Simple Summary
Ophthalmic malignancies refer to rare, highly diversified, aggressive neoplasms. Unlike other types of solid tumors, tissue biopsy is not recommended for intraocular malignancies so a limited number of tissue samples are available for translational research programs. To date, very few biobanks dedicated to ophthalmic malignancies have been reported. The aim of this article was to present the challenges raised by ophthalmic malignancies in order to obtain biospecimens for research purposes, and the biobank dedicated to ophthalmic malignancies set up in our institution (Côte d’Azur University, Nice, France) was detailed. There is an urgent need to develop ocular malignancy biobanks to enhance translational research projects, develop international collaborations, and, ultimately, optimize customized medicine for the treatment of these tumors.
Abstract
Ophthalmic malignancies include various rare neoplasms involving the conjunctiva, the uvea, or the periocular area. These tumors are characterized by their scarcity as well as their histological, and sometimes genetic, diversity. Uveal melanoma (UM) is the most common primary intraocular malignancy. UM raises three main challenges highlighting the specificity of ophthalmic malignancies. First, UM is a very rare malignancy with an estimated incidence of 6 cases per million inhabitants. Second, tissue biopsy is not routinely recommended due to the risk of extraocular dissemination. Third, UM is an aggressive cancer because it is estimated that about 50% of patients will experience metastatic spread without any curative treatment available at this stage. These challenges better explain the two main objectives in the creation of a dedicated UM biobank. First, collecting UM samples is essential due to tissue scarcity. Second, large-scale translational research programs based on stored human samples will help to better determine UM pathogenesis with the aim of identifying new biomarkers, allowing for early diagnosis and new targeted treatment modalities. Other periocular malignancies, such as conjunctival melanomas or orbital malignancies, also raise specific concerns. In this context, the number of biobanks worldwide dedicated to ocular malignancies is very limited. The aims of this article were (i) to describe the specific challenges raised by a dedicated ocular malignancy biobank, (ii) to report our experience in setting up such a biobank, and (iii) to discuss future perspectives in this field.
1. Introduction
Ophthalmic malignancies include a wide spectrum of highly variable tumors involving the conjunctiva, the uvea, the eyelids, and the orbit. Most of these tumors are considered rare and there is no consensus standard of care [1]. Uveal melanoma (UM) is the most common primary intraocular malignancy, with an incidence of only 6 cases per million inhabitants in Western countries [2]. Therefore, UM is considered a rare but aggressive malignancy [3]. The last decade has been marked by dramatic molecular and genetic improvements related to UM. However, the daily clinical management of patients is lacking and it is estimated that 50% of UM patients will experience metastatic spread [4]. To date, there is no curative treatment available for metastatic UM. Current animal models are scarce, especially metastatic models, and these do not allow for the generalization of the findings to humans [5]. Therefore, there is an urgent need to develop customized targeted treatments through collaborative translational and clinical research based on robust clinical, imaging, biological, and genetic data obtained from patients [6].
In 1996, the term “biobank” was first introduced and rapidly popularized [7]. Most human malignancies now have dedicated biobanks [8]. However, ophthalmic malignancy biobanks are poorly reported in the literature, thus limiting data sharing and international collaboration [9]. Since 2013, the Laboratory of Clinical and Experimental Pathology of Nice University (France) has maintained an accredited biobank dedicated to ophthalmic malignancies that belongs to the Cote d’Azur Biobank (BB-0033-00025, https://biobank-cotedazur.fr, accessed on 1 January 2023).
The aims of this article were to detail the Nice Ophthalmic MAligancy (NOMA) biobank and to highlight the current challenges, objectives, and limitations encountered when setting up such a dedicated ophthalmic malignancy biobank.
2. What Are the Specific Challenges Associated with Ophthalmic Malignancies?
2.1. A Wide Variety of Malignancies
The periocular area is a unique and exceptional example of melanoma diversity in the human body. Several types of melanomas may be found in clinical practice, such as primary or metastatic cutaneous melanomas, conjunctival melanomas (CM), UM, and primary or secondary orbital melanomas (Figure 1). For example, although anatomically close (only a few millimeters), CM and UM are very different genetically [10]. It is now well-established that UM and primary orbital melanoma result from driver mutations in the GNAQ and GNA11 genes, whereas cutaneous melanoma and CM result from mutations in the BRAF, NRAS, NF1, and c-Kit genes [11]. These molecular differences explain why CM has benefited from the therapeutic opportunities developed for cutaneous melanoma with anti-BRAF and anti-MEK targeted therapies unlike UM [1,12]. Another major difference is the role of the immune system in UM, CM, and cutaneous melanoma. Due to the “immune privilege”, most conventional anti-PD-1 and anti-CTLA-4 therapies have failed to demonstrate any benefit in UM, while they are effective in cutaneous melanoma and CM [13]. Tebentafusp, a specific anti-GP100 immunotherapy, has recently shown promising results in metastatic UM but a longer follow-up is needed to ascertain its efficacy [14].
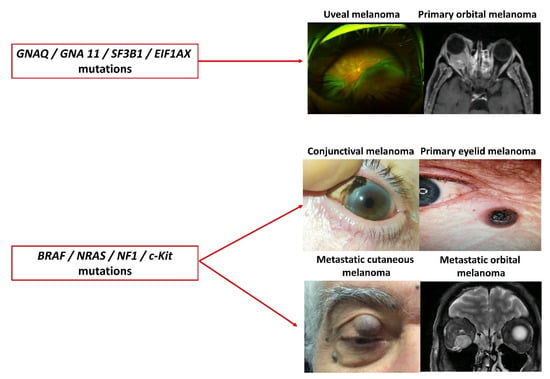
Figure 1.
The spectacular variety of periocular melanomas.
2.2. A Wide Variety of Clinical Presentations and Aggressiveness
In line with other tumors located elsewhere, the clinical presentation may vary greatly from one patient to another. A few decades ago, the prognosis of UM was essentially based on histological findings, and the epithelioid subtype, a high mitotic index, or an extraocular extent were considered poor prognostic factors [15]. More recently, the loss of expression of BAP1 has been shown to be a major pejorative prognostic factor through the induction of tumor dissemination in UM [16] and it is now systematically investigated in daily clinical practice [17]. Lately, chromosomal and genetic findings have revolutionized the prognosis of UM patients by allowing for the distinguishing of patients with a low versus high metastatic risk [16]. Monosomy of chromosome 3 associated with chromosome 8q gain has been shown to be highly predictive of metastatic spread by Trolet et al. [18]. Also, Onken et al. have shown that 15-gene expression profiling in UM allowed for the accurate distinction of class 1 (low risk) and class 2 (high risk) metastatic risk profiles [19].
2.3. Tumor Rarity
Except for eyelid basal cell carcinoma, most ophthalmic malignancies are extremely rare, thus limiting the collection of numerous tissue samples. The annual incidence per million inhabitants of several ophthalmic malignancies is summarized in Table 1. A slight increase in CM incidence has been found over the last few decades [20], but this has not been confirmed in UM [15,21].

Table 1.
Incidence of the main ophthalmic malignancies.
2.4. Lack of Tissue Biopsy: The UM Example
Unlike most malignancies, the diagnosis of UM is based on clinical and ocular ultrasound findings with no need for a tissue biopsy. Transscleral or transvitreal UM biopsies are not recommended and are even contraindicated because they are considered unsafe (risk of intravitreal haemorrhage, retinal detachment) with a non-negligible risk of extraocular tumor dissemination [24]. The only interest of tissue biopsy would be to assess tumor prognosis. However, because there is no curative treatment for disseminated UM, this argument is widely discussed, and the benefit/risk balance of UM biopsy does not appear to be favorable. About 90% of UM cases will be treated conservatively with proton therapy, radiosurgery, or brachytherapy with favorable local outcomes [15]. Only large or diffuse intraocular disseminated tumors are treated with enucleation. The last decade has been marked by an increase in eye-sparing indications and even large UM are increasingly eligible for conservative treatments without major side effects [25]. Taken together, these data explain why UM samples are usually not collected to build biobanks.
2.5. Emergence of Liquid Biopsies
Because most ophthalmic malignancies are rare and since tissue biopsy is not routinely performed in these latter tumors, liquid biopsies (LBs) have recently been popularized [26]. LB in the onco-ophtalmology domain mainly refers to the collection of any potential tumor fluids, such as venous blood or aqueous humor (AH). Several features may be monitored, including circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), circulating tumor RNA (ctRNA), circulating vesicles such as exosomes, and tumor-educated platelets [26]. The advantages of LB include: (i) its non-invasiveness, (ii) the fact that it may be repeated as often as required, thus allowing for disease monitoring, (iii) the large amounts of fluid available, (iv) the fact that it better reflects the metastatic spread of the disease in the case of venous LB, and (v) the fact that it allows the establishment of a reliable prognosis in various malignancies [26]. Thus, two types of LBs are currently being investigated in the field of ophthalmic malignancies.
2.5.1. Venous Liquid Biopsies: The UM Example
CTC and ctDNA have been studied in various prospective studies in UM. CTCs were first investigated in UM in 2008 by Ulmer et al. [27]. CTCs appear to be tumor-specific since they have never been identified in healthy patients and benign lesions. Higher CTC counts have been found in metastatic versus primary UM and in class 2 versus class 1 tumors [26]. It is still unclear whether CTC detection in primary UM might predict further metastatic spread. This feature is of strong interest because it could justify the use of systemic adjuvant treatment for the management of primary UM. The limitations include the use of a wide variety of CTC isolation and identification methods [26].
Higher ctDNA levels have also been found in metastatic UM compared to primary UM. Only one study has compared the CTC counts and ctDNA levels in 40 patients with metastatic UM, and CTCs were detected using the CellSearch® (Menarini Silicon Biosystems, Florence, Italy) device in only 30% of patients, while the ctDNA was detected in 84% of patients [28]. Interestingly, only the ctDNA significantly correlated with the progression-free survival and overall survival in the multivariate analysis [28].
2.5.2. Aqueous Humor (AH) Biopsies
AH collection is a simple, minimally invasive procedure usually performed on an outpatient basis. AH LBs have been mainly studied in retinoblastomas which are the most common primary intraocular malignancy found in childhood. The body of evidence indicates that AH collection is useful for the diagnosis and monitoring of treatment response [29]. It has been shown that AH collection is much more reliable than venous LB [30]. Only a few studies have investigated AH LBs in UM and they have suggested that the AH could be used as a diagnostic and prognostic biomarker in the future [31,32].
3. Legal, Economic, and Technical Aspects
3.1. Legal Considerations
The NOMA biobank is part of the hospital-integrated Cote d’Azur Biobank stored and managed by Nice University Hospital (France). The Cote d’Azur Biobank has developed several platforms, including tissue, LB, and molecular pathology platforms, located in the Laboratory of Clinical and Experimental Pathology (www.biobank-cotedazur.fr, accessed on 1 January 2023) [33]. The main tumors stored have been collected from patients with lung, thyroid, skin, head and neck, and more recently, ocular diseases. The Côte d’Azur Biobank obtained the NF S96-900 certification (National French Standard) in 2010. In 2022, it obtained the ISO 9001 certification as well as the international ISO 20387 standard specific for biobanking activity [34]. These standards guarantee the high quality of the whole biobanking process. Moreover, the LPCE has obtained an accreditation according to the ISO 15189 norm in 2013 for surgical and molecular pathology diagnosis, notably for ophtalmopathology, therefore ensuring the quality of the pre-analytical, analytical, and post-analytical steps before transferring samples into the Côte d’Azur Biobank.
3.2. Funding Considerations
A major issue regarding biobanks, especially biobanks dedicated to a specific pathology, is their financial viability in terms of development and long-term maintenance [9,35,36]. Creating a biobank requires an expert team, various costly processes and equipment, in particular for sample storage, along with computer software [33,37]. The business model of the Côte d’Azur Biobank (BB-0033-00025) is partially supported by French public funding programs funded by the French Ministry of Health, such as the MERRI (“Mission d’Enseignement, Recherche, Reference et Innovation”) and DGOS (“Direction Générale de l’Offre de Soins”) programs. To a lesser extent, different material transfer agreements (MTAs) with public and private institutes allow for biobank funding [33]. These public–public and public–private partnerships were included in the yearly Côte d’Azur Biobank budget [33,38]. In this regard, a costing policy for samples and various associated expertise provided by the biobankers to the scientists, and contracts and MTAs have been elucidated in association with the Research and Innovation Department of Nice University Hospital. These costs and their applications have been assessed according to national and international recommendations while taking into account the share of investment for the biobank and the scientific collaboration level [39]. The contracts signed by the scientists and other partners require mentioning the biobank in their publications in different sections depending on the contribution of the biobank members (co-author, citation of the biobank in the Acknowledgments section).
3.3. Technical, Space, and Computer Considerations
The implementation of the NOMA Biobank is summarized in Table 2. Tissue biopsy, liquid biopsy, and AH puncture were stored in the NOMA biobank in 2013, 2018, and 2020, respectively.

Table 2.
Summary of the NOMA Biobank sample processing (all ocular tumors).
3.3.1. Biobanking Process for UM
The biobanking process, illustrated for UM, is summarized in Figure 2. Briefly, the diagnosis of UM is based on clinical and ultrasound findings obtained by ocular oncologists at Nice University Hospital, one of the two tertiary referral care centers in ocular oncology in France [40]. Preoperative examination includes dilated fundus examination, ultra-wide field retinography (Optomap, Optos PLC, Dunfermline, Scotland, UK), OCT, and ICG angiography, when required. A systemic work-up includes a liver MRI or ultrasonography. Written informed consent is signed preoperatively by the patients in order to be able to include them into the biobank database.
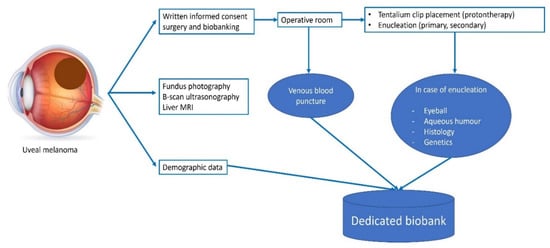
Figure 2.
Whole biobanking process for UM patients.
In UM patients, undergoing conservative surgery (placement of tantalum clips followed by proton therapy) and venous LBs (4 EDTA blood collection tubes of 8 mL) may be performed at the time of initial surgery. We no longer perform transscleral biopsies due to their low yield and the risk of extraocular tumor dissemination. In patients undergoing primary or secondary enucleation, AH collection (once the eye has been enucleated), LB, tissue histology, and genetic analyses may be performed. The NOMA biobank and the LPCE are located in the same department. The operative rooms are closely connected to the laboratory with the use of an intra-hospital pneumatic tube transportation system. Solid and LB samples are usually processed within 15 min of receipt in the laboratory. The processing, amount, and storage conditions for each sample type are summarized in Table 3.

Table 3.
Summary of collected samples in terms of dedicated processing, amount, and storage conditions. (*) The paraffin protocol has been previously described [41]; (**) two consecutive centrifugations at 2000× g at 4 °C; (***) first centrifugation at 1000× g for 20 min in a dedicated Ficoll tube, followed by a second centrifugation at 400× g for 10 min at room temperature. FFPE: formalin-fixed paraffin-embedded. PBMC: peripheral blood mononuclear cells.
Regarding the various procedures for tissue sample collection, the diagnosis was made and the specimens to be collected for the biobank were selected by a pathologist expert in ophthalmology (SL). When possible, the tumor tissue as well as the healthy adjacent tissue were both frozen or fixed in formalin and stored. For each tumor with frozen samples, a mirror sample was embedded in paraffin for quality control and assessment of the percentage of tumor cells, and one fragment was dedicated to the diagnosis while the others were stored for research purposes. Each tissue sample was weighted before freezing. All the samples were processed and stored in appropriate facilities in accordance with standard guidelines for equipment, computing, and safety. Clinical and imaging data are stored in a dedicated accredited database (Synapse medical, Table 4). Synapse software is a specific health data warehouse approved by the CNIL (Commission Nationale de l’Informatique et des Libertés; https://www.cnil.fr/, accessed on 1 January 2023). Biological, histological, and genetic data are saved in another dedicated software belonging to the Cote d’Azur Biobank (https://biobank-cotedazur.fr, accessed on 1 January 2023). We recently acquired MBioLIMS BIOBANKING® software (Modul-bio, Marseille, France) specifically dedicated to biobanking activities. This allows for a complete view of all the samples stored and statistical reports on the biobank activity [42]. Trolet classification was established in 2009 based on tumoral chromosomal abnormalities [18]. Two subtypes based on chromosome 3 disomy (Trolet 1a, 1b) and chromosome 3 monosomy (Trolet 2a, 2b, 2c) have been established. Gain of 8q associated with monosomy 3 was classified as Trolet 2b or 2c and it has been strongly associated with an increased risk of metastatic spread [18,43]. In this work, tumoral DNA extraction was performed on paraffin tissue sections using the Maxwell automate (Promega, Madison, WI, USA) with a dedicated kit. Genomic hybridation and nucleotidic polymorphisms were established by using CGH-array and SNP-array analyses with the Genechip Oncoscan Array (Affymetrix, Santa Clara, CA, USA).

Table 4.
Summary of the data collected in the dedicated SYNAPSE database.
3.3.2. Process for Other Ophthalmic Malignancies
The storage process for other ophthalmic malignancies is similar to that described above. Table 5 summarizes the main data recorded for other ophthalmic malignancies.

Table 5.
Summary of the data collected in the LPCE database (for all tumors except UM).
4. Resources of the NOMA Biobank
4.1. All Tumors
Overall, 207 patients have been included in the NOMA biobank since 2013. As shown in Figure 3, 160 patients had an UM, 31 had a CM, and 16 had an orbital malignancy. Table 6 summarizes the main histological subtypes of the tumors stored in the NOMA biobank. For the 160 patients with an UM, 41% and 77.5% of samples were stored as LBs and solid biopsies, respectively. This preponderance of LB samples is easily explained by the fact that tissue biopsy is not routinely performed in the case of UM and is only obtained when enucleation is performed. The low number of conjunctival and orbital tumors included in the NOMA Biobank is explained by the fact that the tumor size is usually too small to be able to include tissues in a biobank and the samples are only used for clinical diagnostic purposes.
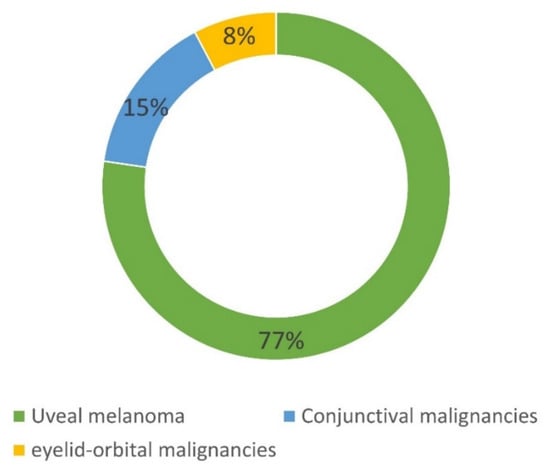
Figure 3.
Composition of the NOMA Biobank samples.

Table 6.
Detailed composition of the NOMA Biobank samples.
4.2. Uveal Melanoma
The number of UM samples available in the NOMA Biobank is summarized in Figure 4. Regarding LBs, most samples were prepared and stored as plasma samples. Only a few AH samples (15 patients) were available in our biobank. This is explained by the fact that we have only systematically collected the AH from enucleated eyeballs since 2020.
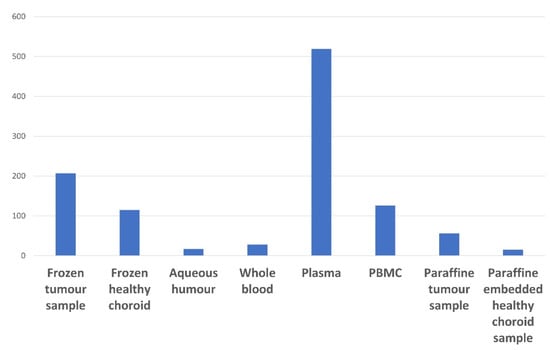
Figure 4.
Summary of the UM tissue and liquid biopsy samples available in the NOMA Biobank.
Among the 160 UM patients included in the NOMA Biobank, 66 (41%) were enucleated, thus allowing for tissue collection (Figure 5).
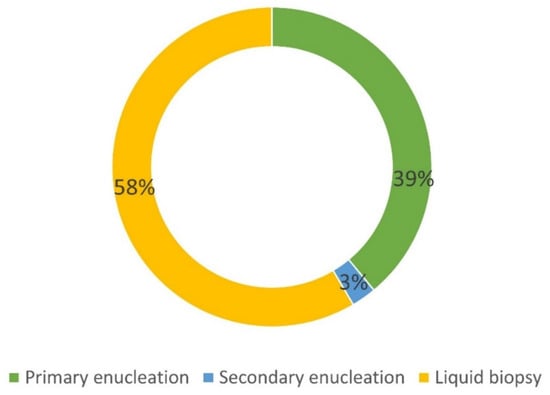
Figure 5.
Details of the origin of UM samples included in the NOMA Biobank. Primary enucleation: no treatment before surgery. Secondary enucleation: treatment before surgery (proton therapy).
The histological features of the enucleated eyeballs are provided in Table 7. Among the patients who underwent secondary enucleation, only those referred for tumor recurrence were included. Patients who underwent secondary enucleation for painful and blind eye due to extensive necrosis of the tumor with proton therapy were not included in the biobank. The cold ischemia time was defined as the time between enucleation and sample freezing for the biobank. The BAP 1 status was assessed by immunohistochemistry. Most of the UM tumors showed histological signs of aggressiveness (67% of cases with epithelioid cells, 20% of cases with extraocular extent, 33% of cases with ciliary body involvement).

Table 7.
Histological features of enucleated UM patients. Cold ischemia time (time between resected specimen collection in the operative room and the freezing procedure); * n = 40 patients.
The pathological Tumor–Node–Metastasis (pTNM) classification of the tumors of enucleated patients is provided in Figure 6. About half of the patients had a pT3 UM.
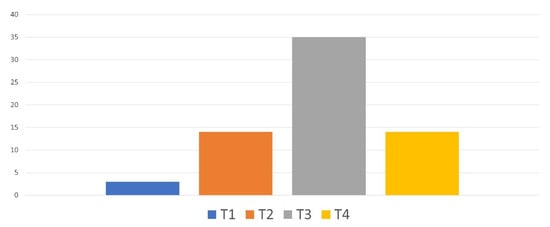
Figure 6.
Pathological TNM classification of the tumors of enucleated patients.
Among the 66 enucleated patients, genetic analyses were performed in 28 (42%) patients. The chromosomal abnormalities as well as the subsequent Trolet classification are shown in Figure 7. Overall, most patients were classified as “poor prognosis” with the loss of a chromosome 3 and a chromosome 8q gain and 64% were classified as “Trolet 2b and 2c”. These findings were consistent with the histological features described above.
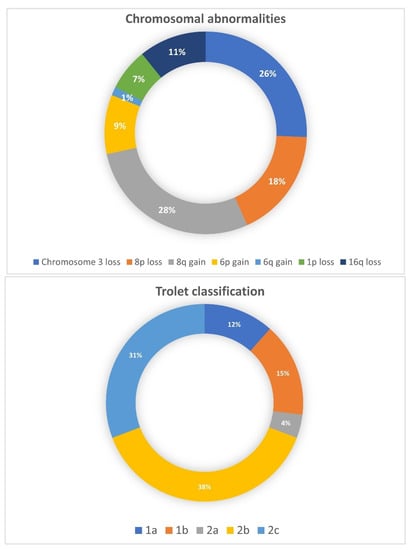
Figure 7.
Chromosomal abnormalities and Trolet classification of 28 enucleated UM patients.
5. Objectives of Setting Up a Dedicated Ophthalmic Malignancy Biobank
5.1. Diagnosis
The diagnosis of a conjunctival or periocular malignancy is routinely made by pathologists. However, in certain circumstances such as UM, a tissue biopsy is not recommended, and treatment is only based on clinical and radiological findings, incurring a risk of diagnostic inaccuracy, such as the enucleation of a non-tumor intraocular mass. AH collection is not routinely performed for the diagnosis of UM. However, it is a minimally invasive procedure performed on an outpatient basis. Several studies have recently shown that the presence of driver mutations in the GNAQ and GNA11 genes could be used as a reliable diagnostic biomarker in UM, thus highlighting the need for a dedicated venous blood and AH biobank in UM [32].
5.2. Translational Research and Precision Medicine
Healthy and tumor tissues as well as LBs from ocular tumors are stored in our biobank, allowing translational research and tumorigenesis exploration. As highlighted above, very rare samples, such as AH samples, are systematically collected prospectively. AH samples should permit the identification of biomarkers for the diagnosis of UM and retinoblastoma follow-up [31]. To date, no targeted therapy has been found to be effective in the treatment of metastatic UM nor the prevention of metastatic spread [12]. In 2018, the tyrosine kinase inhibitor sunitinib administrated in adjuvant therapy in high risk (loss of chromosome 3, gain 8q, Class 2, T3–T4 by American Joint Committee on Cancer classification) primary UM was considered promising due to improvements in overall survival [44]. However, the study was not randomized (since the control group was an historical cohort) and results were not subsequently validated in an independent cohort. However, this study highlights the urgent need to discover new targeted therapies that can be administered, and make room for adjuvant therapeutic decision-making during the primary treatment of ocular malignant tumors. In this context, the systematic storage of UM samples exhibits a strong value for translational research by identifying future therapeutic targets.
5.3. Impact of Radiotherapy on UM Genetics
The storing of secondary enucleation samples following radiotherapy is of high interest and has been minimally studied until now. The impact of radiotherapy on genetic abnormalities and subsequent prognosis is still debated since controversial studies on this topic have been reported. Genetic abnormalities have been found to be more prevalent and more complex in previously irradiated tumors [45]. In contrast, another study found that chromosome 3 status and subsequent prognosis were not modified by radiotherapy [46]. There is also a debate regarding the difficulty of assessing genetic status (especially chromosome 3) in previously irradiated UM. Furthermore, karyotyping and FISH detection were found to be significantly altered by radiotherapy [45], whereas in another study, the detection of chromosome 3 microsatellite analysis was successfully determined [47]. In our experience, genetic testing depends on the etiology of secondary enucleation. Secondary enucleations performed for blind and/or painful eyes are associated with a high rate of post-radiotherapy necrosis, leading genetic testing to be inconclusive. Conversely, secondary enucleation performed for tumor recurrence can be more easily processed. However, it is not always clear whether genetic testing in this case is carried out on a recurrent tumor (i.e., tumor resistance to radiotherapy) or on a primary tumor located outside the field of irradiation.
5.4. Pretreatment Screening
A biobank may also be useful to investigate whether a patient might be eligible for a given treatment. Until recently, there was not standard of care for metastatic UM. Tebentafusp, a specific anti-GP100 immunotherapy, has recently been approved by the Food and Drug Administration but only in HLA-A*02:01 UM patients [48]. The HLA status of patients with cryopreserved blood samples can be easily determined and might help clinicians determine whether or not a patient is a good candidate for tebentafusp treatment.
5.5. Scientific Output
A biobank dedicated to a specific pathology should be managed by an expert team involved in the setting up and development of research projects in tight collaboration with physicians, surgical and molecular pathologists, and researchers. Thus, a specific biobank should be acknowledged and cited in scientific publications. As shown in Figure 8, the number of publications related to ocular oncology published by our department has markedly increased over the last few years, both for fundamental and clinical research programs, mainly through the use of ocular resources from the biobank. Several articles based on the NOMA biobank resources have recently been published in peer-reviewed journals with high impact factors [1,6,49,50,51].
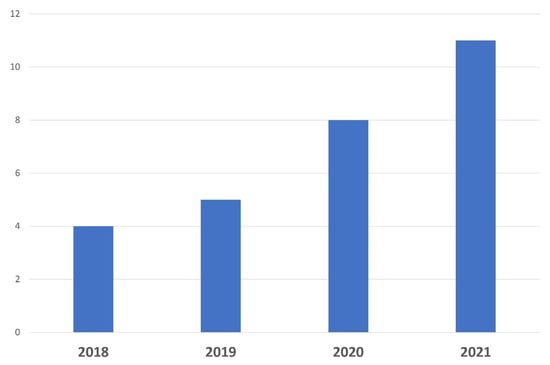
Figure 8.
Number of scientific publications related to ocular oncology and translational research published over the last 4 years by Nice University Hospital thanks to the use of samples.
5.6. National and International Collaborations
Data sharing enabled Nice University Hospital to collaborate with several internationally recognized ocular oncology departments, as summarized in Table 8. Of the 369 FFPE samples stored in the NOMA biobank, 44 (11.9%) have been used for collaborative scientific projects. A specific scientific and ethics committee is mandated to assess each public or private request to use samples according to the current regulations.

Table 8.
Current national and international collaborations.
5.7. Information for Patients and Other Health Professionals
Because clear and accurate information is mandatory before obtaining any written informed consent, our Ocular Oncology Department has recently developed a dedicated website for patients referred to our tertiary care center (https://www.cancerdesyeux.fr, accessed on 1 January 2023). This website provides valuable information on patients’ care and follow-up, as well as research projects. A dedicated website has also been created by the Clinical and Experimental Pathology Laboratory Department of Nice University Hospital to provide useful information to patients and health professionals (http://biobank-cotedazur.fr/, accessed on 1 January 2023). Scientific congresses and relevant learned societies such as the ISOO (International Society of Ocular Oncology), OOG (Ocular Oncology Group), ESOPRS (European Society of Ophthalmic Plastic and Reconstructive Surgery), and SFO (Société Francaise d’Ophtalmologie) allow for the diffusion of knowledge about ophthalmic malignancies, including biobanks.
5.8. Education and Training
A Master in Science (MSc) degree entitled “Biobanks and complex data management” has been set up by the Côte d’Azur University (Nice, France). Developed by recognized experts in the field, this program is the first European MSc degree in the field offered by a public university (https://univ-cotedazur.eu/msc/biobanks-complex-data-management, accessed on 1 January 2023). Dedicated to Biological Sciences, this unique program is intended for students aiming to acquire professional skills in order to provide evolving biobank services, meet regulatory and user requirements, and manage biobanks.
The “Biobanks and Complex Data Management” MSc allows international exchanges through a wide network of leading academic and industry partners.
6. Strengths of the NOMA Biobank
The strengths of our biobank are as follows:
- (i)
- The NOMA biobank is certified (ISO 9001, NF-96S-900) and accredited (ISO 20387) and belongs to an already well-established biobank (Cote d’Azur Biobank) (www.biobank-cotedazur.fr, accessed on 1 January 2023),
- (ii)
- It is stored in a university pathology laboratory (LPCE, Nice) accredited for clinical and molecular pathology according to the ISO 15189 standard,
- (iii)
- Clinical, imaging, histological, biological, and genetic data are collected for each patient,
- (iv)
- Its business model is supported by public funding programs,
- (v)
- It has allowed for an increase in the amount of translational research conducted,
- (vi)
- It allows for an increase in the number of scientific publications and national and international collaborations,
- (vii)
- A master’s degree entitled “Biobanks and Complex Data Management” has been set up by the Côte d’Azur University (Nice, France) to train students to become biobankers.
7. Limitations
However, the NOMA Biobank also has several limitations. First, its small sample size could be explained by the fact that both UM and CM are rarely found in daily clinical practice. In addition, as already mentioned previously, a tissue biopsy is not routinely performed in UM. Despite this, this dedicated ophthalmic biobank is larger than other published ocular malignancy biobanks [9]. Second, this biobank does not currently include metastases, in particular, liver metastases in UM. Also, there is no retinoblastoma samples included in the biobank because the treatment is centralized at the Curie Institute in Paris. Third, there are high numbers of patients with a poor prognosis and aggressive UM (most tumors classified as pT3, high rate of extraocular extent, loss of chromosome 3, most tumors classified as Trolet 2b and 2c). This could be explained by the fact that more advanced UM are better managed with enucleation rather than with proton therapy. This could limit the possibilities of translational research in early UM stages. Fourth, samples are mainly collected at the time of initial tumor management and the collection is not repeated during the follow-up, thus limiting the ability of our biobank to monitor treatment response. Finally, a common challenge for most biobanks is the difficulty of synthesizing all clinical, histological, biological, and genetic data in a single database. In our case, clinical and imaging data are stored in one database (Synapse), while biological, histological, and genetic data are stored in another database.
8. Conclusions
Dedicated, secured, and accredited biobanks are essential to collect high-quality samples to promote translational and multicentric ocular oncology research with the final aim of offering customized medicine. This is especially true for ophthalmic malignancies due to their scarcity and the lack of tissue biopsies performed in intraocular malignancies. Setting up such biobanks is time-, staff-, and cost-consuming and should be financially supported. The existing biobanks need to be promoted and new biobanks need to be created to develop outstanding international collaborations.
Author Contributions
Conceptualization, A.M., S.N.-E., S.L. (Sandra Lassalle) and P.H.; Methodology: A.M., K.W., O.B., M.S., V.T., K.Z., J.F., V.L., M.A., L.G., S.L. (Salome Lalvee), P.H. and S.B.; Validation: L.G., S.L. (Sandra Lassalle), C.B. (Christelle Bonnetaud), S.B., B.M. and P.H.; Writing—Original Draft Preparation: A.M., C.B. (Corine Bertolotto), S.L. (Sandra Lassalle), P.H. and B.M.; Writing—Review and editing: S.L. (Sandra Lassalle), P.H., S.N.-E., L.G., B.M. and P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the GIRCI (Groupement Interregional de Recherche Clinique et d’Innovation) Méditerranée “VALO DATA” 2021 (https://gircimediterranee.fr/, accessed on 1 January 2023).
Acknowledgments
Fondation des Aveugles de Guerre. The authors thank F. Pedeutour from the genetics department of the University Hospital of Nice, France.
Conflicts of Interest
The authors declare not conflict of interest.
References
- Nahon-Estève, S.; Bertolotto, C.; Picard-Gauci, A.; Gastaud, L.; Baillif, S.; Hofman, P.; Groulier, A.; Maschi, C.; Caujolle, J.-P.; Lassalle, S.; et al. Small but Challenging Conjunctival Melanoma: New Insights, Paradigms and Future Perspectives. Cancers 2021, 13, 5691. [Google Scholar] [CrossRef] [PubMed]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.-H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal Melanoma. Nat. Rev. Dis. Primer 2020, 6, 24. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L. Uveal Melanoma: Relatively Rare but Deadly Cancer. Eye Lond. Engl. 2017, 31, 241–257. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic Disease from Uveal Melanoma: Treatment Options and Future Prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Uner, O.E.; Gandrakota, N.; Azarcon, C.P.; Grossniklaus, H.E. Animal Models of Uveal Melanoma. Ann. Eye Sci. 2022, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Lamas, N.J.; Martel, A.; Nahon-Estève, S.; Goffinet, S.; Macocco, A.; Bertolotto, C.; Lassalle, S.; Hofman, P. Prognostic Biomarkers in Uveal Melanoma: The Status Quo, Recent Advances and Future Directions. Cancers 2021, 14, 96. [Google Scholar] [CrossRef]
- Hewitt, R.; Watson, P. Defining Biobank. Biopreserv. Biobank 2013, 11, 309–315. [Google Scholar] [CrossRef]
- Clavreul, A.; Soulard, G.; Lemée, J.-M.; Rigot, M.; Fabbro-Peray, P.; Bauchet, L.; Figarella-Branger, D.; Menei, P. FGB network The French Glioblastoma Biobank (FGB): A National Clinicobiological Database. J. Transl. Med. 2019, 17, 133. [Google Scholar] [CrossRef]
- Gao, Z.; Tan, J.; Wang, S.; Yu, H.; Zhou, Z.; Zhang, Y.; Zhou, M.; Xia, X.; Yao, F.; Huang, J. The Xiangya Ocular Tumor Bank: A Disease-Specific Biobank for Advancing Translational Research into Ocular Tumors. Front. Med. 2021, 8, 774624. [Google Scholar] [CrossRef]
- Rodrigues, M.; de Koning, L.; Coupland, S.E.; Jochemsen, A.G.; Marais, R.; Stern, M.-H.; Valente, A.; Barnhill, R.; Cassoux, N.; Evans, A.; et al. So Close, yet so Far: Discrepancies between Uveal and Other Melanomas. A Position Paper from UM Cure 2020. Cancers 2019, 11, 1032. [Google Scholar] [CrossRef]
- Pandiani, C.; Béranger, G.E.; Leclerc, J.; Ballotti, R.; Bertolotto, C. Focus on Cutaneous and Uveal Melanoma Specificities. Genes Dev. 2017, 31, 724–743. [Google Scholar] [CrossRef]
- Reichstein, D.; Brock, A.; Lietman, C.; McKean, M. Treatment of Metastatic Uveal Melanoma in 2022: Improved Treatment Regimens and Improved Prognosis. Curr. Opin. Ophthalmol. 2022, 33, 585–590. [Google Scholar] [CrossRef]
- Komatsubara, K.M.; Carvajal, R.D. Immunotherapy for the Treatment of Uveal Melanoma: Current Status and Emerging Therapies. Curr. Oncol. Rep. 2017, 19, 45. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.-F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal Melanoma: Trends in Incidence, Treatment, and Survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Smit, K.N.; Jager, M.J.; de Klein, A.; Kiliç, E. Uveal Melanoma: Towards a Molecular Understanding. Prog. Retin. Eye Res. 2020, 75, 100800. [Google Scholar] [CrossRef]
- Stålhammar, G.; Grossniklaus, H.E. Intratumor Heterogeneity in Uveal Melanoma BAP-1 Expression. Cancers 2021, 13, 1143. [Google Scholar] [CrossRef]
- Trolet, J.; Hupé, P.; Huon, I.; Lebigot, I.; Decraene, C.; Delattre, O.; Sastre-Garau, X.; Saule, S.; Thiéry, J.-P.; Plancher, C.; et al. Genomic Profiling and Identification of High-Risk Uveal Melanoma by Array CGH Analysis of Primary Tumors and Liver Metastases. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2572–2580. [Google Scholar] [CrossRef]
- Onken, M.D.; Worley, L.A.; Char, D.H.; Augsburger, J.J.; Correa, Z.M.; Nudleman, E.; Aaberg, T.M.; Altaweel, M.M.; Bardenstein, D.S.; Finger, P.T.; et al. Collaborative Ocular Oncology Group Report Number 1: Prospective Validation of a Multi-Gene Prognostic Assay in Uveal Melanoma. Ophthalmology 2012, 119, 1596–1603. [Google Scholar] [CrossRef]
- Brouwer, N.J.; Verdijk, R.M.; Heegaard, S.; Marinkovic, M.; Esmaeli, B.; Jager, M.J. Conjunctival Melanoma: New Insights in Tumour Genetics and Immunology, Leading to New Therapeutic Options. Prog. Retin. Eye Res. 2022, 86, 100971. [Google Scholar] [CrossRef]
- Aronow, M.E.; Topham, A.K.; Singh, A.D. Uveal Melanoma: 5-Year Update on Incidence, Treatment, and Survival (SEER 1973–2013). Ocul. Oncol. Pathol. 2018, 4, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Höllhumer, R.; Williams, S.; Michelow, P. Ocular Surface Squamous Neoplasia: Management and Outcomes. Eye 2021, 35, 1562–1573. [Google Scholar] [CrossRef]
- Quigley, C.; Deady, S.; Hughes, E.; McElnea, E.; Zgaga, L.; Chetty, S. National Incidence of Eyelid Cancer in Ireland (2005–2015). Eye 2019, 33, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, L.; Midena, E.; Trainiti, S.; Londei, D.; Bonaldi, L.; Bini, S.; Parrozzani, R. Uveal Melanoma Biopsy: A Review. Cancers 2019, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Bensoussan, E.; Thariat, J.; Maschi, C.; Delas, J.; Schouver, E.D.; Hérault, J.; Baillif, S.; Caujolle, J.-P. Outcomes after Proton Beam Therapy for Large Choroidal Melanomas in 492 Patients. Am. J. Ophthalmol. 2016, 165, 78–87. [Google Scholar] [CrossRef]
- Martel, A.; Baillif, S.; Nahon-Esteve, S.; Gastaud, L.; Bertolotto, C.; Roméo, B.; Mograbi, B.; Lassalle, S.; Hofman, P. Liquid Biopsy for Solid Ophthalmic Malignancies: An Updated Review and Perspectives. Cancers 2020, 12, 3284. [Google Scholar] [CrossRef]
- Ulmer, A.; Beutel, J.; Süsskind, D.; Hilgers, R.-D.; Ziemssen, F.; Lüke, M.; Röcken, M.; Rohrbach, M.; Fierlbeck, G.; Bartz-Schmidt, K.-U.; et al. Visualization of Circulating Melanoma Cells in Peripheral Blood of Patients with Primary Uveal Melanoma. Clin. Cancer Res. 2008, 14, 4469–4474. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Madic, J.; Mariani, P.; Piperno-Neumann, S.; Rampanou, A.; Servois, V.; Cassoux, N.; Desjardins, L.; Milder, M.; Vaucher, I.; et al. Detection Rate and Prognostic Value of Circulating Tumor Cells and Circulating Tumor DNA in Metastatic Uveal Melanoma. Int. J. Cancer 2014, 134, 1207–1213. [Google Scholar] [CrossRef]
- Ghiam, B.K.; Xu, L.; Berry, J.L. Aqueous Humor Markers in Retinoblastoma, a Review. Transl. Vis. Sci. Technol. 2019, 8, 13. [Google Scholar] [CrossRef]
- Berry, J.L.; Xu, L.; Polski, A.; Jubran, R.; Kuhn, P.; Kim, J.W.; Hicks, J. Aqueous Humor Is Superior to Blood as a Liquid Biopsy for Retinoblastoma. Ophthalmology 2020, 127, 552–554. [Google Scholar] [CrossRef]
- Wierenga, A.P.A.; Cao, J.; Mouthaan, H.; van Weeghel, C.; Verdijk, R.M.; van Duinen, S.G.; Kroes, W.G.M.; Dogrusöz, M.; Marinkovic, M.; van der Burg, S.S.H.; et al. Aqueous Humor Biomarkers Identify Three Prognostic Groups in Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4740–4747. [Google Scholar] [CrossRef]
- Im, D.H.; Peng, C.-C.; Xu, L.; Kim, M.E.; Ostrow, D.; Yellapantula, V.; Bootwalla, M.; Biegel, J.A.; Gai, X.; Prabakar, R.K.; et al. Potential of Aqueous Humor as a Liquid Biopsy for Uveal Melanoma. Int. J. Mol. Sci. 2022, 23, 6226. [Google Scholar] [CrossRef]
- Washetine, K.; Heeke, S.; Bonnetaud, C.; Kara-Borni, M.; Ilié, M.; Lassalle, S.; Butori, C.; Long-Mira, E.; Marquette, C.H.; Cohen, C.; et al. Establishing a Dedicated Lung Cancer Biobank at the University Center Hospital of Nice (France). Why and How? Cancers 2018, 10, 220. [Google Scholar] [CrossRef]
- De Blasio, P.; Biunno, I. New Challenges for Biobanks: Accreditation to the New ISO 20387:2018 Standard Specific for Biobanks. BioTech 2021, 10, 13. [Google Scholar] [CrossRef]
- De Souza, Y.G. Sustainability of Biobanks in the Future. Adv. Exp. Med. Biol. 2015, 864, 29–35. [Google Scholar] [CrossRef]
- Vaught, J.; Rogers, J.; Carolin, T.; Compton, C. Biobankonomics: Developing a Sustainable Business Model Approach for the Formation of a Human Tissue Biobank. J. Natl. Cancer Inst. Monogr. 2011, 2011, 24–31. [Google Scholar] [CrossRef]
- Harati, M.D.; Williams, R.R.; Movassaghi, M.; Hojat, A.; Lucey, G.M.; Yong, W.H. An Introduction to Starting a Biobank. In Methods Molecular Biology; Humana Press: Clifton, NJ, USA, 2019; Volume 1897, pp. 7–16. [Google Scholar] [CrossRef]
- Hofman, P.; Bréchot, C.; Zatloukal, K.; Dagher, G.; Clément, B. Public-Private Relationships in Biobanking: A Still Underestimated Key Component of Open Innovation. Virchows Arch. Int. J. Pathol. 2014, 464, 3–9. [Google Scholar] [CrossRef]
- Clément, B.; Yuille, M.; Zaltoukal, K.; Wichmann, H.-E.; Anton, G.; Parodi, B.; Kozera, L.; Bréchot, C.; Hofman, P.; Dagher, G.; et al. Public Biobanks: Calculation and Recovery of Costs. Sci. Transl. Med. 2014, 6, 261fs45. [Google Scholar] [CrossRef]
- Mathis, T.; Cassoux, N.; Tardy, M.; Piperno, S.; Gastaud, L.; Dendale, R.; Maschi, C.; Nguyen, A.-M.; Meyer, L.; Bonnin, N.; et al. Management of uveal melanomas, guidelines for oncologists. Bull. Cancer 2018, 105, 967–980. [Google Scholar] [CrossRef]
- Hofman, V.; Ilie, M.; Long, E.; Lassalle, S.; Butori, C.; Bence, C.; Washetine, K.; Lalvee, S.; Hofman, P. Immunohistochemistry and personalised medicine in lung oncology: Advantages and limitations. Bull. Cancer 2014, 101, 958–965. [Google Scholar] [CrossRef]
- Jacotot, L.; Woodward, M.; de Montalier, A.; Vaglio, P. Utilizing Modular Biobanking Software in Different Types of Biobanking Activities. Biopreservation Biobanking 2022, 20, 417–422. [Google Scholar] [CrossRef] [PubMed]
- van Beek, J.G.M.; Koopmans, A.E.; Vaarwater, J.; de Rooi, J.J.; Paridaens, D.; Naus, N.C.; de Klein, A.; Verdijk, R.M.; Kiliç, E. The Prognostic Value of Extraocular Extension in Relation to Monosomy 3 and Gain of Chromosome 8q in Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, M.E.; Orloff, M.; Sato, R.; Chervoneva, I.; Shields, C.L.; Shields, J.A.; Mastrangelo, M.J.; Sato, T. Adjuvant Sunitinib in High-Risk Patients with Uveal Melanoma: Comparison with Institutional Controls. Ophthalmology 2018, 125, 210–217. [Google Scholar] [CrossRef]
- Dogrusöz, M.; Kroes, W.G.M.; van Duinen, S.G.; Creutzberg, C.L.; Versluis, M.; Bleeker, J.C.; Marinkovic, M.; Luyten, G.P.M.; Jager, M.J. Radiation Treatment Affects Chromosome Testing in Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5956–5964. [Google Scholar] [CrossRef] [PubMed]
- Coupland, S.E.; Kalirai, H.; Ho, V.; Thornton, S.; Damato, B.E.; Heimann, H. Concordant Chromosome 3 Results in Paired Choroidal Melanoma Biopsies and Subsequent Tumour Resection Specimens. Br. J. Ophthalmol. 2015, 99, 1444–1450. [Google Scholar] [CrossRef]
- Thornton, S.; Coupland, S.E.; Heimann, H.; Hussain, R.; Groenewald, C.; Kacperek, A.; Damato, B.; Taktak, A.; Eleuteri, A.; Kalirai, H. Effects of Plaque Brachytherapy and Proton Beam Radiotherapy on Prognostic Testing: A Comparison of Uveal Melanoma Genotyped by Microsatellite Analysis. Br. J. Ophthalmol. 2020, 104, 1462–1466. [Google Scholar] [CrossRef]
- Dhillon, S. Tebentafusp: First Approval. Drugs 2022, 82, 703–710. [Google Scholar] [CrossRef]
- Lassalle, S.; Nahon-Esteve, S.; Frouin, E.; Boulagnon-Rombi, C.; Josselin, N.; Cassoux, N.; Barnhill, R.; Scheller, B.; Baillif, S.; Hofman, P. PD-L1 Expression in 65 Conjunctival Melanomas and Its Association with Clinical Outcome. Int. J. Mol. Sci. 2020, 21, 9147. [Google Scholar] [CrossRef]
- Pandiani, C.; Strub, T.; Nottet, N.; Cheli, Y.; Gambi, G.; Bille, K.; Husser, C.; Dalmasso, M.; Béranger, G.; Lassalle, S.; et al. Single-Cell RNA Sequencing Reveals Intratumoral Heterogeneity in Primary Uveal Melanomas and Identifies HES6 as a Driver of the Metastatic Disease. Cell Death Differ. 2021, 28, 1990–2000. [Google Scholar] [CrossRef]
- Krossa, I.; Strub, T.; Martel, A.; Nahon-Esteve, S.; Lassalle, S.; Hofman, P.; Baillif, S.; Ballotti, R.; Bertolotto, C. Recent Advances in Understanding the Role of HES6 in Cancers. Theranostics 2022, 12, 4374–4385. [Google Scholar] [CrossRef]
- Martel, A.; Baillif, S.; Nahon-Esteve, S.; Gastaud, L.; Bertolotto, C.; Lassalle, S.; Lagier, J.; Hamedani, M.; Poissonnet, G. Orbital Exenteration: An Updated Review with Perspectives. Surv. Ophthalmol. 2021, 66, 856–876. [Google Scholar] [CrossRef]
- Martel, A.; Lassalle, S.; Picard-Gauci, A.; Gastaud, L.; Montaudie, H.; Bertolotto, C.; Nahon-Esteve, S.; Poissonnet, G.; Hofman, P.; Baillif, S. New Targeted Therapies and Immunotherapies for Locally Advanced Periocular Malignant Tumours: Towards a New “Eye-Sparing” Paradigm? Cancers 2021, 13, 2822. [Google Scholar] [CrossRef]
- Monjanel, B.; Baillif, S.; Lagier, J.; Gastaud, L.; Poissonnet, G.; Martel, A. Efficacy and Safety of an Artificial Dermal Graft for the Reconstruction of Exenterated Sockets: A Preliminary Report. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2827–2835. [Google Scholar] [CrossRef]
- Strub, T.; Martel, A.; Nahon-Esteve, S.; Baillif, S.; Ballotti, R.; Bertolotto, C. Translation of Single-Cell Transcriptomic Analysis of Uveal Melanomas to Clinical Oncology. Prog. Retin. Eye Res. 2021, 85, 100968. [Google Scholar] [CrossRef]
- Bellini, L.; Strub, T.; Habel, N.; Pandiani, C.; Marchetti, S.; Martel, A.; Baillif, S.; Bailly-Maitre, B.; Gual, P.; Ballotti, R.; et al. Endoplasmic Reticulum Stress Mediates Resistance to BCL-2 Inhibitor in Uveal Melanoma Cells. Cell Death Discov. 2020, 6, 22. [Google Scholar] [CrossRef]
- Thariat, J.; Martel, A.; Matet, A.; Loria, O.; Kodjikian, L.; Nguyen, A.-M.; Rosier, L.; Herault, J.; Nahon-Estève, S.; Mathis, T. Non-Cancer Effects Following Ionizing Irradiation Involving the Eye and Orbit. Cancers 2022, 14, 1194. [Google Scholar] [CrossRef]
- Martel, A.; Baillif, S.; Thomas, P.; Almairac, F.; Galatoire, O.; Hamedani, M.; Fontaine, D.; Lanteri-Minet, M. Phantom Vision after Eye Removal: Prevalence, Features and Related Risk Factors. Br. J. Ophthalmol. 2022, 106, 1603–1609. [Google Scholar] [CrossRef]
- Martel, A.; Oberic, A.; Moulin, A.; Tieulie, N.; Hamedani, M. Clinical, radiological, pathological features, treatment and follow-up of periocular and/or orbital amyloidosis: Report of 6 cases and literature review. J. Fr. Ophtalmol. 2018, 41, 492–506. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).