Simple Summary
There is a correlation between quality of life (QoL) scores and treatment outcomes in patients receiving head and neck cancer (HNC) treatment. Higher QoL scores have been associated with improved survival yet there are considerable differences in the assessment of QoL in clinical trials. The aim of this systematic scoping review is to evaluate the variability of QoL reporting in clinical trials investigating anti-EGFR treatment. Our study confirms no standard method for reporting QoL data in clinical trials for HNC patients. QoL benchmarks are assessed and reported differently between studies. Therefore, these metrics are difficult to evaluate on a larger scale, preventing quantitative analysis. This review identifies the need to standardize the method for QoL assessment.
Abstract
In patients receiving treatment for head and neck cancer (HNC), there is a correlation between quality of life (QoL) scores and treatment outcomes. Higher QoL scores have been associated with improved survival. Despite this, the assessment of QoL in clinical trials varies considerably. Three databases (Scopus, PubMed, and Cinahl) were queried for articles published in English between 2006 and 2022. Two reviewers (SRS and ANT) performed study screening, data extraction, and risk of bias assessment. The authors identified 21 articles that met the inclusion criteria. A total of 5961 patients were evaluated. QoL was reported as average scores for specific variables across five different surveys in 12 included articles. Supplemental QoL data were available in 10 included studies. Critical appraisal of studies indicated a high risk of bias due to the inclusion of trials. There is no standard method for reporting QoL data in clinical trials for HNC patients undergoing treatment with anti-EGFR inhibitors. Future clinical trials should standardize their method for assessing and reporting quality-of-life data to increase patient-centered care and refine treatment choices to optimize survival.
1. Introduction
As one of the most common forms of cancer in the United States [1], head and neck cancer (HNC) is a debilitating disease with detrimental effects on patients, both functionally and emotionally. The clinical trials that have transformed the treatment of HNC often provide detailed survival-based outcomes [2,3], yet their assessment of quality of life (QoL) does not appear to be standardized [4,5].
This poses a problem for all HNC treatment modalities, especially for anti-epidermal growth factor receptors (EGFR) monoclonal antibodies such as cetuximab [6,7,8]. Despite their efficacy [9], these medications have significant side effects that negatively impact patient QoL and are associated with toxicities including an acneiform rash, nausea, dyspnea, and fatigue [10,11]. Cetuximab is less toxic than cisplatin, which was the basis for the De-ESCALaTE HPV and RTOG 1016 trials [2,12]. Both of these trials concluded that there was no improvement in toxicity compared with cisplatin [2,12]. Additionally, the intensity of adverse reactions from anti-EGFR medications has been shown to influence QoL and treatment adherence [13,14], yet the assessment of these effects is not consistent in clinical trials.
Trials that assess HNC treatment with anti-EGFR inhibitors demonstrate variable results on their QoL impact. Most trials note that the QoL in patients undergoing treatment for HNC decreases significantly during chemoradiation therapy [2,15,16,17,18,19] but improves by the end of the trial follow-up period. Based on this transient decrease and the inconsistent reporting in studies, it appears that QoL has not been thoroughly investigated. Multimodal treatment regimens for HNC using anti-EGFR inhibitors typically lasts for 6–7 weeks [20], and any reduction in QoL can be difficult for patients to endure, affecting treatment adherence.
The initial objective of this study was to evaluate the impact of anti-EGFR medications on QoL. However, after performing a systematic review, it was impossible to quantitatively assess QoL data due to significant variation in the available, reported data. Appropriate QoL assessment predicts treatment success and decreased morbidity and mortality [21], yet it is not assessed uniformly in the clinical trials for anti-EGFR treatments. Therefore, it is necessary to evaluate QoL changes to provide patient-centered care and ensure optimal adherence to treatment. This systematic scoping review aims to update the scientific community on the variability of QoL reporting in clinical trials investigating anti-EGFR treatment for HNC. A secondary aim is to highlight the importance of addressing QoL concerns while patients are actively being treated.
2. Methods
A systematic scoping review methodology was chosen due to its strengths in summarizing and disseminating findings on a broad topic [22]. The review protocol was not registered or published in peer-reviewed publications. The review followed the five-stage framework outlined by Arksey and O’Malley (2005) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRIMSA-ScR; see Figure 1) [23].
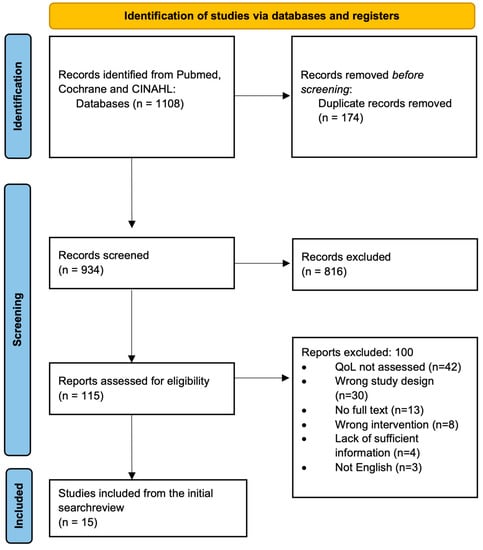
Figure 1.
PRIMSA flow chart of study selection.
2.1. Stage 1: Identify the Research Question
This review sought to answer the following research questions: What is the variability of QoL reporting in clinical trials investigating anti-EGFR treatment for HNC? Moreover, what is the importance of addressing QoL concerns while patients are actively being treated?
2.2. Stage 2: Identify Relevant Literature
An academic librarian assisted in developing the search strategy, which was executed by the two primary authors, SRS and ANT. A systematic review was performed in accordance with PRISMA guidelines [23] using the PubMed, Scopus (Elsevier), and CINAHL (EBSCO) databases from the date of inception to August 2022. The search aimed to identify all English articles related to head and neck cancer treated with anti-EGFR medications. Another goal of the search was to identify articles that assessed these treatments using QoL instruments. Articles written in English were chosen to identify studies and QoL instruments in a widely applicable and universal language, which also avoids validation uncertainty, cross-cultural differences, and inaccuracies in translation. QOL instruments are also primarily developed in English before being translated into other languages [24]. The initial search was made for PubMed and utilized Medical Subject Headings [MESH] and text word [tw] and title and abstract [tiab]. This search was then modified for use in the other databases (Scopus and CINAHL). Key terms for this search were related to head and neck cancer, quality of life evaluation, and the antineoplastic use of EGFR inhibitors such as cetuximab.
2.3. Stage 3: Study Selection
At least two authors (SRS and ANT) independently screened records by title and abstract, then by full text using Covidence, a review management software. Disagreements were resolved with all authors. The inclusion criteria required included articles to be full-text, primary studies, English-language, and published in a peer-reviewed journal. Other aspects of the inclusion criteria were as follows: studies needed to evaluate using an anti-EGFR inhibitor within the clinical trial. The population evaluated had head and neck carcinoma. Each included study needed to directly evaluate patient QoL using a validated assessment tool. Included assessment tools included: EORTC QLQ-30, QLQ-H&N-35, FHNSI-10, FACT H&N, VF, HNQOL, and UW-QOL. The full names and characteristics of these surveys are detailed below. All patients were greater than or equal to the age of 18 years old and all studies were published in English. Articles were excluded if they were not specific to head and neck cancer treatment, or if the wrong intervention was assessed. For example, if the immunotherapy evaluated was not anti-EGFR specifically. Articles were also excluded if the study failed to directly evaluate QOL using a validated survey. Additionally, articles were eliminated if they were non-human studies, or had the wrong study design (i.e., case reports, case series, and review articles). Studies that evaluated endocrine neoplasms were also excluded.
2.3.1. Procedure
Search Process
This review was conducted in compliance with PRISMA-ScR scoping review methods [23]. The initial search yielded 1108 articles which were uploaded into Covidence, a review management software, for screening. There were 174 duplicate articles. After duplicates were removed, 934 titles and abstracts were screened for relevance independently by two reviewers (SRS and ANT). Authors included titles and abstracts if they mentioned quality of life, if the authors felt there was a high likelihood that quality of life would be mentioned in the full text. There were 816 articles excluded at this stage.
A total of 115 full texts were assessed for eligibility by the same reviewers (SRS and ANT). Articles were included for synthesis following full-text review if they had an appropriate study design, they evaluated the use of anti-EGFR inhibitors, and they provided sufficient information on QoL assessment during the trial so that the data could be utilized to compare to other studies during the synthesis stage. For example, if a study mentioned QoL early in the manuscript but did not report a method for collecting these data or QoL-specific results, they were excluded under this criterion. There were 42 references that were excluded at the full-text stage because quality of life was not assessed in the manuscript. Thirty references had the wrong study design and therefore were excluded. A total of 13 title and abstract entries did not have associated full texts, 8 articles had the wrong intervention, and 4 articles needed to provide more information on QoL to be compared to the other manuscripts. There were 8 articles that assessed the wrong intervention, where anti-EGFR inhibitors were not assessed, and 3 studies were not written in the English language. Following a full-text review, 15 articles met the inclusion criteria and were selected for synthesis. Each study’s references were screened, and 6 additional articles were included, leading to 21 articles. Several articles were published using data from the same trial. To prevent duplication, the first article published was included with the remaining articles excluded. The PRISMA flow diagram is outlined in Figure 1.
2.4. Stage 4: Charting the Data
2.4.1. Data Extraction
For the 21 included studies, general information was collected, including author, year of publication, country of study, sample size, study aims, and study design. Data extraction was performed independently by two reviewers (SRS and ANT) and conflicts were resolved by a third reviewer (SAN). The methodology for QoL assessment was collected. This included the validated surveys used, assessment frequency during the treatment period (i.e., at what time points QoL was assessed during the trial and follow-up), patient survey response rate, presentation of QoL assessment (graph, table, both), and whether supplementary data on QoL was available. The supplementary data were collected to assess the reporting of raw QoL data, as mean QoL scores were often presented in the text of included studies.
2.4.2. Level of Evidence and Risk of Bias
References were exported into the review management software (Covidence; Veritas Health Innovation) for study selection. The level of evidence was assessed independently by the two reviewers (SRS and ANT). Conflicts related to the level of evidence for each article were first resolved by a discussion between the two reviewers (SRS and ANT) and then by a third reviewer (SAN) when necessary. Articles were critically appraised to assess the level of evidence per the criteria of the Oxford Center for Evidence-Based Medicine [25]. The risk of bias in each included article was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions (version 6.2) [26]. Two authors (SRS and ANT) performed a pilot assessment on 3 studies to check for consistency of assessment and then performed independent risk assessments on the remaining studies. All disagreements were resolved once both authors came to a consensus. The risk of bias items for randomized trials included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcomes assessment, incomplete outcome data, selective reporting, and other bias. The risk of bias for each aspect was graded as low, unclear, or high. Articles were not excluded based on quality since this is not required within the Arksey and O’Malley (2005) framework.
2.5. Stage 5: Collating, Summarizing, and Reporting Results
Review results are presented through descriptive statistics of included studies and a narrative summary of findings. Implications of the analysis are then discussed.
3. Results
3.1. Study Characteristics
All 21 studies were clinical trials [2,3,12,15,16,17,18,19,27,28,29,30,31,32,33,34,35,36,37,38,39] that were published between the years 2005 to 2021. Articles selected for inclusion were level 2 studies which included both comparative and randomized clinical studies based on the Oxford Level of Evidence. Critical appraisal of studies indicated a low risk of bias or unclear risk of bias for the majority of included studies (Figures S1 and S2). The potential sources of bias that were most pronounced were in the domains of “blinding of participants and personnel,” “blinding of outcome assessment,” and “other bias.” A list of the included studies, the author, the year, the number of patients evaluated, the treatment modalities evaluated in each study, and the availability of supplemental data are reported in Table 1. The specific surveys that were used in each study, the reporting method for QoL (graph, table, or both), and the timeline of QoL assessment are represented in Table 1 as well as Figure 2.

Table 1.
Prevalence of quality-of-life data reported per article.
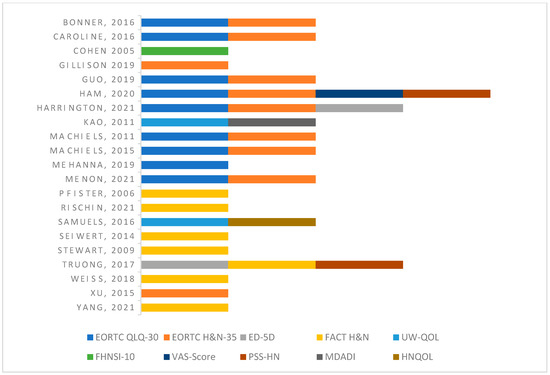
Figure 2.
QoL assessment tool used per study [2,3,12,15,16,17,18,19,27,28,29,30,31,32,33,34,35,36,37,38,39].
The most common assessment tool was the European Organization for Research and Treatment of Cancer Quality of Life Head and Neck Module (EORTC QLQ-H&N-35), which was used by 10 studies. The European Organization for Research and Treatment of Cancer Quality of Life (EORTC QLQ-C30) was used by nine studies and the Functional Assessment of Cancer Therapy- Head and Neck (FACT-HN) was used by seven studies. Two studies used the following surveys to evaluate quality of life: The Performance Status Scale for Head and Neck, the University of Washington Quality of Life Questionnaire (UW-QOL), and the EuroQol (EQ-5D). The Functional Assessment of Cancer Therapy Head & Neck Cancer Symptom Index (FHNSI-10), Visual Analog Scale (VAS-scale), Head and Neck Quality of Life Instrument (HNQOL), and MD Anderson Dysphagia Inventory (MDADI) were each used by one article. Of note, the majority of included studies used multiple tools to assess QoL.
3.2. Quality of Life Measures
There are several authenticated surveys that are used to assess quality of life in patients undergoing HNC treatment. A brief overview of the most common tools in this review is detailed below. The quality-of-life assessment tools used by each study are represented in Figure 2.
3.3. EORTC QLQ-30
The European Organization for Research and Treatment of Cancer (EORTC) developed the QLQ-C30 tool to measure quality of life in cancer patients. The questionnaire measures quality of life with scales in nine categories: three symptom scales (fatigue, pain, and nausea/vomiting), five functional scales (physical, role, cognitive, emotional, and social), and a scale for global health and quality of life. This tool has been a validated measure of quality of life in cancer patients since 1993 [40,41].
3.4. QLQ-H&N-35
This assessment tool is used to measure quality of life in head and neck cancer patients exclusively [42,43]. Seven scales are used to assess pain, ability to swallow, taste and smell, speech, social eating, sexuality, and social contact. Problems associated with dentition, dry mouth, mouth-opening, saliva thickness, coughing, and feeling ill are also assessed. It has been validated for use in conjunction with the QLQ-30 [44].
3.5. UW-QOLR
The University of Washington Quality of Life Questionnaire (UW-QOL) is a self-administered form for patients who have undergone treatment for head and neck cancer. The different domains include pain, appearance, activity, swallowing, and speech, among others. Patients also rate the importance of each domain. This tool measures the global quality of life in head and neck cancer patients [45].
3.6. FACT-HN
The Functional Assessment of Cancer Therapy—Head and Neck (FACT-HN) is a survey that consists of 28 general and 11 head and neck-related topics scored using a Likert-type scale. The themes assessed are physical well-being, social and family well-being, relationship with their physician, emotional well-being, functional well-being, and head and neck-related symptoms [46,47,48].
3.7. Timeline of QOL Assessment during Treatment
Each included study assessed the quality of life at varying times based on their specific treatment protocol. Most studies assessed QOL at baseline, and periodically during treatment and in the months following treatment [2,3,12,15,16,17,18,19,27,28,30,31,34,35,38,39]. The exact timeline for assessing QOL for each study is outlined in Table 1.
4. Discussion
HNC is a profound disease that affects approximately 900,000 individuals annually and causes roughly 400,000 deaths per year [1]. Fortunately, survival outcomes are improving as treatments continue to advance [49]. QoL assessment is a patient-reported outcome (PRO) that is utilized to gauge the impact of cancer and treatment on a patient’s daily life. It evaluates a patient’s overall well-being and contentment while they battle to survive both their disease and treatment. Therefore, it is an integral evaluation for clinical trials that assess therapeutic agents with negative side effects, such as anti-EGFR inhibitors. Additionally, previous studies have identified QoL as an independent predictor for overall survival, regardless of cancer staging [50]. Given the clinical implications of these findings, this scoping review was conducted to identify current gaps in QoL appraisal and to highlight the importance of standardizing this assessment in clinical trials.
The results of this review identify both limited and varied QoL data in the included clinical trials. A total of 11 different PRO surveys were utilized among the 21 studies to assess the QoL and functional status of the patients. The most common assessment was the QLQ-30 combined with the H&N-35, which was seen in nine studies. However, even in those nine studies, variation in the timeline of assessment as well as the reporting method was seen. For example, the clinical trial conducted by Bonner et al. [19] used these two surveys at baseline, week 4, week 8, month 8, and month 12. Their QoL results were reported using both graphs and tables. Using the same tools, the Caroline study [27] evaluated QoL at baseline, when the patient completed radiotherapy, and again at 6 weeks and 24 weeks post-radiotherapy. This study did not report quality of life data using a graph or table and provided no supplemental data. Therefore, QoL data could not be compared between these two studies despite the use of the same modalities. This is just one example of the variation found in the included studies.
The most common tools were the QLQ-30 and/or H&N-35, used in approximately 52% of studies, and the FACT-H&N, used in 33% of the included articles. There is currently no method to assess how the results of these surveys compare to each other, prohibiting analysis on a larger scale [51]. Additionally, one included article only assessed quality of life using the H&N-35, which is not considered a validated assessment tool unless used with QLQ-30 [42]. These findings illustrate the incoherent method of QoL data collection and reporting.
Given the improvements seen in survival outcomes [49], the current paradigm for successful treatment needs to expand and include patient-centered outcomes. The justification for this change is exhibited by a study that examined different preferences for quality of life versus the longevity of life (LoL) in HNC treatment [52]. Younger HNC patients selected aggressive treatment to increase LoL whereas older patients preferred treatments to improve QoL. A similar study found that QoL has a direct impact on treatment adherence in patients with gastrointestinal carcinomas [53]. These findings convey that patients are driven by different motivators, demonstrating the need for patient-centered care when selecting and monitoring appropriate treatment regimens. Treatment with anti-EGFR inhibitors is associated with severe side effects, and the patient’s ability to tolerate them should be balanced with survival goals.
Additionally, although not for HNC specifically, QoL assessment during brain cancer treatment has been identified as a tool that improves the physician–patient relationship and patient communication [54]. The findings of this study serve as a reminder that measuring QoL outcomes can lead to PRO improvement by simply administering the survey. This discovery provides additional incentive for why QoL should be assessed during oncological treatment for head and neck tumors.
This scoping review identified multiple studies that reported a negative impact on QoL using anti-EGFR inhibitors [2,15,16,17,18,19]. Yet, these findings appear to be overlooked as they are transient and are not prominent beyond several months after treatment. Active treatment usually spans 6–7 weeks [20], and this period is distressing for patients. Despite improvement in QoL after treatment, research indicates that patient care and comfort can be improved with increased attention to PROs during active treatment [54]. Significant negative impacts, even when limited to active treatment, should not be overlooked. This is an area where current clinical trials in this field have room to improve.
There is a myriad of challenges that prohibit the standardized use of QoL assessment tools in clinical trials. These obstacles are derived from the subjective and variable nature of QoL reporting. It is difficult to evaluate QoL with accurate measurements and make inter-patient comparisons. Furthermore, there is no data to confirm which validated survey is the best to use nor is there evidence on how to compare scores of one tool to those of another [55]. This creates uncertainty regarding survey selection and the most appropriate timeline for QoL assessment in clinical trial designs [56].
In this review, the clinical trials utilized different surveys for QoL, and some only reported their significant survey findings which were different among the studies [19,30]. If all outcomes regardless of significance were reported, the QoL data could have been pooled to identify which QoL outcomes were most affected and how those categories could be better addressed in the future. Most of the studies identified in this scoping review utilize multiple QoL assessment tools for a holistic appraisal. This strategy creates respondent fatigue for patients, which has been shown to increase the amount of missing data and poor reporting of PROs in clinical trials [57]. This is an additional complication in the assessment of QoL for clinical trials.
A notable strength of this review was the comprehensive approach to EGFR inhibitors and QoL measurements. The thorough search strategy allowed us to be reasonably confident in capturing all relevant QoL instruments and studies in the English language. This study is important in evaluating the most current literature on QoL assessment in HNC but has its own limitations. This scoping review was limited to articles that evaluated anti-EGFR inhibitors and does not evaluate QoL for other commonly utilized medications such as PD-1 inhibitors. Another limitation of our study is the repetition of multiple studies analyzing the same results from one clinical trial. To avoid duplication of PROs, only the first published article from each trial was included in our review. It is possible that other studies related to the same clinical trial assessed quality of life more thoroughly. Although the aim of this scoping review was to assess all articles that reported cetuximab, this created some additional restrictions. Due to the nature of a scoping review, some included articles evaluated cetuximab treatment for metastatic or recurrent disease. In this scenario, the impact of anti-EGFR treatments on quality of life is confounded by other therapies. Additionally, it is difficult to pool QoL scores when the treatment modalities are not exactly the same. This was another limitation as the majority of included studies evaluated anti-EGFR inhibitors in conjunction with other treatment modalities. Finally, a limitation of this review is that articles in the grey literature or non-English articles and QoL instruments were excluded. The reasons for this are described earlier. However, we are aware of non-English manuscripts and QoL instruments that may exist.
The initial intention of this study was to perform a meta-analysis of the QoL scores among patients undergoing HNC treatment with anti-EGFR inhibitors. This antineoplastic agent was chosen for its debilitating side effect profile [58,59,60]. It was found that studies utilizing the same assessment tools differed in their reporting methods, and this prevented doing a pooled analysis. This reiterates a similar conclusion made in 2001 [5] and 2016 by Rogers et al. who identified the need for a strict standard for QoL assessment and reporting in clinical trials [4]. When considering the inconsistent nature of the assessment, it is concerning that progress has not been made in the past twenty years and suggests that little is known about the impact of novel cancer treatments on QoL at a population level. A standardized evaluation will facilitate a patient-centered approach to therapy and ultimately improve therapeutic success. Future directions of this research should aim to assess the QoL assessment methods in other treatment modalities for head and neck cancer such as PD-1 inhibitors.
5. Conclusions
The findings of this systematic scoping review restate the urgency to standardize the reporting system for QoL in HNC clinical trials. Important gaps in knowledge have been identified. Current HNC-specific QoL instruments are few. Objective appraisal criteria for measurement properties and clinical and pragmatic considerations are required to recommend the best QoL instruments. It is crucial to overcome the presented challenges associated with the validated surveys and collectively decide how to utilize these tools best as a field. Uniform QOL data will convey any important findings that are found from different treatment options and allow clinicians to implement a more patient-centered approach to treatment decisions in HNC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15092475/s1, Risk of bias assessment graphs are included as supplementary materials. Figure S1: Risk of bias summary: review authors’ judgments about each risk of bias item for each included study. Figure S2: Risk of bias graph: review authors’ decisions about each risk of bias item presented as percentages across all included studies.
Author Contributions
Conceptualization, S.A.N., J.G.N. and A.E.K.; Methodology, S.R.S. and A.N.T.; Formal Analysis and Investigation, S.R.S., A.N.T. and S.A.N.; Data Curation, S.R.S. and A.N.T.; Writing—Original Draft Preparation, S.R.S., S.A.N. and J.G.N.; Writing—Review and Editing, S.R.S., A.N.T., A.E.K., J.M.K., W.G.A. and A.E.K.; Supervision, J.G.N. and A.E.K. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was required to produce this manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created for this study. Additional information will be provided upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Murray, Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [PubMed]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; King, M.; Kenny, L.; Porceddu, S.; Wratten, C.; Macann, A.; Jackson, J.E.; Bressel, M.; Herschtal, A.; Fisher, R.; et al. Randomized Trial of Radiation Therapy With Weekly Cisplatin or Cetuximab in Low-Risk HPV-Associated Oropharyngeal Cancer (TROG 12.01)—A Trans-Tasman Radiation Oncology Group Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 876–886. [Google Scholar] [CrossRef]
- Rogers, S.N. Improving quality-of-life questionnaires in head and neck cancer. Expert Rev. Qual. Life Cancer Care 2016, 1, 61–71. [Google Scholar] [CrossRef]
- Schwartz, S.; Patrick, D.L.; Yueh, B. Quality-of-Life Outcomes in the Evaluation of Head and Neck Cancer Treatments. Arch. Otolaryngol.–Head Neck Surg. 2001, 127, 673–678. [Google Scholar] [CrossRef]
- Cohen, M.H.; Chen, H.; Shord, S.; Fuchs, C.; He, K.; Zhao, H.; Sickafuse, S.; Keegan, P.; Pazdur, R. Approval summary: Cetuximab in combination with cisplatin or carboplatin and 5-fluorouracil for the first-line treatment of patients with recurrent locoregional or metastatic squamous cell head and neck cancer. Oncologist 2013, 18, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J. The EGFR as a target for anticancer therapy—Focus on cetuximab. Eur. J. Cancer 2001, 37 (Suppl. S4), S16–S22. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. Anti-epidermal growth factor receptor drugs in cancer therapy. Expert Opin. Investig. Drugs 2002, 11, 755–768. [Google Scholar]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Orditura, M.; De Vita, F.; Galizia, G.; Lieto, E.; Vecchione, L.; Vitiello, F.; Martinelli, E.; Ciardiello, F. Correlation between efficacy and skin rash occurrence following treatment with the epidermal growth factor receptor inhibitor cetuximab: A single institution retrospective analysis. Oncol. Rep. 2009, 21, 1023–1028. [Google Scholar] [CrossRef]
- Specenier, P.; Vermorken, J.B. Cetuximab: Its unique place in head and neck cancer treatment. Biol. Targets Ther. 2013, 7, 77–90. [Google Scholar]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.; Vincent, M. Adverse events associated with anti-EGFR therapies for the treatment of metastatic colorectal cancer. Curr. Oncol. 2010, 17 (Suppl. S1), S18–S30. [Google Scholar] [CrossRef]
- Mitchell, E.P.; Perez-Soler, R.; Van Cutsem, E.; E LaCouture, M. Clinical presentation and pathophysiology of EGFRI dermatologic toxicities. Oncology 2007, 21, 4–9. [Google Scholar] [PubMed]
- Kao, J.; Genden, E.M.; Gupta, V.; Policarpio, E.L.; Burri, R.J.; Rivera, M.; Gurudutt, V.; Som, P.M.; Teng, M.; Packer, S.H. Phase 2 trial of concurrent 5-fluorouracil, hydroxyurea, cetuximab, and hyperfractionated intensity-modulated radiation therapy for locally advanced head and neck cancer. Cancer 2011, 117, 318–326. [Google Scholar] [CrossRef]
- Samuels, S.E.; Tao, Y.; Lyden, T.; Haxer, M.; Spector, M.; Malloy, K.M.; Prince, M.E.; Bradford, C.R.; Worden, F.P.; Schipper, M.; et al. Comparisons of dysphagia and quality of life (QOL) in comparable patients with HPV-positive oropharyngeal cancer receiving chemo-irradiation or cetuximab-irradiation. Oral Oncol. 2016, 54, 68–74. [Google Scholar] [CrossRef]
- Weiss, J.; Gilbert, J.; Deal, A.M.; Weissler, M.; Hilliard, C.; Chera, B.; Murphy, B.; Hackman, T.; Liao, J.J.; Olson, J.G.; et al. Induction chemotherapy with carboplatin, nab-paclitaxel and cetuximab for at least N2b nodal status or surgically unresectable squamous cell carcinoma of the head and neck. Oral Oncol. 2018, 84, 46–51. [Google Scholar] [CrossRef]
- Xu, T.; Liu, Y.; Dou, S.; Li, F.; Guan, X.; Zhu, G. Weekly cetuximab concurrent with IMRT aggravated radiation-induced oral mucositis in locally advanced nasopharyngeal carcinoma: Results of a randomized phase II study. Oral Oncol. 2015, 51, 875–879. [Google Scholar] [CrossRef]
- Bonner, J.; Giralt, J.; Harari, P.; Spencer, S.; Schulten, J.; Hossain, A.; Shao-Chun, C.; Chin, S.; Baselga, J.; Chang, S.-C. Cetuximab and Radiotherapy in Laryngeal Preservation for Cancers of the Larynx and Hypopharynx: A Secondary Analysis of a Randomized Clinical Trial. JAMA Otolaryngol.-Head Neck Surg. 2016, 142, 842–849. [Google Scholar] [CrossRef]
- Mehra, R.; Cohen, R.B.; Burtness, B.A. The role of cetuximab for the treatment of squamous cell carcinoma of the head and neck. Clin. Adv. Hematol. Oncol. 2008, 6, 742–750. [Google Scholar]
- Karvonen-Gutierrez, C.A.; Ronis, D.L.; Fowler, K.E.; Terrell, J.E.; Gruber, S.B.; Duffy, S.A. Quality of life scores predict survival among patients with head and neck cancer. J. Clin. Oncol. 2008, 26, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. Theory Pract. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D. Translation and validation of study instruments for cross-cultural research. Gastroenterology 2004, 126, S124–S128. [Google Scholar] [CrossRef]
- The Oxford Levels of Evidence 2. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 20 July 2021).
- Higgins, J.P.T.; James; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Chichester, UK, 2019. [Google Scholar]
- Caroline, B.; Sundus, Y.; Dawn, D.; Carol, G.; Susan, M. Cost analysis of cetuximab (Erbitux) plus radiotherapy (ERT) versus concomitant cisplatin plus radiotherapy (CRT) within an NHS oncology unit (single institution): A pilot study. Br. J. Radiol. 2016, 89, 20160105. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.; Kane, M.A.; List, M.A.; Brockstein, B.E.; Mehrotra, B.; Huo, D.; Mauer, A.M.; Pierce, C.; Dekker, A.; Vokes, E.E. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2005, 11, 8418–8424. [Google Scholar] [CrossRef]
- Guo, Y.; Ahn, M.J.; Chan, A.; Wang, C.H.; Kang, J.H.; Kim, S.B.; Bello, M.; Arora, R.S.; Zhang, Q.; He, X.; et al. Afatinib versus methotrexate as second-line treatment in Asian patients with recurrent or metastatic squamous cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 3): An open-label, randomised phase III trial. Ann. Oncol. 2019, 30, 1831–1839. [Google Scholar]
- Ham, J.C.; van Meerten, E.; Fiets, W.E.; Beerepoot, L.V.; Jeurissen, F.J.F.; Slingerland, M.; Jonker, M.A.; Husson, O.; van der Graaf, W.T.A.; van Herpen, C.M.L. Methotrexate plus or minus cetuximab as first-line treatment in a recurrent or metastatic (R/M) squamous cell carcinoma population of the head and neck (SCCHN), unfit for cisplatin combination treatment, a phase Ib-randomized phase II study Commence. Head Neck 2020, 42, 828–838. [Google Scholar] [CrossRef]
- Harrington, K.J.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.F.; Ahn, M.J.; Soria, A.; Machiels, J.P.H.; Mach, N.; Mehra, R.; et al. Quality of Life with Pembrolizumab for Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: KEYNOTE-040. J. Natl. Cancer Inst. 2021, 113, 171–181. [Google Scholar] [CrossRef]
- Machiels, J.P.; Subramanian, S.; Ruzsa, A.; Repassy, G.; Lifirenko, I.; Flygare, A.; Sørensen, P.; Nielsen, T.; Lisby, S.; Clement, P.M. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: An open-label, randomised phase 3 trial. Lancet Oncol. 2011, 12, 333–343. [Google Scholar] [CrossRef]
- Machiels, J.P.; Haddad, R.I.; Fayette, J.; Licitra, L.F.; Tahara, M.; Vermorken, J.B.; Clement, P.M.; Gauler, T.; Cupissol, D.; Grau, J.J.; et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. Lancet Oncol. 2015, 16, 583–594. [Google Scholar] [PubMed]
- Menon, N.; Patil, V.; Noronha, V.; Joshi, A.; Bhattacharjee, A.; Satam, B.J.; Mathrudev, V.; Laskar, S.G.; Prabhash, K. Quality of life in patients with locally advanced head and neck cancer treated with concurrent chemoradiation with cisplatin and nimotuzumab versus cisplatin alone—Additional data from a phase 3 trial. Oral Oncol. 2021, 122, 105517. [Google Scholar] [CrossRef]
- Pfister, D.G.; Su, Y.B.; Kraus, D.H.; Wolden, S.L.; Lis, E.; Aliff, T.B.; Zahalsky, A.J.; Lake, S.; Needle, M.N.; Shaha, A.R.; et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: A pilot phase II study of a new combined-modality paradigm. J. Clin. Oncol. 2006, 24, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Fayette, J.; Cupissol, D.; Del Campo, J.M.; Clement, P.M.; Hitt, R.; Degardin, M.; Zhang, W.; Blackman, A.; Ehrnrooth, E.; et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann. Oncol. 2014, 25, 1813–1820. [Google Scholar] [CrossRef]
- Stewart, J.S.; Cohen, E.E.; Licitra, L.; Van Herpen, C.M.; Khorprasert, C.; Soulieres, D.; Vodvarka, P.; Rischin, D.; Garin, A.M.; Hirsch, F.R.; et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2009, 27, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Truong, M.T.; Zhang, Q.; Rosenthal, D.I.; List, M.; Axelrod, R.; Sherman, E.; Weber, R.; Nguyen-Tân, P.F.; El-Naggar, A.; Konski, A.; et al. Quality of Life and Performance Status From a Substudy Conducted Within a Prospective Phase 3 Randomized Trial of Concurrent Accelerated Radiation Plus Cisplatin With or Without Cetuximab for Locally Advanced Head and Neck Carcinoma: NRG Oncology Radiation Therapy Oncology Group 0522. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 687–699. [Google Scholar] [PubMed]
- Yang, P.; Liu, J.; Yong, H.; Ma, J.; Gao, X. Effect of cetuximab combined with IMRT and concurrent chemotherapy in treating locally advanced nasopharyngeal carcinoma. J. Balk. Union Oncol. 2021, 26, 138–144. [Google Scholar]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Kaasa, S.; Bjordal, K.; Aaronson, N.; Moum, T.; Wist, E.; Hagen, S.; Kvikstad, A. The EORTC core quality of life questionnaire (QLQ-C30): Validity and reliability when analysed with patients treated with palliative radiotherapy. Eur. J. Cancer 1995, 31a, 2260–2263. [Google Scholar] [CrossRef]
- Bjordal, K.; Hammerlid, E.; Ahlner-Elmqvist, M.; de Graeff, A.; Boysen, M.; Evensen, J.F.; Biörklund, A.; de Leeuw, J.R.; Fayers, P.M.; Jannert, M.; et al. Quality of life in head and neck cancer patients: Validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J. Clin. Oncol. 1999, 17, 1008–1019. [Google Scholar]
- Bjordal, K.; de Graeff, A.; Fayers, P.M.; Hammerlid, E.; van Pottelsberghe, C.; Curran, D.; Ahlner-Elmqvist, M.; Maher, E.J.; Meyza, J.W.; Brédart, A.; et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. Eur. J. Cancer 2000, 36, 1796–1807. [Google Scholar]
- Singer, S.; Wollbrück, D.; Wulke, C.; Dietz, A.; Klemm, E.; Oeken, J.; Meister, E.F.; Gudziol, H.; Bindewald, J.; Schwarz, R. Validation of the EORTC QLQ-C30 and EORTC QLQ-H&N35 in patients with laryngeal cancer after surgery. Head Neck 2009, 31, 64–76. [Google Scholar] [PubMed]
- Hassan, S.J.; Weymuller, E.A., Jr. Assessment of quality of life in head and neck cancer patients. Head Neck 1993, 15, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J.; et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, L.L.; Zimmerman, G.J.; Cella, D.F.; Long, S.A. Quality of life and functional status measures in patients with head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 482–487. [Google Scholar] [CrossRef]
- List, M.A.; D’Antonio, L.L.; Cella, D.F.; Siston, A.; Mumby, P.; Haraf, D.; Vokes, E. The Performance Status Scale for Head and Neck Cancer Patients and the Functional Assessment of Cancer Therapy-Head and Neck Scale: A study of utility and validity. Cancer 1996, 77, 2294–2301. [Google Scholar] [CrossRef]
- Pulte, D.; Brenner, H. Changes in survival in head and neck cancers in the late 20th and early 21st century: A period analysis. Oncologist 2010, 15, 994–1001. [Google Scholar] [CrossRef]
- Efficace, F.; Collins, G.S.; Cottone, F.; Giesinger, J.M.; Sommer, K.; Anota, A.; Schlussel, M.M.; Fazi, P.; Vignetti, M. Patient-Reported Outcomes as Independent Prognostic Factors for Survival in Oncology: Systematic Review and Meta-Analysis. Value Health 2021, 24, 250–267. [Google Scholar] [CrossRef]
- Iravani, K.; Jafari, P.; Akhlaghi, A.; Khademi, B. Assessing whether EORTC QLQ-30 and FACT-G measure the same constructs of quality of life in patients with total laryngectomy. Health Qual. Life Outcomes 2018, 16, 183. [Google Scholar] [CrossRef]
- Shrestha, A.; Martin, C.; Burton, M.; Walters, S.; Collins, K.; Wyld, L. Quality of life versus length of life considerations in cancer patients: A systematic literature review. Psychooncology 2019, 28, 1367–1380. [Google Scholar] [CrossRef]
- Cheville, A.L.; Alberts, S.R.; Rummans, T.A.; Basford, J.R.; Lapid, M.I.; Sloan, J.A.; Satele, D.V.; Clark, M.M. Improving Adherence to Cancer Treatment by Addressing Quality of Life in Patients With Advanced Gastrointestinal Cancers. J. Pain Symptom Manag. 2015, 50, 321–327. [Google Scholar] [CrossRef] [PubMed]
- King, S.; Exley, J.; Parks, S.; Ball, S.; Bienkowska-Gibbs, T.; MacLure, C.; Harte, E.; Stewart, K.; Larkin, J.; Bottomley, A.; et al. The use and impact of quality of life assessment tools in clinical care settings for cancer patients, with a particular emphasis on brain cancer: Insights from a systematic review and stakeholder consultations. Qual. Life Res. 2016, 25, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.; Aranha, A.M.F.; Borges, A.H.; Volpato, L.E.R. Head and Neck Cancer Patients’ Quality of Life: Analysis of Three Instruments. J. Dent. 2020, 21, 31–41. [Google Scholar]
- Vickers, A.J. Multiple assessment in quality of life trials: How many questionnaires? How often should they be given? J. Soc. Integr. Oncol. 2006, 4, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Aiyegbusi, O.L.; Roydhouse, J.; Rivera, S.C.; Kamudoni, P.; Schache, P.; Wilson, R.; Stephens, R.; Calvert, M. Key considerations to reduce or address respondent burden in patient-reported outcome (PRO) data collection. Nat. Commun. 2022, 13, 6026. [Google Scholar] [CrossRef]
- Aggarwal, H.; Punekar, R.S.; Li, L.; Carter, G.C.; Walker, M.S. Quality of life analysis of patients treated with cetuximab or cisplatin for locoregionally advanced squamous cell carcinoma of head and neck in the United States. Health Qual. Life Outcomes 2020, 18, 195. [Google Scholar] [CrossRef]
- Boone, S.L.; Rademaker, A.; Liu, D.; Pfeiffer, C.; Mauro, D.J.; Lacouture, M.E. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: Survey results. Oncology 2008, 72, 152–159. [Google Scholar] [CrossRef]
- Li, T.; Perez-Soler, R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target. Oncol. 2009, 4, 107–119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).