and decreased survival, characterized by anorexia and unintended weight loss [8] and increased serum or tumor microenvironment concentrations of certain cytokines, such as tumor necrosis factor-α, interleukin (IL)-6, IL-8, and growth differentiation factor-15. It also increases the concentration of bone marrow-derived suppressor cells in plasma or tumor microenvironment [19]. Cachexia is also associated with immune system dysfunction and increased susceptibility to infection [20], both of which are presumed to weaken the efficacy of ICI therapy, leading to poor OS and PFS.
Weight loss often occurs during cancer pharmacotherapy [21]; however, the frequency of weight loss in patients with NSCLC receiving ICI plus chemotherapy remains unclear. Additionally, although many studies have focused on pre-treatment body weight status [22,23,24,25,26], no study has addressed the significance of body weight loss during therapy. Therefore, this study investigated these issues and, for the first time, found that 38.1% of patients experienced weight loss during ICI plus chemotherapy and that body weight loss during therapy was associated with poor outcomes.
Because it was previously reported that a lower pre-treatment BMI was associated with poor outcomes in ICI monotherapy [27], this study investigated the negative impact of body weight loss during therapy according to pre-treatment BMI. Interestingly, the impact of body weight loss during therapy differed according to the pre-treatment BMI. While the current study revealed that body weight loss during therapy led to inferior survival in patients with a pre-treatment standard or high BMI, such a negative effect was not observed in patients with a low BMI (Figure 2). Patients with a low BMI pre-treatment in the non-WL group showed numerically shorter survival than those with standard or high BMI (OS; 17.1 vs. 31.1 or 25.4 months. PFS; 5.9 vs. 13.1 or 10.7 months). The therapeutic effect in such patients may be likely already diminished, regardless of subsequent weight loss. However, it has been reported that the favorable prognosis associated with a high BMI is offset by the negative impact of weight loss before treatment [28], which is consistent with our current data. Collectively, these data suggest that even if a patient’s baseline weight is standard or higher, attention must be paid to the occurrence of weight loss.
However, the efficacy of nutritional interventions for weight loss in patients with cancer remains unclear [29]. Furthermore, whether preventing body weight loss during therapy through nutritional management improves the survival of patients with NSCLC remains unclear. There is evidence that nutritional support improves the QoL of patients receiving radiotherapy [30]; however, these results have not been confirmed in patients receiving pharmacotherapy [31]. Although little evidence is currently available to support the efficacy of nutritional interventions for patients with cancer, the European Society for Clinical Nutrition and Metabolism recommends identifying patients with cancer at nutritional risk through early screening, followed by nutritional counseling and nutritional support [32]. A multicenter randomized trial showed a reduced risk of short-term mortality and improved QoL with active nutritional support compared to usual hospital foods for patients with non-terminal cancer [33]. Further research is necessary to determine whether aggressive nutritional therapy improves the prognosis of patients with cancer treated with ICI plus chemotherapy.
This study had a few limitations. First, it was a retrospective cohort study with heterogeneous data. There were older patients in the WL group, which is potentially associated with poor prognosis. However, the multivariate analysis included age and showed that WL was an independent factor for poor survival. Secondly, there was no available data on body weight loss during chemotherapy or ICI therapy alone. Therefore, it remains unknown whether body weight loss during ICI plus chemotherapy is due to ICI treatment, chemotherapy, or both. Third, this study lacked data on weight loss before the initiation of ICI plus chemotherapy. Considering the possibility that there might have been more patients with cachexia who lost weight before the initiation of ICI plus chemotherapy in the WL group, the pre-treatment BMIs were compared between the WL and non-WL groups. No significant differences were observed between the two groups. Finally, this study did not reveal whether nutritional management during ICI plus chemotherapy improves patient survival. Further clinical studies are required to address this issue.
5. Conclusions
Patients with NSCLC who experienced weight loss during ICI plus chemotherapy had a shorter OS and PFS than those without weight loss. A 5% weight loss during ICI plus chemotherapy serves as an early indicator of suboptimal therapeutic response. These findings emphasize the importance of routine monitoring of body weight and nutritional status during treatment. Clinicians should consider incorporating nutritional assessments into standard care to effectively identify and manage at-risk patients. Additionally, future studies should explore the role of nutritional status, specifically weight change and nutritional support, in responsiveness to ICI plus chemotherapy.
Author Contributions
M.T.: Writing—Original draft preparation, Software, Data curation, Investigation; E.I.: Conceptualization, Methodology, Writing—Original draft preparation, Writing—Reviewing and Editing; I.O.: Formal analysis, Writing—Reviewing and Editing; K.K.: Supervision, Writing—Reviewing and Editing; Y.M.: Supervision, Writing—Reviewing and Editing; K.H.: Supervision, Writing—Reviewing and Editing; others: Investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethical review board (Okayama University Hospital Ethics Committee, K1805-019, approved on 17 August 2018).
Informed Consent Statement
The study was retrospectively conducted on anonymous clinical data; opt-out was adopted for obtaining consent.
Data Availability Statement
The datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank all patients and their families for participating in this study.
Conflicts of Interest
Masataka Taoka received honoraria from AstraZeneca K.K. Eiki Ichihara has received honoraria from AstraZeneca K.K., Novartis Pharma K.K., Janssen Pharmaceutical K.K., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Pfizer Japan Inc., Bristol-Myers Squibb K.K., ONO Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, and Boehringer Ingelheim. Eiki Ichihara received research funding from Janssen Pharmaceutical K.K.; Pfizer Japan Inc.; Bristol-Myers Squibb K.K.; ONO Pharmaceutical Co., Ltd.; and Takeda Pharmaceutical Company, Ltd. Toshihide Yokoyama received honoraria from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Bristol-Myers Squibb K.K., ONO Pharmaceutical Co., Ltd., MSD K.K., and Nippon Kayaku Co., Ltd. Toshihide Yokoyama received grants from AstraZeneca K.K., Boehringer Ingelheim, Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., MSD K.K., Daiichi Sankyo Co., Ltd., and Parexel International Inc. Tomoki Tamura received honoraria from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., and Boehringer Ingelheim. Hirohisa Kano received honoraria from Chugai Pharmaceutical Co., Ltd., MSD K.K., AstraZeneca K.K., Bristol-Myers Squibb K.K., and ONO Pharmaceutical Co., Ltd. Haruyuki Kawai has received honoraria from Glaxo Smith Kline, Meiji Seika Pharma Co., Ltd., Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., MSD K.K., Nippon Kayaku Co., Ltd., AstraZeneca K.K., Bristol-Myers Squibb K.K., Boehringer Ingelheim, and Novartis Pharma K.K. Masaaki Inoue received honoraria from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., MSD K.K., Bristol-Myers Squibb K.K., Daiichi-Sankyo Co., Ltd., and Nippon Kayaku Co., Ltd. Nobuaki Fujimoto received honoraria from ONO Pharmaceutical Co., Ltd., AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., MSD K.K., and Nippon Kayaku Co., Ltd. Nobuaki Fujimoto received consulting fees from AstraZeneca K.K. Katsuyuki Kiura has received honoraria from Merck Biopharma, Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, Boehringer Ingelheim, Eli Lilly Japan K.K., Pfizer Japan Inc., and Bristol-Myers Squibb K.K. Katsuyuki Kiura has received grants from Teijin Pharma Limited, Shionogi Pharmaceutical Co., Ltd., Boehringer Ingelheim, Nippon Kayaku Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Katsuyuki Kiura received consulting fees from Nippon Kayaku Co., Ltd. and Nipro K. K. Yoshinobu Maeda has received honoraria from Asahi Kasei Pharma Corporation, Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., KYORIN Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, Chugai Pharmaceutical Co., Ltd., Teijin Pharma Limited, Japan Blood Products Organization, NIPPON KAYAKU Co., Ltd., Nippon Shinyaku Co., Ltd., Mallinckrodt Pharma K.K., and REGiMMUNE Co., Ltd. Yoshinobu Maeda received grants from Asahi Kasei Pharma Corporation, Astellas Pharma Inc., AstraZeneca K.K., Amgen K.K., AbbVie GK, Eisai Co., Ltd., Viatris Inc., Otsuka Pharmaceutical Co., Ltd., ONO Pharmaceutical Co., Ltd., KYORIN Pharmaceutical Co., Ltd., KISSEI Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Gilead Sciences, Inc., KONICA MINOLTA, Inc., Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd., JCR Pharmaceuticals Co., Ltd., Celgene Corporation, CSL Behring K.K., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Company Limited, TERUMO Corporation, Chugai Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., Bayer Yakuhin, Ltd., Bristol-Myers Squibb K.K., Novartis Pharma K.K., Pfizer Japan Inc., Pharma Essentia Corp., Mundipharma K.K., Human Life CORD Japan Inc., Meiji Seika Pharma Co., Ltd., Medical Review Co., Ltd., Janssen Pharmaceutical K.K., and Yakult Honsha Co., Ltd. Yoshinobu Maeda received grants from research funds under contracts with Chugai Pharmaceutical Co., Ltd. and Nippon Shinyaku Co., Ltd. Katsuyuki Hotta received honoraria from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Bristol-Myers Squibb K.K., ONO Pharmaceutical Co., Ltd., MSD K.K., Nippon Kayaku Co., Ltd., Amgen K.K., Taiho Pharmaceutical Co., Ltd., Merck, and Boehringer Ingelheim. Katsuyuki Hotta received research funding from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., ONO Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, MSD K.K., Eli Lilly Japan K.K., and AbbVie GK. The authors have no conflicts of interest to declare.
References
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab Plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab Plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in Combination with Carboplatin Plus Nab-Paclitaxel Chemotherapy Compared with Chemotherapy Alone as First-Line Treatment for Metastatic Non-Squamous Non-Small-Cell Lung Cancer (IMpower130): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-Line Nivolumab Plus Ipilimumab Combined with Two Cycles of Chemotherapy in Patients with Non-Small-Cell Lung Cancer (CheckMate 9LA): An International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Gogishvili, M.; Melkadze, T.; Makharadze, T.; Giorgadze, D.; Dvorkin, M.; Penkov, K.; Laktionov, K.; Nemsadze, G.; Nechaeva, M.; Rozhkova, I.; et al. Cemiplimab Plus Chemotherapy versus Chemotherapy Alone in Non-Small Cell Lung Cancer: A Randomized, Controlled, Double-Blind Phase 3 Trial. Nat. Med. 2022, 28, 2374–2380. [Google Scholar] [CrossRef]
- Turner, D.C.; Kondic, A.G.; Anderson, K.M.; Robinson, A.G.; Garon, E.B.; Riess, J.W.; Jain, L.; Mayawala, K.; Kang, J.; Ebbinghaus, S.W.; et al. Pembrolizumab Exposure-Response Assessments Challenged by Association of Cancer Cachexia and Catabolic Clearance. Clin. Cancer Res. 2018, 24, 5841–5849. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Scott, H.R.; McMillan, D.C.; Forrest, L.M.; Brown, D.J.F.; McArdle, C.S.; Milroy, R. The Systemic Inflammatory Response, Weight Loss, Performance Status and Survival in Patients with Inoperable Non-Small Cell Lung Cancer. Br. J. Cancer 2002, 87, 264–267. [Google Scholar] [CrossRef]
- Shepshelovich, D.; Xu, W.; Lu, L.; Fares, A.; Yang, P.; Christiani, D.; Zhang, J.; Shiraishi, K.; Ryan, B.M.; Chen, C.; et al. Body Mass Index (BMI), BMI Change, and Overall Survival in Patients with SCLC and NSCLC: A Pooled Analysis of the International Lung Cancer Consortium. J. Thorac. Oncol. 2019, 14, 1594–1607. [Google Scholar] [CrossRef]
- Degens, J.H.R.J.; Dingemans, A.M.C.; Willemsen, A.C.H.; Gietema, H.A.; Hurkmans, D.P.; Aerts, J.G.; Hendriks, L.E.L.; Schols, A.M.W.J. The Prognostic Value of Weight and Body Composition Changes in Patients with Non-Small-Cell Lung Cancer Treated with Nivolumab. J. Cachexia Sarcopenia Muscle 2021, 12, 657–664. [Google Scholar] [CrossRef]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; De Van Der Schueren, M.A.E.; Den Braver, N.R.; Berkhof, J.; Langius, J.A.E.; Verheul, H.M.W. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef]
- Ando, C.; Ichihara, E.; Yokoyama, T.; Inoue, K.; Tamura, T.; Fujiwara, K.; Oda, N.; Kano, H.; Kishino, D.; Watanabe, K.; et al. More than One-Third of Advanced Non-Small-Cell Lung Cancer Patients Do Not Receive Immunochemotherapy Due to Intolerance. J. Cancer Res. Clin. Oncol. 2023, 149, 4933–4938. [Google Scholar] [CrossRef]
- Nishimura, T.; Ichihara, E.; Yokoyama, T.; Inoue, K.; Tamura, T.; Sato, K.; Oda, N.; Kano, H.; Kishino, D.; Kawai, H.; et al. The Effect of Pleural Effusion on Prognosis in Patients with Non-Small Cell Lung Cancer Undergoing Immunochemotherapy: A Retrospective Observational Study. Cancers 2022, 14, 6184. [Google Scholar] [CrossRef]
- Tokunaga, T.; Matsuzawa, Y.; Kotani, K.; Keno, Y.; Kobatake, T.; Fujioka, S.; Tarui, S. Ideal Body Weight Estimated from the Body Mass Index with the Lowest Morbidity. Int. J. Obes. 1991, 15, 2010254. [Google Scholar]
- Shiroyama, T.; Nagatomo, I.; Koyama, S.; Hirata, H.; Nishida, S.; Miyake, K.; Fukushima, K.; Shirai, Y.; Mitsui, Y.; Takata, S.; et al. Impact of Sarcopenia in Patients with Advanced Non-Small Cell Lung Cancer Treated with PD-1 Inhibitors: A Preliminary Retrospective Study. Sci. Rep. 2019, 9, 2447. [Google Scholar] [CrossRef]
- Yang, R.; Cheung, M.C.; Pedroso, F.E.; Byrne, M.M.; Koniaris, L.G.; Zimmers, T.A. Obesity and Weight Loss at Presentation of Lung Cancer Are Associated with Opposite Effects on Survival. J. Surg. Res. 2011, 170, e75–e83. [Google Scholar] [CrossRef]
- Martin, L.; Senesse, P.; Gioulbasanis, I.; Antoun, S.; Bozzetti, F.; Deans, C.; Strasser, F.; Thoresen, L.; Jagoe, R.T.; Chasen, M.; et al. Diagnostic Criteria for the Classification of Cancer-Associated Weight Loss. J. Clin. Oncol. 2015, 33, 90–99. [Google Scholar] [CrossRef]
- Rounis, K.; Makrakis, D.; Tsigkas, A.P.; Georgiou, A.; Galanakis, N.; Papadaki, C.; Monastirioti, A.; Vamvakas, L.; Kalbakis, K.; Vardakis, N.; et al. Cancer Cachexia Syndrome and Clinical Outcome in Patients with Metastatic Non-Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors: Results from a Prospective, Observational Study. Transl. Lung Cancer Res. 2021, 10, 3538–3549. [Google Scholar] [CrossRef]
- de Matos-Neto, E.M.; Lima, J.D.C.C.; de Pereira, W.O.; Figuerêdo, R.G.; Riccardi, D.M.D.R.; Radloff, K.; das Neves, R.X.; Camargo, R.G.; Maximiano, L.F.; Tokeshi, F.; et al. Systemic Inflammation in Cachexia—Is Tumor Cytokine Expression Profile the Culprit? Front. Immunol. 2015, 6, 629. [Google Scholar] [CrossRef]
- Fukahori, M.; Shibata, M.; Hamauchi, S.; Kasamatsu, E.; Machii, K. A Retrospective Cohort Study to Investigate the Incidence of Cancer-Related Weight Loss During Chemotherapy in Gastric Cancer Patients. Support. Care Cancer 2021, 29, 341–348. [Google Scholar] [CrossRef]
- Miyawaki, T.; Naito, T.; Yabe, M.; Kodama, H.; Nishioka, N.; Miyawaki, E.; Mamesaya, N.; Kobayashi, H.; Omori, S.; Wakuda, K.; et al. Impact of Weight Loss on Treatment with PD-1/PD-L1 Inhibitors Plus Chemotherapy in Advanced Non-Small-Cell Lung Cancer. Support. Care Cancer 2022, 30, 1633–1641. [Google Scholar] [CrossRef]
- Bonomi, P.D.; Crawford, J.; Dunne, R.F.; Roeland, E.J.; Smoyer, K.E.; Siddiqui, M.K.; McRae, T.D.; Rossulek, M.I.; Revkin, J.H.; Tarasenko, L.C. Mortality Burden of Pre-Treatment Weight Loss in Patients with Non-Small-Cell Lung Cancer: A Systematic Literature Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 1226–1239. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A Multicenter Study of Body Mass Index in Cancer Patients Treated with Anti-PD-1/PD-L1 Immune Checkpoint Inhibitors: When Overweight Becomes Favorable. J. Immunother. Cancer 2019, 7, 57. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of Body-Mass Index and Outcomes in Patients with Metastatic Melanoma Treated with Targeted Therapy, Immunotherapy, or Chemotherapy: A Retrospective, Multicohort Analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical Effects of Obesity on T Cell Function During Tumor Progression and PD-1 Checkpoint Blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef]
- Ichihara, E.; Harada, D.; Inoue, K.; Sato, K.; Hosokawa, S.; Kishino, D.; Watanabe, K.; Ochi, N.; Oda, N.; Hara, N.; et al. The Impact of Body Mass Index on the Efficacy of Anti-PD-1/PD-L1 Antibodies in Patients with Non-Small Cell Lung Cancer. Lung Cancer 2020, 139, 140–145. [Google Scholar] [CrossRef]
- Antoun, S.; Lanoy, E.; Ammari, S.; Farhane, S.; Martin, L.; Robert, C.; Planchard, D.; Routier, E.; Voisin, A.L.; Messayke, S.; et al. Protective Effect of Obesity on Survival in Cancers Treated with Immunotherapy Vanishes When Controlling for Type of Cancer, Weight Loss and Reduced Skeletal Muscle. Eur. J. Cancer 2023, 178, 49–59. [Google Scholar] [CrossRef]
- Baguley, B.J.; Edbrooke, L.; Denehy, L.; Prado, C.M.; Kiss, N. A Rapid Review of Nutrition and Exercise Approaches to Managing Unintentional Weight Loss, Muscle Loss, and Malnutrition in Cancer. Oncologist 2024, oyae261. [Google Scholar] [CrossRef]
- Langius, J.A.E.; Zandbergen, M.C.; Eerenstein, S.E.J.; van Tulder, M.W.; Leemans, C.R.; Kramer, M.H.H.; Weijs, P.J.M. Effect of Nutritional Interventions on Nutritional Status, Quality of Life and Mortality in Patients with Head and Neck Cancer Receiving (Chemo)Radiotherapy: A Systematic Review. Clin. Nutr. 2013, 32, 671–678. [Google Scholar] [CrossRef]
- Baldwin, C.; Spiro, A.; McGough, C.; Norman, A.R.; Gillbanks, A.; Thomas, K.; Cunningham, D.; O’Brien, M.; Andreyev, H.J.N. Simple Nutritional Intervention in Patients with Advanced Cancers of the Gastrointestinal Tract, Non-Small Cell Lung Cancers or Mesothelioma and Weight Loss Receiving Chemotherapy: A Randomised Controlled Trial. J. Hum. Nutr. Diet. 2011, 24, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Practical Guideline: Clinical Nutrition in Cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Bargetzi, L.; Brack, C.; Herrmann, J.; Bargetzi, A.; Hersberger, L.; Bargetzi, M.; Kaegi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; et al. Nutritional Support During the Hospital Stay Reduces Mortality in Patients with Different Types of Cancers: Secondary Analysis of a Prospective Randomized Trial. Ann. Oncol. 2021, 32, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).