Prevalence and Effect of Low Skeletal Muscle Mass among Hepatocellular Carcinoma Patients Undergoing Systemic Therapy: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Literature Selection and Data Extraction
2.4. Assessment of Methodological Quality
2.5. Statistical Analysis
3. Results
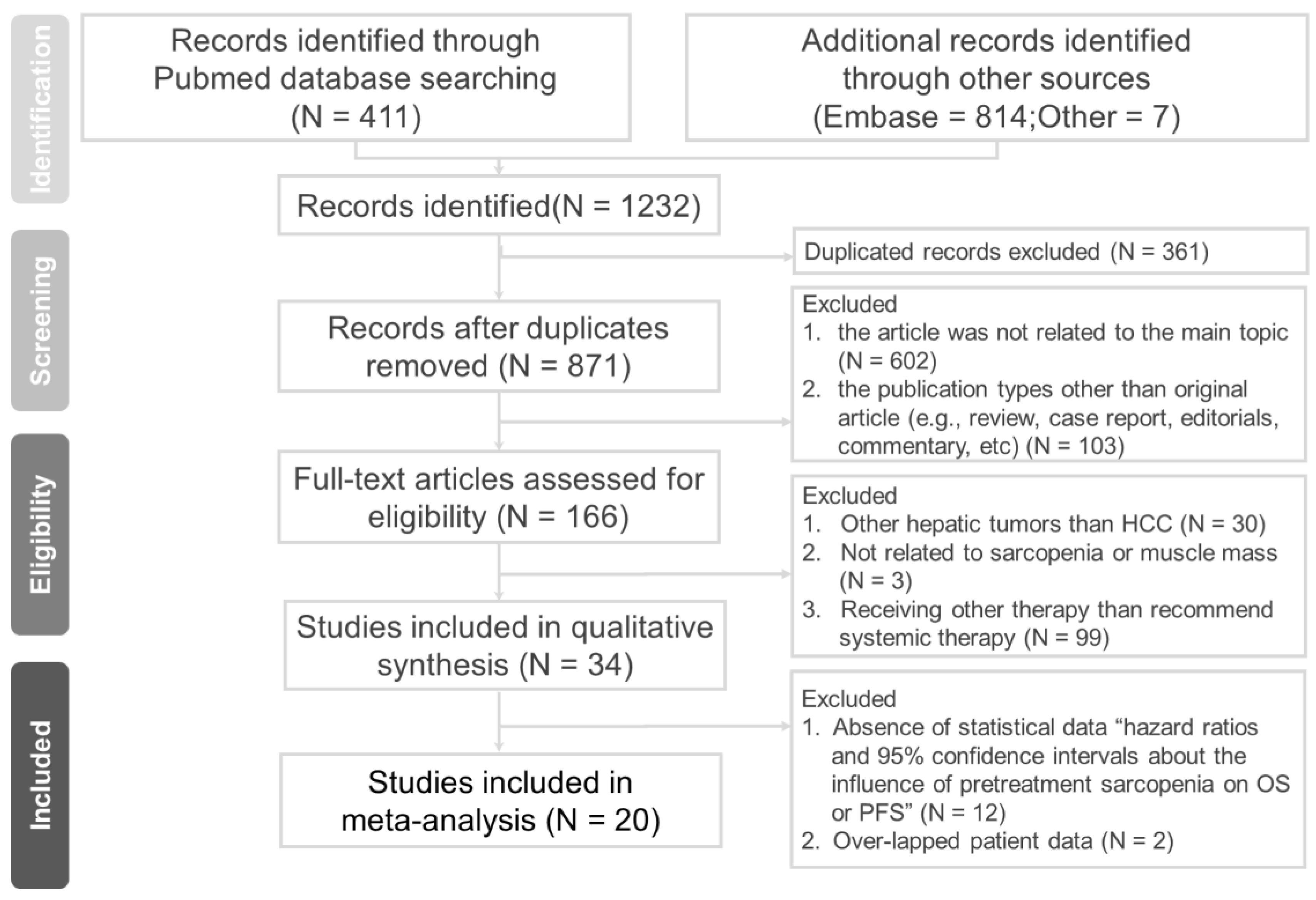
3.1. Literature Search and Study Selection
3.2. Characteristics of Included Studies
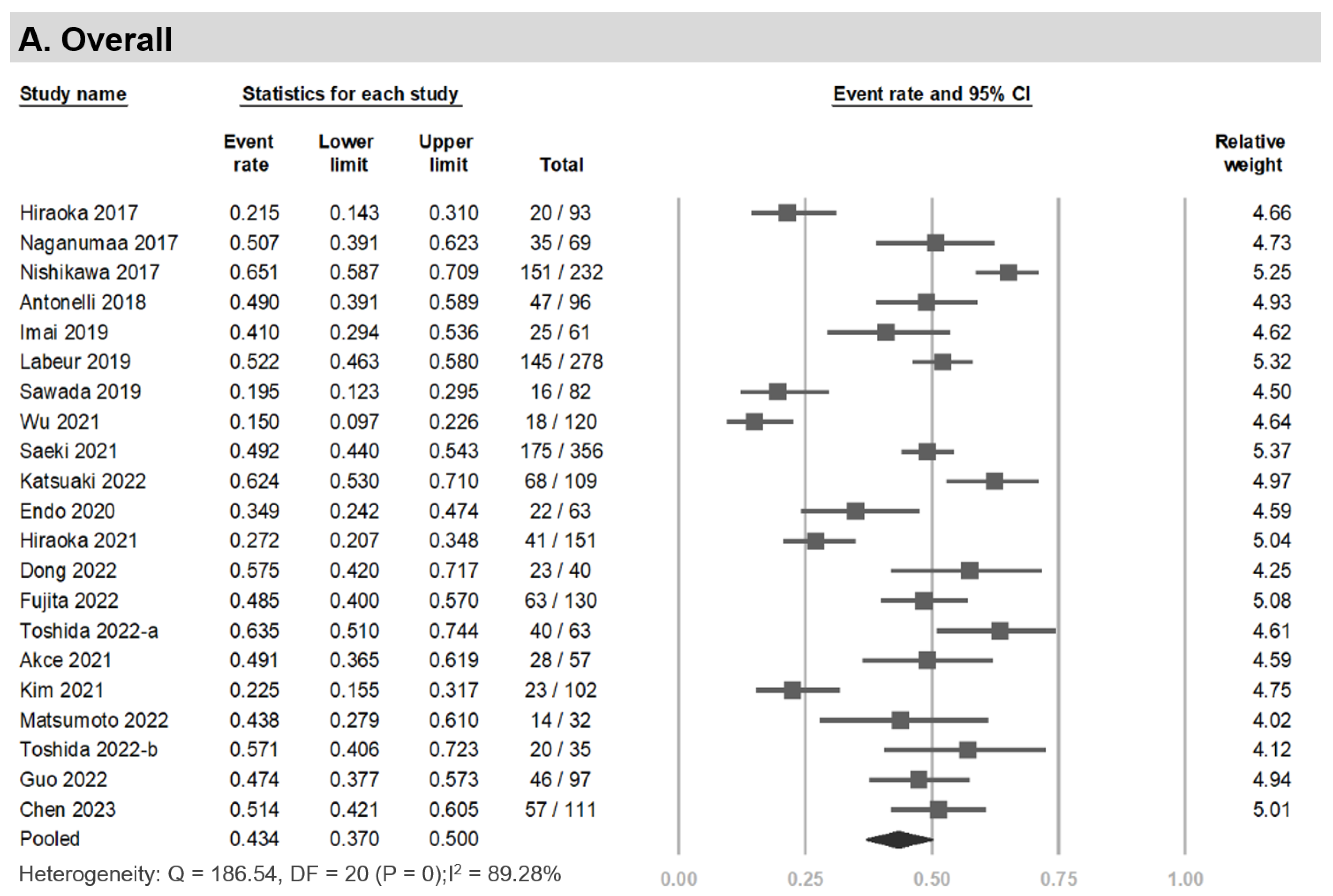
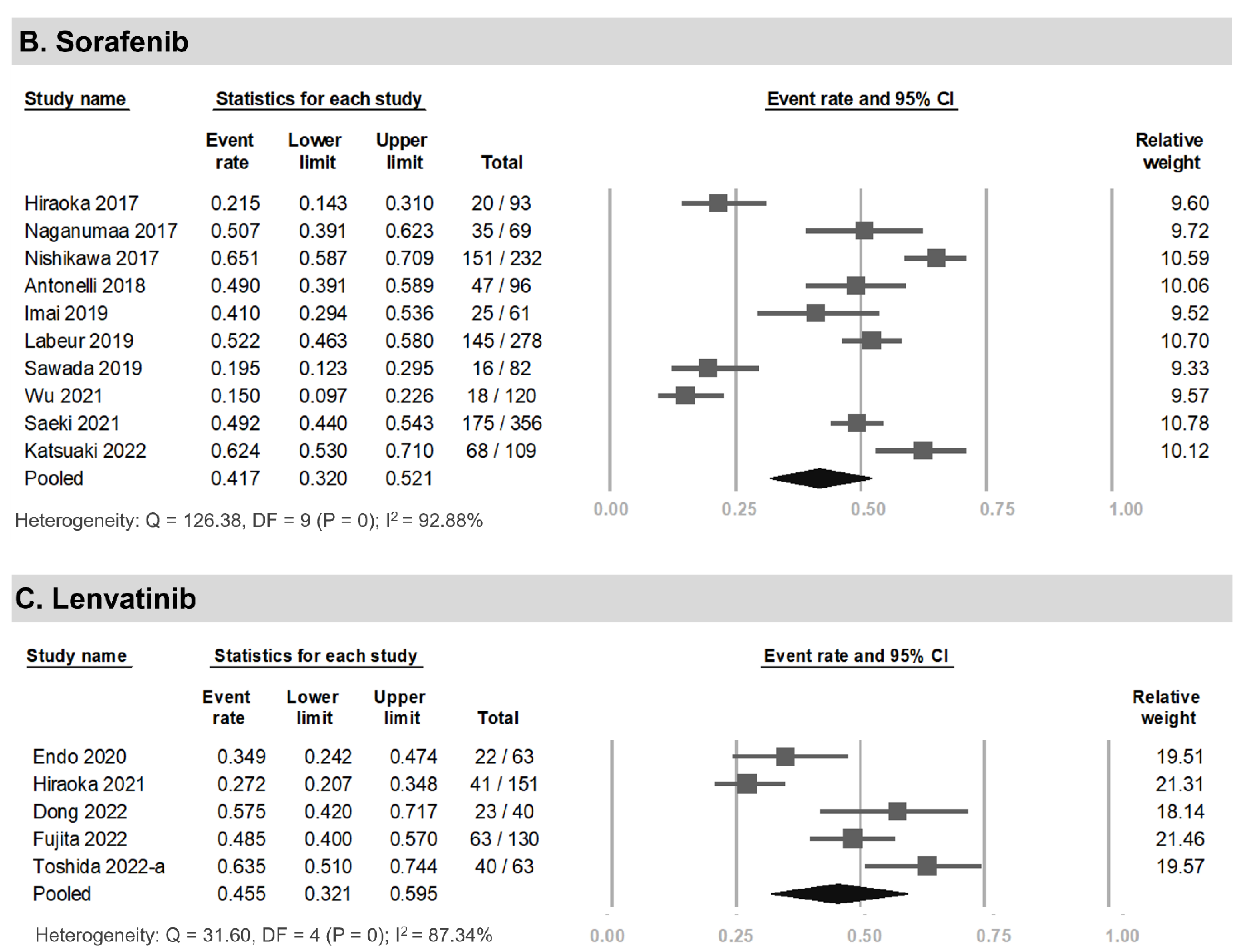
3.3. Prevalence of LSMM among HCC Patients Undergoing Systemic Therapy
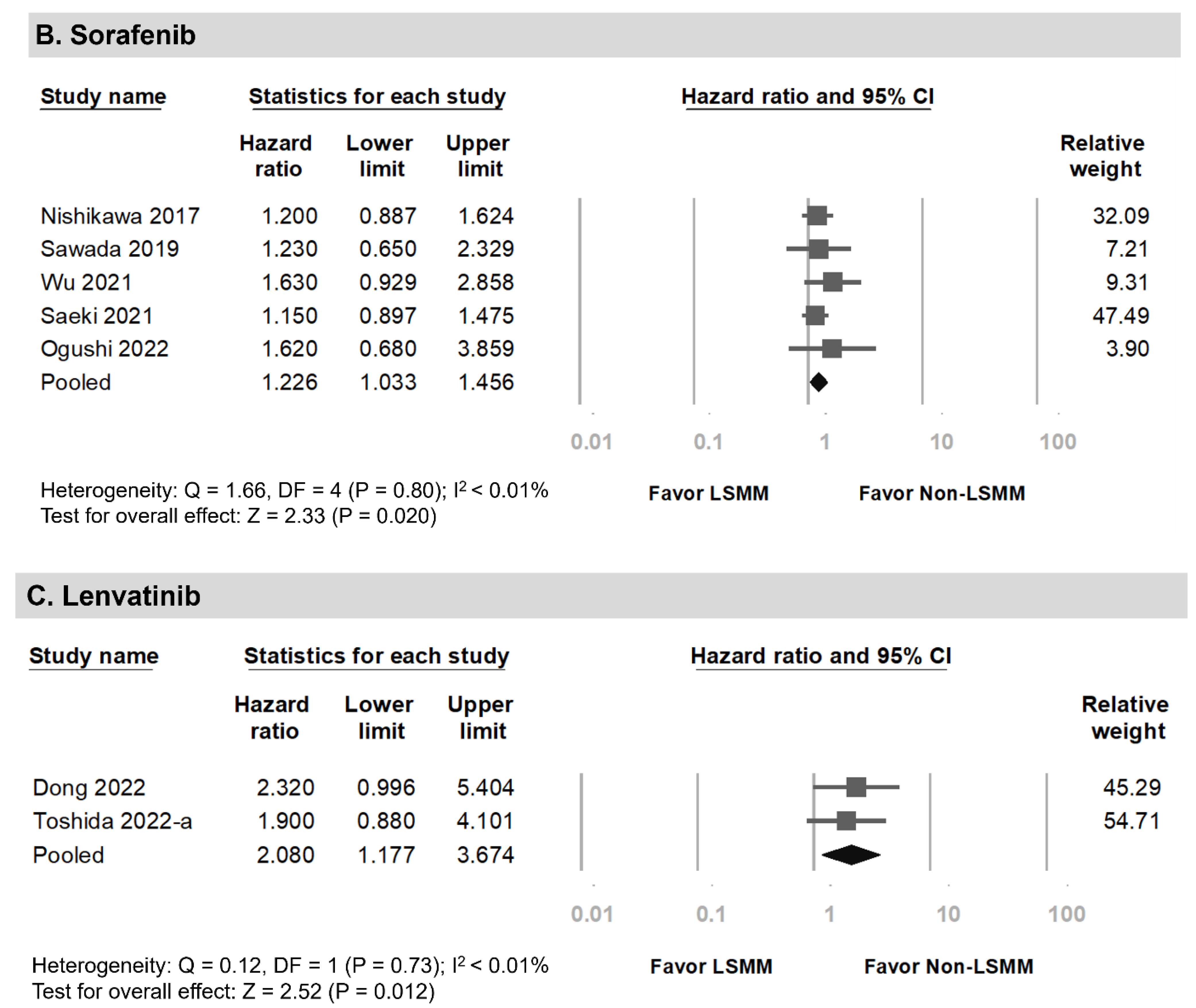
3.4. Overall Survival among HCC Patients Undergoing Systemic Therapy with Versus without LSMM
3.5. Progression-Free Survival among HCC Patients Undergoing Systemic Therapy with and without LSMM
3.6. Publication Bias and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491. [Google Scholar] [CrossRef]
- Sidali, S.; Trépo, E.; Sutter, O.; Nault, J. New concepts in the treatment of hepatocellular carcinoma. United Eur. Gastroenterol. J. 2022, 10, 765–774. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system: The 2022 update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef]
- Perisetti, A.; Goyal, H.; Yendala, R.; Chandan, S.; Tharian, B.; Thandassery, R.B. Sarcopenia in hepatocellular carcinoma: Current knowledge and future directions. World J. Gastroenterol. 2022, 28, 432–448. [Google Scholar] [CrossRef]
- Yamasaki, T.; Saeki, I.; Yamauchi, Y.; Matsumoto, T.; Suehiro, Y.; Kawaoka, T.; Uchikawa, S.; Hiramatsu, A.; Aikata, H.; Kobayashi, K.; et al. Management of Systemic Therapies and Hepatic Arterial Infusion Chemotherapy in Patients with Advanced Hepatocellular Carcinoma Based on Sarcopenia Assessment. Liver Cancer 2022, 11, 329–340. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Fu, W.; Zhang, G.; Feng, X.; Wang, X.; Wang, F.; Zhang, L.; Deng, Y. Association between sarcopenia and prognosis of hepatocellular carcinoma: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 978110. [Google Scholar] [CrossRef]
- March, C.; Omari, J.; Thormann, M.; Pech, M.; Wienke, A.; Surov, A. Prevalence and role of low skeletal muscle mass (LSMM) in hepatocellular carcinoma. A systematic review and meta-analysis. Clin. Nutr. ESPEN 2022, 49, 103–113. [Google Scholar] [CrossRef]
- Stuck, A.K.; Basile, G.; Freystaetter, G.; de Godoi Rezende Costa Molino, C.; Lang, W.; Bischoff-Ferrari, H.A. Predic-tive validity of current sarcopenia definitions (EWGSOP2, SDOC, and AWGS2) for clinical outcomes: A scoping review. J. Cachexia Sarcopenia Muscle 2023, 14, 71–83. [Google Scholar] [CrossRef]
- Gallo, P.; Silletta, M.; De Vincentis, A.; Prinzi, F.L.; Terracciani, F.; Di Fazio, G.; Flagiello, V.; Gentilucci, U.V.; Incalzi, R.A.; Picardi, A. Sarcopenia in Hepatocellular Carcinoma: Pathogenesis and Man-agement. Chemotherapy 2022, 67, 152–163. [Google Scholar] [CrossRef]
- Wu, C.H.; Liang, P.C.; Hsu, C.H.; Chang, F.T.; Shao, Y.Y.; Ting-Fang Shih, T. Total skeletal, psoas and rectus abdomi-nis muscle mass as prognostic factors for patients with advanced hepatocellular carcinoma. J. Formos. Med. Assoc. 2021, 120, 559–566. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Kariyama, K.; Tada, T.; Tani, J.; Fukunishi, S.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; et al. Clinical importance of muscle volume in lenvatinib treatment for hepatocellular carcinoma: Analysis adjusted with inverse probability weighting. J. Gastroenterol. Hepatol. 2020, 36, 1812–1819. [Google Scholar] [CrossRef]
- Saeki, I.; Yamasaki, T.; Maeda, M.; Kawano, R.; Hisanaga, T.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T.; Takami, T.; et al. No Muscle Depletion with High Visceral Fat as a Novel Beneficial Bi-omarker of Sorafenib for Hepatocellular Carcinoma. Liver Cancer 2018, 7, 359–371. [Google Scholar] [CrossRef]
- Antonelli, G.; Gigante, E.; Iavarone, M.; Begini, P.; Sangiovanni, A.; Iannicelli, E.; Biondetti, P.; Pellicelli, A.M.; Miglioresi, L.; Marchetti, P.; et al. Sarcopenia is associated with reduced survival in patients with advanced hepatocellular carcinoma undergoing sorafenib treatment. United Eur. Gastroenterol. J. 2018, 6, 1039–1048. [Google Scholar] [CrossRef]
- Nishikawa, H.; Nishijima, N.; Enomoto, H.; Sakamoto, A.; Nasu, A.; Komekado, H.; Nishimura, T.; Kita, R.; Kimura, T.; Iijima, H.; et al. Prognostic significance of sarcopenia in patients with hepato-cellular carcinoma undergoing sorafenib therapy. Oncol. Lett. 2017, 14, 1637–1647. [Google Scholar] [CrossRef]
- Naganuma, A.; Hoshino, T.; Suzuki, Y.; Uehara, D.; Kudo, T.; Ishihara, H.; Sato, K.; Kakizaki, S.; Yamada, M.; Takagi, H. Association between Skeletal Muscle Depletion and Sorafenib Treatment in Male Patients with Hepatocellular Carcinoma: A Retrospective Cohort Study. Acta Med. Okayama 2017, 71, 291–299. [Google Scholar]
- Hiraoka, A.; Hirooka, M.; Koizumi, Y.; Izumoto, H.; Ueki, H.; Kaneto, M.; Kitahata, S.; Aibiki, T.; Tomida, H.; Miyamoto, Y.; et al. Muscle volume loss as a prognostic marker in hepatocellular car-cinoma patients treated with sorafenib. Hepatol. Res. 2017, 47, 558–565. [Google Scholar] [CrossRef]
- Guan, J.; Yang, Q.; Chen, C.; Wang, G.; Zhu, H. Prognostic value of low skeletal muscle mass in hepatocellular carcinoma patients treated with sorafenib or lenvatinib: A meta-analysis. Excli J. 2021, 20, 1–16. [Google Scholar]
- Chen, B.B.; Liang, P.C.; Shih, T.T.F.; Liu, T.H.; Shen, Y.C.; Lu, L.C.; Lin, Z.Z.; Hsu, C.; Hsu, C.H.; Cheng, A.L.; et al. Sarcopenia and myosteatosis are associated with survival in patients re-ceiving immunotherapy for advanced hepatocellular carcinoma. Eur. Radiol. 2023, 33, 512–522. [Google Scholar] [CrossRef]
- Toshida, K.; Itoh, S.; Tomiyama, T.; Morinaga, A.; Kosai, Y.; Tomino, T.; Kurihara, T.; Nagao, Y.; Morita, K.; Harada, N.; et al. Comparison of the prognostic effect of sarcopenia on atezolizumab plus bevacizumab and lenvatinib therapy in hepatocellular carcinoma patients. JGH Open 2022, 6, 477–486. [Google Scholar] [CrossRef]
- Matsumoto, H.; Tsuchiya, K.; Nakanishi, H.; Hayakawa, Y.; Yasui, Y.; Uchihara, N.; Suzuki, K.; Tanaka, Y.; Miyamoto, H.; Ishido, S.; et al. Clinical Usefulness of Monitoring Muscle Volume during Atezolizumab Plus Bevacizumab Therapy in Patients with Unresectable Hepatocellular Carcinoma. Cancers 2022, 14, 3551. [Google Scholar] [CrossRef]
- Fujita, M.; Abe, K.; Kuroda, H.; Oikawa, T.; Ninomiya, M.; Masamune, A.; Okumoto, K.; Katsumi, T.; Sato, W.; Iijima, K.; et al. Influence of skeletal muscle volume loss during lenvatinib treatment on prognosis in unresectable hepatocellular carcinoma: A multicenter study in Tohoku, Japan. Sci. Rep. 2022, 12, 6479. [Google Scholar] [CrossRef]
- Dong, D.; Shi, J.Y.; Shang, X.; Liu, B.; Xu, W.L.; Cui, G.Z.; Wang, N.Y. Prognostic significance of sarcopenia in patients with hepatocellular carci-noma treated with lenvatinib: A retrospective analysis. Medicine 2022, 101, e28680. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Yu, J.I.; Park, H.C.; Yoo, G.S.; Choi, C.; Hong, J.Y.; Lim, H.Y.; Lee, J.; Choi, M.S.; Lee, J.E.; et al. Incorporating sarcopenia and inflammation with radiation therapy in patients with hepatocellular carcinoma treated with nivolumab. Cancer Immunol. Immunother. 2020, 70, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Akce, M.; Liu, Y.; Zakka, K.; Martini, D.J.; Draper, A.; Alese, O.B.; Shaib, W.L.; Wu, C.; Wedd, J.P.; Sellers, M.T.; et al. Impact of Sarcopenia, BMI, and Inflammatory Biomarkers on Survival in Advanced Hepatocellular Carcinoma Treated with Anti-PD-1 Antibody. Am. J. Clin. Oncol. 2020, 44, 74–81. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, Y.; Wu, F.; Dong, X.; Zheng, C. Prognostic impact of sarcopenia in patients with hepatocellular carcinoma treated with PD-1 inhibitor. Therap. Adv. Gastroenterol. 2022, 15, 17562848221142417. [Google Scholar] [CrossRef] [PubMed]
- Su, G.L.; Altayar, O.; O’shea, R.; Shah, R.; Estfan, B.; Wenzell, C.; Sultan, S.; Falck-Ytter, Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022, 162, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.C.; Lai, C.C.; Chen, Y.H.; Lai, E.C.; Hung, M.J.; Chi, C.C. Associations of thiazide use with skin cancers: A systematic review and meta-analysis. BMC Med. 2022, 20, 228. [Google Scholar] [CrossRef]
- Kuo, L.-T.; Shao, S.-C.; Chi, C.-C. Ten essential steps for performing a systematic review: A quick tutorial. Dermatol. Sin. 2022, 40, 204–206. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 December 2021).
- Imai, K.; Takai, K.; Miwa, T.; Taguchi, D.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Rapid Depletions of Subcutaneous Fat Mass and Skeletal Muscle Mass Pre-dict Worse Survival in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Cancers 2019, 11, 1206. [Google Scholar] [CrossRef]
- Labeur, T.A.; van Vugt, J.L.; Cate, D.W.T.; Takkenberg, R.B.; Ijzermans, J.N.; Koerkamp, B.G.; de Man, R.A.; van Delden, O.M.; Eskens, F.A.; Klümpen, H.-J. Body Composition Is an Independent Predictor of Outcome in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Liver Cancer 2018, 8, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Saitho, Y.; Hayashi, H.; Hasebe, T.; Nakajima, S.; Ikuta, K.; Fujiya, M.; Okumura, T. Skeletal muscle mass is associated with toxicity, treatment tolerability, and additional or subsequent therapies in patients with hepatocellular carcinoma receiving sorafenib treatment. JGH Open 2019, 3, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Kuroda, H.; Kanazawa, J.; Sato, T.; Fujiwara, Y.; Abe, T.; Sato, H.; Kooka, Y.; Oikawa, T.; Sawara, K.; et al. Impact of Grip Strength in Patients with Unresectable Hepatocellular Carcinoma Treated with Lenvatinib. Cancers 2020, 12, 2146. [Google Scholar] [CrossRef] [PubMed]
- Saeki, I.; Yamasaki, T.; Yamauchi, Y.; Takami, T.; Kawaoka, T.; Uchikawa, S.; Hiramatsu, A.; Aikata, H.; Kawano, R.; Kobayashi, K.; et al. Skeletal Muscle Volume Is an Independent Predictor of Survival af-ter Sorafenib Treatment Failure for Hepatocellular Carcinoma. Cancers 2021, 13, 2247. [Google Scholar] [CrossRef] [PubMed]
- Ogushi, K.; Chuma, M.; Numata, K.; Nozaki, A.; Moriya, S.; Uojima, H.; Kondo, M.; Morimoto, M.; Maeda, S. Impact of psoas muscle index assessed by a simple measurement method on tolerability and duration of continued treatment with sorafenib in hepatocellular carcinoma pa-tients. Eur. J. Gastroenterol. Hepatol. 2022, 34, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Parise, H.; A Payette, H.; Abad, L.W.; D’Agostino, R.; Jacques, P.F.; Wilson, P.W.; A Dinarello, C.; Harris, T.B. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: The Framingham Heart Study. Am. J. Med. 2003, 115, 429–435. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa e Silva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its patho-physiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef]
- Yang, M.; Shen, Y.; Tan, L.; Li, W. Prognostic Value of Sarcopenia in Lung Cancer: A Systematic Review and Meta-analysis. Chest 2019, 156, 101–111. [Google Scholar] [CrossRef]
- Xu, X.-T.; He, D.-L.; Tian, M.-X.; Wu, H.-J.; Jin, X. Prognostic Value of Sarcopenia in Patients with Diffuse Large B-Cell Lymphoma Treated With R-CHOP: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 816883. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Dou, Q.-L.; Zeng, Y.; Yang, Y.; Cheng, A.S.K.; Zhang, W.-W. Sarcopenia as a predictor of mortality in women with breast cancer: A meta-analysis and systematic review. BMC Cancer 2020, 20, 172. [Google Scholar] [CrossRef]
- Mir, O.; Coriat, R.; Blanchet, B.; Durand, J.-P.; Boudou-Rouquette, P.; Michels, J.; Ropert, S.; Vidal, M.; Pol, S.; Chaussade, S.; et al. Sarcopenia Predicts Early Dose-Limiting Toxicities and Pharmacokinetics of Sorafenib in Patients with Hepatocellular Carcinoma. PLoS ONE 2012, 7, e37563. [Google Scholar] [CrossRef]
- Edinger, A.L.; Thompson, C.B. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell 2002, 13, 2276–2288. [Google Scholar] [CrossRef]
- Huemer, F.; Schlintl, V.; Hecht, S.; Hackl, H.; Melchardt, T.; Rinnerthaler, G.; Greil, R.; Weiss, L. Regorafenib Is Associated with Increased Skeletal Muscle Loss Com-pared to TAS-102 in Metastatic Colorectal Cancer. Clin. Color. Cancer 2019, 18, 159–166. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sar-copenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.; Hartshorn, K.; Rahma, O.; Lin, N.; Snyder-Cappione, J.E. Aging, immune senescence, and immunotherapy: A comprehensive review. Semin. Oncol. 2018, 45, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Park, S.; Han, S.; Ahn, J.H.; Kim, S.; Sinn, D.H.; Jeong, W.K.; Ko, J.S.; Gwak, M.S.; Kim, G.S. Sarcopenia as a predictor of post-transplant tumor recurrence after living donor liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Sci. Rep. 2018, 8, 7157. [Google Scholar] [CrossRef]
- Waning, D.L.; Mohammad, K.S.; Reiken, S.; Xie, W.; Andersson, D.C.; John, S.; Chiechi, A.; Wright, L.E.; Umanskaya, A.; Niewolna, M.; et al. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat. Med. 2015, 21, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Flint, T.R.; Janowitz, T.; Connell, C.M.; Roberts, E.W.; Denton, A.E.; Coll, A.P.; Jodrell, D.I.; Fearon, D.T. Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab. 2016, 24, 672–684. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel III, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFbeta attenuates tumour response to PD-L1 blockade by con-tributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]



| First Author (Year) | Country | Setting | Treatment Regimen | Patients, n (Male/ Female) | Age, Years | Method Used to Estimate Muscle Mass | Cut-off Value for Pretreatment LSMM | LSMM (%) Yes/No | Study Period (Year) | Into OS MA | OS Adjustment Factors | OS HR (95% CI) | Into PFS MA | PFS Adjustment Factors | PFS HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sorafenib | |||||||||||||||
| Hiraoka (2017) [17] | Japan | multi-center | sorafenib 800/400/200 mg/day | 93 (81/12) | 68.3 # | L3-PMI | M: 4.24 cm2/m2; F: 2.50 cm2/m2 | 21.5% (20/73) | 4 | Yes | age, sex, DCP > 100 mAU/mL, positive for bone metastases | 2.16 (1.07–4.36) | NR | NR | NR |
| Nishikawa (2017) [15] | Japan | multi-center | sorafenib 800 mg/day | 232 (181/51) | 72 * | L3-SMI | M: 36.2 cm2/m2; F: 29.6 cm2/m2 | 65.1% (151/81) | 5.5 | Yes | age, sex, initial dose, ECOG-PS extrahepatic metastases, portal vein invasion, tumor burden ≥ 50%, AST, ALP, ascites, serum albumin level, serum AFP, DCP | 2.74 (1.92–3.92) | Yes | univariate | 1.20 (0.89–1.63) |
| Naganumaa (2017-M) (2017-F) [16] | Japan | single-center, Takasaki | sorafenib (100–800 mg/day) | 69 (51/18) | 72 * | L3-SMI | <42 cm2/m2 | 51% (35/34) | 5.5 | Yes | age, Child–Pugh score, clinical stage, AFP | 2.32 (1.13–4.77) | NR | NR | NR |
| Antonelli (2018) [14] | Rome | multi-center | sorafenib | 96 (75/21) | 69 # | L3-SMI | M: BMI > 25 : 53cm2/m2 BMI < 25: 43 cm2/m2; F: 41 cm2/m2 | 49% (47/49) | 2.1 | Yes | age, sex, vascular invasion, MELD score, | 1.63 (1.05–2.53) | NR | NR | NR |
| Imai (2019) [31] | Japan | single-center, Gifu | sorafenib 800 mg/day | 61 (54/7) | 67.3 # | L3-SMI | M: 42 cm2/m2; F: 38 cm2/m2 | 41% (25/36) | 4.1 | Yes | age, sex, L3SMI, DSFMI, therapeutic effect | 2.45 (1.27–4.73) | NR | NR | NR |
| Labeur (2019) [32] | Netherlands | multi-center | sorafenib | 278 (220/58) | 64 * | L3-SMI | M: 52.4 cm2/m2; F: 38.5 cm2/m2 | 52% (145/133) | 4 | Yes | univariate | 1.20 (0.94–1.54) | NR | NR | NR |
| Sawada (2019) [33] | Japan | single-center, Asahikawa | sorafenib | 82 (67/15) | 69 # | L3-SMI | M: 36.2 cm2/m2; F: 29.6 cm2/m2 | 20% (16/66) | 4.1 | Yes | age, sex, AFP ≥ 100 ng/mL, BCLC stage C, additional/subsequent therapies, low skeletal muscle mass, positive invasion of hepatic vessels, duration of Sorafenib treatment | 1.15 (0.54–2.47) | Yes | univariate | 1.23 (0.65–2.33) |
| Wu (2021) (males only) [11] | Taiwan | single-center, Taipei | first-line sorafenib-containing therapy or combined with tegafur/uracil | 120 | NA | L3-SMI | 39.1 cm2/m2 | 15% (18/102) | 10 | Yes | age, LSMM of TSM; elderly; underweight; HBsAg; anti-HCV; ALBI grade 2; AFP ≥ 400 ng/mL; macrovascular invasion; extrahepatic metastasis; BCLC C; CLIP score ≥ 3; ECOG PS ≥ 1; combination therapy (vs. sorafenib alone) | 2.12 (1.13–3.97) | Yes | age, sex, body weight, HBsAg, HCV, ALBI group, AFP, macro vascular invasion, extrahepatic metastasis, BCLC C, CLIP score, ECOG PS, combination therapy | 1.63 (0.93–2.86) |
| Saeki (2021) [35] | Japan | multi-center | sorafenib 800 mg/day | 356 (287/69) | 69.5 * | L3-SMI | M < 45 cm2/m2; F < 38 cm2/m2 | 49% (175/181) | 7.5 | Yes | age, sex, BMI, ECOG-PS, Child–Pugh class, tumor number, tumor size, macrovascular invasion, extrahepatic spread | 1.50 (1.13–2.00) | Yes | age, sex, BMI, ECOG-PS, Child–Pugh class, tumor number, tumor size, macrovascular invasion, extrahepatic spread | 1.15 (0.90–1.48) |
| Ogushi (2022) [36] | Japan | single-center, Yokohama | sorafenib 800/400 mg/day | 109 (84/25) | 73 * | L3-PMI | M: 7.038 cm2/m2; F: 4.400 cm2/m2 | 62% (68/41) | 6.7 | Yes | age, sex, HCV or HBV, BMI, Child–Pugh score, PS, BCLC stage, past history of TACE, AFP, DCP | 1.48 (0.90–2.45) | Yes | age, sex, HCV or HBV, BMI, Child–Pugh score, PS, BCLC stage, past history of TACE, AFP, DCP | 1.62 (0.68–1.86) |
| Lenvatinib | |||||||||||||||
| Endo (2020) [34] | Japan | single-center, Iwate | lenvatinib (8 mg/day < 60 kg or 12 mg/day > 60 kg) | 63 (53/10) | 71 * | L3-SMI | M < 42 cm2/m2; F < 38 cm2/m2 | 35% (22/47) | 0.7 | Yes | univariate | 1.06 (0.43–2.56) | NR | NR | NR |
| Hiraoka (2021) [12] | Japan | multi-center | lenvatinib (8 mg/day < 60 kg or 12 mg/day > 60 kg) | 151 (116/35) | NA | L3-PMI | M: 4.24 cm2/m2; F: 2.50 cm2/m2 | 27% (41/110) | 2 | Yes | age, sex, AFP, BCLC stage (C and D), BMI | 1.65 (1.02–2.69) | NR | NR | NR |
| Dong (2022) [23] | China | single-center, Changchun | lenvatinib (8 mg/day < 60 kg or 12 mg/day > 60 kg) | 40 (37/3) | 59 * | L3-SMI | M: 42 cm2/m2; F: 38 cm2/m2 | 57.5% (23/17) | 0.8 | Yes | age, Alb, maximum tumor diameter, portal vein thrombosis | 3.89 (1.26–12.05) | Yes | univariate | 2.32 (1.00–5.41) |
| Fujita (2022) [22] | Japan | multi-center | lenvatinib 4 mg/8 mg/12 mg based on their body weight and liver function reserve | 130 (107/23) | 70 * | L3-PMI | M: 6 cm2/m2; F: 3.4 cm2/m2 | 48% (63/67) | 2.5 | Yes | univariate | 1.43 (0.83–2.47) | NR | NR | NR |
| Toshida (2022-a) [20] | Japan | single-center, Fukuoka | lenvatinib (8 mg/day < 60 kg or 12 mg/day > 60 kg | 63 (43/20) | 69–75 * | L3-SMI | M: 42 cm2/m2; F: 38 cm2/m2 | 68.2% (40/23); ATZ/BEV, 57.1% (20/15); LEN, 63.5% (40/23) | 3.8 | Yes | age, sex, LMR < 4.0, ALBI grade, best response | 2.86 (1.11–7.33) | Yes | ALBI grade, 2/3 (vs. 1) | 1.90 (0.88–4.10) |
| Immunotherapy | |||||||||||||||
| Akce (2021) [25] | Georgia | single-center | anti-PD1 antibody-containing regimens | 57 (44/13) | 66 * | L3-SMI | M: 43 cm2/m2; F: 39 cm2/m2 | 49.1% (28/29) | 3 | Yes | age, sex, BCLC stage (B and C vs. A); Inflammation biomarkers | 1.71 (0.73–4.00) | Yes | sex, Child–Pugh score, inflammation biomarkers | 0.99 (0.54–1.85) |
| Kim (2021) [24] | Korea | single-center, Seoul | intravenous nivolumab 3 mg/kg | 102 (87/15) | 61.3 * | L3-SMI | M: 42 cm2/m2; F: 38 cm2/m2 | 22.5% (23/79) | 2 | Yes | age, sex, ECOG PS, ALBI group, AFP, intrahepatic tumor burden, surgery, RT, ALC and NLR risk group | 1.11 (0.62–1.97) | Yes | univariate | 1.31 (0.80–2.14) |
| Matsumoto (2022) [21] | Japan | single-center, Tokyo | ATZ 1200 mg + BEV 15 mg/kg Q3W | 32 (19/13) | 77 * | L3-SMI | M: 42 cm2/m2; F: 38 cm2/m2 | 53% (17/15) | 1.5 | NR | NR | NR | Yes | univariate | 1.10 (0.40–3.10) |
| Toshida (2022-b) [20] | Japan | single-center, Fukuoka | ATZ 1200 mg + BEV 15 mg/kg Q3W | 35(28/7) | 72 * | L3-SMI | M: 42 cm2/m2; F: 38 cm2/m2 | 57.1% (20/15) | 3.8 | Yes | univariate | 1.30 (0.29–5.86) | Yes | A+B+L: ALBI grade, 2/3 (vs. 1), sarcopenia, LEN:ALBI grade, 2/3(vs1), sarcopenia | 1.26 (0.44–4.20) |
| Guo (2022) [26] | China | single-center, Hubei | camrelizumab | 97 (79/18) | 52 # | L3-SMI | M: 37.7 cm2/m2; F: 34.3 cm2/m2 | 47.4% (46/51) | 1.3 | NR | NR | NR | number of tumors, Child-Pugh class, macrovascular invasion, extrahepatic spread, ECOG performance, tumor size, PLR, NLR | 1.97 (1.17–3.33) | |
| Chen (2023) [19] | Taiwan | single-center, Taipei | immunotherapy | 111 (97/14) | 59 # | L3-SMI | M: 40.8 cm2/m2; F: 34.9 cm2/m2 | 51.3% (57/54) | 5 | Yes | Age, sex, multinodular or massive, Child–Pugh, myosteatosis | 2.09 (1.29–3.39) | Yes | univariate | 1.50 (1.00–2.25) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, M.-H.; Tseng, C.-W.; Hsu, C.-S.; Chen, Y.-C.; Kao, I.-T.; Wu, C.-Y.; Shao, S.-C. Prevalence and Effect of Low Skeletal Muscle Mass among Hepatocellular Carcinoma Patients Undergoing Systemic Therapy: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 2426. https://doi.org/10.3390/cancers15092426
Kuo M-H, Tseng C-W, Hsu C-S, Chen Y-C, Kao I-T, Wu C-Y, Shao S-C. Prevalence and Effect of Low Skeletal Muscle Mass among Hepatocellular Carcinoma Patients Undergoing Systemic Therapy: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(9):2426. https://doi.org/10.3390/cancers15092426
Chicago/Turabian StyleKuo, Meng-Hsuan, Chih-Wei Tseng, Ching-Sheng Hsu, Yen-Chun Chen, I-Ting Kao, Chen-Yi Wu, and Shih-Chieh Shao. 2023. "Prevalence and Effect of Low Skeletal Muscle Mass among Hepatocellular Carcinoma Patients Undergoing Systemic Therapy: A Systematic Review and Meta-Analysis" Cancers 15, no. 9: 2426. https://doi.org/10.3390/cancers15092426
APA StyleKuo, M.-H., Tseng, C.-W., Hsu, C.-S., Chen, Y.-C., Kao, I.-T., Wu, C.-Y., & Shao, S.-C. (2023). Prevalence and Effect of Low Skeletal Muscle Mass among Hepatocellular Carcinoma Patients Undergoing Systemic Therapy: A Systematic Review and Meta-Analysis. Cancers, 15(9), 2426. https://doi.org/10.3390/cancers15092426







