Simple Summary
Peripheral T-cell lymphomas are a group of rare cancers of T cells or natural killer cells, most often with a poor prognosis. In recent years, significant progress has been made through the development of more specific therapies. This review aims to provide an up-to-date overview of current treatments in nodal PTCL.
Abstract
Peripheral T-cell lymphomas (PTCLs) are a heterogeneous group of rare neoplasms of mature T cells or natural killer (NK) cell. PTCLs usually have an aggressive course and a poor outcome. In recent years, significant progress has been made in the knowledge of the molecular lymphomagenesis of PTCLs, and through the development of new, more specific therapeutic molecules, one can hope in the coming years for more personalized medicine and improved patient prognosis. This review aims to provide an up-to-date overview of the current therapeutic approaches in nodal PTCLs.
1. Introduction
Peripheral T-cell lymphomas (PTCLs) are neoplasms of mature T cells or natural killer (NK) cells. PTCLs are a heterogeneous group of rare lymphoid malignancies, comprising 31 entities in the updated 2017 World Health Organization (WHO) classification [1]), which can be classified into 4 groups: nodal, extranodal, leukemic and primary cutaneous (Figure 1). PTCLs account for only 5–10% of all noncutaneous lymphomas, with geographic and ethnic variations [2,3,4]. The pathogenesis and the mechanisms of PTCL transformation are complex and have begun to be deciphered in recent years [5].
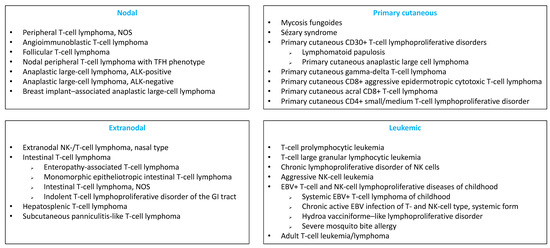
Figure 1.
Classification of mature T- and NK-cell neoplasms according to the 2017 WHO classification.
PTCLs usually have an aggressive course and a poor outcome. The rarity and heterogeneity of these diseases limit the study of new treatments in clinical trials. Until recent years, few therapies have been developed specifically for PTCLs. The situation has now changed, with many new drugs, but too few patients available to test them. Over recent years, our understanding of PTCLs molecular pathogenesis has advanced remarkably, and we can expect the development of specific treatments for individualized PTCL entities, or the targeting of a specific protein or biological pathway shared by certain entities. This review provides an update on therapies for nodal PTCLs.
2. Frontline Treatment of PTCLs
Given the rarity of PTCLs, there is a lack of randomized studies, and most therapeutic approach paradigms for PTCLs have been derived from treatments developed for aggressive B-cell non-Hodgkin lymphomas (NHLs). As a result, a cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen was considered the standard therapy despite results showing that it was suboptimal (except in ALK+ anaplastic large cell lymphoma (ALCL)). Thus, for the main nodal PTCLs (AITL, PTCL-NOS, and ALK− ALCL), the 2- and 5-year progression-free survival (PFS) is approximately 35% and 25%, and the 2- and 5-year overall survival (OS) is 45% and 35%, respectively [6,7,8,9,10,11,12,13].
2.1. How to Improve CHOP?
For more than 20 years, several studies have attempted to improve the results of CHOP in PTCLs, but very few have succeeded. Here, we present these studies as well as the unresolved questions.
2.1.1. CHOEP and Other Intensive Chemotherapy Regimens
Adding etoposide to CHOP (CHOEP) was one of the first attempts to improve PTCL prognosis. Although there have been no randomized studies comparing CHOP and CHOEP regimens in PTCLs, a number of retrospective or phase 2 prospective studies have suggested a benefit of CHOEP. Due to the increased toxicity of CHOEP, this regimen is usually preferred in patients less than 60–65 years old. Table 1 summarizes the main studies that evaluated CHOEP in PTCLs [7,9,12,14,15,16,17,18,19]. Apart from a Korean retrospective study which did not find any benefit of CHOEP over CHOP, suggesting that Asian patients would not benefit from the addition of etoposide, most studies suggested an advantage of CHOEP over CHOP in PFS and/or OS. This benefit is most evident in patients with ALK+ ALCL, with two retrospective studies dedicated to this entity showing a benefit in PFS and OS, independent of the international prognostic index (IPI) [12,19]. In the absence of a randomized study, the real benefit of using CHOEP in PTCLs is still a matter of debate.

Table 1.
Published studies of frontline CHOEP in PTCLs.
Besides the CHOEP regimen, some intensive polychemotherapies have been studied, such as dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (DA-EPOCH) [20,21], Mega-CHOEP [22], and cyclophosphamide, vincristine, doxorubicin, dexamethasone/methotrexate, cytarabine (HyperCVAD) [23,24]. A total of 2 phase 2 studies reported the results of DA-EPOCH in PTCLs. The first 1 enrolled 24 patients with systemic ALCL (ALK+, n = 15; ALK−, n = 9; median age 38 years), and reported, after a median follow-up of 14.4 years, event-free survival (EFS) probabilities of 72% and 62.5%, and OS probabilities of 78% and 87.5%, in ALK+ and ALK− ALCL patients, respectively [20]. In the Japanese study, 41 patients (mostly PTCL-NOS and AITL) were treated with DA-EPOCH. Their 2-year PFS and OS were 53% and 73%, respectively [21]. The German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) reported the results of MegaCHOEP in 33 PTCL patients (no ALK+ ALCL) [22]. A total of 22 out of 33 patients (66.7%) received all of the planned therapy. The main reasons for the early discontinuation of treatment were progressive or stable disease in 4 patients (12%), and extensive toxicity in 6 patients (18%), with 2 therapy-related deaths. The 3-year EFS and OS were 26% and 45%, respectively. The Australasian Leukaemia and Lymphoma Group reported a phase 2 study of a modified hyperCVAD frontline therapy in 26 PTCL patients [23]. This regimen resulted in similar outcomes to CHOP-like chemotherapy and was associated with significant toxicity. Similar results were reported by the MD Anderson Cancer Center in a retrospective study [24]. Overall, these intensive regimens do not seem to provide any benefit compared to CHOP/CHOEP, and their toxicity is often more marked.
2.1.2. Combination of CHOP with Novel Agents
With the development of new drugs, a number of prospective phase 1 and 2 studies have evaluated CHOP associated with monoclonal antibodies, small molecule inhibitors, epigenetic modifiers, or antimetabotite [25,26,27,28,29,30,31,32,33,34]. Table 2 summarizes these studies. Only 3 of these combinations (brentuximab vedotin (BV) + cyclophosphamide, doxorubicin, and prednisone (CHP), alemtuzumab-CHOP, and romidepsin-CHOP) were subsequently evaluated in a randomized, phase 3 trial versus CHOP. A phase 2 study was recently presented at the 2021 ASH meeting, assessing the addition of etoposide to BV-CHP (BV-CHEP regimen) in adults with newly diagnosed PTCL (including ALK+ ALCL with IPI ≥ 2) and CD30 expression ≥ 1% on tumor cells by immunohistochemistry [35]. Patients received BV consolidation and autoSCT was allowed. The primary endpoint was the CR rate after BV-CHEP. Overall, 48 patients were enrolled (including 3 ALK+ ALCL). The CR rate was 80% (ORR 91%), and the treatment was tolerable. The BV-CHEP regimen could be of special interest in patients with ALK+ ALCL, in whom etoposide and BV have demonstrated a benefit.

Table 2.
Phase 1 and 2 studies combining CHOP/CHOP-like with novel agents (published studies).
2.1.3. Randomized Studies Challenging CHOP
Very few randomized studies have tried to challenge CHOP in PTCLs. Three of them combined CH(O)P with a novel agent, and two others each evaluated a new chemotherapy regimen (etoposide, ifosfamide, cisplatin, doxorubicin, bleomycin, vinblastine and dacarbazine (VIP-reinforced-ABVD); gemcitabine, cisplatin, and methylprednisolone (GEM-P)) [11,13,36,37,38,39]. Only ECHELON-2 study assessing BV-CHP reached its primary endpoint. Table 3 summarizes the main results of the 5 studies.

Table 3.
Randomized (published) studies in frontline treatment of PTCLs.
LTP-95 is a randomized, phase 3 study that compared the etoposide, ifosfamide, cisplatin alternating with doxorubicin, bleomycin, vinblastine, dacarbazine (VIP-reinforced-ABVD; VIP-rABVD) regimen to CHOP as frontline treatment in PTCLs. The primary objective was the 2-year EFS (events were cessation of treatment for toxicity or progression, relapse, or death from any cause). A total of 88 patients were randomized (43 to VIP-rABVD and 45 to CHOP), including 10 with ALK+ ALCL. After a median follow-up of 9 years, the 2-year EFS was 45% with VIP-rABVD and 41% with CHOP, which was not statistically different.
CHEMO-T is a randomized, phase 2 study that compared the gemcitabine, cisplatin, and methylprednisolone (GEM-P) regimen with CHOP in PTCL patients ≥ 18 years [11]. ALK+ ALCL were not included. The primary endpoint was complete response (CR)/CR unconfirmed (CRu) rates on completion of chemotherapy. After the inclusion of 87 patients (44 to GEM-P and 43 to CHOP), a planned review of efficacy data by the independent data monitoring committee (IDMC) showed that fewer patients had achieved a CR/CRu with GEM-P (46%) than with CHOP (62%). The IDMC concluded there was a high likelihood that GEM-P would be inferior to CHOP at the end of the trial, and the trial was closed early.
ECHELON-2 is a randomized, phase 3 study comparing BV-CHP with CHOP in patients ≥ 18 years with CD30+ PTCLs [37]. Enrollment targeted 75% of patients with systemic ALCL (sALCL) to ensure the secondary endpoint of PFS in this subgroup could be appropriately assessed. Key inclusion criteria were as follows: age ≥ 18 years, eligible histology according to WHO 2008 classification (ALK+ ALCL with IPI ≥ 2, ALK− ALCL, PTCL-not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), adult T-cell leukemia/lymphoma (ATLL), enteropathy-associated T-cell lymphoma (EATL), or hepatosplenic T-cell lymphoma (HSTL)), performance status (PS) 0–2; CD30 should be ≥10% of neoplastic cells (in cases where enumeration of neoplastic cells was not possible, total lymphocytes may have been used) by local review. Primary endpoint was modified PFS by independent central review, the events of which were relapse/progression, death from any cause, or receipt of subsequent systemic chemotherapy for failure of frontline treatment. Randomization was stratified by histological subtype according to local pathology assessment (ALK+ ALCL vs. all other histologies) and IPI score (0–1 vs. 2–3 vs. 4–5). Patients received 6 or 8 cycles (at the investigator’s discretion at registration) of either BV-CHP or CHOP. Consolidative stem-cell transplantation or radiotherapy after treatment was permitted at the investigator’s discretion (stem-cell transplantation intent was prespecified before the first cycle of chemotherapy). In all, 226 patients were assigned to each arm. Median PFS was 48.2 months in the BV-CHP arm vs. 20.8 months in the CHOP arm (hazard ratio (HR) 0.71 (95% confidence interval (CI) 0.54–0.93), p = 0.01), with 3-year PFS of 57% vs. 44%. OS was also significantly improved in the BV-CHP arm, with 3-year OS of 77% vs. 69% (HR 0.66 (95% CI 0.46–0.95), p = 0.02). Incidence and severity of febrile neutropenia and peripheral neuropathy were similar between arms. Based on ECHELON-2, the Food and Drug Administration approved brentuximab vedotin for the treatment of adult patients with previously untreated sALCL or other CD30-expressing PTCLs, including AITL and PTCL-NOS, in combination with cyclophosphamide, doxorubicin, and prednisone. The European Medicines Agency (EMA)’s approval was more restricted, brentuximab vedotin being indicated in combination with cyclophosphamide, doxorubicin, and prednisone for adult patients with previously untreated sALCL. The EMA’s decision was based on the fact that the subgroup analysis of AITL (n = 54) and PTCL-NOS (n = 72) showed no benefit of BV-CHP in PFS and OS compared to CHOP. Although the study was not powered to demonstrate a benefit in these subgroups, the observed confidence interval makes the existence of a clinically relevant difference between BV-CHP and CHOP unlikely in AITL and PTCL-NOS.
An updated analysis was recently published after prolonged follow-up [38]. The estimated 5-year PFS was 51% for the BV-CHP arm versus 43% for the CHOP arm (p = 0.0077; HR 0.70 (0.53–0.91)), and the estimated 5-year OS was 70% for the BV-CHP arm versus 61% for the CHOP arm (p = 0.0424; HR 0.72 (0.53–0.99)). In subgroup analyses, the PFS improvement was significant only for sALCL (by pooling ALK+ and ALK− sALCL, but not in each individual entity).
Thus, ECHELON-2 mainly demonstrated a benefit of BV-CHP on CHOP in ALCL. However, some questions remain unanswered. The choice of CHOP as the control arm rather than CHOEP could be questioned. Indeed, as indicated previously, two retrospective studies have shown a benefit of CHOEP over CHOP in PFS and OS in ALK+ ALCL [12,19], and the question remains open for ALK− ALCL. Furthermore, since ALK+ ALCL IPI 0–1 were not included in ECHELON-2, we support the claim that CHOEP should be the preferred option for these patients, given the lack of data for BV-CHP.
Another point of discussion concerns the rearrangement of DUSP22 (DUSP22-R), which is present in 20 to 30% of all ALK− ALCL. Until recently, the prognostic impact of DUSP22-R was uncertain, with 5-year OS ranging from 40% to 90% in the 2 main studies, including 12 and 22 patients, respectively [40,41]. Recently, a LYSA and TENOMIC study compared the outcome of 45 ALK− ALCL patients with DUSP22-R and 55 non-rearranged (DUSP22 non-R) cases [42]. The 5-year PFS was 46% and 21% for the DUSP22-R and non-R patients, respectively (p < 0.01), and 5-year OS was 57% and 45% for the DUSP22-R and non-R patients, respectively (p = 0.32). Since ECHELON-2 did not analyze DUSP22-R, it is not known whether the distribution of DUSP22-R was balanced between BV-CHP and CHOP arms, and to what extent this could have impacted the PFS difference observed between BV-CHP and CHOP arms in patients with ALK− ALCL.
The last questionable point concerns the correlation between the CD30 expression and the efficacy of BV in non-ALCL subtypes (ALCL tumor cells are always positive for CD30 by definition). In ECHELON-2 inclusion criteria, by local review, CD30 should be ≥10% of neoplastic cells, but in cases where enumeration of neoplastic cells was not possible, total lymphocytes may have been used. This means that CD30 expression by non-tumor cells could be used (such cells can be seen in PTCLs, notably in AITL), which complicates the evaluation of the correlation between expression and efficacy. Moreover, after central review, some patients had <10% CD30+, and even no expression. Finally, a study of CD30 expression above vs. below median (or at 10%) did not predict response to BV-CHP in ECHELON-2 non-ALCL subtypes, as responses were seen across CD30 levels [43].
In summary, ECHELON-2 is an important study in the field of PTCLs. As the study did not report survival curves for each PTCL entity, comparison with other studies is difficult. Questions regarding the comparison between BV-CHP and CHOEP, especially in ALK+ ALCL, the impact of DUSP22-R in ALK− ALCL, and the optimal CD30 expression level need to be clarified.
ACT-2 is a randomized, phase 3 study that compared alemtuzumab-CHOP (A-CHOP) to CHOP in 116 PTCL patients (58 in each arm), aged 61–80 years [39]. ALK+ ALCL were not included. The primary endpoint was 3-year EFS. CR/CRu rates were 60% and 43% for A-CHOP and CHOP, respectively. The 3-year EFS, PFS, and OS were 27%, 28%, and 37% for A-CHOP, vs. 24%, 29%, and 56% for CHOP, respectively, with no significant differences. A-CHOP increased hematotoxicity, resulting in more grade ≥3 infections (40% vs. 21%) and death due to infections (4 vs. 1) than CHOP. In summary, A-CHOP increased CR rates, but it did not improve survival due to toxicity.
The Ro-CHOP randomized, phase 3 study compared romidepsin-CHOP vs. CHOP in PTCL patients aged 18–80 years [13]. ALK+ ALCL were not included. The primary end point was PFS. For the 421 enrolled patients (Ro-CHOP, n = 211; CHOP, n = 210), median PFS was 12.0 vs. 10.2 months, and 2-year PFS was 43% vs. 36% for Ro-CHOP vs. CHOP (p = 0.096), respectively. The 2-year OS was 64% vs. 63% for Ro-CHOP vs. CHOP (p = 0.48), respectively. Preplanned PFS analyses were conducted in subgroups with potential prognostic/predictive factors for the intent-to-treat population. There was no statistically significant difference in PFS between the Ro-CHOP and CHOP arms for any subgroup analyzed. Exploratory analysis in centrally-confirmed AITL and other nodal lymphomas of T follicular helper (TFH) cell origin showed prolonged PFS in the Ro-CHOP arm compared to the CHOP arm, with a median PFS of 19.5 months vs. 10.6 months ((HR 0.69 (95% CI 0.48–1.00; p = 0.046)), respectively. Grade 3/4 cytopenias were more frequent in the Ro-CHOP arm, with thrombocytopenia (50% vs. 10%), neutropenia (49% vs. 33%), and anemia (47% vs. 17%). In summary, the addition of romidepsin to CHOP did not improve PFS nor OS, and increased toxicity. The suggested benefit in TFH-like lymphomas (AITL and TFH PTCL) warrants further investigation.
2.2. The role of Consolidative Hematopoietic Stem-Cell Transplantation
2.2.1. Autologous Stem-Cell Transplantation
Prospective Studies
A total of 5 prospective phase 2 studies, one of which was subsequently updated, assessed upfront autologous stem-cell transplantation (autoSCT) in PTCLs [14,44,45,46,47,48]. Most of these studies excluded ALK+ ALCL, which has a better prognosis. Table 4 summarizes the main results of these studies.

Table 4.
Prospective, published, phase 2 studies of consolidative autoSCT in PTCL.
The largest of these trials, the NLG-T-01 study, was conducted by the Nordic Lymphoma Group [14], and was the basis for the current European guidelines [49]. Overall, 160 patients with histopathologically confirmed PTCL (excluding ALK+ ALCL) were treated with 6 cycles of CHOEP-14 (etoposide was omitted in patients > 60 years). Patients in CR or partial response (PR) proceeded to consolidation with a carmustine, etoposide, cytarabine, and melphalan (BEAM) conditioning regimen, followed by autoSCT. A total of 115 patients (72%) underwent autoSCT. Treatment-related mortality was 4%. After a median follow-up of 60.5 months, 5-year PFS and OS rates were 44% and 51%, respectively. Subtype-specific analysis revealed the highest PFS and OS occurring in patients with ALK− ALCL (5-year PFS and OS of 61% and 70%, respectively). Patients with AITL had 5-year PFS and OS of 49% and 52%, respectively; patients with PTCL-NOS had 5-year PFS and OS of 38% and 47%, respectively; and patients with EATL had 5-year PFS and OS of 38% and 48%, respectively.
The COMPLETE prospective, multicenter cohort study reported the outcome of 119 patients with nodal PTCL in CR1 [50]. Among them, 36 patients underwent autoSCT in CR1. In multivariate analysis, autoSCT was independently associated with improved OS (HR, 0.37; 95% CI, 0.15–0.89).
Retrospective Studies
The largest retrospective studies, which included at least 100 patients, are presented in Table 5 with their main results [9,12,51].

Table 5.
Selected, published retrospective studies of consolidative autoSCT in PTCLs, including at least 100 patients.
2.2.2. Allogeneic Stem-Cell Transplantation
The majority of allogeneic stem-cell transplantation (alloSCT) studies in PTCLs concern relapsed or refractory patients, and few frontline data are available. Here we present the most relevant frontline studies.
Prospective Studies
In 2014, the FIL group reported the results of a phase 2 study assessing intensified chemo-immunotherapy, with or without stem-cell transplantation, in newly diagnosed patients with PTCL [52]. On the basis of donor availability, patients ≤ 60 years old in response received alloSCT or autoSCT. Among 61 patients ≤ 60 years old, 38 (62%) responded and received alloSCT (n = 23) or autoSCT (n = 14), and one patient in CR was not transplanted. After a median follow-up of 40 months, the 4-year OS, PFS and disease-free survival (DFS) rates were 49%, 44%, and 65%, respectively. The study was not powered to evaluate the differences between autoSCT and alloSCT.
The randomized AATT phase 3 trial was recently published [17]. This study is the only phase 3 study assessing a transplant strategy in the frontline treatment of PTCLs. It was hypothesized that alloSCT could improve outcomes over autoSCT. Key inclusion criteria were as follows: age 18–60, stage II-IV or age-adjusted IPI (aaIPI) > 0, PS 0–3, and a nodal or extranodal PTCL excluding ALK+ ALCL. Patients were randomized upfront to receive 4 cycles of CHOEP-14, 1 cycle of DHAP (dexamethasone, cytarabine, and cisplatin or carboplatin), and autoSCT or alloSCT. In the absence of progressive disease (PD) at the time of restaging, patients continued in the study and were scheduled to receive either BEAM high-dose chemotherapy followed by autoSCT or myeloablative conditioning with fludarabine, busulfan, and cyclophosphamide, followed by alloSCT. The primary end point was EFS at 3 years. Overall, 104 patients were included. The data safety and monitoring board, in agreement with the study steering committee, stopped randomization and recruitment because a planned interim analysis had shown that the study was highly unlikely to meet the primary end point. The transplant-related mortality rate (TRM) contributed to this decision. After a median follow-up of 42 months, the 3-year EFS after alloSCT was 43% vs. 38% after autoSCT. The 3-year OS was 57% vs. 70% after alloSCT or autoSCT, respectively, without significant differences between treatment arms. Interestingly, there was no relapse among the 21 responding patients who underwent alloSCT, as opposed to 13 of 36 patients (36%) proceeding to autoSCT. However, 8 of the 26 patients (31%) died of transplant-related toxicity after alloSCT vs. none of the 41 patients after autoSCT.
Retrospective Studies
The Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) recently reported a registry study which analyzed 285 patients with PTCL who underwent alloSCT between 2006 and 2014 [53]. For the 138 patients who underwent frontline alloSCT in CR1 or PR1, the 4-year OS was 63%, the cumulative incidence of relapse was 19% at 2 years, and the TRM was 24% at 4 years.
2.3. What Can Be Learned from Studies Dedicated to A Single Nodal PTCL Entity?
The majority of studies on PTCLs have mixed the different histological entities, complicating the evaluation of prognostic factors and the impact of treatment for each PTCL subtype. A few studies, more often retrospective than prospective, have focused on a specific entity. These studies are presented in Table 6 [12,18,19,54,55,56,57,58,59,60,61,62].

Table 6.
Published studies focused on a single nodal PTCL entity.
Regarding AITL, a retrospective LYSA study which analyzed patients included in several clinical trials did not find any significant difference between CHOP and more intensive treatments such as doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (ACVBP) [54]. A Japanese study found similar survival (PFS and OS) with CHOP and pirarubicin, cyclophosphamide, vincristine, and prednisolone (THP-COP) regimens [55]. Two prospective phase 2 studies conducted by the LYSA did not show any benefit to the addition of rituximab or lenalidomide to CHOP compared to historical controls [56,58]. Finally, a study from the prospective International T-cell Project (ITCP) found that patients who underwent consolidative transplantation in CR1 had significantly better outcomes compared with transplant-eligible patients (age ≤ 65 years) who did not undergo transplantation, with 5-year PFS of 79% vs. 31%, and 5-year OS of 89% vs. 52%, respectively [59]. On the other hand, there was no significant difference in 5-year OS for patients receiving chemotherapy regimens with and without etoposide (50% vs. 43%, respectively).
Considering PTCL-NOS, the retrospective study from the IIL lymphoma registry did not find any benefit from bone marrow autologous transplantation [60]. In the retrospective study from the International Peripheral T-Cell Lymphoma (IPTCL) Project, there was no survival advantage for patients who received anthracycline-based combination chemotherapy compared with those receiving combination chemotherapy without anthracycline [61]. In the recent study from the prospective ITCP on ALK− ALCL, regimens containing both anthracycline and etoposide were associated with superior OS but not PFS [62].
Regarding ALK+ ALCL, a retrospective study from the Nordic Lymphoma Group and an individual-patient data pooled analysis from 6 studies (including 263 patients) support that the integration of etoposide into the CHOP (CHOEP) may be associated with important improvements in PFS and OS [12,19]. Of note, the benefit of CHOEP over CHOP was independent of IPI in both studies. Finally, in the pooled analysis, 34 patients underwent consolidative autoSCT (all were <60 years), and for patients <60 years in CR or PR, in stratified Cox models including etoposide-based induction, IPI and consolidative autoSCT, only the etoposide-based induction and the IPI remained independently prognostic for PFS and OS, without impact of autoSCT.
A proposed treatment algorithm for newly diagnosed nodal PTCLs is shown in Figure 2.
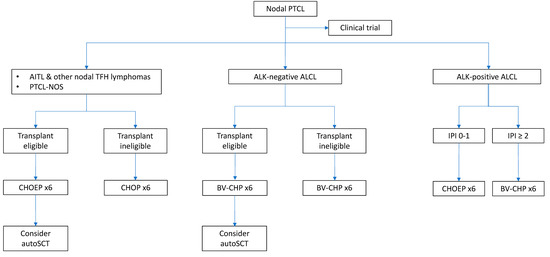
Figure 2.
Proposed algorithm of frontline treatment in nodal PTCLs. This algorithm is based on histological subtype, transplant-eligibility, and prognostic factors.
3. Treatment of Relapsed/Refractory PTCLs
3.1. How Many Patients Relapse or Progress?
In the ITCP study, among 937 patients enrolled between 2006 and 2015 who received first-line treatment, 633 (68%) either progressed (47%) or relapsed (21%) [63]. Relapsed disease was defined as relapse at least 1 month from completion of front-line therapy in patients who achieved a CR or a satisfactory PR. Among the 197 relapsed patients, 125 (63%) had an early relapse (≤12 months), and 72 (37%) had a late relapse (>12 months).
In a retrospective cohort of 775 mainly nodal PTCL patients from the Mayo Clinic and the Swedish Lymphoma Registry treated with curative intent between 2000 and 2012, 582 (75%) had an event of relapse/progression, retreatment, or death from any cause [10]. A total of 64% of patients progressed/relapsed within the first 24 months.
3.2. What Is the Outcome after the First Event of Progression/Relapse?
The British Columbia Cancer Agency (BCCA) reported the survival of patients with relapsed/refractory (R-R) nodal PTCL diagnosed between 1976 and 2010, who were not transplanted after progression/relapse [64]. For the 153 analyzed patients (79 PTCL-NOS, 38 AITL, 25 ALK− ALCL (including 1 case with unknown ALK status), and 11 ALK+ ALCL), median time from initial diagnosis to progression/relapse after primary therapy was 6.7 months. After progression/relapse, median second PFS and OS were 3.1 and 5.5 months, respectively, without difference by PTCL subtype. Patients who received chemotherapy at relapse (n = 89) had a better median PFS and OS of 3.7 and 6.5 months, respectively, which was statistically significant, but clinically disappointing.
In the prospective ITCP study, the median OS of the 633 R-R patients was 5.8 months, with a 3-year OS of 23% [63]. The outcome was poorer for refractory versus relapsed patients, with median OS of 5 versus 11 months, and 3-year OS of 21% versus 28%, respectively (p < 0.001).
In the study from the Mayo Clinic and the Swedish Lymphoma Registry, patients who progressed/relapsed within the first 24 months had a median OS of 4.9 months and 5-year OS of 11% [10]. In contrast, median OS after achieving EFS24 was not reached, and 5-year OS was 78%.
The retrospective study from the Swedish Lymphoma Registry showed that central nervous system (CNS) involvement at progression/relapse in PTCLs occurred in 4.5% [65]. However, the poor outcome after relapse was largely driven by systemic rather than CNS disease and was not significantly altered by the presence of CNS involvement at relapse.
Overall, these studies showed that progressions/relapses of PTCLs are frequent (approximately 70% of patients), occur most often early (during the first year after initial diagnosis), and have a poor outcome, with a median OS of approximately 6 months. This survival rate did not significantly improve between 1976 and 2015. However, since that time, new treatments have improved results somewhat, at least in some subtypes, as we will see.
3.3. Second-Line Chemotherapy Regimen for PTCL-NOS and AITL
The same salvage combination regimens as for aggressive B lymphomas can be used, such as DHAP, DHAX (dexamethasone, cytarabine, oxaliplatin), ICE (ifosfamide, carboplatin, etoposide), GDP (gemcitabine, cisplatin, dexamethasone), ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin), or GEMOX (gemcitabine, oxaliplatin) [66].
Single chemotherapy agents such as bendamustine or gemcitabine may be used. Bendamustine was assessed in the BENTLY phase 2 study [67]. For the 60 enrolled patients, the objective response rate (ORR) after 3 cycles was 50% (CR/CRu 28%), the median PFS and OS were 3.6 and 6.2 months, respectively. In a subsequent, French, real-life study on 138 R-R PTCLs, ORR was 33% (CR 25%), median PFS and OS were 3.1 and 4.4 months, respectively [68]. In a phase 2 study including 20 PTCL patients treated with gemcitabine, the ORR was 55% (CR 30%) [69]. Among the 6 CR patients, 5 were in continuous CR with a median duration of response of 34 months.
3.4. Treatment of R-R ALCL
In the pre-BV era, a study from the LYSA reported the outcome of ALK+ and ALK− ALCL after the first progression/relapse [70]. Among the 138 (64 ALK+ and 74 ALK− ALCL) adults initially treated in clinical trials, 40 (14 ALK+ and 26 ALK− ALCL) had a first progression or relapse. Most patients received standard salvage chemotherapy (mainly DHAP or ESHAP). The ORR to salvage chemotherapy was 54% (ALK+, 71%; ALK−, 47%; CR 46%, PR 8%). Median follow-up from the first R-R events was 12.5 years. For ALK+ and ALK− patients, respectively, median time between inclusion in first-line clinical trials and first R-R events was 6 and 11.1 months; median PFS after the first-R/R events were 3.8 and 5.3 months; median OS were 13.6 and 8.1 months, and 5-y OS were 36% and 19%, respectively.
A phase 2 study evaluated the safety and efficacy of BV in patients with R-R sALCL [71]. The primary endpoint was the ORR according to the 2007 revised response criteria. BV (1.8 mg/kg) was administered every 3 weeks for up to 16 cycles. Median observation time was 6 years from the start of treatment. Overall, 58 patients (16 ALK+ and 42 ALK− ALCL) were enrolled. A total of 50 patients achieved a best objective response (ORR 86%), including 38 (66%) CR and 12 (20%) PR. The median PFS was not reached for CR patients, 4.5 months for PR patients, 2.5 months for SD patients, and 1.1 for PD patients, meaning that CR should be the goal of BV treatment. Positron emission tomography (PET) after 4 cycles was highly predictive of outcome. In ALK+ and ALK− ALCL patients, 10/16 (63%) and 28/42 (67%) were in CR, respectively. Among the 10 ALK+ patients in CR, 6 underwent alloSCT, 1 underwent autoSCT, and 3 were not transplanted. At last follow-up, 4 alloSCT and 1 non-transplanted patients were still alive and in continuous CR. Among the 28 ALK− patients in CR, 2 underwent alloSCT, 7 underwent autoSCT, and 19 no transplantation. At last follow-up, 1 alloSCT, 5 autoSCT, and 11 non-transplanted patients were still alive and in continuous CR. For ALK+ and ALK− patients, the 5-year PFS rates were 37% and 39%, and the 5-year OS rates were 56% and 61%, respectively.
Overall, although this study was not randomized, its results showed better outcomes than historical controls in terms of response, PFS, and OS, and BV has become the standard treatment for R-R ALCL. Recently, a population-based study described the efficacy of BV monotherapy in R-R sALCL using Public Health England data [72]. For the 127 patients treated between 2014 and 2019, the 2-year OS was 47%, without significant difference according to ALK status. The majority of deaths occurred within 18 months, with very few events after this period.
For R-R ALK+ ALCL, some ALK inhibitors have been evaluated in children and adults. In adults, ALK inhibitors are mainly used after failure of BV treatment. Table 7 summarizes prospective studies [73,74,75,76,77,78].

Table 7.
Prospective studies on ALK inhibitors for R-R ALK+ ALCL.
3.5. What Is the Role of Hematopoietic Stem-Cell Transplantation in R-R PTCLs?
As previously shown, the BCCA reported the survival of patients with R-R nodal PTCLs who were not transplanted after progression/relapse [64]. Patients who received chemotherapy at relapse (n = 89) had a median PFS and OS of 3.7 and 6.5 months, respectively. The goal of transplantation is therefore to improve these results.
3.5.1. Autologous Stem-Cell Transplantation
Studies on autoSCT in adults with R-R PTCLs are retrospective. A prospective pediatric study, the ALCL-relapse trial, assessed a risk-stratified treatment (alloSCT, autoSCT, or vinblastine monotherapy) of children with R-R ALK+ ALCL [79]. Table 8 summarizes studies that included at least 50 patients [63,80,81,82,83] and the pediatric ALCL-relapse trial. Overall, after autoSCT in CR/PR adults, the 3- to 5-y OS is approximately 50%.

Table 8.
Published retrospective studies of autoSCT in R-R PTCLs (of at least 50 patients).
3.5.2. Allogeneic Stem-Cell Transplantation
There are only 1, small, prospective phase 2 study in adults, 1 prospective pediatric study in ALK+ ALCL, and retrospective studies mainly from registries [53,79,82,84,85,86,87]. Table 9 summarizes the prospective studies and the retrospective studies that included at least 50 patients.

Table 9.
Published studies of alloSCT in R-R PTCLs (of at least 50 patients for retrospective studies).
Overall, after alloSCT, the 3 to 5-y OS is approximately 50%. Progressive disease at time of alloSCT is the dominant adverse factor. However, even in this situation, prolonged survival is observed in about 35% of cases. An altered performance status is the second factor associated with a reduced OS. Finally, in recent years, the non-relapse mortality (NRM) has improved and is now approximately 20% at 3 years.
3.5.3. Autologous or Allogeneic Stem-Cell Transplantation?
Based on non-randomized studies, autoSCT and alloSCT in R-R PTCLs improved the outcome compared to no transplantation. The question is whether it is better to perform an autoSCT or an alloSCT. In the absence of a randomized study, this question cannot be formally answered. Recently, a systematic review and meta-analysis compared the effectiveness and safety of autoSCT vs. alloSCT in patients with R-R PTCLs [88]. A total of 30 studies (including 885 patients who underwent autoSCT and 880 who underwent alloSCT) were analyzed. In the autoSCT group, 5-year PFS and OS rates were 40% and 53%, respectively, and the 3-year TRM was 7%. In the alloSCT group, 5-year PFS and OS rates were 48% and 54%, respectively, and the 3-year TRM was 32%. The conclusion was that PFS and OS were similar in the autoSCT and alloSCT groups; however, alloSCT was associated with specific survival benefits.
Concerning ALK+ ALCL, the prospective pediatric ALCL-relapse trial assessed a risk-stratified treatment (alloSCT, autoSCT, or vinblastine monotherapy) of children with R-R ALK+ ALCL [79]. AlloSCT was highly efficacious for patients with a high-risk early relapse (within the first year after initial diagnosis) or refractory disease, whereas autoSCT did not prevent additional relapse in early relapsed ALCL. The main conclusion of the study was that autoSCT is not indicated for R-R ALK+ ALCL. Except for low-risk patients (with a late relapse) for whom vinblastine monotherapy is an efficacious treatment option, all other R-R patients should be referred for alloSCT. In the absence of prospective data in adults, this approach could be applied in adults with R-R ALK+ ALCL.
3.6. Novel Agents
Several novel agents have been assessed in R-R PTCLs, and, while some have received approval for clinical use, approval is not uniform across countries. BV and ALK inhibitors have been previously described. Table 10 and Table 11 summarize the prospective studies of other single agents [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108] and combinations [109,110,111,112,113,114,115,116,117], respectively.

Table 10.
Published, prospective studies of novel agents in R-R PTCLs (single agent studies).

Table 11.
Published, prospective studies of novel agents in R-R PTCLs (combination studies).
Overall, with single agents, ORR ranged from 0 to 50%, CR rates from 0 to 27%, and median PFS was most often less than 6 months. Combination studies included small numbers of patients, making it difficult to interpret the results on both efficacy and toxicity. Larger studies are therefore necessary.
A study from the COMPLETE registry compared treatment results for patients with R-R PTCLs who received single agents to those who received combination chemotherapy [118]. Single agents were as follows: romidepsin, belinostat, BV, alisertib, lenalidomide, denileukin diftitox, bendamustine, or pralatrexate. Combination included any multiagent chemotherapy regimen excluding the above single agents. At first retreatment, 31 patients received single agents and 26 received combination therapy. The CR rate was higher with single agents vs. combination therapy (41% vs. 19%; p = 0.02). After a median follow-up of 2 years, median PFS (11.2 vs. 6.7 months; p = 0.02), and OS (38.9 vs. 17.1 months; p = 0.02) were longer with single agents. This study suggests better results with single agent treatment, but in the absence of randomized study, the debate remains open.
4. Conclusions
The management of PTCL patients continues to be a challenge for physicians, and its treatment is still an unmet medical need. The historical “one-size-fits-all” PTCL treatment approach has been shown to be ineffective and should no longer be used. Recent advances in the biology of PTCLs have led to the identification of new targets, and to the establishment of biomarker-driven treatments such as BV for ALCL and ALK inhibitors for ALK+ ALCL. Given the differences between PTCL entities in terms of biology, lymphomagenesis, response to treatment, and outcome, future clinical trials should be oriented primarily by PTCL entity, and possibly by a common molecular target. This will require multicentric collaboration to facilitate participation in clinical trials.
Funding
This research received no external funding.
Conflicts of Interest
The author wishes to disclose the following professional affiliations: consultancies with AbbVie, iQone Healthcare, Janssen, Roche, and Takeda.
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.; Stein, H.; Thiele, J. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues, Revised 4th ed.; IARC: Lyon, France, 2017. [Google Scholar]
- International T-Cell Lymphoma Project. International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study: Pathology Findings and Clinical Outcomes. J. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Baron, M.; Amara, N.; Haioun, C.; Dandoit, M.; Maynadié, M.; Parrens, M.; Vergier, B.; Copie-Bergman, C.; Fabiani, B.; et al. Impact of Expert Pathologic Review of Lymphoma Diagnosis: Study of Patients From the French Lymphopath Network. J. Clin. Oncol. 2017, 35, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Hsi, E.D.; Horwitz, S.M.; Carson, K.R.; Pinter-Brown, L.C.; Rosen, S.T.; Pro, B.; Federico, M.; Gisselbrecht, C.; Schwartz, M.; Bellm, L.; et al. Analysis of Peripheral T-cell Lymphoma Diagnostic Workup in the United States. Clin. Lymphoma Myeloma Leuk. 2017, 17, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Fiore, D.; Cappelli, L.V.; Broccoli, A.; Zinzani, P.L.; Chan, W.C.; Inghirami, G. Peripheral T cell lymphomas: From the bench to the clinic. Nat. Cancer 2020, 20, 323–342. [Google Scholar] [CrossRef]
- Savage, K.J.; Harris, N.L.; Vose, J.M.; Ullrich, F.; Jaffe, E.S.; Connors, J.M.; Rimsza, L.; Pileri, S.A.; Chhanabhai, M.; Gascoyne, R.D.; et al. ALK− anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: Report from the International Peripheral T-Cell Lymphoma Project. Blood 2008, 111, 5496–5504. [Google Scholar] [CrossRef]
- Schmitz, N.; Trümper, L.; Ziepert, M.; Nickelsen, M.; Ho, A.D.; Metzner, B.; Peter, N.; Loeffler, M.; Rosenwald, A.; Pfreundschuh, M. Treatment and prognosis of mature T-cell and NK-cell lymphoma: An analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood 2010, 116, 3418–3425. [Google Scholar] [CrossRef] [Green Version]
- Sibon, D.; Fournier, M.; Brière, J.; Lamant, L.; Haioun, C.; Coiffier, B.; Bologna, S.; Morel, P.; Gabarre, J.; Hermine, O.; et al. Long-Term Outcome of Adults With Systemic Anaplastic Large-Cell Lymphoma Treated Within the Groupe d’Étude des Lymphomes de l’Adulte Trials. J. Clin. Oncol. 2012, 30, 3939–3946. [Google Scholar] [CrossRef]
- Ellin, F.; Landström, J.; Jerkeman, M.; Relander, T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: A study from the Swedish Lymphoma Registry. Blood 2014, 124, 1570–1577. [Google Scholar] [CrossRef] [Green Version]
- Maurer, M.J.; Ellin, F.; Srour, L.; Jerkeman, M.; Bennani, N.N.; Connors, J.M.; Slack, G.W.; Smedby, K.E.; Ansell, S.M.; Link, B.K.; et al. International Assessment of Event-Free Survival at 24 Months and Subsequent Survival in Peripheral T-Cell Lymphoma. J. Clin. Oncol. 2017, 35, 4019–4026. [Google Scholar] [CrossRef]
- Gleeson, M.; Peckitt, C.; To, Y.M.; Edwards, L.; Oates, J.; Wotherspoon, A.; Attygalle, A.D.; Zerizer, I.; Sharma, B.; Chua, S.; et al. CHOP versus GEM-P in previously untreated patients with peripheral T-cell lymphoma (CHEMO-T): A phase 2, multicentre, randomised, open-label trial. Lancet Haematol. 2018, 5, e190–e200. [Google Scholar] [CrossRef] [Green Version]
- Sibon, D.; Nguyen, D.-P.; Schmitz, N.; Suzuki, R.; Feldman, A.L.; Gressin, R.; Lamant, L.; Weisenburger, D.D.; Rosenwald, A.; Nakamura, S.; et al. ALK−positive anaplastic large-cell lymphoma in adults: An individual patient data pooled analysis of 263 patients. Haematologica 2019, 104, e562–e565. [Google Scholar] [CrossRef] [PubMed]
- Bachy, E.; Camus, V.; Thieblemont, C.; Sibon, D.; Casasnovas, R.-O.; Ysebaert, L.; Damaj, G.; Guidez, S.; Pica, G.M.; Kim, W.S.; et al. Romidepsin Plus CHOP Versus CHOP in Patients With Previously Untreated Peripheral T-Cell Lymphoma: Results of the Ro-CHOP Phase III Study (Conducted by LYSA). J. Clin. Oncol. 2022, 40, 242–251. [Google Scholar] [CrossRef] [PubMed]
- d’Amore, F.; Relander, T.; Lauritzsen, G.F.; Jantunen, E.; Hagberg, H.; Anderson, H.; Holte, H.; Österborg, A.; Merup, M.; Brown, P.; et al. Up-Front Autologous Stem-Cell Transplantation in Peripheral T-Cell Lymphoma: NLG-T-01. J. Clin. Oncol. 2012, 30, 3093–3099. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Byun, J.M.; Park, K.; Ae, K.Y.; Lee, D.; Kim, D.S.; Yoon, S.-S.; Koh, Y. Redefining the role of etoposide in first-line treatment of peripheral T-cell lymphoma. Blood Adv. 2017, 1, 2138–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janikova, A.; Chloupkova, R.; Campr, V.; Klener, P.; Hamouzova, J.; Belada, D.; Prochazka, V.; Pytlik, R.; Pirnos, J.; Duras, J.; et al. First-line therapy for T cell lymphomas: A retrospective population-based analysis of 906 T cell lymphoma patients. Ann. Hematol 2019, 98, 1961–1972. [Google Scholar] [CrossRef]
- Schmitz, N.; Truemper, L.; Bouabdallah, K.; Ziepert, M.; Leclerc, M.; Cartron, G.; Jaccard, A.; Reimer, P.; Wagner, E.; Wilhelm, M.; et al. A randomized phase 3 trial of autologous vs. allogeneic transplantation as part of first-line therapy in poor-risk peripheral T-NHL. Blood 2021, 137, 2646–2656. [Google Scholar] [CrossRef]
- Shustov, A.; Cabrera, M.E.; Civallero, M.; Bellei, M.; Ko, Y.H.; Manni, M.; Skrypets, T.; Horwitz, S.M.; De Souza, C.A.; Radford, J.A.; et al. ALK−negative anaplastic large cell lymphoma: Features and outcomes of 235 patients from the International T-Cell Project. Blood Adv. 2021, 5, 640–648. [Google Scholar] [CrossRef]
- Cederleuf, H.; Bjerregård Pedersen, M.; Jerkeman, M.; Relander, T.; d’Amore, F.; Ellin, F. The addition of etoposide to CHOP is associated with improved outcome in ALK+ adult anaplastic large cell lymphoma: A Nordic Lymphoma Group study. Br. J. Haematol. 2017, 178, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Dunleavy, K.; Pittaluga, S.; Shovlin, M.; Roschewski, M.; Lai, C.; Steinberg, S.M.; Jaffe, E.S.; Wilson, W.H. Phase II trial of dose-adjusted EPOCH in untreated systemic anaplastic large cell lymphoma. Haematologica 2015, 101, e27–e29. [Google Scholar] [CrossRef] [Green Version]
- Maeda, Y.; Nishimori, H.; Yoshida, I.; Hiramatsu, Y.; Uno, M.; Masaki, Y.; Sunami, K.; Masunari, T.; Nawa, Y.; Yamane, H.; et al. Dose-adjusted EPOCH chemotherapy for untreated peripheral T-cell lymphomas: A multicenter phase II trial of West-JHOG PTCL0707. Haematologica 2017, 102, 2097–2103. [Google Scholar] [CrossRef] [Green Version]
- Nickelsen, M.; Ziepert, M.; Zeynalova, S.; Glass, B.; Metzner, B.; Leithaeuser, M.; Mueller-Hermelink, H.K.; Pfreundschuh, M.; Schmitz, N. High-dose CHOP plus etoposide (MegaCHOEP) in T-cell lymphoma: A comparative analysis of patients treated within trials of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Ann. Oncol. 2009, 20, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Hapgood, G.; Stone, J.M.; Zannino, D.; George, A.; Marlton, P.; Prince, H.M.; Hui, C.-H.; Prosser, I.; Lewis, I.D.; Bradstock, K.; et al. A phase II study of a modified hyper-CVAD frontline therapy for patients with adverse risk diffuse large B-cell and peripheral T-cell non-Hodgkin lymphoma. Leuk. Lymphoma 2018, 60, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Escalón, M.P.; Liu, N.S.; Yang, Y.; Hess, M.; Walker, P.L.; Smith, T.L.; Nam, H.; Dang, M.D. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: The, M.D. Anderson Cancer Center experience. Cancer 2005, 103, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A.; Zaja, F.; Patti, C.; Billio, A.; Specchia, M.R.; Tucci, A.; Levis, A.; Manna, A.; Secondo, V.; Rigacci, L.; et al. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: Results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood 2007, 110, 2316–2323. [Google Scholar] [CrossRef] [Green Version]
- Roswarski, J.; Roschewski, M.; Melani, C.; Pittaluga, S.; Lucas, A.; Steinberg, S.M.; Waldmann, T.A.; Wilson, W.H. Phase 1/2 study of alemtuzumab with dose-adjusted EPOCH in untreated aggressive T and NK cell lymphomas. Leuk. Lymphoma. 2019, 60, 2062–2066. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yoon, D.H.; Kang, H.J.; Kim, J.S.; Park, S.K.; Kim, H.J.; Lee, J.; Ryoo, B.-Y.; Ko, Y.H.; Huh, J.; et al. Bortezomib in combination with CHOP as first-line treatment for patients with stage III/IV peripheral T-cell lymphomas: A multicentre, single-arm, phase 2 trial. Eur. J. Cancer 2012, 48, 3223–3231. [Google Scholar] [CrossRef]
- Foss, F.M.; Sjak-Shie, N.; Goy, A.; Jacobsen, E.; Advani, R.; Smith, M.; Komrokji, R.; Pendergrass, K.; Bolejack, V. A multicenter phase II trial to determine the safety and efficacy of combination therapy with denileukin diftitox and cyclophosphamide, doxorubicin, vincristine and prednisone in untreated peripheral T-cell lymphoma: The CONCEPT study. Leuk. Lymphoma 2013, 54, 1373–1379. [Google Scholar] [CrossRef]
- Oki, Y.; Younes, A.; Copeland, A.; Hagemeister, F.; Fayad, L.E.; McLaughlin, P.; Shah, J.; Fowler, N.; Romaguera, J.; Kwak, L.; et al. Phase I study of vorinostat in combination with standard CHOP in patients with newly diagnosed peripheral T-cell lymphoma. Br. J. Haematol. 2013, 162, 138–141. [Google Scholar] [CrossRef]
- Dupuis, J.; Morschhauser, F.; Ghesquières, H.; Tilly, H.; Casasnovas, O.; Thieblemont, C.; Ribrag, V.; Bossard, C.; LeBras, F.; Bachy, E.; et al. Combination of romidepsin with cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated patients with peripheral T-cell lymphoma: A non-randomised, phase 1b/2 study. Lancet Haematol. 2015, 2, e160–e165. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, D.-Y.; Kim, J.S.; Yoon, D.H.; Lee, W.S.; Lee, H.; Do, Y.R.; Kang, H.J.; Eom, H.S.; Ko, Y.H.; et al. A phase II study of everolimus (RAD001), an mTOR inhibitor plus CHOP for newly diagnosed peripheral T-cell lymphomas. Ann. Oncol. 2016, 27, 712–718. [Google Scholar] [CrossRef]
- Advani, R.H.; Ansell, S.M.; Lechowicz, M.J.; Beaven, A.W.; Loberiza, F.; Carson, K.R.; Evens, A.; Foss, F.; Horwitz, S.; Pro, B.; et al. A phase II study of cyclophosphamide, etoposide, vincristine and prednisone (CEOP) Alternating with Pralatrexate (P) as front line therapy for patients with peripheral T-cell lymphoma (PTCL): Final results from the T- cell consortium trial. Br. J. Haematol. 2015, 172, 535–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanale, M.A.; Horwitz, S.M.; Forero-Torres, A.; Bartlett, N.L.; Advani, R.; Pro, B.; Chen, R.W.; Davies, A.; Illidge, T.; Uttarwar, M.; et al. Five-year outcomes for frontline brentuximab vedotin with CHP for CD30-expressing peripheral T-cell lymphomas. Blood 2018, 131, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.B.; Cashen, A.F.; Nikolinakos, P.G.; Beaven, A.W.; Barta, S.K.; Bhat, G.; Hasal, S.J.; De Vos, S.; Oki, Y.; Deng, C.; et al. Belinostat in combination with standard cyclophosphamide, doxorubicin, vincristine and prednisone as first-line treatment for patients with newly diagnosed peripheral T-cell lymphoma. Exp. Hematol. Oncol. 2021, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.F.; Zain, J.; Savage, K.J.; Feldman, T.A.; Brammer, J.E.; Chen, L.; Popplewell, L.L.; Budde, L.E.; Mei, M.; Leslie, L.A.; et al. Brentuximab Vedotin Plus Cyclophosphamide, Doxorubicin, Etoposide, and Prednisone (CHEP-BV) Followed By BV Consolidation in Patients with CD30-Expressing Peripheral T-Cell Lymphomas. Blood 2021, 128, 133. [Google Scholar] [CrossRef]
- Simon, A.; Peoch, M.; Casassus, P.; Deconinck, E.; Colombat, P.; Desablens, B.; Tournilhac, O.; Eghbali, H.; Foussard, C.; Jaubert, J.; et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br. J. Haematol. 2010, 151, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.; Christensen, J.H.; Morschhauser, F.; Domenech, E.D.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2018, 393, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Trümper, L.; Iyer, S.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann. Oncol. 2022, 33, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Wulf, G.G.; Altmann, B.; Ziepert, M.; D’Amore, F.; Held, G.; Greil, R.; Tournilhac, O.; Relander, T.; Viardot, A.; Wilhelm, M.; et al. Alemtuzumab plus CHOP versus CHOP in elderly patients with peripheral T-cell lymphoma: The DSHNHL2006-1B/ACT-2 trial. Leukemia 2021, 35, 143–155. [Google Scholar] [CrossRef]
- Parrilla Castellar, E.R.; Jaffe, E.S.; Said, J.W.; Swerdlow, S.H.; Ketterling, R.P.; Knudson, R.A.; Sidhu, J.S.; Hsi, E.D.; Karikehalli, S.; Jiang, L.; et al. ALK−negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 2014, 124, 1473–1480. [Google Scholar] [CrossRef] [Green Version]
- Hapgood, G.; Ben-Neriah, S.; Mottok, A.; Lee, D.G.; Villa, D.; Sehn, L.H.; Connors, J.M.; Gascoyne, R.D.; Feldman, A.L.; Farinha, P.; et al. Identification of high-risk DUSP22-rearranged ALK−negative anaplastic large cell lymphoma. Br. J. Haematol. 2019, 186, e28–e31. [Google Scholar] [CrossRef] [Green Version]
- Sibon, D.; Bisig, B.; Bonnet, C.; Bachy, E.; Cavalieri, D.; Fataccioli, V.; Drieux, F.; Bruneau, J.; Lemonnier, F.; Bossard, C.; et al. Impact of dusp22 rearrangement on the prognosis of systemic alk-negative anaplastic large cell lymphoma: A lysa and tenomic study. Hematol. Oncol. 2021, 39. [Google Scholar] [CrossRef]
- Illidge, T.; Horwitz, S.; Iyer, S.; Bartlett, N.L. Response to brentuximab vedotin plus CHP according to CD30 expression in the ECHELON-2 trial. Br. J. Haematol. 2020, 189, 111–112. [Google Scholar]
- Corradini, P.; Tarella, C.; Zallio, F.; Dodero, A.; Zanni, M.; Valagussa, P.; Gianni, A.M.; Rambaldi, A.; Barbui, T.; Cortelazzo, S. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia 2006, 20, 1533–1538. [Google Scholar] [CrossRef]
- Rodríguez, J.; Conde, E.; Gutiérrez, A.; Arranz, R.; León, Á.; Marín, J.; Bendandi, M.; Albo, C.; Caballero, M.D.; Grupo Español de Linfomas/Trasplante Autólogo de Médula Ósea. Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: A prospective study from The Gel-Tamo Study Group. Eur. J. Haematol. 2007, 79, 32–38. [Google Scholar] [CrossRef]
- Mercadal, S.; Briones, J.; Xicoy, B.; Pedro, C.; Escoda, L.; Estany, C.; Camos, M.; Colomo, L.; Espinosa, Í.; Martinez, S.; et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann. Oncol. 2008, 19, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Reimer, P.; Rüdiger, T.; Geissinger, E.; Weissinger, F.; Nerl, C.; Schmitz, N.; Engert, A.; Einsele, H.; Müller-Hermelink, H.K.; Wilhelm, M. Autologous Stem-Cell Transplantation As First-Line Therapy in Peripheral T-Cell Lymphomas: Results of a Prospective Multicenter Study. J. Clin. Oncol. 2009, 27, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Smetak, M.; Reimer, P.; Geissinger, E.; Ruediger, T.; Metzner, B.; Schmitz, N.; Engert, A.; Schaefer-Eckart, K.; Birkmann, J. First-line therapy of peripheral T-cell lymphoma: Extension and long-term follow-up of a study investigating the role of autologous stem cell transplantation. Blood Cancer J. 2016, 6, e452. [Google Scholar] [CrossRef]
- d’Amore, F.; Gaulard, P.; Trümper, L.; Corradini, P.; Kim, W.-S.; Specht, L.; Pedersen, M.B.; Ladetto, M. Peripheral T-cell lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v108–v115. [Google Scholar] [CrossRef] [Green Version]
- Park, S.I.; Horwitz, S.M.; Foss, F.M.; Pinter-Brown, L.C.; Carson, K.R.; Rosen, S.T.; Pro, B.; Hsi, E.D.; Federico, M.; Gisselbrecht, C.; et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: Report from COMPLETE, a prospective, multicenter cohort study. Cancer 2019, 125, 1507–1517. [Google Scholar] [CrossRef]
- Fossard, G.; Broussais, F.; Coelho, I.; Bailly, S.; Nicolas-Virelizier, E.; Toussaint, E.; Lancesseur, C.; Le Bras, F.; Willems, E.; Tchernonog, E.; et al. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: An analysis of patients from LYSA centers. Ann. Oncol. 2018, 29, 715–723. [Google Scholar] [CrossRef]
- Corradini, P.; Vitolo, U.; Rambaldi, A.; Miceli, R.; Patriarca, F.; Gallamini, A.; Olivieri, A.; Benedetti, F.; Todeschini, G.; Rossi, G.; et al. Intensified chemo-immunotherapy with or without stem cell transplantation in newly diagnosed patients with peripheral T-cell lymphoma. Leukemia 2014, 28, 1885–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamez, A.-C.; Dupont, A.; Blaise, D.; Chevallier, P.; Forcade, E.; Ceballos, P.; Mohty, M.; Suarez, F.; Beguin, Y.; De Latour, R.P.; et al. Allogeneic stem cell transplantation for peripheral T cell lymphomas: A retrospective study in 285 patients from the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). J. Hematol. Oncol. 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Mourad, N.; Mounier, N.; Brière, J.; Raffoux, E.; Delmer, A.; Feller, A.; Meijer, C.J.L.M.; Emile, J.-F.; Bouabdallah, R.; Bosly, A.; et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood 2008, 111, 4463–4470. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T.; Shimada, K.; Yamamoto, K.; Chihara, D.; Ichihashi, T.; Oshima, R.; Tanimoto, M.; Iwasaki, T.; Isoda, A.; Sakai, A.; et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: A multicenter cooperative study in Japan. Blood 2012, 119, 2837–2843. [Google Scholar] [CrossRef] [PubMed]
- Delfau-Larue, M.-H.; De Leval, L.; Joly, B.; Plonquet, A.; Challine, D.; Parrens, M.; Delmer, A.; Salles, G.; Morschhauser, F.; Delarue, R.; et al. Targeting intratumoral B cells with rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma. A clinicobiological study of the GELA. Haematologica 2012, 97, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Federico, M.; Rudiger, T.; Bellei, M.; Nathwani, B.N.; Luminari, S.; Coiffier, B.; Harris, N.L.; Jaffe, E.; Pileri, S.A.; Savage, K.J.; et al. Clinicopathologic Characteristics of Angioimmunoblastic T-Cell Lymphoma: Analysis of the International Peripheral T-Cell Lymphoma Project. J. Clin. Oncol. 2013, 31, 240–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemonnier, F.; Safar, V.; Beldi-Ferchiou, A.; Cottereau, A.-S.; Bachy, E.; Cartron, G.; Fataccioli, V.; Pelletier, L.; Robe, C.; Letourneau, A.; et al. Integrative analysis of a phase 2 trial combining lenalidomide with CHOP in angioimmunoblastic T-cell lymphoma. Blood Adv. 2021, 5, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Advani, R.; Skrypets, T.; Civallero, M.; Spinner, M.A.; Manni, M.; Kim, W.; Shustov, A.; Horwitz, S.M.; Hitz, F.; Cabrera, M.E.; et al. Outcomes and Prognostic Factors in Angioimmunoblastic T cell Lymphoma: Final Report from the International TCell Project. Blood 2021, 138, 213–220. [Google Scholar] [CrossRef]
- Gallamini, A.; Stelitano, C.; Calvi, R.; Bellei, M.; Mattei, D.; Vitolo, U.; Morabito, F.; Martelli, M.; Brusamolino, E.; Iannitto, E.; et al. Peripheral T-cell lymphoma unspecified (PTCL-U): A new prognostic model from a retrospective multicentric clinical study. Blood 2004, 103, 2474–2479. [Google Scholar] [CrossRef] [Green Version]
- Weisenburger, D.D.; Savage, K.J.; Harris, N.L.; Gascoyne, R.D.; Jaffe, E.; MacLennan, K.A.; Rüdiger, T.; Pileri, S.; Nakamura, S.; Nathwani, B.; et al. Peripheral T-cell lymphoma, not otherwise specified: A report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood 2011, 117, 3402–3408. [Google Scholar] [CrossRef] [Green Version]
- Federico, M.; Bellei, M.; Marcheselli, L.; Schwartz, M.; Manni, M.; Tarantino, V.; Pileri, S.; Ko, Y.-H.; Cabrera, M.E.; Horwitz, S.; et al. Peripheral T cell lymphoma, not otherwise specified (PTCL-NOS). A new prognostic model developed by the International T cell Project Network. Br. J. Haematol. 2018, 181, 760–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellei, M.; Foss, F.M.; Shustov, A.R.; Horwitz, S.M.; Marcheselli, L.; Kim, W.S.; Cabrera, M.E.; Dlouhy, I.; Nagler, A.; Advani, R.H.; et al. The outcome of peripheral T-cell lymphoma patients failing first-line therapy: A report from the prospective, International T-Cell Project. Haematologica 2018, 103, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Mak, V.; Hamm, J.; Chhanabhai, M.; Shenkier, T.; Klasa, R.; Sehn, L.H.; Villa, D.; Gascoyne, R.D.; Connors, J.M.; Savage, K.J. Survival of Patients With Peripheral T-Cell Lymphoma After First Relapse or Progression: Spectrum of Disease and Rare Long-Term Survivors. J. Clin. Oncol. 2013, 31, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Ellin, F.; Landström, J.; Jerkeman, M.; Relander, T. Central nervous system relapse in peripheral T-cell lymphomas: A Swedish Lymphoma Registry study. Blood 2015, 126, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, S.M.; Ansell, S.; Ai, W.Z.; Barnes, J.; Barta, S.K.; Clemens, M.W.; Dogan, A.; Goodman, A.M.; Goyal, G.; Guitart, J.; et al. NCCN Guidelines Insights: T-Cell Lymphomas, Version 1.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Canc. Netw. 2020, 18, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Damaj, G.; Gressin, R.; Bouabdallah, K.; Cartron, G.; Choufi, B.; Gyan, E.; Banos, A.; Jaccard, A.; Park, S.; Tournilhac, O.; et al. Results From a Prospective, Open-Label, Phase II Trial of Bendamustine in Refractory or Relapsed T-Cell Lymphomas: The BENTLY Trial. J. Clin. Oncol. 2013, 31, 104–110. [Google Scholar] [CrossRef]
- Reboursiere, E.; Le Bras, F.; Herbaux, C.; Gyan, E.; Clavert, A.; Morschhauser, F.; Malak, S.; Sibon, D.; Broussais, F.; Braun, T.; et al. Bendamustine for the treatment of relapsed or refractory peripheral T cell lymphomas: A French retrospective multicenter study. Oncotarget 2016, 7, 85573–85583. [Google Scholar] [CrossRef] [Green Version]
- Zinzani, P.L.; Venturini, F.; Stefoni, V.; Fina, M.; Pellegrini, C.; Derenzini, E.; Gandolfi, L.; Broccoli, A.; Argnani, L.; Quirini, F.; et al. Gemcitabine as single agent in pretreated T-cell lymphoma patients: Evaluation of the long-term outcome. Ann. Oncol. 2009, 21, 860–863. [Google Scholar] [CrossRef]
- Morel, A.; Brière, J.; Lamant, L.; Loschi, M.; Haioun, C.; Delarue, R.; Tournilhac, O.; Bachy, E.; Sonet, A.; Amorim, S.; et al. Long-term outcomes of adults with first-relapsed/refractory systemic anaplastic large-cell lymphoma in the pre-brentuximab vedotin era: A LYSA/SFGM-TC study. Eur. J. Cancer 2017, 83, 146–153. [Google Scholar] [CrossRef]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood 2017, 130, 2709–2717. [Google Scholar] [CrossRef] [Green Version]
- Halligan, S.J.; Grainge, M.J.; Martinez-Calle, N.; Fox, C.P.; Bishton, M.J. Population-based cohort study of the efficacy of brentuximab vedotin in relapsed systemic anaplastic large-cell lymphoma using Public Health England data. Br. J. Haematol. 2021, 196, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Brugières, L.; Houot, R.; Cozic, N.; De La Fouchardière, C.; Morschhauser, F.; Brice, P.; Arakelyan Laboure, N.; Auvrignon, A.; Aladjidi, N.; Kolb, B.; et al. Crizotinib in Advanced ALK+ Anaplastic Large Cell Lymphoma in Children and Adults: Results of the Acs© Phase II Trial. Blood 2017, 130, 2831. [Google Scholar] [CrossRef]
- Mossé, Y.P.; Voss, S.D.; Lim, M.; Rolland, D.; Minard, C.G.; Fox, E.; Adamson, P.; Wilner, K.; Blaney, S.M.; Weigel, B.J. Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children’s Oncology Group Study. J. Clin. Oncol. 2017, 35, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Gambacorti-Passerini, C.; Orlov, S.; Zhang, L.; Braiteh, F.; Huang, H.; Esaki, T.; Horibe, K.; Ahn, J.-S.; Beck, J.T.; Edenfield, W.J.; et al. Long-term effects of crizotinib in ALK−positive tumors (excluding NSCLC): A phase 1b open-label study. Am. J. Hematol. 2018, 93, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Bossi, E.; Aroldi, A.; Brioschi, F.A.; Steidl, C.; Baretta, S.; Renso, R.; Verga, L.; Fontana, D.; Sharma, G.G.; Mologni, L.; et al. Phase two study of crizotinib in patients with anaplastic lymphoma kinase ( ALK )-positive anaplastic large cell lymphoma relapsed/refractory to chemotherapy. Am. J. Hematol. 2020, 95, E319–E321. [Google Scholar] [CrossRef] [PubMed]
- Fukano, R.; Mori, T.; Sekimizu, M.; Choi, I.; Kada, A.; Saito, A.M.; Asada, R.; Takeuchi, K.; Terauchi, T.; Tateishi, U.; et al. Alectinib for relapsed or refractory anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: An open-label phase II trial. Cancer Sci. 2020, 111, 4540–4547. [Google Scholar] [CrossRef]
- Fischer, M.; Moreno, L.; Ziegler, D.S.; Marshall, L.V.; Zwaan, C.M.; Irwin, M.S.; Casanova, M.; Sabado, C.; Wulff, B.; Stegert, M.; et al. Ceritinib in paediatric patients with anaplastic lymphoma kinase-positive malignancies: An open-label, multicentre, phase 1, dose-escalation and dose-expansion study. Lancet Oncol. 2021, 22, 1764–1776. [Google Scholar] [CrossRef]
- Knörr, F.; Brugières, L.; Pillon, M.; Zimmermann, M.; Ruf, S.; Attarbaschi, A.; Mellgren, K.; Burke, G.A.A.; Uyttebroeck, A.; Wróbel, G.; et al. Stem Cell Transplantation and Vinblastine Monotherapy for Relapsed Pediatric Anaplastic Large Cell Lymphoma: Results of the International, Prospective ALCL-Relapse Trial. J. Clin. Oncol. 2020, 38, 3999–4009. [Google Scholar] [CrossRef]
- Rodríguez, J.; Caballero, M.D.; Gutiérrez, A.; Marín, J.; Lahuerta, J.J.; Sureda, A.; Carreras, E.; León, A.; Arranz, R.; de Sevilla, A.F.; et al. High-dose chemotherapy and autologous stem cell transplantation in peripheral T-cell lymphoma: The GEL-TAMO experience. Ann. Oncol. 2003, 14, 1768–1775. [Google Scholar] [CrossRef]
- Nademanee, A.; Palmer, J.M.; Popplewell, L.; Tsai, N.-C.; Delioukina, M.; Gaal, K.; Cai, J.-L.; Kogut, N.; Forman, S.J. High-Dose Therapy and Autologous Hematopoietic Cell Transplantation in Peripheral T Cell Lymphoma (PTCL): Analysis of Prognostic Factors. Biol. Blood Marrow Transplant. 2011, 17, 1481–1489. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Burns, L.J.; Van Besien, K.; LeRademacher, J.; He, W.; Fenske, T.S.; Suzuki, R.; Hsu, J.W.; Schouten, H.C.; Hale, G.A.; et al. Hematopoietic Cell Transplantation for Systemic Mature T-Cell Non-Hodgkin Lymphoma. J. Clin. Oncol. 2013, 31, 3100–3109. [Google Scholar] [CrossRef] [PubMed]
- El-Asmar, J.; Reljic, T.; Ayala, E.; Hamadani, M.; Nishihori, T.; Kumar, A.; Kharfan-Dabaja, M.A. Efficacy of High-Dose Therapy and Autologous Hematopoietic Cell Transplantation in Peripheral T Cell Lymphomas as Front-Line Consolidation or in the Relapsed/Refractory Setting: A Systematic Review/Meta-Analysis. Biol. Blood Marrow Transplant. 2015, 22, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Corradini, P.; Dodero, A.; Zallio, F.; Caracciolo, D.; Casini, M.; Bregni, M.; Narni, F.; Patriarca, F.; Boccadoro, M.; Benedetti, F.; et al. Graft-Versus-Lymphoma Effect in Relapsed Peripheral T-Cell Non-Hodgkin’s Lymphomas After Reduced-Intensity Conditioning Followed by Allogeneic Transplantation of Hematopoietic Cells. J. Clin. Oncol. 2004, 22, 2172–2176. [Google Scholar] [CrossRef] [PubMed]
- Le Gouill, S.; Milpied, N.; Buzyn, A.; Peffault De Latour, R.; Vernant, J.-P.; Mohty, M.; Moles, M.-P.; Bouabdallah, K.; Bulabois, C.-E.; Dupuis, J.; et al. Graft-Versus-Lymphoma Effect for Aggressive T-Cell Lymphomas in Adults: A Study by the Société Française de Greffe de Moëlle et de Thérapie Cellulaire. J. Clin. Oncol. 2008, 26, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Spina, F.; Narni, F.; Patriarca, F.; Cavattoni, I.; Benedetti, F.; Ciceri, F.; Baronciani, D.; Scimè, R.; Pogliani, E.; et al. Allogeneic transplantation following a reduced-intensity conditioning regimen in relapsed/refractory peripheral T-cell lymphomas: Long-term remissions and response to donor lymphocyte infusions support the role of a graft-versus-lymphoma effect. Leukemia 2011, 26, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Epperla, N.; Ahn, K.W.; Litovich, C.; Ahmed, S.; Battiwalla, M.; Cohen, J.B.; Dahi, P.; Farhadfar, N.; Farooq, U.; Freytes, C.O.; et al. Allogeneic hematopoietic cell transplantation provides effective salvage despite refractory disease or failed prior autologous transplant in angioimmunoblastic T-cell lymphoma: A CIBMTR analysis. J. Hematol. Oncol. 2019, 12, 6. [Google Scholar] [CrossRef]
- Du, J.; Yu, D.; Han, X.; Zhu, L.; Huang, Z. Comparison of Allogeneic Stem Cell Transplant and Autologous Stem Cell Transplant in Refractory or Relapsed Peripheral T-Cell Lymphoma: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e219807. [Google Scholar] [CrossRef]
- Coiffier, B.; Pro, B.; Prince, H.M.; Foss, F.; Sokol, L.; Greenwood, M.; Caballero, D.; Borchmann, P.; Morschhauser, F.; Wilhelm, M.; et al. Results From a Pivotal, Open-Label, Phase II Study of Romidepsin in Relapsed or Refractory Peripheral T-Cell Lymphoma After Prior Systemic Therapy. J. Clin. Oncol. 2012, 30, 631–636. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Horwitz, S.; Masszi, T.; Van Hoof, A.; Brown, P.D.N.; Doorduijn, J.; Hess, G.; Jurczak, W.; Knoblauch, P.; Chawla, S.; et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J. Clin. Oncol. 2015, 33, 2492–2499. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Dong, M.; Hong, X.; Zhang, W.; Feng, J.; Zhu, J.; Yu, L.; Ke, X.; Huang, H.; Shen, Z.; et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann. Oncol. 2015, 26, 1766–1771. [Google Scholar] [CrossRef]
- Barr, P.M.; Li, H.; Spier, C.; Mahadevan, D.; Leblanc, M.; Haq, M.U.; Huber, B.D.; Flowers, C.R.; Wagner-Johnston, N.D.; Horwitz, S.M.; et al. Phase II Intergroup Trial of Alisertib in Relapsed and Refractory Peripheral T-Cell Lymphoma and Transformed Mycosis Fungoides: SWOG 1108. J. Clin. Oncol. 2015, 33, 2399–2404. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, O.A.; Özcan, M.; Jacobsen, E.D.; Roncero, J.M.; Trotman, J.; Demeter, J.; Masszi, T.; Pereira, J.; Ramchandren, R.; Beaven, A.; et al. Randomized Phase III Study of Alisertib or Investigator’s Choice (Selected Single Agent) in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Morschhauser, F.; Bouabdallah, K.; Bron, D.; Cunningham, D.; Assouline, S.E.; Verhoef, G.; Linton, K.; Thieblemont, C.; Vitolo, U.; et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann. Oncol. 2017, 28, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Koch, R.; Porcu, P.; Oki, Y.; Moskowitz, A.; Perez, M.; Myskowski, P.; Officer, A.; Jaffe, J.D.; Morrow, S.N.; et al. Activity of the PI3K-δ,γ inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood 2018, 131, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Huen, A.; Haverkos, B.M.; Zain, J.; Radhakrishnan, R.; Lechowicz, M.J.; Devata, S.; Korman, N.J.; Pinter-Brown, L.; Oki, Y.; Barde, P.J.; et al. Phase I/Ib Study of Tenalisib (RP6530), a Dual PI3K δ/γ Inhibitor in Patients with Relapsed/Refractory T-Cell Lymphoma. Cancers 2020, 12, 2293. [Google Scholar] [CrossRef]
- Kumar, A.; Vardhana, S.; Moskowitz, A.J.; Porcu, P.; Dogan, A.; Dubovsky, J.A.; Matasar, M.J.; Zhang, Z.; Younes, A.; Horwitz, S.M. Pilot trial of ibrutinib in patients with relapsed or refractory T-cell lymphoma. Blood Adv. 2018, 2, 871–876. [Google Scholar] [CrossRef]
- Moskowitz, A.J.; Ghione, P.; Jacobsen, E.; Ruan, J.; Schatz, J.H.; Noor, S.; Myskowski, P.; Vardhana, S.; Ganesan, N.; Hancock, H.; et al. A phase 2 biomarker-driven study of ruxolitinib demonstrates effectiveness of JAK/STAT targeting in T-cell lymphomas. Blood 2021, 138, 2828–2837. [Google Scholar] [CrossRef]
- Umakanthan, J.M.; Iqbal, J.; Batlevi, C.L.; Bouska, A.; Smith, L.M.; Shostrom, V.; Nutsch, H.; William, B.M.; Bociek, R.G.; Lunning, M.; et al. Phase I/II study of dasatinib and exploratory genomic analysis in relapsed or refractory non-Hodgkin lymphoma. Br. J. Haematol. 2018, 184, 744–752. [Google Scholar] [CrossRef]
- Morschhauser, F.; Fitoussi, O.; Haioun, C.; Thieblemont, C.; Quach, H.; Delarue, R.; Glaisner, S.; Gabarre, J.; Bosly, A.; Lister, J.; et al. A phase 2, multicentre, single-arm, open-label study to evaluate the safety and efficacy of single-agent lenalidomide (Revlimid®) in subjects with relapsed or refractory peripheral T-cell non-Hodgkin lymphoma: The EXPECT trial. Eur. J. Cancer 2013, 49, 2869–2876. [Google Scholar] [CrossRef]
- Toumishey, E.; Prasad, A.; Dueck, G.; Chua, N.; Finch, D.; Johnston, J.; Van Der Jagt, R.; Stewart, D.; White, D.; Belch, A.; et al. Final report of a phase 2 clinical trial of lenalidomide monotherapy for patients with T-cell lymphoma. Cancer 2014, 121, 716–723. [Google Scholar] [CrossRef]
- Barta, S.K.; Zain, J.; MacFarlane, A.W.; Smith, S.M.; Ruan, J.; Fung, H.C.; Tan, C.R.; Yang, Y.; Alpaugh, R.K.; Dulaimi, E.; et al. Phase II Study of the PD-1 Inhibitor Pembrolizumab for the Treatment of Relapsed or Refractory Mature T-cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2019, 19, 356–364.e3. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wu, J.; Wang, Z.; Zhang, L.; Wang, Z.; Zhang, M.; Cen, H.; Peng, Z.; Li, Y.; Fan, L.; et al. Efficacy and safety of geptanolimab (GB226) for relapsed or refractory peripheral T cell lymphoma: An open-label phase 2 study (Gxplore-002). J. Hematol. Oncol. 2021, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Ribrag, V.; Caballero, L.; Fermé, C.; Zucca, E.; Arranz, R.; Briones, J.; Gisselbrecht, C.; Salles, G.; Gianni, A.M.; Gomez, H.; et al. Multicenter phase II study of plitidepsin in patients with relapsed/refractory non-Hodgkin’s lymphoma. Haematologica 2012, 98, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Losada, A.; Muñoz-Alonso, M.J.; García, C.; Sánchez-Murcia, P.A.; Martínez-Leal, J.F.; Dominguez, J.M.; Lillo, M.P.; Gago, F.; Galmarini, C.M. Translation Elongation Factor eEF1A2 is a Novel Anticancer Target for the Marine Natural Product Plitidepsin. Sci. Rep. 2016, 6, 35100. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, O.A.; Pro, B.; Pinter-Brown, L.; Bartlett, N.; Popplewell, L.; Coiffier, B.; Lechowicz, M.J.; Savage, K.J.; Shustov, A.R.; Gisselbrecht, C.; et al. Pralatrexate in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results From the Pivotal PROPEL Study. J. Clin. Oncol. 2011, 29, 1182–1189. [Google Scholar] [CrossRef]
- Boonstra, P.S.; Polk, A.; Brown, N.; Hristov, A.C.; Bailey, N.; Kaminski, M.S.; Phillips, T.; Devata, S.; Mayer, T.; Wilcox, R.A. A single center phase II study of ixazomib in patients with relapsed or refractory cutaneous or peripheral T-cell lymphomas. Am. J. Hematol. 2017, 92, 1287–1294. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, M.; Bociek, R.G.; Fanale, M.; Iyer, S.P.; Lechowicz, M.J.; Bierman, P.J.; Armitage, J.O.; Lunning, M.; Kallam, A.; Vose, J.M. Phase 1 trial of carfilzomib in relapsed/refractory peripheral T-cell lymphoma. Ann. Hematol. 2021, 101, 335–340. [Google Scholar] [CrossRef]
- Hopfinger, G.; Nösslinger, T.; Lang, A.H.; Linkesch, W.; Melchardt, T.; Weiss, L.; Egle, A.; Greil, R. Lenalidomide in combination with vorinostat and dexamethasone for the treatment of relapsed/refractory peripheral T cell lymphoma (PTCL): Report of a phase I/II trial. Ann. Hematol. 2014, 93, 459–462. [Google Scholar] [CrossRef]
- Tan, D.; Phipps, C.; Hwang, W.Y.K.; Tan, S.-Y.; Yeap, C.H.; Chan, Y.H.; Tay, K.; Lim, S.T.; Lee, Y.S.; Kumar, S.G.; et al. Panobinostat in combination with bortezomib in patients with relapsed or refractory peripheral T-cell lymphoma: An open-label, multicentre phase 2 trial. Lancet Haematol. 2015, 2, e326–e333. [Google Scholar] [CrossRef]
- Pellegrini, C.; Dodero, A.; Chiappella, A.; Monaco, F.; Degl’Innocenti, D.; Salvi, F.; Vitolo, U.; Argnani, L.; Corradini, P.; Zinzani, P.L.; et al. A phase II study on the role of gemcitabine plus romidepsin (GEMRO regimen) in the treatment of relapsed/refractory peripheral T-cell lymphoma patients. J. Hematol. Oncol. 2016, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Holkova, B.; Yazbeck, V.; Kmieciak, M.; Bose, P.; Ma, S.; Kimball, A.; Tombes, M.B.; Shrader, E.; Wan, W.; Weir-Wiggins, C.; et al. A phase 1 study of bortezomib and romidepsin in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma, indolent B-cell lymphoma, peripheral T-cell lymphoma, or cutaneous T-cell lymphoma. Leuk. Lymphoma 2017, 58, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.E.; Lichtenstein, R.; Lue, J.; Sawas, A.; Deng, C.; Lichtenstein, E.; Khan, K.; Atkins, L.; Rada, A.; Kim, H.A.; et al. A phase 1 study of romidepsin and pralatrexate reveals marked activity in relapsed and refractory T-cell lymphoma. Blood 2018, 131, 397–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strati, P.; Chihara, D.; Oki, Y.; Fayad, L.E.; Fowler, N.; Nastoupil, L.; Romaguera, J.E.; Samaniego, F.; Garg, N.; Feng, L.; et al. A phase I study of romidepsin and ifosfamide, carboplatin, etoposide for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. Haematologica 2018, 103, e416–e418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, O.A.; Falchi, L.; Lue, J.K.; Marchi, E.; Kinahan, C.; Sawas, A.; Deng, C.; Montanari, F.; Amengual, J.E.; Kim, H.A.; et al. Oral 5-azacytidine and romidepsin exhibit marked activity in patients with PTCL: A multicenter phase 1 study. Blood 2019, 134, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Martin, P.; Somasundaram, N.; Lim, C.; Tao, M.; Poon, E.; Yunon, M.J.; Toh, S.Q.; Yan, S.X.; Farid, M.; et al. Phase I study of selinexor in combination with dexamethasone, ifosfamide, carboplatin, etoposide chemotherapy in patients with relapsed or refractory peripheral T-cell or naturalkiller/T-cell lymphoma. Haematologica 2020, 106. [Google Scholar] [CrossRef]
- Yhim, H.-Y.; Kim, T.; Kim, S.; Shin, H.-J.; Koh, Y.; Kim, J.; Park, J.; Park, G.; Kim, W.; Moon, J.; et al. Combination treatment of copanlisib and gemcitabine in relapsed/refractory PTCL (COSMOS): An open-label phase I/II trial. Ann. Oncol. 2020, 32, 552–559. [Google Scholar] [CrossRef]
- Stuver, R.N.; Khan, N.; Schwartz, M.; Acosta, M.; Federico, M.; Gisselbrecht, C.; Horwitz, S.M.; Lansigan, F.; Pinter-Brown, L.C.; Pro, B.; et al. Single agents vs combination chemotherapy in relapsed and refractory peripheral T-cell lymphoma: Results from the comprehensive oncology measures for peripheral T-cell lymphoma treatment (COMPLETE) registry. Am. J. Hematol. 2019, 94, 641–649. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).