Simple Summary
The bone scan (BS) is widely used in follow-up to detect bone metastasis (BM) in breast cancer (BC) patients presenting bone-related symptoms after surgery. However, it remains controversial whether asymptomatic BS (intensive postoperative BS) screening could be translated into a survival benefit. Therefore, we conducted this multicenter real-world study to understand the prognostic impact of intensive postoperative BS screening among 1059 Chinese patients with BM during the years 2005–2013. This study showed that intensive postoperative BS screening was an independent prognostic factor and prolonged the survival in patients with BC with BM. The prognostic value of intensive BS screening was consistently favorable for survival in patients at clinical high-risk. These findings suggested that intensive BS screening was important for improving survival, and should be recommended for postoperative surveillance, especially for patients with a high risk of recurrence and metastasis.
Abstract
The prognostic value of intensive postoperative bone scan (BS) screening, which is performed in asymptomatic patients with breast cancer (BC) after surgery, remained unclear. Patients diagnosed with BC with bone metastasis (BM) from five medical centers in China during the years 2005–2013 were retrospectively collected. Propensity score matching (PSM) was performed to balance the baseline characteristics. The survival outcomes were overall survival (OS) and overall survival after BM (OSABM). Among 1059 eligible patients, 304 underwent intensive postoperative BS while 755 did not. During a median follow-up of 6.67 years (95%CI 6.45, 7.21), intensive postoperative BS prolonged the median OS by 1.63 years (Log-Rank p = 0.006) and OSABM by 0.66 years (Log-Rank p = 0.002). Intensive postoperative BS was an independent prognostic factor for both OS (adjusted HR 0.77, 95%CI 0.64, 0.93, adjusted p = 0.006) and OSABM (adjusted HR 0.71, 95%CI 0.60, 0.86, adjusted p < 0.001). The prognostic value of intensive postoperative BS was consistently favorable for OS among clinical high-risk patients, including those with ages younger than 50, stage II, histology grade G3 and ER-Her2- subtype. This multicenter real-world study showed that intensive postoperative BS screening improved survival for BC patients with BM and should probably be recommended for postoperative surveillance, especially for patients at clinical high-risk.
1. Introduction
Breast cancer (BC) is the most commonly diagnosed malignant cancer in women [1], and bone is the most common distant metastatic site [2,3,4]. The bone scan (BS), a conventional and cost-effective modality for detecting the entire skeleton in one examination [5,6], is widely used in postoperative follow-up for surveillance of bone metastasis (BM) in BC patients presenting related symptoms after surgery. However, current guidelines do not recommend intensive BS screening, which is referred to BS screening in asymptomatic patients, without specific findings on clinical examination before a diagnosis of BM.
The prognostic value of intensive postoperative BS remains unclear. Two well-designed randomized controlled trials, GIVIO (Interdisciplinary Group for Cancer Care Evaluation) trials [7], as well as Rosselli del Turco trials [8], and the Cochrane meta-analysis [9] demonstrated that intensive follow-up (imaging examinations including BS and laboratory tests) does not improve overall survival compared to clinical follow-up (physical examinations and annual mammography). Hence the American Society of Clinical Oncology (ASCO) [10], National Comprehensive Cancer Network (NCCN) [11] and European Society for Medical Oncology (ESMO) [12] do not recommend an intensive follow-up including BS.
It is important to note that the two trials were conducted almost three decades ago when advanced postoperative screening methods and palliative therapeutic options were scarce. Moreover, oncologists at that time lacked an adequate understanding of the intrinsic biological characteristics of BC. Recently, new regimens of systemic chemotherapy [13,14] and endocrine therapy [15] have made considerable progress in increasing patients’ survival with far-advanced cancer. Anti-Her2 (human epidermal growth factor receptor 2) therapy increased the prognosis of patients with Her2-positive metastatic BC [16,17]. Bone-modifying agents, such as bisphosphonates [18] and denosumab [19], slowed down the progression of skeletal-related events, thus promoting the quality of life.
It is possible that recent improvements in diagnostics and treatments could promote earlier detection and effective treatment of BM, important for improving survival. Therefore, we conducted this multicenter real-world study to understand the prognostic factors of BC patients with BM, especially the prognostic impact of an intensive postoperative BS after initial diagnosis of BC.
2. Materials and Methods
2.1. Design and Patients
According to Chinese Society of Breast Surgery (CSBrS), this multicenter real-world study was conducted by five medical centers in China. This study has been registered in Clinicaltrials.gov as NCT03924609 on 23 April 2019 and approved by the Ethics Committee of the People’s Hospital of Peking University (No. 2021PHB071-001). As this study was a retrospective study and all data were performed anonymously, the need for informed consent from patients was waived. All data generated or analyzed during the study are included in the published paper.
Patients eligible were required to have a histology-confirmed diagnosis of invasive BC and undergo curative-intent primary therapy. The diagnosis of BM must be supported by pathological or imaging evidence. The following cases were not eligible: (1) with other malignant primary cancer; (2) de novo stage IV BC; (3) incomplete and ambiguous clinical and pathological records.
2.2. Clinicopathological Factors
Clinicopathological factors of eligible patients were extracted from the standardized case report forms. Intensive postoperative BS was defined as at least one asymptomatic postoperative BS screening after initial diagnosis of BC before a diagnosis of BM. Clinical postoperative BS was referred to postoperative BS screening only performed in patients presenting bone-related symptoms. Primary tumor staging was defined according to the criteria of the TNM (tumor-nodal-metastasis) staging system by AJCC (American Joint Committee on Cancer) [20]. The histology type of BC was defined according to criteria from the WHO (World Health Organization) [21]. The molecular subtypes of BC were classified based on the expression of the estrogen receptor (ER) and Her2 according to ASCO/CAP (American Society of Clinical Oncology/College of American Pathologists) [22,23]. Based on the timing of BM and visceral metastasis (VM), the pattern of distant metastasis was mainly divided into the following types: (1) BM only: only diagnosed with BM; (2) BM with VM: diagnosed with BM and VM simultaneously; (3) BM to VM: first diagnosed with BM, followed by VM; (4) VM to BM: first diagnosed with VM, followed by BM.
2.3. Follow-Up and Outcomes Definition
Follow-up was conducted by telephone or clinical visit from the date of diagnosis of BM until death. The follow-up information was obtained from the databases of the participating medical centers. The survival endpoints were overall survival (OS), which was calculated from the date BC was diagnosed to the date of death, and overall survival after diagnosis of bone metastasis (OSABM), which was calculated from the date BM was diagnosed to the date of death. The length of bone-metastasis free interval (BMFI) was also retrospectively observed, which was calculated as the time from diagnosis of BC to initial BM.
2.4. Propensity Score Matching (PSM)
When comparing survival between patients who underwent an intensive postoperative BS and those who underwent a clinical postoperative BS, propensity score matching was used to balance the baseline characteristics. We performed a 1:2 nearest-neighbor matching procedure within a caliper of 0.02 and all clinic and pathological factors were included in the matching model. Balance between the two groups before and after matching was assessed using standardized mean differences (SMD) and p-value by chi-square test or t test. SMD > 0.20 or p-value < 0.05 were considered imbalanced.
2.5. Statistical Analysis
Continuous variables were reported as mean and standard deviation, whereas categorical variables were reported as percentage. Statistical differences in the distribution of continuous and categorical variables were conducted by t-test and chi-square test, respectively. The statistical differences in the distribution of BMFI in various subgroups according to TNM stage and molecular subtype of BC were tested using the Kruskal–Wallis method.
Survival analysis was performed using the Kaplan–Meier method before and after PSM, thus median survival time was estimated and the Log-rank test was used for comparisons between groups. After PSM, univariate and multivariate Cox proportional hazards regression analyses and associated 95% confidence intervals (95%CI) were used to assess whether the hazard risks of survival endpoints in patients varied by certain clinical or pathological factors. Factors that showed a univariate connection with survival (p-value < 0.20) or considered clinically relevant were entered into the multivariate Cox proportional hazard regression model. Interaction terms were tested using the qualitative method and the univariate stratified Cox proportional hazard regression model, which were used to investigate whether the association between postoperative follow-up strategies and survival outcomes differed according to all clinical and pathological factors. Two-tailed p-values < 0.05 were considered statistically significant. All analyses were conducted using R*64 4.0.0 (Beijing, China, http://Rproject.org, accessed on 10 January 2022) and IBM SPSS Statistics 25.
3. Results
3.1. Patient Characteristics
From February 2005 to December 2013, we retrospectively identified 1425 patients with BC with BM from five medical centers in China. Excluding 239 patients with de novo stage IV BC and 127 with incomplete clinicopathological records, 1059 eligible patients were included in the analyses. The flow chart of the process of patients’ enrollment and analyses is presented in Figure 1.
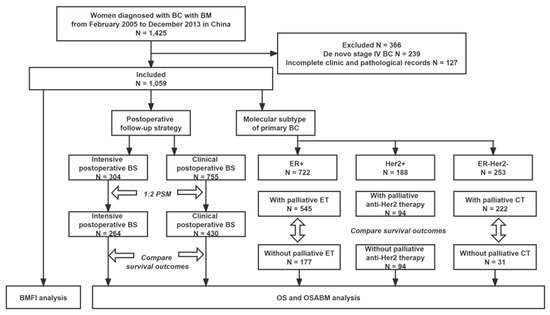
Figure 1.
Flowchart of the process of patient’s enrollment and analyses. Abbreviations: BC = breast cancer; BM = bone metastasis; BMFI = bone metastasis-free interval; BS = bone scan; CT = chemotherapy; ER = estrogen receptor; Her2 = Human epidermal growth factor receptor 2; ET = endocrine therapy; OS = overall survival; OSABM = overall survival after diagnosis of bone metastasis; PSM = propensity scores matching.
Among 1059 eligible patients, 304 underwent an intensive postoperative BS while 755 underwent a clinical postoperative BS. The median time when a patient received the first intensive postoperative BS was 2.5 years after initial diagnosis of BC. Baseline characteristics in the two groups stratified by postoperative follow-up strategy were balanced after PSM (shown in Table 1).

Table 1.
Clinicopathological characteristics of eligible patients (N = 1059) stratified by postoperative follow-up strategy (Clinical postoperative BS vs. Intensive postoperative BS) before and after PSM.
3.2. The Impact of an Intensive Postoperative BS on Survival
Follow-up was regularly performed until December 2018. During a median follow-up of 6.67 years (95%CI 6.45, 7.21), 759 out of 1059 eligible patients were dead: 197 in the intensive postoperative BS group and 562 in the clinical postoperative BS group. Before PSM, both median OS and OSABM of patients with an intensive postoperative BS were longer than those with a clinical postoperative BS (median OS, 7.99 vs. 6.61 years, Log-Rank p = 0.003, Figure 2A; median OSABM, 3.16 vs. 2.57 years, Log-Rank p = 0.003, Figure 2C). After PSM, both OS and OSABM benefits were still statistically significant in patients with an intensive postoperative BS (median OS, 7.88 vs. 6.25 years, Log-Rank p = 0.006, Figure 2B; median OSABM, 3.16 vs. 2.50 years, Log-Rank p = 0.002, Figure 2D).
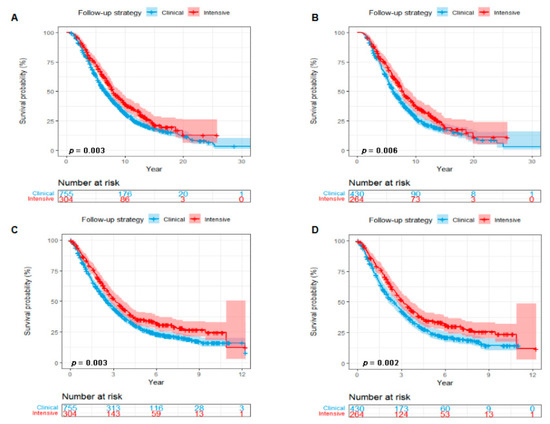
Figure 2.
Kaplan–Meier curves showing a comparison of survival among patients with breast cancer with BM according to postoperative follow-up strategy (Intensive postoperative BS vs. Clinical postoperative BS). OS curves before (A) and after (B) PSM. OSABM curves before (C) and after (D) PSM. Abbreviations: BS = bone scan; BM = bone metastasis; OS = overall survival; OSABM = overall survival after diagnosis of bone metastasis; PSM = propensity scores matching.
3.3. Univariate and Multivariate Analysis of Factors Influencing Survival
When adjusting clinicopathological covariates after PSM, intensive postoperative BS was a favorable prognostic factor for both OS and OSABM of patients with BC with BM and reduced the risk of mortality by 23% (OS, adjusted HR 0.77, 95%CI 0.64, 0.93, adjusted p = 0.006; OSABM, adjusted HR 0.71, 95%CI 0.60, 0.86, adjusted p < 0.001). Histology type, TNM stage, distant metastatic pattern and palliative endocrine therapy were also independent prognostic factors for both OS and OSABM. Additionally, BMFI and age at diagnosis of BM were independent prognostic factors of OS and OSABM, respectively. The results of univariate and multivariate analysis of clinicopathological factors affecting OS and OSABM among eligible patients after PSM are listed in Table 2.

Table 2.
Univariate and multivariate analysis of clinicopathological factors affecting OS and OSABM among eligible patients (N = 694) after PSM.
3.4. Interaction and Univariate Stratified Analysis of the Impact of an Intensive Postoperative BS on Survival
As shown in Figure 3, eligible patients were stratified by all clinicopathological factors and palliative treatment methods on BM to explore the relationship between postoperative follow-up strategy and survival after PSM. The prognostic value of an intensive postoperative BS was consistently favorable for OS among BC patients at clinical high-risk, including an age at diagnosis of BM younger than 50, TNM stage II, histology grade G3 and ER-Her2-subtype (Figure 3A). Similarly, as for OSABM, the favorable prognostic value of an intensive postoperative BS was also significant in patients at clinical high-risk, including TNM stage II, histology grade G3 and ER-Her2-subtype (Figure 3B).
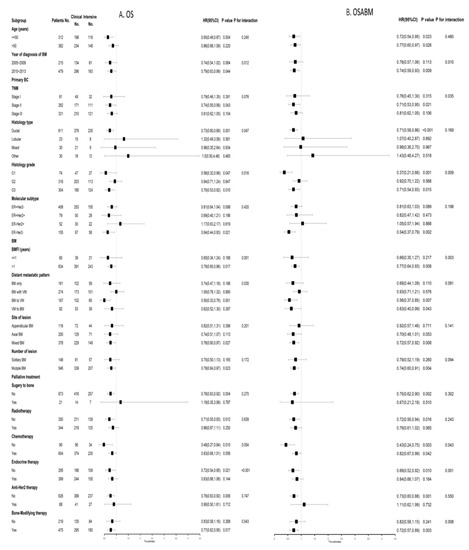
Figure 3.
Forest plots of interaction and univariate subgroup analyses on the association between postoperative follow-up strategies (Intensive postoperative BS vs. Clinical postoperative BS) and (A) OS and (B) OSABM of patients with breast cancer with BM after PSM. Abbreviations: BS = bone scan; BM = bone metastasis; BMFI = bone metastasis-free interval; 95%CI = 95% confidence interval; ER = estrogen receptor; Her2 = Human epidermal growth factor receptor 2; HR = hazard risk; No. = Numbers of patients; OS = overall survival; OSABM = overall survival after bone metastasis; PSM = propensity scores matching.
3.5. The Impact of Palliative Treatments on Survival Stratified by Molecular Subtype
From the point of molecular subtypes of BC, we observed the association between palliative treatments and survival of patients with BM. For patients with a Her2+ BC, 50% (94/188) received palliative anti-Her2 therapy. Palliative anti-Her2 therapy prolonged median OS by 2.4 years (Log-Rank p = 0.002; HR 0.60, 95%CI 0.43, 0.83; Figure 4A) and OSABM by 1.6 years (Log-Rank p < 0.001; HR 0.49, 95%CI 0.35, 0.68; Figure 4B) among Her2+ patients. For patients with an ER + BC, 75.5% (545/722) underwent palliative endocrine therapy. Palliative endocrine therapy improved both OS (Log-Rank p < 0.001; HR 0.70, 95%CI 0.57, 0.86; Figure 4C) and OSABM (Log-Rank p = 0.007; HR 0.75, 95%CI 0.61, 0.92; Figure 4D) for this subgroup of patients. In addition, 87.7% (222/253) of patients with an ER-HER2-BC received palliative chemotherapy. However, palliative chemotherapy converted into neither OS (Log-Rank p = 0.300; HR 0.81, 95%CI 0.53, 1.24; Figure 4E) nor OSABM (Log-Rank p = 0.070; HR 0.68, 95%CI 0.45, 1.04; Figure 4F) benefits for ER-Her2-patients.
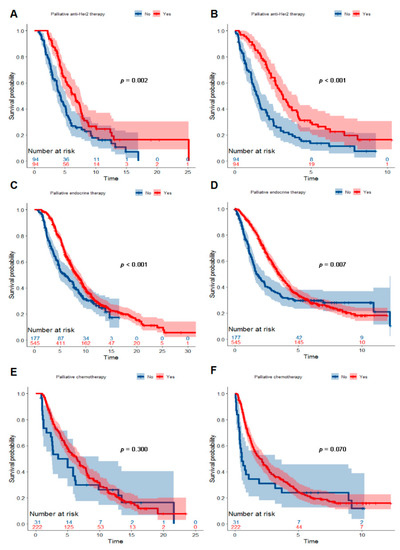
Figure 4.
Kaplan–Meier curves showing a comparison of survival time among patients with breast cancer with BM according to molecular subtype and palliative treatment. Curves for OS (A) and OSABM (B) of patients with a Her2+ breast cancer stratified by palliative anti-Her2 therapy. Curves for OS (C) and OSABM (D) of patients with an ER+ breast cancer stratified by palliative endocrine therapy. Curves for OS € (E) and OSABM (F) of patients with an ER-Her2-breast cancer stratified by palliative chemotherapy. Abbreviations: BM = bone metastasis; ER = estrogen receptor; Her2 = Human epidermal growth factor receptor 2; OS = overall survival; OSABM = overall survival after diagnosis of bone metastasis.
3.6. The Association of BMFI with BC Stage and Molecular Subtype
The median BMFI was 3.08 years for 1059 eligible patients. However, as shown in Figure 5, BC patients with a different TNM stage and molecular subtype presented specific distributions of the length of BMFI. The median BMFI was 3.29 years for patients at stage I-II and 2.13 years for patients at stage III (p < 0.001, Figure 5C). The annual incidence of BM reached a peak at the second year after initial diagnosis of BC among patients at stage III (24.5%, 120/489), the third year among patients at stage II (19.0%, 84/443), while the fourth year among patients at stage I (18.1%, 23/127, Figure 5A). The median BMFI was 3.38, 2.88 and 2.30 years for patients with an ER+, ER-Her2- and Her2+ BC, respectively (p < 0.001, Figure 5D). Compared with ER+ and ER-Her2-, patients with a Her2+ BC progressed to BM more rapidly. The cumulative incidence of BM (two years after initial diagnosis of BC) was 26.6% (192/722) for ER+ patients and 34.4% (87/253) for ER-Her2-patients; however, it was 42.0% (79/188) for Her2+ patients (Figure 5B).
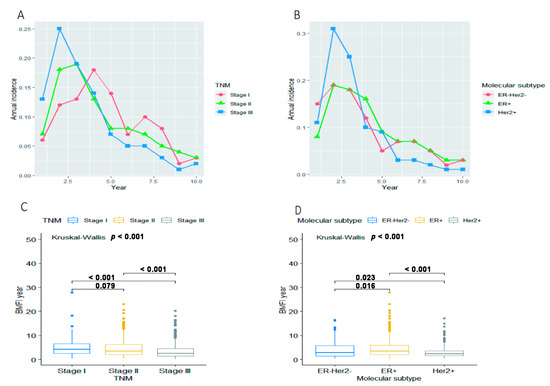
Figure 5.
Annual incidence of BM for overall eligible patients (N = 1059) in groups stratified by (A) TNM stage and (B) molecular subtype. The distribution of BMFI for overall eligible patients (N = 1059) in groups stratified by (C) TNM stage and (D) molecular subtype. Abbreviations: BM = bone metastasis; BMFI = bone metastasis-free survival; ER = estrogen receptor; Her2 = Human epidermal growth factor receptor 2.
4. Discussion
This multicenter real-world study showed an intensive postoperative BS improved survival for BC patients with BM. In the point of molecular subtypes of BC, palliative anti-Her2 therapy and endocrine therapy improved both OS and OSABM among patients with a Her2+ and ER+ BC, respectively. These results indicated that the intensive postoperative BS and phenotype-specific palliative systemic treatments were important for improving survival of patients with BM.
Currently, ASCO, NCCN and ESMO guidelines do not recommend an intensive postoperative BS for BC patients [10,11,12]. However, in clinical practice, there are substantial variations in adherence to guideline recommendations. Intensive follow-up is a widespread reality that costs 2.2–3.6 times more than follow-up suggested by guidelines [24]. In a large population-based retrospective longitudinal study (n = 11,219) of women in Canada, 8.7–14.6% of women underwent BS screening in each follow-up year, and about half of them had greater than ASCO guideline-recommended surveillance imaging for metastatic diseases [25]. In line with these results, Surveillance, Epidemiology, and End Results (SEER)-Medicare database showed that 13.3% of 37,967 patients underwent at least one BS screening in the first year of follow-up [26]. Similarly, in our study, 28.7% (304/1059) of patients received an intensive postoperative BS. There are several possible reasons for the overuse of intensive BS imaging. First, the patient-driven anxiety and the feeling of reassurance induced by intensive postoperative surveillance, including the BS. Stemmler et al. have examined 801 questionnaires of German women with a history of BC and reported that more than 47.8% of them needed an intensive schedule, which increased their feeling of security [27]. Second, patients with early or limited metastatic recurrence may be curable; thus, the monitoring of asymptomatic patients could result in better efficacy of BC treatment, at least in theory, when tumor burden is low [26]. Third, all the high-level evidence was conducted almost 30 years ago in an era of outdated technology and limited therapeutic options. Current evidence demonstrated that improvements in diagnostics and treatments could improve the survival of patients with metastatic BC, especially with more detailed subtype classification and corresponding efficient target therapies [13,14,15,17]. However, there are no current well-designed trials to verify this issue. To the best of our knowledge, this is the first study that observed the prognostic value of an intensive postoperative BS in patients with BC with BM.
In our study, an intensive postoperative BS resulted in an independent prognostic factor of OS and OSABM among patients with BC with BM. It was worth noticing that 85.4% (904/1059) of patients received palliative chemotherapy, and 66.1% (700/1059) received bone-modifying therapy. In addition, 75.5% (545/722) of ER+ patients received palliative endocrine therapy and 50% (94/188) of Her2+ patients received palliative anti-Her2 therapy. The strength of these treatments was much stronger than it was decades ago. Palliative endocrine therapy had been identified as an independent prognostic factor for OS as well as OSABM, and palliative anti-Her2 therapy also improved OS and OSABM of patients with Her2+ BC. For ER-Her2-patients, palliative systemic chemotherapy increased 5-year OS by 14.3% (57.7% vs. 43.4%) and 2-year OSABM by 18.7% (49.7% vs. 31.0%) compared with the patients who did not receive palliative chemotherapy. This evidence suggested that intensive detection and effective phenotype-specific systemic intervention for BM could be translated into a survival benefit.
In order to make intensive postoperative BSs more cost-effective, we selected high-risk patients based on stratified analysis. A higher tumor burden led to a higher risk of distant metastasis [28,29,30,31]. Our study showed that the patients at stage II-III progressed to BM more rapidly compared with those at stage I. It was worth nothing that an intensive postoperative BS particularly improved survival of patients at stage II. Consequently, it was rational to suggest patients with a heavy local tumor burden receive intensive postoperative BS screening. From an intrinsic biological point of view, early BC presents special metastatic behaviors [32,33], so postoperative monitoring strategies should vary accordingly. The ER-Her2-subtype, with a dramatically increased risk of distant relapse [34], accounted for 23.9% (253/1059) of patients in our study. An intensive postoperative BS improved OS as well as OSABM among ER-Her2-patients. Thus, we assumed that an intensive postoperative BS for ER-Her2-patients might be of significance. However, an intensive postoperative BS did not convert into a survival benefit in Her2+ patients. It is possible that this was due to limited Her2 status detection techniques and therapeutic options, even though early postoperative detection of BM was performed. In our study, 367 out of 1059 patients were diagnosed with BM before 2009, when Her2 status detection techniques were not commonly used in China, and trastuzumab was not widely implemented for relapse patients.
It is also worth noting that for all eligible patients, 26.5% (281/1059) were diagnosed with BM only, 37.6% (398/1059) were BM with VM, 23.6% (250/1059) were BM followed by VM, and 12.3% (130/1059) were VM followed by BM. There is probably a certain percent of patients classified as BM with VM who developed BM first and then progressed to VM but were not detected when simple BM originated. Previous studies showed that 26% to 50% of patients with early BC developed bone metastasis as the first site of distant relapse [4]. Consequently, early detection and treatment of BM may prolong the interval to visceral metastasis. As predicted, according to interaction and univariate stratified subgroup analysis, an intensive postoperative BS could improve OS for patients with ‘’BM to VM”, thus supporting the idea that early detection and early treatment are effective.
This multicenter real-world study showed that an intensive postoperative BS should probably be recommended as a follow-up strategy for patients with BC with BM. The main limitation of the present study is the retrospective study design. When evaluating the prognostic value of an intensive postoperative BS, cost-effectiveness and quality of life were not included in the analyses. Future studies with a randomized design are warranted to get an explicit estimation.
5. Conclusions
This multicenter real-world study showed that intensive postoperative BS screening improved survival for BC patients with BM, and should be recommended for postoperative surveillance, especially for patients at clinical high-risk.
Author Contributions
Conceptualization, G.L. and S.W.; methodology, L.Y., W.D., T.H., G.L. and S.W.; software, L.Y., W.D. and T.H.; validation, G.L. and S.W.; formal analysis, L.Y., W.D. and T.H.; investigation, G.L. and S.W.; resources, L.Y., W.D., M.L., L.C., Z.Y., Q.L., G.L. and S.W.; data curation, L.Y., G.L. and S.W.; writing—original draft preparation, L.Y.; writing—review and editing, L.Y., G.L. and S.W.; visualization, L.Y. and T.H.; supervision, G.L. and S.W.; project administration, G.L. and S.W.; funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of Science and Technology of People’s Republic of China (Grant No. 2016YFC0901302), the National Natural Science Foundation of China (Grant No. 92059105, 82002979), and the Beijing Municipal Natural Science Foundation (Grant No. 7202212).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the People’s Hospital of Peking University (No. 2021PHB071-001).
Informed Consent Statement
As this study was a retrospective study and all data analyses were performed anonymously, the need for patient consent was waived.
Data Availability Statement
All data generated or analyzed during the study are included in the published paper.
Acknowledgments
The authors would like to acknowledgement the member units of CSBrS for data collection: Department of Breast Oncology, Harbin Medical University Cancer Hospital; Department of Breast Surgery, Sun Yai-Sen Memorial Hospital; Department of Breast Surgery, Fudan University Shanghai Cancer Center; Department of Breast Surgery, The Second Hospital of Shandong University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Cetin, K.; Christiansen, C.F.; Svaerke, C.; Jacobsen, J.B.; Sørensen, H.T. Survival in patients with breast cancer with bone metastasis: A Danish population-based cohort study on the prognostic impact of initial stage of disease at breast cancer diagnosis and length of the bone metastasis-free interval. BMJ Open 2015, 5, e007702. [Google Scholar] [CrossRef]
- Hamaoka, T.; Madewell, J.E.; Podoloff, D.A.; Hortobagyi, G.N.; Ueno, N.T. Bone imaging in metastatic breast cancer. J. Clin. Oncol. 2004, 22, 2942–2953. [Google Scholar] [CrossRef]
- Hildebrandt, M.G.; Gerke, O.; Baun, C.; Falch, K.; Hansen, J.A.; Farahani, Z.A.; Petersen, H.; Larsen, L.B.; Duvnjak, S.; Buskevica, I.; et al. [18F]Fluorodeoxyglucose (FDG)-Positron Emission Tomography (PET)/Computed Tomography (CT) in Suspected Recurrent Breast Cancer: A Prospective Comparative Study of Dual-Time-Point FDG-PET/CT, Contrast-Enhanced CT, and Bone Scintigraphy. J. Clin. Oncol. 2016, 34, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.J.; Azad, G.K.; Goh, V. Imaging Bone Metastases in Breast Cancer: Staging and Response Assessment. J. Nucl. Med. 2016, 57 (Suppl. 1), 27S–33S. [Google Scholar] [CrossRef]
- Ghezzi, P.; Magnanini, S.; Rinaldini, M.; Berardi, F.; Di Biagio, G.; Testare, F.; Tavoni, N.; Schittulli, F.; D’Amico, C.; Pedicini, T.; et al. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. The GIVIO Investigators. JAMA 1974, 271, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Palli, D.; Russo, A.; Saieva, C.; Ciatto, S.; Del Turco, M.R.; Distante, V.; Pacini, P. Intensive vs clinical follow-up after treatment of primary breast cancer: 10-year update of a randomized trial. National Research Council Project on Breast Cancer Follow-up. JAMA 1999, 281, 1586. [Google Scholar] [CrossRef]
- Moschetti, I.; Cinquini, M.; Lambertini, M.; Levaggi, A.; Liberati, A. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst. Rev. 2016, 5, CD001768. [Google Scholar] [CrossRef]
- Khatcheressian, J.L.; Hurley, P.; Bantug, E.; Esserman, L.J.; Grunfeld, E.; Halberg, F.; Hantel, A.; Henry, N.L.; Muss, H.B.; Smith, T.J.; et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 961–965. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2020, 18, 452–478. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Kalinsky, K.; Diamond, J.; Vahdat, L.; Tolaney, S.; Juric, D.; O’Shaughnessy, J.; Moroose, R.; Mayer, I.; Abramson, V.; Goldenberg, D.; et al. Sacituzumab govitecan in previously treated hormone receptor- positive/HER2-negative metastatic breast cancer: Final results from a phase I/II, single-arm, basket trial. Ann. Oncol. 2020, 31, 1709–1718. [Google Scholar] [CrossRef]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.-A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Kim, S.-B.; Cortés, J.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Knott, A.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013, 14, 461–471. [Google Scholar] [CrossRef]
- Perez, E.A.; Barrios, C.; Eiermann, W.; Toi, M.; Im, Y.-H.; Conte, P.; Martin, M.; Pienkowski, T.; Pivot, X.; Burris, H.A.; et al. Trastuzumab Emtansine with or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: Primary Results from the Phase III MARIANNE Study. J. Clin. Oncol. 2017, 35, 141–148. [Google Scholar] [CrossRef]
- O’Carrigan, B.; Wong, M.H.; Willson, M.L.; Stockler, M.R.; Pavlakis, N.; Goodwin, A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2017, 10, Cd003474. [Google Scholar] [CrossRef]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Edge, S.B.; Hortobagyi, G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018, 25, 1783–1785. [Google Scholar] [CrossRef]
- Frank, G.A.; Danilova, N.V.; Andreeva, I.I.; Nefedova, N.A. WHO classification of tumors of the breast, 2012. Arkh. Patol. 2013, 75, 53–63. [Google Scholar]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 2020, 144, 545–563. [Google Scholar] [CrossRef]
- Mille, D.; Roy, T.; Carrère, M.-O.; Ray, I.; Ferdjaoui, N.; Späth, H.-M.; Chauvin, F.; Philip, T. Economic impact of harmonizing medical practices: Compliance with clinical practice guidelines in the follow-up of breast cancer in a French Comprehensive Cancer Center. J. Clin. Oncol. 2000, 18, 1718–1724. [Google Scholar] [CrossRef]
- Grunfeld, E.; Hodgson, D.C.; Del Giudice, M.E.; Moineddin, R. Population-based longitudinal study of follow-up care for breast cancer survivors. J. Oncol. Pract. 2010, 6, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Keating, N.L.; Landrum, M.B.; Guadagnoli, E.; Winer, E.P.; Ayanian, J.Z. Surveillance testing among survivors of early-stage breast cancer. J. Clin. Oncol. 2007, 25, 1074–1081. [Google Scholar] [CrossRef]
- Hans-Joachim, S.; Dorit, L.; Petra, S.; Ingo, B.; Steffen, K.; Alexander, F.P.; Wilhelm, B.M.; Margrit, G.; Ursula, G.-P.; Verena, H.; et al. The reality in the surveillance of breast cancer survivors-results of a patient survey. Breast Cancer 2008, 1, 17–23. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, S.-S.; Han, W.; Kim, S.W.; Shin, H.J.; Choe, K.J.; Oh, S.K.; Youn, Y.-K.; Noh, N.-Y.; Kim, S.-W. The clinical use of staging bone scan in patients with breast carcinoma: Reevaluation by the 2003 American Joint Committee on Cancer staging system. Cancer 2005, 104, 499–503. [Google Scholar] [CrossRef]
- Lee, Y.T. Bone scanning in patients with early breast carcinoma: Should it be a routine staging procedure? Cancer 1981, 47, 486–495. [Google Scholar] [CrossRef]
- Brar, H.S.; Sisley, J.F.; Johnson, R.H. Value of preoperative bone and liver scans and alkaline phosphatase in the evaluation of breast cancer patients. Am. J. Surg. 1993, 165, 221–223. [Google Scholar] [CrossRef]
- Lewin, A.A.; Moy, L.; Baron, P.; Didwania, A.D.; Diflorio-Alexander, R.M.; Hayward, J.H.; Le-Petross, H.T.; Newell, M.S.; Rewari, A.; Scheel, J.R.; et al. ACR Appropriateness Criteria(®) Stage I Breast Cancer: Initial Workup and Surveillance for Local Recurrence and Distant Metastases in Asymptomatic Women. J. Am. Coll. Radiol. 2017, 14, S282–S292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buonomo, O.C.; Caredda, E.; Portarena, I.; Vanni, G.; Orlandi, A.; Bagni, C.; Petrella, G.; Palombi, L.; Orsaria, P. New insights into the metastatic behavior after breast cancer surgery, according to well-established clinicopathological variables and molecular subtypes. PLoS ONE 2017, 12, e0184680. [Google Scholar] [CrossRef] [PubMed]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.U.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Vanderplas, A.; Hughes, M.E.; Theriault, R.L.; Edge, S.B.; Wong, Y.-N.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; Weeks, J.C. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012, 118, 5463–5472. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).