Simple Summary
About 35% of patients with multiple myeloma are ≥75 years old at diagnosis, and their treatment should be individualized according to their fragility score. Fragility assessment scales include the International Myeloma Working Group Palumbo Fragility Scale, the Revised Initial Myeloma Comorbidity Index, and the Mayo score. However, they are time-consuming, but more importantly, there are no convincing data from a real world experience supporting their usefulness in everyday clinical practice. Patients with multiple myeloma over 75 years of age are instantly classified as intermediate fit or frail. These patients should be offered non-intensive treatment in reduced doses or palliative care. In the prospective observational, multicenter study, we showed that most patients over 75 years of age qualified for treatment are safely treated with three-drug regimens regardless of their fragility categorization. Current fragility scores for patients with MM over 75 years old have limited value in daily practice.
Abstract
Fragility scales are intended to help in therapeutic decisions. Here, we asked if the fragility assessment in MM patients ≥ 75 years old qualified for treatment by the local physician correlates with the choice of treatment: a two- or three-drug regimens. Between 7/2018 and 12/2019, we prospectively enrolled 197 MM patients at the start of treatment from the 13 Polish Myeloma Group centers. The data to assess fragility were prospectively collected, but centrally assessed fragility was not disclosed to the local center. The activity of daily living (ADL) could be assessed in 192 (97.5%) and was independent in 158 (80.2%), moderately impaired in 23 (11.7%), and 11 (5.6%) in completely dependent. Patients with more than three comorbidities made up 26.9% (53 patients). Thus, according to the Palumbo calculator, 43 patients were in the intermediate fitness group (21.8%), and the rest belonged to the frailty group (153, 77.7%). Overall, 79.7% of patients (157) received three-drug regimens and 20.3% (40) received two-drug regimens. In each ECOG group, more than three out of four patients received three-drug regimens. According to the ADL scale, 82.3% of the independent 65.2% of moderately impaired, and 81.8% of the dependent received three-drug regimens. Out of 53 patients with at least four comorbidities, 71.7% received three-drug regimens, and the rest received two-drug regimens. Thirty-four patients from the intermediate fit group (79.0%), and 123 (79.9%) from the frail group received three-drug regimens. Early mortality occurred in 25 patients (12.7%). No one discontinued treatment due to toxicity. To conclude, MM patients over 75 are mainly treated with triple-drug regimens, not only in reduced doses, regardless of their frailty scores. However, the absence of prospective fragility assessment did not negatively affect early mortality and the number of treatment discontinuations, which brings into question the clinical utility of current fragility scales in everyday practice.
1. Introduction
It is estimated that the percentage of people over 75 years of age will increase up to 22% in developed countries by 2023, which means that, as described in 1976 by Isaac Bernard, “great geriatric problems” experienced by frail people will impact the treatment of cancer [1]. Fragility is a multidimensional state of reduced reserves of energy, fitness, cognitive ability, and health that makes individuals more vulnerable to stressors and can reduce resistance to cancer and cancer treatment. Understanding areas of vulnerability, such as frailty, comorbidities, and disability, is believed to be important for tailoring treatment to optimize outcomes for individual patients [2,3,4,5].
The Polish Gerontological Society recommends that individuals over 60 years old should undergo a comprehensive geriatric assessment. The comprehensive geriatric assessment is a multi-directional diagnostic process for obtaining data on a patient’s medical, psychosocial, and functional well-being. It includes functional fitness, mental health, socio-environmental conditions, and physical health. This approach is achieved through valorized research in the form of questionnaires and tests that take approximately 20 to 40 min to complete. It helps understand the patient and their needs and indicates the possibilities for social functioning and therapeutic disease processes. Moreover, it enables identifying patients who, despite their age, can receive full oncological treatment without an increased risk of complications and those who only qualify for supportive and symptomatic treatment [4,6]. The intermediate group consists of patients requiring therapy modification to reduce side effects and maintain quality of life.
Most patients with multiple myeloma (MM) are over 65 years of age (60% of patients). Modern treatment of MM contains two to four agents: steroids with either alkylating agents or proteasome inhibitors, or/and immunomodulatory agents, and recently monoclonal antibodies. Multiple agent schemas are usually more effective but also more toxic. This is particularly important for elderly patients who are more sensitive to complications as they have smaller organ reserves, less ability to repair cellular damage, comorbidities, polypharmacy, and slower pharmacokinetics and pharmacodynamics of drugs. Using scales to assess their fragility to optimize therapy is reasonable. Frailty assessment of the elderly was initially based on age and performance status, according to Karnofsky and the Eastern Cooperative Oncology Group (ECOG). However, this proved to be insufficient over time. The first scale proposed by the International Myeloma Working Group (IWMG) to assess myeloma patients from three different groups was the Geriatric Assessment scale (GA) introduced by Palumbo et al., which has been validated based on four prospective studies of 869 patients [4,7]. The GA scale includes the activities of daily living (ADL, Katz scale), instrumental ADL (I-ADL, Lawton scale), and the Charlson Comorbidity Index (CCI), in addition to age and ECOG performance status, and distinguishes three groups of patients: fit—fit, intermediately fit—intermediate fit, and weak (frail)—frail. Of these groups, the frail group is associated with a more than three-fold higher risk of death, progression, and adverse events, regardless of International Staging System (ISS) prognosis, genetic disorders, and type of treatment (Supplementary Materials Tables S1–S3).
The authors suggest that patients from the fit group could receive the full dose of recommended treatments and may benefit from autologous transplantation; patients from the intermediate group may benefit from treatment with modified doses or a two-drug treatment (Supplementary Materials Table S4). In contrast, frail patients may benefit from palliative, symptomatic, or reduced treatment doses after carefully analyzing possible side effects [4] (Supplementary Materials Tables S3 and S4). An important observation in developing the GA fitness calculator is that 80 years of age should be the limit for defining a “weak state” [4,7].
The second scale evaluating the efficiency of patients with MM is the Revised Initial Myeloma Comorbidity Index (R-MCI), which was validated by a retrospective evaluation of 801 patients (1997–2012) from a center in Germany [8]. The scale uses five points concerning the patient’s condition to determine a score, which includes kidney disease, lung disease, Karnofsky’s fitness rating scale, age, and fitness [9]. Patients were divided into three groups with a clear relationship between fitness and treatment tolerance and significantly different overall survival (OS) times. In the fit group, OS was 10.1 years, while it was 4.4 years in the intermediately fit group and 1.2 years in the frail (weak) group. Lung and kidney diseases significantly affect treatment tolerance regardless of the treatment used. However, the greatest limitation of the R-MCI scale is that the therapies used in this study are currently not recommended (Supplementary Materials Table S4).
The third proposed prognostic scale used for people over 65 is the Mayo Risk Score (MRS) [10], assessing the ADL, CCI, ECOG performance status, and Revised-ISS (R-ISS) stage. In addition, N-terminal pro-brain natriuretic peptide (NT-proBNP) protein levels were measured. The study confirmed that three parameters are sufficient to identify the “weak” group for whom treatment may be detrimental and shorten overall survival (OS). The components of the scale are age, ECOG, and N-terminal pro B-type natriuretic peptide (NT-proBNP). NT-proBNP indicates cardiac and renal function and a threshold of 300 ng/L corresponds to a well-established age-independent cut-off point for excluding acute heart failure [11]. Patients over 70 years of age with ECOG performance ≥ 2 and NT-proBNP ≥ 300 ng/L belonged to the high group, and their OS time was 18 months, with only 18% of them receiving any therapy. The second group of patients met two out of three criteria and had an OS of 28 months, while OS was 58 months in patients with one point [10].
The presented fragility assessment scales are valuable, but are challenging to use in everyday clinical practice. Indeed, the scale proposed by the IMWG is time-consuming, taking around 40 min to complete. The R-MCI scale seems simpler and less time-consuming. The patient completes the test themselves, which may therefore be biased. The MRS, based on three parameters, is the most straightforward tool in everyday practice. However, the lack of an objective assessment of the patient’s functioning in everyday life is a limitation of this tool. Patients over 75 are automatically classified as immediately fit or frail according to the IMWG scale. According to the guidelines, these patients should be offered non-intensive treatment at reduced doses. Palliative care and supportive treatment are indicated in the fragile group, and treatment should include reduced doses of at most two-drug regimens [11] (Supplementary Materials Table S3).
Despite the above recommendation, no data from real-world evidence (RWE) studies support the clinical utility of current fragility scales in everyday practice. Therefore, we asked if the subjective clinical assessment of the local treating physician and the subsequent choice of the intensity and type of treatment: two- versus three-agent schema, corresponds to the prospective assessment of the fragility score in MM patients over 75 years. We also assess whether more intensive treatment was associated with worse tolerance and first-line mortality.
2. Materials and Methods
2.1. Study Participants
The prospective, cross-sectional observational study performed between July 2018 and December 2019 enrolled 197 MM patients ≥ 75 years at the start of treatment from the 13 Polish Myeloma Group centers. The palliative patients were not included. The patients were followed for a fixed 12-month period.
2.2. The Fragility Assessment
The centers were asked to collect the following data at the time of diagnosis: clinical stage of the disease according to the ISS and Durie and Salmon classification, CCI, performance status according to the ECOG scale, ADL (Katz scale) and I-ADL (Lawton scale), treatment method, and primary blood test results, including lactate dehydrogenase (LDH), C-reactive protein (CRP), hemoglobin, platelets, NT-pro-BNP, ẞ2-microglobulin, albumin, calcium, and creatinine. The frailty scores were assessed centrally and were not disclosed to the local centers. Primary physicians did not perform the frailty scores assessment. In the second part of the study, data on patient management, assessments of the response to treatment, survival, and post-treatment complications were collected 12 months after the initiation of treatment.
2.3. Statistical Analysis
Categorical variables were summarized as frequencies and percentages. Pairwise comparisons between patient subgroups employed Fisher’s exact test. The existence of a monotonic trend was assessed with the Cochran–Armitage test. The correlation of treatment regimen with frailty scores was investigated using the rcompanion (v. 2.4.30) and psych (v. 2.3.6) packages (Cramer’s V or Phi correlation was applied accordingly). All the analyses were performed using the R software package version 4.0.1 (R Foundation for Statistical Computing, http://www.r-project.org (accessed on 15 June 2020)). A two-sided p-value ≤ 0.05 was considered statistically significant. All data were entered into a Microsoft Office Excel spreadsheet.
3. Results
3.1. Patients Characteristics
The cohort included 100 males and 97 females at a median age 77 (75–90) years. One third of patients were over the age of 80 (65, 33.0%). The clinical characteristics are shown in Table 1. Most patients were diagnosed with myeloma of the immunoglobulin (Ig)G subtype (69.0%), followed by IgA (16.2%) and light chains (10.2%), IgM (2.0%), IgD (1.0%), and biclonal type (0.5%).

Table 1.
Clinical characteristics.
3.2. Physical Fitness and Comorbidities
ECOG 0-2 was documented in 171 (86.8%) patients (Table 2. The simple geriatric scale of basic vital functions according to the ADL was assessed in 192 (97.5%) patients, and most of them (82.3%) were independent (Table 2). All patients were evaluated with the CCI, which revealed that only 53 (26.9%) had more than three comorbidities. The most common comorbidities were hypertension (160, 81.2%), ischemic heart disease (58, 29.4%), heart failure (50, 25.4%), kidney disease (48, 24.4%), respiratory disease (41, 20.8%), diabetes (40, 20.3%), gastritis/gastroesophageal reflux disease (29, 14.7%), other cancers (28, 14.2%), hepatic dysfunction (22, 11.2%), and cerebral circulation disorders (18, 9.1%). Less frequent comorbidities included dementia (6, 3.0%), connective tissue disease (4, 2.0%), and hemiparesis (4, 2.0%).

Table 2.
Assessment of the type of treatment and selected parameters.
3.3. Evaluation of the First-Line Treatment
A three-drug regimen was used in 157 (79.7%) of patients. The most frequent schemas were CTD 54 (27.4%) and VMP 39 (19.8%), then MPT, VTD and VCD: 31 (15.7%), 22 (11.2%), 10 (5.1%), respectively (Table 3). There was no preference for any two-drug regimen (Table 3).

Table 3.
Types of first-line treatments and the number of deaths during the 1st line treatment.
Modification of the scheme consisted of reducing the dose of at least one drug. Among the three-drug regimens, 22.3% (35 patients) were modified, as were 47.5% (19 patients) of the two-drug regimens. The most frequently modified regimen was the VTD (13 out of 22) and VCD (3 out of 10) regimens, while among the two-drug regimens, the modification was similar in percentage in each regimen.
Of the patients, intermediate fit (43; 21.8%) and frailty (154; 78.2%) according to the IMWG scale, the modification (reduction of doses) in three-component therapies was 27.2% for intermediate fit, and 22.8% for frailty. It is noteworthy that 47.5% of patients treated with two-component regimens (19 of 40; 20.3%) had reduced dose regimens, and similarly in both groups: intermediate fit 44.4%, and fragile 49.4% (Supplementary Materials Tables S6 and S7).
It is worth noting that among the patients over 80 years of age (65 patients, 33.0%), 50 (76.9%) also received the three-drug therapy (and 30% of them received a modified regimen).
No case of treatment discontinuation due to unacceptable toxicity or due to patient withdrawal from therapy was reported.
3.4. First-Line Treatment Analysis
During the 12 months of prospective observation, 107 (54.3%) patients received one line of treatment, 84 (42.6%) two lines of treatment, and 6 (3.0%) three lines of treatment. Next, we analyzed whether the functioning status and comorbidities correlated with the type of first-line therapy (Supplementary Materials Tables S5, S6 and S10). Over three-quarters of patients in each ECOG fitness group received a three-drug regimen (Table 2). No relationship was found between the general performance status and the type of regimens (two- vs. three-drugs) (Table 2). However, higher creatinine levels (above 1.9 mg/dL) determined the choice of treatment: individuals with elevated creatinine levels were more likely to receive two-drug regimens (OR = 3.05, 95%CI [1.33, 6.92]; p = 0.005) (Table 2).
Regarding performance status, according to the ADL, 82.3% of the fit patients and, surprisingly, 81.8% of the completely dependent ones received three-drug regimens (Table 2). The I-ADL could be recorded on the day of enrollment, and after 12 months, for only 59 patients (29.9%). In almost all patients (58, 98.3%), the deterioration of complex activities of daily living was documented, of whom 52 (88.1%) received a three-drug regimen. The correlation between the choice of treatment regimen and the frailty scale (IMWG) was not statistically significant (Table 2).
We also asked if the number of comorbidities affected the choice of treatment. The median number of comorbidities was 2 (0–9). Every fourth person had at least four comorbidities, and 71.7% of the patients in this group received a three-drug treatment. The percentages of patients with a lower number of comorbidities receiving three-drug schemas were similar and comparable to patients with a higher number of comorbidities (Figure 1). The Cochran–Armitage test showed no significant trend (p = 0.335) (Figure 1) between the number of comorbidities and the applied treatment regimen (two-drug or three-drug regimen) and additionally, Fisher’s exact test showed no relationship between these variables (p = 0.395) (Supplementary Materials Table S8).
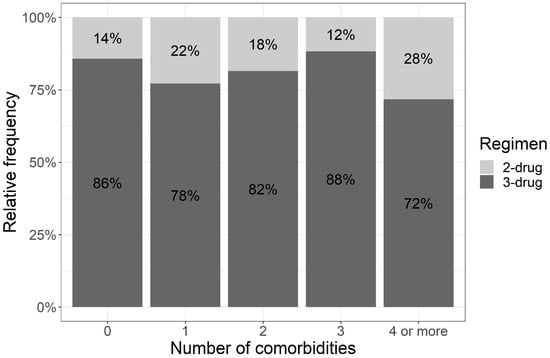
Figure 1.
Trend between the number of comorbidities and the treatment protocol selected (test Cochran–Armitage).
No statistically significant relationship was found when analyzing the relationship between the type of comorbidity and the selected regimens (Supplementary Materials Table S8).
3.5. Evaluation of the Response to First-Line Treatment
Almost three-quarters (143, 72.6%) of patients responded to the first line treatment regardless of the two- or three-drug regimen. Three-drug regimens allowed for a better response (CR + VGPR + PR vs. SD + PD: OR = 2.09, 95% CI [0.93, 4.58]; p = 0.072) since patients who received a two-drug regimen achieved more often only disease stabilization (SD vs. rest: OR = 3.304, 95% CI [1.37, 7.76]; p = 0.005) (Table 4).

Table 4.
Responses according to the 2- or 3-drug regimen.
Bortezomib used in the first-line treatment in 83 patients (42.1%) did not bring any benefit in terms of better responses (n = 197, p = 0.11) (Supplementary Materials Table S9).
3.6. Evaluation of the Response Performance Status and Fragility
The response to treatment after first-line treatment was analyzed according to ECOG performance status, ADL, and the presence of the most common comorbidities (Table 5). None except one of the 26 patients with ECOG > 2 achieved CR or VGPR. Similar trend was observed for the ADL (Table 5). None of the 11 completely dependent patients achieved CR or VGPR. Moreover, many of them (36.4%) experienced disease progression, and only 45.5% responded to treatment, regardless of the treatment used (Table 5). The response rates obtained in patients with at least four comorbidities were no worse than in the other groups, and there was no relationship between the number of comorbidities and the response (p = 0.156).

Table 5.
Relationship of responses to treatment to ECOG performance status, Katz scale, and the most frequently reported comorbidities.
3.7. Analysis of Deaths
During the first-line treatment, fourteen patients died, with six (42.8%) due to disease progression, five (35.7%) to infection, one (7.1%) to heart failure, and one (7.1%) due to other malignancy, while the cause of death was not established in one patient (7.1%) (Supplementary Materials Tables S11 and S12). Of the 14 deceased persons, 12 (85.7%) received a three-drug treatment (four patients with modified treatment, 33.3%), which is 7.6% of all the patients receiving three-drug regimens, and the remaining two had a two-drug regimens (one patient was treated by modified regimen) (Table 2). During the second line of treatment, eleven people died: nine due to disease progression and two in the course of an infection. At the end of the study, 25 (12.7%) patients died in total, 15 (7.6%) due to disease progression and 7 (3.6%) due to infection (Supplementary Materials Table S12), after 12 months from the diagnosis of the disease.
4. Discussion
This study suggests that the available frailty scales for patients with MM over 75 who were qualified for treatment are not useful in clinical practice regarding the choice of two- vs. three-drug regimens. The early mortality and the number of treatment discontinuations were not affected by the absence of a prospective fragility assessment by the local physician. This surprising observation may result from the fact that our patients, who qualified for treatment despite advanced age (>75 years old), were relatively fit, with the majority being in ECOG 0-2. However, age itself plays a very important role in the fragility assessment. According to the recommended frailty scale developed by the International Myeloma Working Group [7,9,11,12], patients over 75 years can only be categorized as intermediate fit or fragile depending on the number of comorbidities (two or more than two) and patients over 80 years are always categorized as frail. Consequently, all patients from our study should be treated either with a modified drug doses regimen (intermediate fit) or should be referred to palliative treatment or at most, reduced dose two-drug regimens (fragile) [7,11,12,13,14].
Our data showed that even very old frail MM patients might benefit from the three-drug regimens administered by an experienced primary oncologist or hematologist. Nevertheless, the management of these patients still remains a challenge, primarily be-cause there are no clear recommendations on how to treat patients over 75 years of age. So far, there have been no prospective clinical trials dedicated to this group of patients. In addition, in many studies for patients with newly diagnosed myeloma, age over 80 years is an exclusion criterion. Based on the presented RWD, neither age nor the number and/or type of comorbidities (except for renal impairment) affect the choice of treatment and the start of therapy. These observations suggest that age and the number of comorbidities should not be the major determinants of frailty.
Our results are in line with other current RWE that showed that prior to the daratumumab era, most of the MM patients older than >75 were treated with three-drug regimens, most commonly with steroids (dexamethasone at a dose < 20 mg/dose), immunomodulatory drugs (lenalidomide or thalidomide—at the best tolerated dose, starting treatment with the full recommended doses) and cyclophosphamide or bortezomib (both at the best-tolerated dose, starting treatment with the recommended doses of cyclophosphamide and bortezomib of one per week at full dose). The advantage of the CTD and MPT regimen is their oral administration. Therefore, it might be a preferred choice for the physician and the patient and their family/carers alike. We showed that the use of bortezomib did not improve the response, but its use requires more frequent visits to treatment centers, which may pose a logistic problem for elderly patients.
Our cohort included 22% intermediate-fit patients and 78% frail patients. None of them underwent autotransplantation, but three-drug regimens (also VTD and VCD) were used in about 80% of patients in each group. It should be noted that the modification of drug doses in the 3-component regimens affected every 4th patient in the intermediate-fit group and every 5th patient in the frail group (Supplementary Materials Table S7). There-fore, our group of patients was treated more intensively than the accepted recommendations, and the choice was not influenced by objective scales or other parameters. In our study, regimens were used according to the recommendations of the IMWG and the Polish Myeloma Group [12]. However, only 22.3% of patients treated with the three drug regimens required drug modification. The most often modified regimens included VTD, VCD, and CTD. This translated to a better tolerance, although at the expense of a worse treatment response. We demonstrated that the safest three-component regimens are VCD, MPT and CTD, and the least safe are VMP, followed by VTD (based on the number of fatal events in the first and second line of treatment) (Table 3). Notably, VRD was used in only one patient since this schema was not reimbursed at the time of the study.
It is worth noting that patients with impaired renal function or kidney disease were referred for treatment with a three-drug regimen less frequently and were treated with regimens containing bortezomib more often (50%). Renal impairment usually indicates more aggressive and advanced diseases not always well-reflected by the ISS score [15]. Therefore two drug regimens, especially daratumumab with dexamethasone, might be insufficient for some patients on dialysis, patients with advanced kidney disease, or those with advanced vascular thromboembolism. The results of the NCT04151667 study will probably provide the answer to this question.
In our analysis, during 12 months of treatment, no patient discontinued treatment due to toxicity or self-decision. It is much lower compared to the Palumbo study where the cumulative incidence of treatment discontinuation at 12 months was 20.8% in the intermediate fitness group and 31.2% in the frail patient cohort [4]. If death due to infection or exacerbation of comorbidities can be perceived as the toxicity leading to treatment discontinuation, one should add ten patients (5.1%) who died, of which nine received treatment with three drugs (4.6%) and eight were in the frailty group. This observation indicates that three-drug regimens should be used with caution in frail patients.
In contrast, our data suggest that three-drug regimens can be safely administered: early mortality (12.7%) was much lower than the 35.3% reported from NCI SEER data from 1993–2010 [16,17] and comparable to the results from newer studies: 9.1% in the study by McQuilten and colleagues [18], and 12.6% in an observational study of the Asian population [19] with the exception of a study by Lee et al., who reported 30% mortality in this group of patients [20]. Additionally, it was shown in our analysis that mortality was similar in both groups, regardless of the treatment intensity (for the three-drug regimen as the first line therapy it was: 7.6%, and for the two-drug regimen it was 5.0%).
Existing data suggest that mortality during the first-line treatment is mainly related to the exacerbation of comorbidities [21]. In the presented analysis, mortality from exacerbation of comorbidities during therapy occurred only in two patients; both received three-component treatment (1%) (heart failure and lung cancer progression) (Supplementary Materials Table S12). This may suggest some selection bias for “better” patients referred to our centers. The high number of patients with ECOG 0-2 corroborates this notion.
The lack of a clear relationship between the current fragility assessment in MM patients may also result from the fact that new drugs registered during the last five years have a significantly different toxicity profile and its effect on older and frail patients. In the MAIA study, in which 44% of the patients over 75 (9% intermediate fit, 32.9% frail), daratumumab (anti-CD38 antibody) along with Rd (Dara-RD) not only extended the median PFS from 34.4 months to 61.9 months and the OS (under development), but also showed good tolerance [22]. Early mortality (up to 12 months) in the MAIA study was 5.8% of patients in the frailty group in the D-Rd arm and 5.3% in the Rd arm, while in the intermediate fit group in the D-Rd arm it was 0.8%, and 2.5% in the Rd arm. On the other hand, the reduction of doses in the frail group in the study arm (Dara-Rd) affected 64.3% of cases, and 44% in the control arm (Rd). Unfortunately, the available data lack information on the subgroup over 75 years of age. Moreover, the complications of this treatment were no greater than those in the control group. Of note, all patients enrolled to the MAIA trial were in ECOG 0-2. This observation, similarly to our findings, suggests that patients with MM over 75 with good performance status (ECOG 0-2) could be safely treated with the three-drug regimen, even though in our study, daratumumab was not used as the first line. However, an additional fourth agent, such as in the ALCYONE study (daratumumab + VMP), resulted in higher toxicity, mainly in terms of infection, and a shorter PFS of 36.4 months [23]. Therefore, four-drug regimens may be too toxic in this group of patients. Currently, the Dara-Rd regimen is the most effective treatment and has an acceptable safety profile. The results of a trial of daratumumab with VRd vs. VRd (NCT03652064) for transplant ineligible patients are eagerly expected with the hope they would answer questions regarding the safety and efficacy of a four-drug regimen in elderly patients.
New treatment options such as chimeric antigen receptor (CAR)-T therapies and bispecific antibodies (T-cell redirecting therapies) need careful evaluation in patients over 75 years old. The CARTITUDE 5 trial using ciltacabtagene autoleucel (ciltacel) includes patients not eligible for transplantation, but it precludes patients over 80 years old (NCT04923893). CAR-T therapy is well tolerated, and toxicity appears predictable and manageable. The safety data are so far optimistic and demonstrate acceptable rates of Grade 3 and 4 events [24,25], which suggests that this therapy might be also considered in elderly patients, without any age limit, after prior careful selection in order to prevent possible adverse events. In contrast to CAR-T, bispecific antibodies are available “off the shelf” and are most often administered cyclically subcutaneously (every 1–4 weeks). The recruitment to trials for non-transplant eligible patients in initial line of treatment, with no age limit, has recently started (EudraCT Number 2021-000803-20). The safety of bispecific antibodies is even better than CAR-T and patients over 75 could benefit from this therapy. The question of fragility should be addressed again in the era of new therapies.
5. Conclusions
To conclude, our study showed that in everyday practice, the management of patients over 75 who qualified for treatment was not dependent on the performance of geriatric scales and the patient’s fragility assessment. The choice of the type of therapy did not correlate with the fragility score. However, most patients had a relatively good performance status (ECOG 0-2). They were treated with three-drug regimens regardless of the number of comorbidities. Despite that, mortality in the first line was acceptable and comparable to other studies. Impaired renal function was the only factor leading to a more frequent choice of two-drug regimens. Our results therefore question the need for a formal geriatric assessment used in its present form in daily practice and suggest that a holistic clinical assessment by an experienced physician is no worse for the appropriate choice of treatment, especially in patients with good performance status. However, a new era in multiple myeloma treatment may require us to re-address the utility of the frailty scores. Future frailty scores should be based on more objective data and not on “physician feeling”, especially in view of the potential severe toxicities of new treatment options (immunotherapy and CAR T cells). A big limitation of our study is the lack of cytogenetic results and its possible effect on the choice of therapy. Moreover, our study did not include a questionnaire assessing the quality of life, which could help to better understand the patient’s views on the chosen treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15133469/s1; Table S1. Fragility subgroup analysis for the FIRST study. Adapted from [14,16]; Table S2. Fragility scale according to the International Myeloma Working Group; Table S3. International Myeloma Working Group scale for patients over 75; Table S4. Comparison of fitness scales for the elderly with multiple myeloma; Table S5. Response to first line treatment depending on whether drugs were modified or not; Table S6. Use of regimens with or without drug modification; Table S7. Use of regimens with or without drug modification according of frailty; Table S8. The relationship between the number of comorbidities identified in each patient and the treatment protocol selected; Table S9. Responses to bortezomib use in first-line treatment; Table S10. The relationship between co-existing disease and the treatment protocol selected; Table S11. The second-line treatment used; Table S12. Analysis of deaths.
Author Contributions
Conceptualization, A.T. and J.M.Z.; Methodology, A.T., J.M.Z., M.K.K. and A.J.C.; Software, M.K.K.; Bioinformatical and statistical analyses, M.K.K. and A.J.C.; The acquisition of the clinical data/Resources, A.T., A.J., K.G., H.C., E.S., J.H., A.K.d.N., A.W., A.Ś., A.W.-G., J.D.-S., R.G.-K., A.P., W.K.-P., J.K., P.R., D.W., H.S., G.W.B., B.Z. and K.J.; Validation, A.T., J.M.Z., M.K.K. and A.J.C., Investigation, A.T., J.M.Z., M.K.K., A.J.C. and K.W.; Data curation A.T. and M.K.K.; Writing—Original Draft Preparation, A.T.; Writing—Review & Editing, J.M.Z., A.T., M.K.K. and A.J.C.; Revising critically, A.T., A.J., K.G., H.C., E.S., J.H., A.K.d.N., A.W., A.Ś., A.W.-G., J.D.-S., R.G.-K., A.P., W.K.-P., J.K., P.R., D.W., H.S., G.W.B., B.Z. and K.J. Visualization, A.T. and M.K.K.; Supervision, J.M.Z. and A.J.C.; Project Administration, J.M.Z. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Independent Bioethics Committee for Scientific Research in Gdansk (Poland) (NKBBN/647/2018–2020, 11 December 2020).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Isaacs, B. An Introduction to Geriatrics; Baillière Tindall & Cassell: London, UK, 1965. [Google Scholar]
- Jolanta Życzkowska, T.G. Frailty—An overview for oncologists. Onkologia w Praktyce Klinicznej 2010, 6, 5. [Google Scholar]
- Łacko, A. Specyfika leczenia chorych na nowotwory w podeszłym wieku. Medycyna Wieku Podeszłego 2012, 2, 5. [Google Scholar]
- Palumbo, A.; Bringhen, S.; Mateos, M.V.; Larocca, A.; Facon, T.; Kumar, S.K.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef]
- Alicia Snowden, T.K. Frailty Assessment in Multiple Myeloma. Haematol. Soc. Aust. N. Z. 2022, 3. [Google Scholar]
- Krzemieniecki, K. Całościowa ocena geriatryczna i jej znaczenie kliniczne w onkologii—Systematyczny przegląd piśmiennictwa. Onkol. Prak. Klin 2010, 6, 5. [Google Scholar]
- Bringhen, S.; Mateos, M.V.; Zweegman, S.; Larocca, A.; Falcone, A.P.; Oriol, A.; Rossi, D.; Cavalli, M.; Wijermans, P.; Ria, R.; et al. Age and organ damage correlate with poor survival in myeloma patients: Meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica 2013, 98, 980–987. [Google Scholar] [CrossRef]
- Engelhardt, M.; Domm, A.S.; Dold, S.M.; Ihorst, G.; Reinhardt, H.; Zober, A.; Hieke, S.; Baayen, C.; Müller, S.J.; Einsele, H.; et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica 2017, 102, 910–921. [Google Scholar] [CrossRef]
- Möller, M.D.; Gengenbach, L.; Graziani, G.; Greil, C.; Wäsch, R.; Engelhardt, M. Geriatric assessments and frailty scores in multiple myeloma patients: A needed tool for individualized treatment? Curr. Opin. Oncol. 2021, 33, 648–657. [Google Scholar] [CrossRef]
- Milani, P.; Vincent Rajkumar, S.; Merlini, G.; Kumar, S.; Gertz, M.A.; Palladini, G.; Lacy, M.Q.; Buadi, F.K.; Hayman, S.R.; Leung, N.; et al. N-terminal fragment of the type-B natriuretic peptide (NT-proBNP) contributes to a simple new frailty score in patients with newly diagnosed multiple myeloma. Am. J. Hematol. 2016, 91, 1129–1134. [Google Scholar] [CrossRef]
- Mina, R.; Bringhen, S.; Wildes, T.M.; Zweegman, S.; Rosko, A.E. Approach to the Older Adult With Multiple Myeloma. Am. Soc. Clin. Oncol. Educ. Book. Am. Soc. Clin. Oncology. Annu. Meet. 2019, 39, 500–518. [Google Scholar] [CrossRef]
- Giannopoulos, K.; Jamroziak, K.; Usnarska-Zubkiewicz, L.; Dytfeld, D.; Jurczyszyn, A.; Walewski, J.; Lech-Marańda, E.; Walter-Croneck, A.; Pieńkowska-Grela, B.; Wróbel, T.; et al. Zalecenia Polskiej Grupy Szpiczakowej dotyczące rozpoznawania i leczenia szpiczaka plazmocytowego oraz innych dyskrazji plazmocytowych na rok 2021. Polska Grupa Szpiczakowa 2021, 49, 157–206. [Google Scholar]
- Facon, T.; Hulin, C.; Dimopoulos, M.A.; Belch, A.; Meuleman, N.; Mohty, M.; Chen, W.M.; Kim, K.; Zamagni, E.; Rodriguez-Otero, P.; et al. A frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated with continuous lenalidomide plus low-dose dexamethasone in the first (MM-020) trial. Haematologica 2016, 101, 258–259. [Google Scholar] [CrossRef]
- Facon, T.; Hulin, C.; Dimopoulos, M.A.; Belch, A.; Meuleman, N.; Mohty, M.; Chen, W.-M.; Kim, K.; Zamagni, E.; Rodriguez-Otero, P.; et al. A Frailty Scale Predicts Outcomes of Patients with Newly Diagnosed Multiple Myeloma Who Are Ineligible for Transplant Treated with Continuous Lenalidomide Plus Low-Dose Dexamethasone on the First Trial. Blood 2015, 126, 4239. [Google Scholar] [CrossRef]
- Çiftçiler, R.; Göker, H.; Demiroğlu, H.; Haznedaroğlu, İ.C.; Sayınalp, N.; Aksu, S.; Özcebe, O.; Büyükaşık, Y. Evaluation of Prognostic Significance of the International Staging System According to Glomerular Filtration Rate in Newly Diagnosed Multiple Myeloma Patients Eligible for Autologous Stem Cell Transplantation. Turk. J. Haematol. 2021, 38, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Brill, I.K.; Omel, J.; Godby, K.; Kumar, S.K.; Brown, E.E. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Adv. 2017, 1, 282–287. [Google Scholar] [CrossRef]
- Costa, L.J.; Gonsalves, W.I.; Kumar, S.K. Early mortality in multiple myeloma. Leukemia 2015, 29, 1616–1618. [Google Scholar] [CrossRef] [PubMed]
- McQuilten, Z.; Wellard, C.; Moore, E.; Augustson, B.; Bergin, K.; Blacklock, H.; Harrison, S.; Ho, P.J.; King, T.; Quach, H.; et al. Predictors of early mortality in multiple myeloma: Results from the Australian and New Zealand Myeloma and Related Diseases Registry (MRDR). Br. J. Haematol. 2022, 198, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.; Lin, T.W.; Gau, J.P.; Yu, Y.B.; Hsiao, L.T.; Tzeng, C.H.; Chen, P.M.; Chiou, T.J.; Liu, J.H.; Liu, Y.C.; et al. Risk of Early Mortality in Patients With Newly Diagnosed Multiple Myeloma. Medicine 2015, 94, e2305. [Google Scholar] [CrossRef]
- Lee, H.C.; Ailawadhi, S.; Gasparetto, C.J.; Jagannath, S.; Rifkin, R.M.; Durie, B.G.M.; Narang, M.; Terebelo, H.R.; Toomey, K.; Hardin, J.W.; et al. Treatment patterns and outcomes in elderly patients with newly diagnosed multiple myeloma: Results from the Connect(®) MM Registry. Blood Cancer J. 2021, 11, 134. [Google Scholar] [CrossRef]
- Mohty, M.; Cavo, M.; Fink, L.; Gonzalez-McQuire, S.; Leleu, H.; Mateos, M.V.; Raab, M.S.; Schoen, P.; Yong, K. Understanding mortality in multiple myeloma: Findings of a European retrospective chart review. Eur. J. Haematol. 2019, 103, 107–115. [Google Scholar] [CrossRef]
- Facon, T.; Cook, G.; Usmani, S.Z.; Hulin, C.; Kumar, S.; Plesner, T.; Touzeau, C.; Bahlis, N.J.; Basu, S.; Nahi, H.; et al. Daratumumab plus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: Frailty subgroup analysis of MAIA. Leukemia 2022, 36, 1066–1077. [Google Scholar] [CrossRef]
- Mateos, M.V.; Cavo, M.; Blade, J.; Dimopoulos, M.A.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): A randomised, open-label, phase 3 trial. Lancet 2020, 395, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Usmani, S.Z.; Berdeja, J.G.; Agha, M.; Cohen, A.D.; Hari, P.; Avigan, D.; Deol, A.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, an Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J. Clin. Oncol. 2023, 41, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).