The Influence of Vaginal HPV Self-Sampling on the Efficacy of Populational Screening for Cervical Cancer—An Umbrella Review
Abstract
Simple Summary
Abstract
1. Introduction
- age;
- multiparity;
- low socioeconomic status;
- unsuitable diet (diet poor in vitamin C),
- family history of cancer [5].
- full anti-HPV vaccination of 90% of girls before the age of 15;
- high-efficiency screening tests being performed in 90% of women at least twice in their lifetime, i.e., at the age of 35 and 45;
- adequate treatment and care being provided to 90% of women with the diagnosis of precancerous lesions and cervical cancer [6].
- Primary and secondary prophylaxis are effective ways of preventing cervical cancer. Primary prevention is based primarily on immunoprophylaxis and all kinds of measures aimed at eliminating the risk factors. Secondary prevention involves screening and treatment of precancerous lesions. Screening examinations include cytology and assays for oncogenic HPV genotypes [3].
2. Objective
Material and Method
- identification of reliable sources of medical information;
- searching for research papers in the full-text version that can be useful for clinical analysis;
- research selection based on inclusion criteria;
- proper processing of research results;
- qualitative synthesis of results, taking into account the analysis of the clinical and statistical significance of the research results.
- population: the general population of adult women;
- intervention: HPV test of a self-collected vaginal sample;
- comparator: no restriction;
- methodology: meta-analyses of randomized and/or observational studies; systematic reviews of randomized and/or observational studies; systematic reviews of cost-effectiveness analyses;
- outcomes: diagnostic accuracy (sensitivity, specificity) of CC screening tests, uptake of CC screening, CC mortality, acceptability of the sampling method, cost-effectiveness of CC screening.
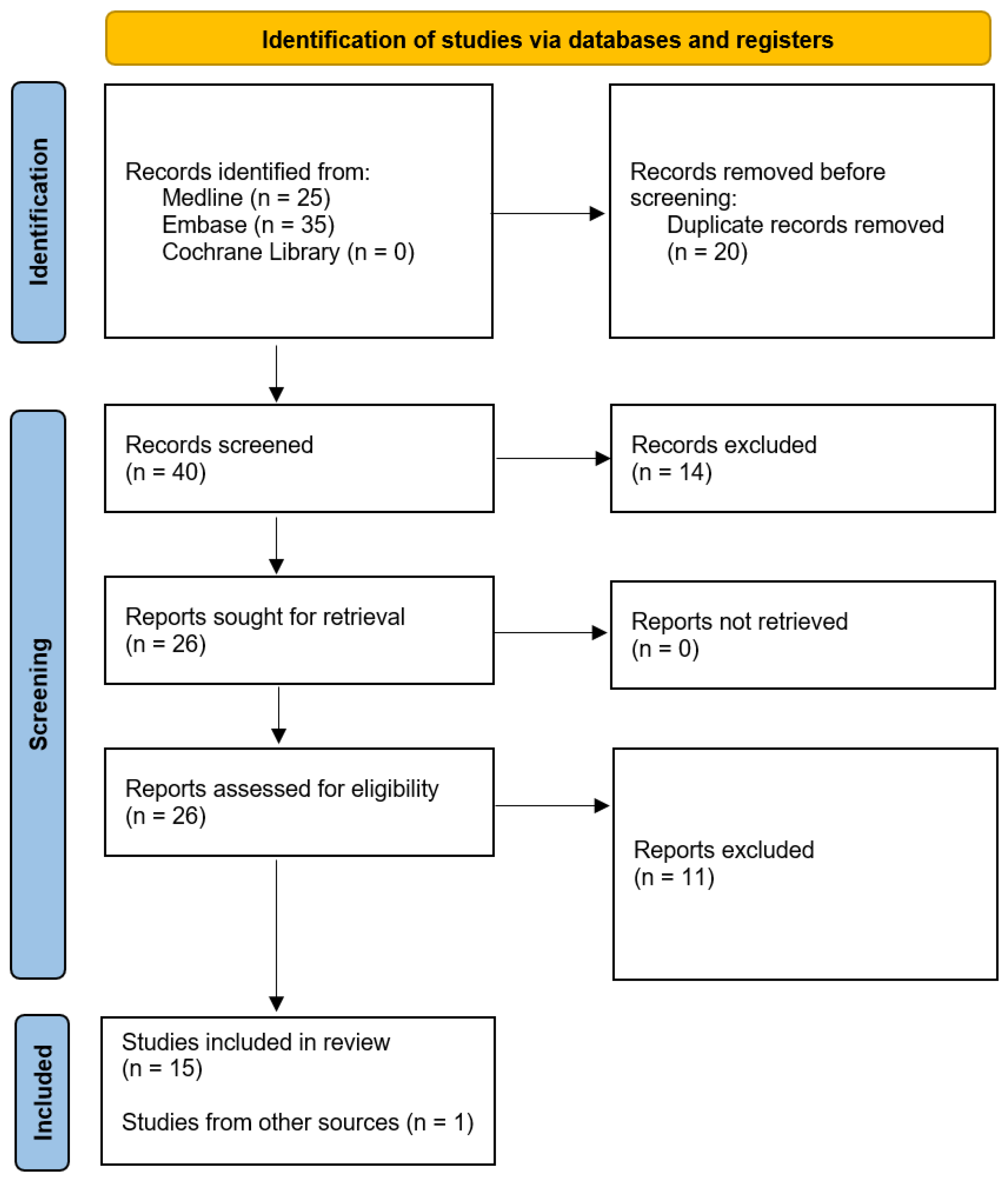
3. Results
- Arbyn 2022—a meta-analysis of 26 diagnostic test accuracy studies including calculation of compatibility parameters between HPV tests in self-collected vs. clinician-collected samples [10];
- Tesfahunei 2021—a meta-analysis of 4 RCTs assessing the efficacy of HPV self-sampling as compared to standard sampling performed by clinicians at healthcare facilities for cervical cancer screening purposes [11];
- Malone 2020—a systematic review of 16 cost-effectiveness analyses assessing the use of HPV self-sampling kits as an intervention to increase uptake of CC screening programs [12];
- Morgan 2019—a systematic review of 19 cross-sectional and 4 qualitative studies aimed at identification of studies assessing the acceptability of self-sampling as compared to sampling performed by a clinician and at determination of preferences and barriers in association with both of these methods [13];
- Yeh 2019—a meta-analysis of 29 RCTs and 4 observational studies designed for the purpose of formulating the WHO guidelines and determining the impact of HPV self-sampling on the uptake of CC screening [14];
- Arbyn 2018—a meta-analysis of 81 observational studies and RCTs assessing the diagnostic accuracy of high-risk HPV (hrHPV) testing in self-sampled tests and the efficacy of the self-sampling approach on the ability of reaching out to women who have never been screened for cervical cancer [15];
- Kelly 2017—a meta-analysis of 8 observational studies comparing the diagnostic accuracy of point-of-care hrHPV tests depending on the sampling method [16];
- Mezei 2017—a systematic review of 19 cost-effectiveness analyses relating to different methods of screening for cervical cancer [17];
- Musa 2017—a meta-analysis of 28 RCTs aimed at determination of the impact of education, physicians’ recommendations to take part in the screening programs, and the availability of HPV self-sampling on the uptake of screening programs among women within the cervical cancer risk group [18];
- Nelson 2017—a meta-analysis of 37 observational and experimental studies assessing patient acceptability and preferences regarding HPV self-sampling as compared to sampling performed by clinicians [19];
- Verdoodt 2015—a meta-analysis of 16 RCTs assessing participation in CC screening following an invitation being sent along with an HPV self-sampling kit as compared to participation in following an invitation to report for a test to be performed in a clinical setting [20];
- Albrow 2014—a systematic review of 4 RCTs assessing interventions aimed at increasing the cervical cancer screening program uptake rates among women aged ≤ 35 years [21];
- Arbyn 2014—a meta-analysis of 36 observational studies and RCTs carried out to verify whether HPV self-sampling is equivalent to clinician sampling [22];
- Camilloni 2013—a meta-analysis of 69 observational and experimental studies assessing the effectiveness of interventions aimed at increasing uptake of established populational screening programs [23];
- Racey 2013—a meta-analysis of 9 RCTs and 1 observational study assessing the impact of HPV self-sampling on the increase in the participation in screening programs among women who had not previously been screened for cervical cancer [24];
- Zhao 2012—a meta-analysis of 5 populational studies comparing the diagnostic accuracy of HPV tests in self-collected samples with the accuracy of HPV tests in clinician-collected samples [25].
3.1. Relationship between the Sampling Method and the Diagnostic Accuracy of HPV Test-Based Cervical Cancer Screening
3.2. Uptake of CC Screening
3.3. The Influence of Self-Sampling on CC Detection and Mortality Rates
3.4. The Acceptability of Self-Sampling among Patients
3.5. Cost-Effectiveness Analyses
4. Discussion
5. Limitations of the Review
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Institute for Health Metrics and Evaluation. GBD Results Tool: Cervical Cancer. 2022. Available online: https://ghdx.healthdata.org/gbd-results-tool (accessed on 3 August 2022).
- World Health Organization. Cervical Cancer. 2022. Available online: https://www.who.int/health-topics/cervical-cancer#tab=tab_1 (accessed on 4 August 2022).
- Zhang, Q.; Xie, W.; Wang, F.; Li, R.H.; Cui, L.; Wang, H.; Fu, X.; Song, J. Epidemiological Investigation and Risk Factors for Cervical Lesions: Cervical Cancer Screening Among Women in Rural Areas of Henan Province China. Med. Sci. Monit. 2016, 22, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cervical Cancer Causes, Risk Factors, and Prevention. 2022. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/8600.00.pdf (accessed on 5 August 2022).
- World Health Organization. Global strategy to Accelerate The elimination of Cervical Cancer as a Public Health Problem. 2022. Available online: https://www.who.int/publications/i/item/9789240014107 (accessed on 5 August 2022).
- World Health Organization. WHO Recommendations on Self-Care Interventions: Human Papillomavirus (HPV) Self-Sampling as Part of Cervical Cancer Screening. 2022. Available online: https://apps.who.int/iris/handle/10665/332333 (accessed on 5 August 2022).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2; The Cochrane Collaboration in London: London, UK, 2021; ISBN 9781119536659. [Google Scholar]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include andomized or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Castle, P.E.; Schiffman, M.; Wentzensen, N.; Heckman-Stoddard, B.; Sahasrabuddhe, V.V. Meta-analysis of agreement/concordance statistics in studies comparing self- vs clinician-collected samples for HPV testing in cervical cancer screening. Int. J. Cancer 2022, 151, 308–312. [Google Scholar] [CrossRef]
- Tesfahunei, H.A.; Ghebreyesus, M.S.; Assefa, D.G.; Zeleke, E.D.; Acam, J.; Joseph, M.; Getachew, E.; Kajogoo, V.D.; Bekele, D.; Manyazewal, T. Human papillomavirus self-sampling versus standard clinician-sampling for cervical cancer screening in sub-Saharan Africa: A systematic review and meta-analysis of randomized controlled trials. Infect. Agent Cancer 2021, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Malone, C.; Barnabas, R.V.; Buist, D.S.M.; Tiro, J.A.; Winer, R.L. Cost-effectiveness studies of HPV self-sampling: A systematic review. Prev. Med. 2020, 132, 105953. [Google Scholar] [CrossRef]
- Morgan, K.; Azzani, M.; Khaing, S.L.; Wong, Y.L.; Su, T.T. Acceptability of Women Self-Sampling versus Clinician-Collected Samples for HPV DNA Testing: A Systematic Review. J. Low. Genit. Tract Dis. 2019, 23, 193–199. [Google Scholar] [CrossRef]
- Yeh, P.T.; Kennedy, C.E.; De Vuyst, H.; Narasimhan, M. Self-sampling for human papillomavirus (HPV) testing: A systematic review and meta-Analysis. BMJ Glob. Health 2019, 4, e001351. [Google Scholar] [CrossRef]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P.; Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.; Mayaud, P.; Segondy, M.; Pant Pai, N.; Peeling, R.W. A systematic review and meta-analysis of studies evaluating the performance of point-of-care tests for human papillomavirus screening. Sex. Transm. Infect. 2017, 93, S36–S45. [Google Scholar] [CrossRef]
- Mezei, A.K.; Armstrong, H.L.; Pedersen, H.N.; Campos, N.G.; Mitchell, S.M.; Sekikubo, M.; Byamugisha, J.K.; Kim, J.J.; Bryan, S.; Ogilvie, G.S. Cost-effectiveness of cervical cancer screening methods in low- and middle-income countries: A systematic review. Int. J. Cancer 2017, 141, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Musa, J.; Achenbach, C.J.; O’Dwyer, L.C.; Evans, C.T.; McHugh, M.; Hou, L.; Simon, M.A.; Murphy, R.L.; Jordan, N. Effect of cervical cancer education and provider recommendation for screening on screening rates: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0183924. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.J.; Maynard, B.R.; Loux, T.; Fatla, J.; Gordon, R.; Arnold, L.D. The acceptability of self-sampled screening for HPV DNA: A systematic review and meta-analysis. Sex. Transm. Infect. 2017, 93, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Verdoodt, F.; Jentschke, M.; Hillemanns, P.; Racey, C.S.; Snijders, P.J.; Arbyn, M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: A systematic review and meta-analysis of randomised trials. Eur. J. Cancer 2015, 51, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Albrow, R.; Blomberg, K.; Kitchener, H.; Brabin, L.; Patnick, J.; Tishelman, C.; Törnberg, S.; Sparén, P.; Widmark, C. Interventions to improve cervical cancer screening uptake amongst young women: A systematic review. Acta Oncol. 2014, 53, 445–451. [Google Scholar] [CrossRef]
- Arbyn, M.; Verdoodt, F.; Snijders, P.J.; Verhoef, V.M.; Suonio, E.; Dillner, L.; Minozzi, S.; Bellisario, C.; Banzi, R.; Zhao, F.H.; et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: A meta-analysis. Lancet Oncol. 2014, 15, 172–183. [Google Scholar] [CrossRef]
- Camilloni, L.; Ferroni, E.; Cendales, B.J.; Pezzarossi, A.; Furnari, G.; Borgia, P.; Guasticchi, G.; Giorgi Rossi, P. Methods to increase participation Working Group. Methods to increase participation in organised screening programs: A systematic review. BMC Public Health 2013, 13, 464. [Google Scholar] [CrossRef]
- Racey, C.S.; Withrow, D.R.; Gesink, D. Self-collected HPV testing improves participation in cervical cancer screening: A systematic review and meta-analysis. Can. J. Public Health 2013, 104, e159–e166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.H.; Lewkowitz, A.K.; Chen, F.; Lin, M.J.; Hu, S.Y.; Zhang, X.; Pan, Q.J.; Ma, J.F.; Niyazi, M.; Li, C.Q.; et al. Pooled analysis of a self-sampling HPV DNA Test as a cervical cancer primary screening method. J. Natl. Cancer Inst. 2012, 104, 178–188. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologist. Updated Cervical Cancer Screening Guidelines. 2022. Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/04/updated-cervical-cancer-screening-guidelines (accessed on 7 August 2022).
- American Cancer Society. The American Cancer Society Guidelines for the Prevention and Early Detection of Cervical Cancer. 2022. Available online: https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/cervical-cancer-screening-guidelines.html (accessed on 7 August 2022).
- Hong Kong Centre for Health Protection & Cancer Expert Working Group on Cancer Prevention and Screening. Recommendations on Prevention and Screening for Cervical Cancer for Health Professionals. 2022. Available online: https://www.chp.gov.hk/en/recommendations/34/index.html (accessed on 7 August 2022).
- The Polish Society of Gynecologists and Obstetricians. Schemat Postępowania w Screeningu Raka Szyjki Macicy (RSM)—Polskiego Towarzystwa Ginekologów I Położników (PTGiP)—Wersja XII. 2021. Available online: https://www.ptgin.pl/artykul/schemat-postepowania-w-screeningu-raka-szyjki-macicy-rsm-ptgip-wersja-xii-2021 (accessed on 7 August 2022).
- United States Preventive Services Task Force. Screening for Cervical Cancer US Preventive Services Task Force Recommendation Statement. 2022. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening (accessed on 7 August 2022).
- Women’s Preventive Services Initiative. Screening for Cervical Cancer. 2022. Available online: https://www.womenspreventivehealth.org/recommendations/screening-for-cervical-cancer/ (accessed on 7 August 2022).
- Sawaya, G.F.; Kulasingam, S.; Denberg, T.D.; Qaseem, A.; Clinical Guidelines Committee of American College of Physicians. Cervical Cancer Screening in Average-Risk Women: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians. Ann. Intern. Med. 2015, 162, 851–859. [Google Scholar] [CrossRef]
- European Commission. European Guidelines for Quality Assurance in Cervical Cancer Screening. 2nd ed.: Supplements. 2022. Available online: https://op.europa.eu/pl/publication-detail/-/publication/a41a4c40-0626-4556-af5b-2619dd1d5ddc (accessed on 7 August 2022).
- Polskie Towarzystwo Onkologii Klinicznej. Nowotwory Kobiecego Układu Rozrodczego. 2022. Available online: http://onkologia.zalecenia.med.pl/pdf/zalecenia_PTOK_tom1_06_Nowotwory_kobiecego_ukladu_plciowego_20130301.pdf (accessed on 7 August 2022).
- Canadian Task Force on Preventive Health Care. Recommendations on Screening for Cervical Cancer. 2022. Available online: https://www.cmaj.ca/content/185/1/35.full (accessed on 7 August 2022).
- United Kingdom National Screening Committee. Guidance. Cervical Screening: Programme Overview. 2022. Available online: https://www.gov.uk/guidance/cervical-screening-programme-overview (accessed on 8 August 2022).
- World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention. 2022. Available online: https://www.who.int/publications/i/item/9789240030824 (accessed on 8 August 2022).
- Kyrgiou, M.; Arbyn, M.; Bergeron, C.; Bosch, F.X.; Dillner, J.; Jit, M.; Kim, J.; Poljak, M.; Nieminen, P.; Sasieni, P.; et al. Cervical screening: ESGO-EFC position paper of the European Society of Gynaecologic Oncology (ESGO) and the European Federation of Colposcopy (EFC). Br. J. Cancer 2020, 123, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Cancer Council Australia. National Cervical Screening Program: Guidelines for the Management of Screen-Detected Abnormalities, Screening in Specific Populations and Investigation of Abnormal Vaginal Bleeding. 2022. Available online: https://www.cancer.org.au/clinical-guidelines/cervical-cancer-screening/summary-of-recommendations (accessed on 8 August 2022).
- The Royal Australian College of General Practitioners. Early Detection of Cancers. Cervical Cancer. 2022. Available online: https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/guidelines-for-preventive-activities-in-general-pr/early-detection-of-cancers/cervical-cancer (accessed on 8 August 2022).
- National Cancer Institute. Cervical Cancer Screening (PDQ®)—Health Professional Version. 2022. Available online: https://www.cancer.gov/types/cervical/hp/cervical-screening-pdq (accessed on 8 August 2022).
- Cho, H.W.; Ouh, Y.T.; Hong, J.H.; Min, K.J.; So, K.A.; Kim, T.J.; Paik, E.S.; Lee, J.W.; Moon, J.H.; Lee, J.K. Comparison of urine, self-collected vaginal swab, and cervical swab samples for detecting human papillomavirus (HPV) with Roche Cobas HPV, Anyplex II HPV, and RealTime HR-S HPV assay. J. Virol. Methods 2019, 269, 77–82. [Google Scholar] [CrossRef]
- Cadman, L.; Reuter, C.; Jitlal, M.; Kleeman, M.; Austin, J.; Hollingworth, T.; Parberry, A.L.; Ashdown-Barr, L.; Patel, D.; Nedjai, B.; et al. A Randomized Comparison of Different Vaginal Self-sampling Devices and Urine for Human Papillomavirus Testing-Predictors 5.1. Cancer Epidemiol. Biomarker. Prev. 2021, 30, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Tranberg, M.; Jensen, J.S.; Bech, B.H.; Andersen, B. Urine collection in cervical cancer screening—Analytical comparison of two HPV DNA assays. BMC Infect Dis. 2020, 20, 926. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Cadman, L.; Ahmad, A.S.; Ho, L.; Terry, G.; Kleeman, M.; Lyons, D.; Austin, J.; Stoler, M.H.; Vibat, C.R.T.; et al. Performance and Diagnostic Accuracy of a Urine-Based Human Papillomavirus Assay in a Referral Population. Cancer Epidemiol. Biomarker. Prev. 2017, 26, 1053–1059. [Google Scholar] [CrossRef]
| Author/Year | Population | HPV Testing Method | End Point | Accuracy of CIN2+ Detection | Accuracy of CIN3+ Detection | ||
|---|---|---|---|---|---|---|---|
| Sensitivity (95% CI) (n Studies) | Specificity (95% CI) (n Studies) | Sensitivity (95% CI) (n Studies) | Specificity (95% CI) (n Studies) | ||||
| Arbyn 2018 [15] (MA) | Women who did not do regular screening tests or women who had never had a screening test in the past | hrHPV assays based on signal amplification | Accuracy of the HPV test in self-collected samples as compared to that performed in clinician-collected samples | R = 0.85 (0.80–0.89) (23 OS) | R = 0.96 (0.93–0.98) (23 OS) | R = 0.86 (0.76–0.98) (9 OS) | R = 0.97 (0.95–0.99) (9 OS) |
| hrHPV assays based on PCR | R = 0.99 (0.97–1.02) (17 OS) | R = 0.98 (0.97–0.99) (17 OS) | R = 0.99 (0.96–1.02) (8 OS) | R = 0.98 (0.97–0.99) (8 OS) | |||
| Kelly 2017 [16] (MA) | Sexually active women (including women with HIV infection) included in the screening program | hrHPV assays based on signal amplification | Accuracy of the HPV test in self-collected samples | 74% (0.65–0.81) (4 OS) | 88% (0.79–0.93) (4 OS) | 75% (0.67–0.82) (3 OS) | 91% (0.83–0.95) (3 OS) |
| hrHPV assays based on signal amplification | Accuracy of the HPV test in clinician-collected samples | 88% (0.81; 0.93) (7 OS) | 84% (0.75; 0.90) (7 OS) | 90% (0.84; 0.94) (4 OS) | 85% (0.73; 0.92) (4 OS) | ||
| Arbyn 2014 [22] (MA) | Women participating in a screening program | - | Absolute accuracy of the HPV test in self-collected samples | 76% (0.69–0.82) (14 RCT) | 86% (0.83–0.89) (14 RCT) | 84% (0.72–0.92) (6 RCT) | 87% (0.84–0.90) (6 RCT) |
| - | Absolute accuracy of the HPV test in clinician-collected samples | 91% (0.87–0.94) (14 RCT) | 88% (0.85–0.91) (14 RCT) | 95% (0.91–0.97) (6 RCT) | 89% (0.87–0.92) (6 RCT) | ||
| Women in high-risk groups | - | Absolute accuracy of the HPV test in self-collected samples | 75% (0.58–0.87) (3 RCT) | 86% (0.77–0.92) (3 RCT) | 42% (0.27–0.57) (1 RCT) | 81% (0.76–0.87) (1 RCT) | |
| - | Absolute accuracy of the HPV test in clinician-collected samples | 88% (0.78–0.93) (3 RCT) | 88% (0.81–0.93) (3 RCT) | 80% (0.67–0.93) (1 RCT) | 82% (0.77–0.88) (1 RCT) | ||
| Zhao 2012 [25] (MA) | Women aged 17–56 years participating in the populational screening program | Hybrid Capture 2 assay | Accuracy of the HPV test in self-collected samples | 86.2% (0.829–0.891) (5 OS) | 80.7% (0.756–0.858) (5 OS) | 86.1% (0.814–0.90) (5 OS) | 79.5% (0.741–0.848) (5 OS) |
| Women aged 17–56 years participating in the populational screening program | Hybrid Capture 2 assay | Accuracy of the HPV test in clinician-collected samples | 97% (0.952–0.983) (5 OS) | 82.7% (0.784–0.870) (5 OS) | 97.8% (0.953–0.992) (5 OS) | 81.3% (0.767–0.858) (5 OS) | |
| Author/Year | N of Studies | Population | Intervention | Comparator | End Point | RR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| Population Summary | Population Size (n/N) * | |||||||
| Tesfahunei [11] 2021 (MA) | 4 RCTs | Female residents of sub-Saharan Africa, aged 25–65 | 3192/4561 (I) 1566/3639 (C) | HPV self-sampling offer | Overall | Screening to be performed by a clinician at a health care facility (HPV test or VIA) | Screening uptake | RR = 1.72 (1.58–1.87) |
| 3 RCTs | 2944/4311(I) 1445/3389 (C) | Within some time range | RR = 1.65 (1.58–1.72) | |||||
| 1 RCT | 248/250 (I) 121/250 (C) | Immediately on recruitment | RR = 2.05 (1.80–2.33) | |||||
| Yeh 2019 [14] (MA) | 29 RCTs | Women eligible for CC screening who had not reported for a cytology test. | 307,960 | HPV self-sampling kit delivery | Overall | Invitation for a screening test to be performed by a clinician (cytology, VIA, HPV assay) | Screening uptake | RR = 2.13 (1.89–2.40) |
| 23 RCTs | 276,229 | By mail | RR = 2.27 (1.89–2.71) | |||||
| 5 RCTs | 88,222 | Opt-in strategy | RR = 1.28 (0.90–1.82) | |||||
| 5 RCTs | 32,238 | Door-to-door strategy | RR = 2.37 (1.12–5.03) | |||||
| Arbyn 2018 [15] (MA) | 19 RCTs | Women who did not do regular screening tests or women who had never had a screening test in the past. | Not specified | HPV self-sampling kit delivery | By mail | Invitation for or reminder about a screening test to be performed by a clinician | Participation | RR = 2.33 (1.86–2.91) |
| 6 RCTs | Opt-in strategy | RR = 1.22 (0.93–1.61) | ||||||
| 4 RCTs | Door-to-door strategy | RR = 2.01 (0.66–6.15) | ||||||
| 1 RCT | Social campaign | RR = 2.58 (1.67–3.99) | ||||||
| Musa 2017 [18] (MA) | 8 RCTs | Women eligible for CC screening | 6154/22,256 (I) 5181/18,314 (C) | HPV self-sampling offer | Screening reminder sent by mail | Screening uptake | RR = 1.71 (1.32–2.22) | |
| Verdoodt [20] 2015 (MA) | 13 RCTs | Women who did not do regular screening tests or women who had never had a screening test in the past. | 90,191 (I) 39,253 (C) | HPV self-sampling kit delivery | By mail | Invitation for or reminder about a screening test to be performed by a clinician | Participation | RR = 2.40 (1.73–3.33) |
| 3 RCTs | 11,067 (I) 10,247 (C) | Opt-in strategy | RR = 0.97 (0.65–1.46) | |||||
| 2 RCTs | 12,420 (I) 16,749 (C) | Door-to-door strategy | RR = 2.21 (0.32–15.48) | |||||
| Albrow 2014 [21] (SR) | 1 RCT | Women eligible for CC screening | 110/413 (I) 246/1114 (C) | HPV self-sampling offer | Screening reminder sent by mail | Screening uptake | RR = 1.23 (1.01–1.48) | |
| Camilloni [23] 2013 (MA) | 7 observational studies | Women aged 25–64 and eligible for CC screening who had not reported for a cytology test. | 3357/64,256 (I) 1150/34,496 (C) | HPV self-sampling kit delivered by mail | A reminder regarding cytological examination to be performed at a health center | Screening uptake | RR = 2.37 (1.44–3.90) | |
| Racey 2013 [24] (MA) | 9 RCTs 1 observational study | Women in developed countries who had never had a screening test in the past. | 28,143/74,312 (I) 13,466/28,369 (C) | HPV self-sampling kit delivered by mail or using the door-to-door strategy | Invitation for a screening test to be performed by a clinician (cytology) | Participation | RR = 2.14 (1.30–3.52) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatara, T.; Wnuk, K.; Miazga, W.; Świtalski, J.; Karauda, D.; Mularczyk-Tomczewska, P.; Religioni, U.; Gujski, M. The Influence of Vaginal HPV Self-Sampling on the Efficacy of Populational Screening for Cervical Cancer—An Umbrella Review. Cancers 2022, 14, 5913. https://doi.org/10.3390/cancers14235913
Tatara T, Wnuk K, Miazga W, Świtalski J, Karauda D, Mularczyk-Tomczewska P, Religioni U, Gujski M. The Influence of Vaginal HPV Self-Sampling on the Efficacy of Populational Screening for Cervical Cancer—An Umbrella Review. Cancers. 2022; 14(23):5913. https://doi.org/10.3390/cancers14235913
Chicago/Turabian StyleTatara, Tomasz, Katarzyna Wnuk, Wojciech Miazga, Jakub Świtalski, Dagmara Karauda, Paulina Mularczyk-Tomczewska, Urszula Religioni, and Mariusz Gujski. 2022. "The Influence of Vaginal HPV Self-Sampling on the Efficacy of Populational Screening for Cervical Cancer—An Umbrella Review" Cancers 14, no. 23: 5913. https://doi.org/10.3390/cancers14235913
APA StyleTatara, T., Wnuk, K., Miazga, W., Świtalski, J., Karauda, D., Mularczyk-Tomczewska, P., Religioni, U., & Gujski, M. (2022). The Influence of Vaginal HPV Self-Sampling on the Efficacy of Populational Screening for Cervical Cancer—An Umbrella Review. Cancers, 14(23), 5913. https://doi.org/10.3390/cancers14235913







