Simple Summary
Tumor-infiltrating lymphocytes (TILs) have been reported to contribute to breast cancer (BC) prognosis. The aim of our study was to investigate the prognostic impact of CD8+ TILs and their subtypes in patients with early breast cancer treated with sequential, dose-dense adjuvant chemotherapy. Tumors of 627 patients were examined for total (t), stromal (s), and intratumoral (i) CD8 lymphocyte density (counts/mm2). Our results showed that high expression of sCD8, iCD8, and tCD8 correlated with higher Ki67, TILs density, ER/PgR negativity, and higher histological grade. We confirmed that patients with high iCD8+ and tCD8+ TILs had longer DFS and OS, as compared to those with low counts/mm2. Survival benefit was retained when adjusting for classical clinical and pathological characteristics, but was not correlated to specific BC subtype. More data are needed to empower the prognostic role of TILs and establish their use in clinical practice.
Abstract
Tumor-infiltrating lymphocytes (TILs) contribute to breast cancer (BC) prognosis. We investigated the prognostic impact of CD8+ TILs in patients with early breast cancer treated with adjuvant chemotherapy in a large observational clinical trial. Along with a 10 year follow-up, considering the efficacy and safety, we report the results of the translational part of our study. We examined the patients’ tumors for total (t), stromal (s), and intratumoral (i) CD8 lymphocyte density (counts/mm2) on tissue-microarray cores. The impact of CD8+ TILs counts on DFS and OS, and its correlation with breast cancer subtypes and standard clinicopathological parameters, were investigated, along with efficacy and safety data. Among the 928 eligible patients, 627 had available CD8+ data. Of which, 24.9% had a high expression of sCD8, iCD8, and total CD8, which were correlated with higher Ki67, TILs density, ER/PgR negativity, and higher histological grade. The 5year DFS and OS rates were 86.1% and 91.4%, respectively. Patients with high iCD8 and tCD8 had longer DFS and OS compared to those with low counts/mm2 (DFS: HR = 0.58, p = 0.011 and HR = 0.65, p = 0.034 and OS: HR = 0.63, p = 0.043 and HR = 0.58, p = 0.020, respectively). Upon adjustment for clinicopathological parameters, iCD8 and tCD8 retained their favorable prognostic significance for DFS and OS, whereas high sCD8 was only prognostic for DFS. Menopausal status, tumor size, and nodal status retained their prognostic significance in all examined multivariate models. CD8+ TILs, and especially their intratumoral subset, represent a potential favorable prognostic factor in early BC.
1. Introduction
Female breast cancer (BC) is the most commonly diagnosed cancer globally and represents the leading cause of cancer death among women [1]. Surgery, with the addition of adjuvant/neoadjuvant systemic therapy, is the gold standard for the treatment of early stage breast cancer and leads to an increase in the patients’ survival rate [2]. Traditionally, tumor grade, tumor size, lymph node status, estrogen (ER), progesterone (PgR), and human epidermal growth factor (HER2/neu or c-erbB2) receptor status have represented the main prognostic markers for BC [3]. Despite important improvements in early BC diagnosis and treatment, new biomarkers are needed, to further improve outcomes and reduce recurrence rates.
The tumor immune microenvironment has been reported to play a vital role in cancer spread, progression, and response to treatment [4], and several studies have investigated its role in BC [5,6]. Tumor infiltrating lymphocytes (TILs), an integral element of the tumor immune microenvironment, have been studied in several malignancies, concerning their prognostic/predictive value [7,8]. TILs and their subtypes have been widely investigated in the field of BC and have demonstrated a proven prognostic [9,10] and predictive role [11,12]. A major sub-population of TILs, cytotoxic CD8 + T cells, are reported to be a favorable prognostic factor [13,14], although their impact on prognosis seems to differ between the various clinicopathological subtypes of BC [15,16]. The location of TILs (stromal TILs (TILs) or intratumoral TILs (iTILs)) has also been reported to be an independent factor associated with BC outcomes. Similarly, the location of CD8+ TILs seems to play a prognostic role in certain types of BC [17].
Beyond prognosis, there has been a large research effort to establish TILs as predictive markers and establish their role in daily clinical practice. Losurdo et al. showed that low TILs negatively impact prediction of outcome in TNBC treated with immunotherapy. This information may suggest a need for induction chemotherapy before PD1/PD-L1 [18] inhibitors in this specific subpopulation. Another study suggested that TILs may be useful as a predictive marker of the therapeutic effect of eribulin chemotherapy in TNBC [19]. More recently, TILs have found their place in cancer treatment. Emerging data showed that adoptive cell therapy with TILs can be an effective treatment for metastatic melanoma [20]. Similarly, a recently published study demonstrated that cell therapy with autologous TILs may constitute a new treatment strategy in metastatic lung cancer [21].
Despite these meaningful advantages in the field, the prognostic or predictive value of TILs in BC remains debatable, due to the significant amount of heterogeneity in the experimental design. In particular, the variability of the methods and criteria used to quantify TILs and the interobserver differences between pathologists when evaluating iTILs constitute obstacles to the integration of TILs into clinical practice. In addition, the heterogeneity of BC makes the use of TILs much more complex [22,23,24].
The aim of the present study was to evaluate the prognostic role of CD8+ TILs within a Hellenic Cooperative Oncology Group study, along with a final report on the efficacy and safety data after a 10 year follow-up period.
2. Results
2.1. Patient and Tumor Characteristics
A total of 990 women were enrolled in the study between November 2007 and December 2010. Eleven patients were deemed ineligible and were excluded from the analysis (Figure 1), leading to a total of 979 eligible patients. Among these, 728 (74.4%) had consented to the use of their biological material for future research.
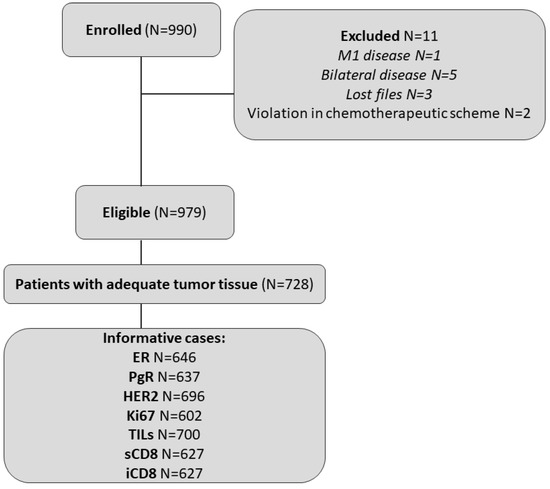
Figure 1.
REMARK diagram.
Selected patient and tumor characteristics are presented in Table 1. The median age at diagnosis was 54 years (range 28–83). About half of the women were postmenopausal (57.6%), had undergone modified radical mastectomy (55.7%), and carried tumors of 2.1–5 cm (51.7%). A central assessment of ER/PgR and HER2 status was available for 88.3% (n = 643) and 95.6% (n = 696) of patients with evaluable FFPEs, respectively. The discordance rate between the local and central assessment was 11.4% for ER/PgR and 8.9% for HER2. Luminal A tumors were classified in 24.5%, luminal B in 44.1%, luminal HER2 in 12.9%, HER2-enriched in 7.1%, and triple-negative (TNBC) in 11.4% of informative cases (n = 588), based on the central assessment. Additionally, CD8 data were available for 86.1% of patients with evaluable FFPEs (n = 627 patients). No significant differences were noted in basic patient and tumor characteristics between the total cohort of eligible patients (n = 979) and patients with evaluable FFPEs for translational research (n = 728).

Table 1.
Patient and tumor characteristics.
2.2. Drug Exposure
The relative dose intensities of all drugs were assessed in the 964 patients (98.5%) with available data who received the study treatment. Of note, five patients received docetaxel instead of paclitaxel, while one additional patient was treated with adriamycin instead of epirubicin. Lastly, another patient received epirubicin followed by the administration of doxorubicin.
The median relative dose intensity (RDI) administered for epirubicin and paclitaxel was 0.997 and 1.00, respectively. This was similar to previously published results by our group [25,26,27]. The median RDI for cyclophosphamide, fluorouracil, and methotrexate was 0.714, 0.714, and 0.785, respectively. Eight patients discontinued treatment with paclitaxel due to allergic reactions/intolerance and continued treatment with docetaxel. Treatment completion was achieved in 902 of 964 patients (93.6%). The main reasons for the discontinuation of study treatment included non-fatal toxicity (n = 35; 56.5%), followed by refusal to continue (n = 11; 17.7%), doctor’s decision (n = 7; 11.3%), moving to another hospital (n = 5; 8.1%), and other reasons (n = 4; 6.5%). Trastuzumab was administered in 214 of 225 patients (95.1%) with HER2-positive disease, according to the local assessment. Additionally, one patient with HER2-negative disease received trastuzumab, due to ambiguous CISH results.
2.3. Immunohistochemical Findings
The median number of sCD8 and iCD8 counts/mm2 was 113.7 and 3.7, respectively (Supplemental Figure S1). Of 627 tumors with available CD8 data, 156 (24.9%) had high expression of sCD8, iCD8, and tCD8 (Figure 2A–D). Patients carrying tumors with high counts/mm2 of all lymphocytic subsets (including stromal, intratumoral, and total CD8) had higher Ki67 levels (all p-values < 0.001) and higher TILs density (all p-values < 0.001). Additionally, tumors with high counts/mm2 of sCD8, iCD8, and tCD8 were more frequently ER/PgR-negative (all p-values < 0.001) and of higher histological grade (all p-values < 0.001) (Supplemental Table S2). TILs density was strongly, positively correlated with sCD8 (rho = 0.46, p < 0.001), iCD8 (rho = 0.50, p < 0.001), and tCD8 (rho = 0.50, p < 0.001). Additionally, a significant positive correlation was observed between TILs density and Ki67, which, however, was not considered strong (rho = 0.32, p < 0.001).
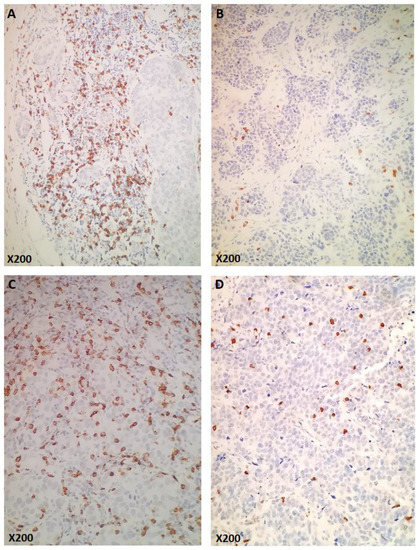
Figure 2.
Examples of CD8 immunohistochemical expression (original magnification as indicated). (A,B): Cases representing high (A) and low (B) numbers of stromal CD8 (sCD8+). (C,D): Cases representing high (C) and low (D) numbers of intratumoral (intraepithelial) CD8 (iCD8+).
2.4. Efficacy
At a median follow-up of 132.5 months (range 6.8–158.8), 253 DFS events (25.8%) had been recorded and 201 deaths (20.5%) had occurred. Information on the cause of death was available for 140 of 148 patients (94.6%) who died upon disease progression following adjuvant E-T-CMF chemotherapy, with 96.4% of them (n = 135) dying of their disease. No deaths were recorded during adjuvant chemotherapy. The median DFS and OS had not yet been reached at the data cut-off date for the analysis (15 June 2021). The 5-year DFS and OS rates were 86.1% (95% CI 84–88%) and 91.4% (95% CI 90–93%), respectively. Four patients (0.4%) developed myelodysplastic syndrome and 33 patients (3.4%) developed other cancers, including colorectal cancer (n = 1), endometrial cancer (n = 3), gastric cancer (n = 2), lung cancer (n = 5), breast cancer (n = 10), ovarian cancer (n = 3), pancreatic cancer (n = 3), melanoma of the skin (n = 3), sarcoma (n = 1), non-Hodgkin lymphoma (n = 1), and multiple myeloma (n = 1). In addition, two patients (0.2%) developed in situ breast carcinoma, seven months and 10 years post-completion of adjuvant chemotherapy, respectively.
Data regarding the site of progression were available for 163 of 185 patients (88.1%) who experienced disease progression. Distant relapses were noted in 151 patients (15.4% of the total cohort; 92.6% of progressors), while 19 patients (1.9% of the total cohort; 11.7% of progressors) presented locoregional relapses. Of note, six patients experienced both locoregional and distant relapses (Supplemental Table S3).
As expected, increasing age, postmenopausal status, radical mastectomy, increased tumor size and higher nodal status were associated with worse patient outcomes in terms of both DFS and OS. In addition, patients treated with adjuvant radiotherapy were at a significantly higher risk of death compared to those who did not receive radiation (Supplemental Table S4).
Patients with high counts/mm2 of iCD8 and tCD8 had longer DFS and OS compared to those with low counts/mm2 (DFS: HR = 0.58, 95% CI 0.39–0.88, p = 0.011 and HR = 0.65, 95% CI 0.44–0.97, p = 0.034 and OS: HR = 0.63, 95% CI 0.40–0.98, p = 0.043 and HR = 0.58, 95% CI 0.37–0.92, p = 0.020, respectively) (Figure 3).
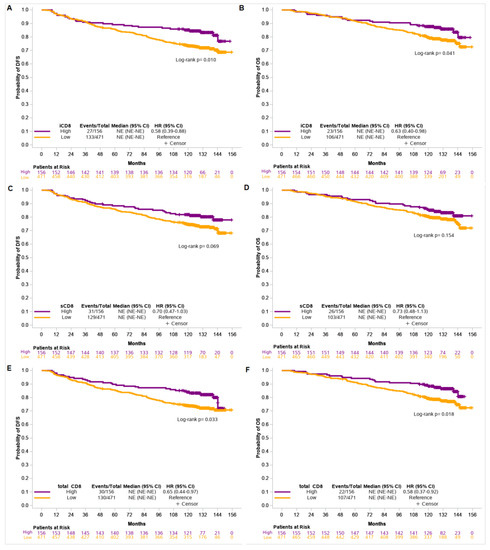
Figure 3.
Disease-free survival (DFS) and overall survival (OS) based on iCD8 (A,B), sCD8 (C,D), and total CD8 (E,F) counts/mm2 in the entire cohort of patients.
TIL density was not associated with patients’ outcome, while only a trend towards improved DFS was observed for tumors with high counts/mm2 of sCD8 (HR = 0.70, 95% CI 0.47–1.03, p = 0.070) (Supplemental Table S5).
Upon adjustment for selected clinicopathological parameters (as described in the statistical analysis section), both iCD8 and tCD8 retained their favorable prognostic significance for DFS and OS, whereas high counts/mm2 of sCD8 were significantly associated with prolonged DFS (Table 2). Of note, menopausal status, tumor size, and nodal status retained their prognostic significance for DFS and OS in all examined multivariate models.

Table 2.
Results of Cox multivariate regression models for DFS and OS in the entire cohort.
We further attempted to assess the prognostic significance of the markers of interest in the subgroups of patients defined by molecular subtype. However, due to the absence of, or the extremely small number of, events of interest in the category of patients with high counts/mm2 of sCD8, iCD8, and tCD8, the evaluation of the impact of CD8 expression on DFS and OS was only feasible among patients with luminal B tumors, but neither CD8 nor TIL density showed prognostic significance in this subpopulation (Supplemental Table S5).
2.5. Safety Profile
The safety profile was assessed in 944 patients with available data (96.4%). Among these, 226 patients reported a total of 325 grade 3–4 events (Table 3). No fatal adverse events were recorded. Neutropenia was the most commonly reported adverse event throughout the study treatment (65 grade 3–4 adverse events), followed by neuropathy (45 severe adverse events).

Table 3.
Incidence of grade 3 and 4 adverse events recorded throughout the study treatment among the 944 patients with available data.
3. Discussion
In the present study, we investigated the impact of CD8+ TIL density on the survival of patients with early BC treated with adjuvant chemotherapy and its correlation with classical clinicopathological characteristics. Simultaneously, we documented the first report of a HeCOG clinical study, regarding the efficacy and safety results after a 10-year follow-up period.
Patients enrolled in this study were treated with an E-T-CMF regimen followed as indicated by one year of trastuzumab treatment. This regimen was used in several studies by our group [25,26,27]; variations existed in the use of taxanes, as at the time of the design of this trial, the optimal dosing schemes and preferable type of taxanes were under investigation [28,29]. Although as dose-dense chemotherapy had already been shown to be superior to conventionally scheduled regimens [30,31], we decided to conduct chemotherapy on a biweekly schedule. Trastuzumab was given for one year in patients with HER2- positive tumors, as it had been recently correlated in several trials with survival benefit in this subgroup [32,33,34].
After a 10 year follow-up, it was found that a DFS event occurred in 25.8% of patients and that 20.5% of patients died of various reasons. The 5 year DFS and OS rates were 86.1% and 94.1%, respectively. These results were consistent with the efficacy data of other reported adjuvant trials by our research group [25,26,27] and with pivotal international randomized trials [30,35,36,37]. Similarly, the rates of secondary malignancies, sites of progression, and disease characteristics associated with worse outcomes were, to a large extent, as expected.
As mentioned above, in this trial, we investigated TIL density and the presence of CD8+ TILs in a series of BC patients, their correlation with specific clinicopathological parameters, and their correlation with patient outcomes. High CD8+ TIL counts, regardless of subtype, were associated with tumors demonstrating higher TIL density, higher ki67, and higher tumor grade. Κi67 index is not T cell specific but an indicator of proliferation activity of tumor cells. Therefore, a high Ki67 index provides an estimation of the growth fraction of tumor cells and reflects the ability of neoplastic cells to highly proliferate. In breast cancer, Ki67 immunohistochemical expression has been extensively investigated and is applied to assess the proliferative activity of cancer cells, taking account of breast cancer molecular subtyping. As it seems that a favorable prognostic factor correlates with poor prognostic parameters, our findings may, in fact, reflect that, in tumors with similar ki67 or the same histological grade, higher TILs can play a favorable prognostic role, if present. In the statistical analysis of this study, ki67 was not examined regarding survival and after multivariate regression, and histological grade was related with improved OS in high CD8+ tumors. The methodology we followed, in combination with the small size of CD8 positive tumors in our study, suggest that the prognostic significance of TILs between Ki67-high and Ki67-low breast cancers remains to be elucidated in a large series of patients. Similarly, negative hormone receptors were associated with higher counts of CD8+ TILs, reflecting the known distinct immunogenic properties of triple-negative and HER2-enriched breast cancers. These findings are consistent with the majority of the literature regarding correlations between TILs and clinicopathological parameters [38,39,40].
Regarding efficacy, this study confirmed the favorable role of high CD8 TILs in BC prognosis. The prognostic role of TILs in BC has been widely reported [41,42,43], with a proven impact on survival [44] and rates of complete response [45]. However, it is not yet clear which subsets of TILs contribute more to this outcome. TILs in BC are largely composed of CD8+ and, to a lesser extent, CD4+ T cells, macrophages, mast cells, regulatory T cells, and plasma cells [46]. CD8+ cytotoxic T cells are known to play a crucial role in the host’s adaptive immune response against cancer and kill cancer cells through several mechanisms [41,47]. In our study, patients with high counts of total CD8+ TILs had longer DFS and OS compared to those with low counts/mm2, reflecting their widely reported positive impact on patient outcomes [15,16].
In recent years, research efforts have focused on establishing the specific population of TILs that could more precisely, and with repeatability, predict clinical outcomes, as the cell type and location of TILs seem to be of great interest. Two TIL locations, stromal and intratumoral, are being investigated separately. This research field is where most discrepancies and ambiguous results appear. In the present study, patients with high counts/mm2 of iCD8 demonstrated DFS and OS benefits. This finding is in line with several published studies, including one by our research group, which studied BC patients in adjuvant settings [40,48,49] and contradicts the results of Catacchio et al. [38]. On the other hand, only a trend towards improved DFS was observed for tumors with high counts/mm2 of sCD8, despite the fact that in most published studies, this lymphocytic subset has been associated with a statistically significant survival benefit. These contradictory results can be explained by a variety of reasons, such as cancer heterogeneity, treatment setting, different methodology, and variability in patients’ populations between studies, along with interobserver discrepancies. In contrast to another published study of our group by Koletsa et al. [40], in the present study, sTIL density did not affect either DFS or OS.
In addition to the above-mentioned factors, the wide distribution of TILs counts/mm2, which led us and other researchers to use binary instead of continuous variables; the cell heterogeneity within a tumor specimen; the selection of tumor areas for evaluation; and the fact that TMAs represent a static snapshot of TILs, while the immune response in tumor stroma is a dynamic situation, are the main limitations of this study that should be considered.
An interesting observation of our study was that high CD8+ TILs are not prognostic in the first two to four years, and their favorable impact on DFS and OS appears later, as demonstrated in the Kaplan–Meier curves in Figure 2. At present, the relationship between intratumoral T cells numbers and patient prognosis is not simply a linear correlation, but rather a complicate phenomenon, in which T cells should be considered as a modifier of tumor growth. It has been shown in mice that T cells and tumor-reactive T cells are prone to gradient differentiation towards a dysfunctional state, and the degree of dysfunctionality may play a crucial role in immunosurveillance [50]. Moreover, Savas et al. [51], using single-cell RNA sequencing, demonstrated that the gene expression significance of CD8+ TRM (T cells with features of tissue -resident memory) was associated with improved survival of patients with breast cancer and specifically in those with a triple-negative phenotype. According to these factors, an explanation of the aforementioned findings could be the gradient degree of immunosurveillance of T cells on prognosis over time. Nevertheless, it could be due to other unidentified reasons or chance, given the relatively small number of patients. Another limitation of this study, which obstructed our attempt to assess the impact of the TIL subset on patient survival according to BC molecular subtypes, was the small number of events of interest in the category of patients with high counts/mm2 of sCD8, iCD8, and total CD8. Only the luminal B subgroup could be analyzed, but without statistically significant results. However, given the strong impact on DFS and OS that we found in the entire patient cohort, we could indirectly suggest that HER2-enriched and triple-negative subgroups were, to a large extent, present in these results. This conjecture is consistent with the known prognostic role of TILs in these subgroups [6,9,13,17].
Although the molecular subtypes did not differ in survival in our study, specific clinicopathological characteristics were associated with improved outcomes in favor of patients with high CD8+ TIL counts and regardless of subsets. These were predominantly tumor size, nodal status, and histological grade, likely following the reported knowledge that the more advanced and aggressive tumors offer a biological background for neoantigen production. This, in turn, enhances a tumor’s immunogenicity, and intratumoral/stromal lymphocytic infiltration is able to predict better responses [52,53]. The role of tumor size and nodal size in prognosis is a well-known issue, and its importance to the high expression of TILs has previously been emphasized in other reports [13,54]. Overall, as more potentially prognostic/predictive factors appear, we need to be cautious about prognosis assessment, as it is not clear if the presence of high TILs could overcome the inferior prognosis that nodal status or tumor size intimate. Even from a biological point of view, high TILs are not always correlated with better outcomes. For instance, it has been reported that CD4+ TILs may change from effectors to suppressors when cancer progresses. This process may coincide with a substantial reduction in antigen expression, resulting in tumor tolerance and, therefore, progression. This negative regulatory role of CD4+ TILs needs to be distinguished from the conventional role of activated CD4+ TILs [44].
As intensified CMF chemotherapy is obsolete in early breast cancer, the regimen used represents another limitation of this study, along with its non-randomized design. On the other hand, we consider the long-term follow-up and the fact that all samples were prospectively collected and centrally assessed as strong points of our study.
In regards to safety, 93.6% of patients completed study treatment with multiple reasons for discontinuation, and no treatment-related deaths were reported. Despite the prophylactic use of G-CSF after every chemotherapy cycle, neutropenia was the most common adverse event, followed by peripheral neuropathy and fatigue. These toxicity data were similar to those previously reported from other randomized adjuvant breast cancer clinical trials conducted by our group [25,26,55].
4. Materials and Methods
4.1. Patients
Eligible patients were at least 18 years old with node-positive early BC, or node-negative disease of intermediate risk according to the 2005 St. Gallen criteria [56]. The patients had undergone breast-conserving surgery or modified radical mastectomy with tumor-free margins, and had adequate hematologic, hepatic, and renal function, a performance status of 0 to 1 on the Eastern Cooperative Oncology Group (ECOG) scale, and presented no evidence of serious cardiac disease (normal left ventricular ejection fraction [LVEF] demonstrated by a multiple gated acquisition (MUGA) scan or echocardiogram was a mandatory procedure).
Before enrollment, each patient signed a written informed consent form for the use of their biological material for future research purposes.
All investigations conducted in this study complied with the principles expressed in the Declaration of Helsinki.
The study protocol was approved by the Bioethics Committee of the Aristotle University of Thessaloniki, School of Medicine (77/10.6.14), by the Institutional Review Board of Papageorgiou Hospital (180/15.7.13), and by the Institutional Review Board of Thermi Clinic (307/2.3.16). The study was registered in the Australian New Zealand Clinical Trials Registry (ANZCTR) and allocated the following Registration Number: ACTRN12615000161527.
Pre-treatment evaluation included medical history, physical examination, imaging examinations according to international guidelines, complete blood count (CBC), and comprehensive biochemistry. Blood tests were obligatory before each chemotherapy cycle and EF, after the completion of chemotherapy, and then every four months during treatment with trastuzumab. Additionally, blood examinations were performed whenever clinically indicated (e.g., in cases of fever over 38 °C, severe stomatitis or diarrhea).
4.2. Treatment
The chemotherapeutic regimen of the present study consisted of three cycles of epirubicin (E, 110 mg/m2) every 2 weeks, followed by 3 cycles of paclitaxel (T, 200 mg/m2) every 2 weeks, followed by 3 cycles of intensified CMF (cyclophosphamide 840 mg/m2, methotrexate 57 mg/m2 and fluorouracil 840 mg/m2) every 2 weeks (E-T-CMF) with G-CSF support. According to the treatment protocol, no patient received preoperative treatment.
Dose modifications were performed as previously described [21]. Toxicity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0. Patients with HER2-positive tumors received 52 weeks of trastuzumab intravenously, initially at a dose of 8 mg/kg as a loading dose, and subsequently 6 mg/kg every three weeks, following the delivery of the last cycle of chemotherapy.
Radiation therapy (RT) was planned for all patients who had undergone partial mastectomy or those with tumor size ≥5 cm and/or more than 4 positive lymph nodes, regardless the type of surgery (conservative or radical), 3–4 weeks following the completion of chemotherapy.
Hormonal therapy was administered to all patients with hormone receptor-positive tumors. Details regarding the administration of hormonotherapy were previously reported [57].
4.3. Immunohistochemistry (IHC)
The immunohistochemical staining of all markers was performed on 3 μm ΤΜA sections using a Bond Max autostainer (Leica Microsystems, Wetzlar, Germany), as previously described [58] and shown in Supplemental Table S1.
IHC for estrogen receptor (ER), progesterone receptor (PgR), HER2, Ki67 labelling index, cytokeratin 5 (CK5), and epidermal growth factor receptor (EGFR) was performed centrally at the Laboratory of Molecular Oncology of the Hellenic Foundation of Cancer Research, Aristotle University of Thessaloniki (Thessaloniki, Greece), as previously described [58]. The results were interpreted by experienced breast cancer pathologists and blinded to the patient’s demographic and clinical data. Tumors were also assessed for these basic pathological characteristics at the local laboratory of the center where each patient was enrolled.
Tumors were classified according to well-known immunohistochemical markers using the IHC4 model [58,59]. They were also categorized into five distinguished molecular subtypes, as previously reported [60,61].
Immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) protocols for ER, PgR, HER2, and Ki67 assessments have previously been described [58]. Briefly, HER2 was scored on a four-point scale from 0–3, with intense membrane staining in >30% invasive tumor cells classified as positive (3+ staining) [62]. Cut-offs for ER and PgR were set at 1% positive nuclei [63] and 14% for Ki67 [64]. The simultaneous staining of ER and PgR was considered as one parameter (hormone receptor status, HRS). Ki67 was scored as a continuous variable (% of positively stained nuclei); the highest score for each TMA core was recorded. FISH evaluation was performed in 20 tumor nuclei [65]. The HER2 gene was categorized as amplified for HER2/CEP17 ratios ≥2.2 [62], or for mean HER2 copy numbers (MB) >6 [66]. The Topoisomerase 2a/Centromere 17 (TOP2A/CEN17) ratio cut-off for TOP2A amplification was ≥2.0 [67]. Lymphocytes expressing CD8 were detected using the monoclonal mouse antihuman antibody CD8 (clone C8/144B, code M7103. dilution 1:80) according to the protocol of the manufacturer (Dako, Glostrup, Denmark).
4.4. Evaluation of CD8+ Lymphocytes
CD8+ lymphocytes were evaluated by two Pathologists without knowledge of the clinical, pathological, and survival data.
Immunostaining for CD8 was estimated in the tumor stroma (sCD8), as well as in the intratumoral/intraepithelial compartment where CD8+ cells were attached to malignant cells (iCD8). In addition, CD8+ cells within the tumor area (total CD8 (tCD8)) defined as the sum of sCD8 and iCD8 was also examined.
For each core (1.5 mm of diameter), CD8+ cells were counted in four fields of magnification (×200) covering the entire area of the core. Therefore, four values per core were recorded. In cases in which the distinction of sCD8 and iCD8 was ambiguous, CD8 positivity was estimated at higher magnification (×400). The density of CD8+ cells in each tumor compartment was assessed as the ratio of cell counts per mm2 surface. Therefore, the stromal tumor area, the tumor area occupied by the malignant cells and the total tumor area were recorded as % increments of the total core area on matched Hematoxylin and Eosin TMA sections. The surface of each compartment in mm2 was calculated based on the percentage of the recorded area % and the total core surface (1.76625 mm2 for 1.5 mm cores). Then, the stromal CD8+ cell count per core was divided by the respective stromal surface, the intratumoral CD8+ cell count by the respective malignant cell surface, and the total tumor cell count by the total tumor surface, to assess the density of CD8 positive cells [36,68]. Average values were recorded in cases of tumors which were evaluated on multiple cores. The obtained values were distributed in an extremely wide range, while multiple outliers were identified for each lymphocytic subset, accounting for a significant percentage of the total sample in each case. These outliers were natural (i.e., they could not be attributed to technical causes). The inclusion of outliers in the continuous lymphocytic subset variables contributed to skewed analyses that were statistically inaccurate. Omitting them from the analysis, however, would have led to misinterpretation of the results. Therefore, we considered the upper quartile (75th percentile) of each distribution to be an appropriate threshold for the classification of tumors into high and low counts/mm2.
4.5. Evaluation of TILs
Whole sections of hematoxylin and eosin stained slides were used to evaluate stromal TILs (sTILs) in accordance to the criteria proposed by the International TILs BC Working Group. The percentage of all mononuclear cells (including lymphocytes and plasma cells) in the stromal tumor component within the border of invasive carcinomas was recorded. Stromal TILs density was assessed as an average of all evaluated ×100 fields per tumor.
4.6. Follow-Up
All patients were followed up at study entry, every six months for the first five years and every year thereafter, with clinical examinations, CBC, biochemistry panels, serological markers, chest X-rays, and abdominal ultrasonography (or CT scans if clinically indicated). Mammography and ultrasonography of the patients’ breasts were performed annually. Bone scans were not routinely performed after the third year, except when clinically indicated.
4.7. Statistical Analysis
Disease-free survival (DFS), calculated from the date of study treatment initiation to the first locoregional/distant relapse, contralateral breast cancer, secondary neoplasm, death from any cause, or last contact, whichever occurred, was the primary endpoint of the study. Secondary endpoints included assessment of overall survival (OS), estimated as the time interval from treatment initiation until death (from any cause) or last contact; the safety profile of the study treatment; and the prognostic significance of the biomarkers of interest for the patient’s outcome.
Due to the wide range of the distribution of stromal CD8 (sCD8), intratumoral (iCD8), and total CD8 (tCD8) and the presence of multiple natural outliers that could not be excluded from the analysis (to avoid misinterpretation of the results), the third quartiles of the respective distributions were used as the cut-off points to dichotomize tumors into high and low counts/mm2. Descriptive statistics with counts (%) and medians (minimum, maximum) values were used to summarize patient and tumor characteristics and the biomarker distributions. Associations between sCD8, iCD8, and tCD8 with selected clinicopathological parameters were assessed using a chi-square test (for categorical variables) and the Wilcoxon rank-sum test (for continuous variables). Spearman correlations were used to evaluate the associations of TILs with Ki67, sCD8, iCD8, and tCD8.
OS and DFS survival rates were obtained via Kaplan–Meier analyses and compared between groups with a two-sided log-rank test. Univariate Cox regression models were applied to evaluate the effect of clinicopathological parameters of interest, CD8 and TILs on DFS and OS. In the multivariate analysis, the effect of each lymphocytic subset marker that was univariately associated with patient outcomes was adjusted for menopausal status (premenopausal, postmenopausal, tumor size (≤2 cm, 2.1–5 cm, >5 cm), nodal status (0–3, ≥4), histological grade (I–II, III), and adjuvant radiotherapy (yes, no). Age and the type of surgery were not included in the multivariate models, due to their correlation with menopausal status and nodal status, respectively. All statistical analyses were performed using the SAS software (SAS version 9.4, SAS Institute Inc. Cary, NC, USA). Statistical significance was set at a two-sided p of 0.050.
5. Conclusions
In conclusion, the present trial showed that dose-dense E-T-CMF is a tolerable chemotherapy regimen for adjuvant treatment of intermediate/high risk breast cancer, with an efficacy comparable to more recent dose-dense schemes. CD8+ TILs, and especially their intratumoral subset, represent a potential favorable prognostic factor and impact survival rates. Although quantifying TILs using histopathology and immunohistochemistry is the most used technique, a variety of methodological factors may confound the results and, therefore, the impact of TILs in prognosis. In addition, the quantification of TILs does not take into account the dynamics and functionality of the tumor microenvironment. Studies in various histological subtypes of breast cancer, along with strict laboratory procedures and the use of novel approaches, such as automated computational assessments, are needed, in order to better understand the role of TILs and their subsets in BC prognosis and treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://files.hecog.gr/Spathas_manuscript_Supplemental_.docx. The supplemental data presented in this study (Supplemental Figure S1 Distribution of sCD8, iCD8 and total CD8.; Supplemental Table S1. Staining protocols and manufacturers for IHC markers. ER1: citric acid-pH6, ER2: EDTA-pH 9, enz2: Proteinase K, RT: Room Temperature; Supplemental Table S2. Association of iCD8, sCD8 and tCD8 with selected clinicopathological parameters of interest; Supplemental Table S3. Sites of relapse in the entire cohort of patients; Supplemental Table S4. Association of clinicopathological parameters of interest with patient outcome; Supplemental Table S5. Association of sCD8, iCD8, and total CD8 with DFS and OS in the entire cohort of patients and among patients with luminal B tumors) are available in https://files.hecog.gr/Spathas_manuscript_Supplemental_.docx.
Author Contributions
Conceptualization N.S., A.C.G. and G.F.; formal analysis, G.-A.K.; investigation, A.C.G., A.B., A.V.C., A.P.-B., M.B., S.C., K.C., I.K., T.K. and P.A.; resources, N.S., H.G., F.Z., A.B., T.K., P.A., D.P., E.G., A.K. (Angelos Koutras), G.Z., E.S., D.B., C.K., I.B., G.A., A.P., E.R. (Evangelia Razis), A.K. (Anna Koumarianou), E.R. (Eleni Res), H.L. and G.F.; writing—original draft, N.S., A.C.G., G.-A.K. and G.F.; writing—review and editing: A.C.G., H.G., F.Z., A.K. (Anna Koumarianou), M.B., S.C., A.P., E.R. (Evangelia Razis) and G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hellenic Society for Medical Oncology (HeSMO) and by an internal Hellenic Cooperative Oncology Group research grant (HE10/08). The funders played no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study protocol was approved by the Bioethics Committee of the Aristotle University of Thessaloniki, School of Medicine (77/10.6.14), by the Institutional Review Board of Papageorgiou Hospital (180/15.7.13), and by the Institutional Review Board of Thermi Clinic (307/2.3.16). The study was registered in the Australian New Zealand Clinical Trials Registry (ANZCTR) and allocated the following Registration Number: ACTRN12615000161527
Informed Consent Statement
Each patient signed a written informed consent form for the use of their biological material for future research purposes. All investigations conducted in this study complied with the principles expressed in the Declaration of Helsinki.
Data Availability Statement
The data presented in this study are available in the article and supplementary material, while further details can be obtained on request from the corresponding author.
Acknowledgments
This work is dedicated with deep respect to the memory of our friend, mentor, and beloved collaborator George Stathopoulos, Medical Oncologist, full member of the Hellenic Cooperative Oncology Group. Part of this study was presented to the ESMO Breast Cancer Congress 3–5 May 2022 in Berlin, Germany. The authors are grateful to all patients and their families for their trust and participation in the study. The authors wish to thank Eneida Jaupaj for tissue sample collection and Maria Moschoni for coordinating the data management.
Conflicts of Interest
S.N.: Honoraria/Speaker: Roche, BMS, Astra-Zeneca, Travel: Sanofi, Amgen, Roche; H.G.: Advisory Role: Bristol-Myers Squibb, MSD Oncology, Amgen, Novartis, Roche, Pierre-Fabre, Honoraria: Bristol-Myers Squibb, MSD Oncology, Roche, Amgen, Novartis, Research Funding (institution): Bristol-Myers Squibb, Roche, MSD Oncology, Travel: Roche, Bristol-Myers Squibb; F.Z.: has received honoraria for lectures and has served in an advisory role for Astra-Zeneca, Daiichi, Eli-Lilly, Merck, MSD, Genesis-Pharma and Roche; D.P.: Advisory Role: Roche, MSD, Astellas. Honoraria: Roche, MSD, Astellas; A.K. (Angelos Koutras): Consulting or advisory role: Novartis, Roche, Genesis, Astra-Zeneca. Speaker’s bureau: GSK. Travel: Sanofi-Aventis, Astellas, Genesis, Amgen, BMS, Merck Serono; G.Z.: Honorary lectures: Amgen, Ipsen, Leo, Merck; G.A.: Advisory Boards: Novartis, BMS, Roche Hellas, Astra Zeneca, Sanofi, Amgen, Genesis Pharma, Merck, Pfizer; A.P.: Consulting or advisory role: Amgen, Merck Serono, Roche, BMS, Astra-Zeneca, MSD, Honoraria: Amgen, Merck Serono, Roche, BMS, Astra-Zeneca, MSD. Research funds: BMS Kura; E.R.: Consulting or advisory role: Novartis; Honoraria: Novartis; Travel: Genesis Pharmaceuticals, Pfizer, Roche, Bristol-Myers Squibb, Genekor; A.K. (Anna Koumarianou): Advisory Role: Genesis Pharma. Honoraria: Pfizer. Speaker’s bureau: Roche. Research Funding: Merck. Travel: MSD. Educational grants: Novartis, Pfizer, Merck, Roche, BMS, MSD, Genesis, and Ipsen; H.L.: Advisory/Consultation Fees from Roche, Astra Zeneca, Novartis, BMS, MSD, Pfizer, Amgen and Merck; G.F.: Advisory Board of Pfizer, Sanofi and Roche. Honoraria from Astra-Zeneca. Stock ownership: Genprex, Daiichi Sankyo, RFL Holdings, Formycon. The rest of the authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Schnitt, S.J. Classification and prognosis of invasive breast cancer: From morphology to molecular taxonomy. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2010, 23 (Suppl. 2), S60–S64. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Galon, J.; Dieu-Nosjean, M.C.; Tartour, E.; Sautès-Fridman, C.; Fridman, W.H. Immune infiltration in human tumors: A prognostic factor that should not be ignored. Oncogene 2010, 29, 1093–1102. [Google Scholar] [CrossRef]
- Katsuta, E.; Rashid, O.M.; Takabe, K. Clinical relevance of tumor microenvironment: Immune cells, vessels, and mouse models. Hum. Cell 2020, 33, 930–937. [Google Scholar] [CrossRef]
- Stovgaard, E.S.; Nielsen, D.; Hogdall, E.; Balslev, E. Triple negative breast cancer—Prognostic role of immune-related factors: A systematic review. Acta Oncol. 2018, 57, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Al-Shibli, K.I.; Donnem, T.; Al-Saad, S.; Persson, M.; Bremnes, R.M.; Busund, L.T. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin. Cancer Res. 2008, 14, 5220–5227. [Google Scholar] [CrossRef]
- Ancuta, E.; Ancuţa, C.; Zugun-Eloae, F.; Iordache, C.; Chirieac, R.; Carasevici, E. Predictive value of cellular immune response in cervical cancer. Rom. J. Morphol. Embryol. Rev. 2009, 50, 651–655. [Google Scholar]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.; Hitre, E.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.; Provenzano, E.; Dawson, S.J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef]
- Mao, Y.; Qu, Q.; Chen, X.; Huang, O.; Wu, J.; Shen, K. The Prognostic Value of Tumor-Infiltrating Lymphocytes in Breast Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0152500. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lachapelle, J.; Leung, S.; Gao, D.; Foulkes, W.D.; Nielsen, T.O. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012, 14, R48. [Google Scholar] [CrossRef]
- Miyashita, M.; Sasano, H.; Tamaki, K.; Hirakawa, H.; Takahashi, Y.; Nakagawa, S.; Watanabe, G.; Tada, H.; Suzuki, A.; Ohuchi, N.; et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: A retrospective multicenter study. Breast Cancer Res. 2015, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, X.; Zhou, E.; Chen, G.; Qian, K.; Wu, X.; Miao, X.; Tang, Z. Intratumoral CD8⁺ cytotoxic lymphocyte is a favorable prognostic marker in node-negative breast cancer. PLoS ONE 2014, 9, e95475. [Google Scholar] [CrossRef]
- Losurdo, A.; De Sanctis, R.; Fernandes, B.; Torrisi, R.; Masci, G.; Agostinetto, E.; Gatzemeier, W.; Errico, V.; Testori, A.; Tinterri, C.; et al. Insights for the application of TILs and AR in the treatment of TNBC in routine clinical practice. Sci. Rep. 2020, 10, 20100. [Google Scholar] [CrossRef]
- Kashiwagi, S.; Asano, Y.; Goto, W.; Takada, K.; Takahashi, K.; Noda, S.; Takashima, T.; Onoda, N.; Tomita, S.; Ohsawa, M.; et al. Use of Tumor-infiltrating lymphocytes (TILs) to predict the treatment response to eribulin chemotherapy in breast cancer. PLoS ONE 2017, 12, e0170634. [Google Scholar] [CrossRef]
- Kristensen, N.P.; Heeke, C.; Tvingsholm, S.A.; Borch, A.; Draghi, A.; Crowther, M.D.; Carri, I.; Munk, K.K.; Holm, J.S.; Bjerregaard, A.M.; et al. Neoantigen-reactive CD8+ T cells affect clinical outcome of adoptive cell therapy with tumor-infiltrating lymphocytes in melanoma. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Creelan, B.C.; Wang, C.; Teer, J.K.; Toloza, E.M.; Yao, J.; Kim, S.; Landin, A.M.; Mullinax, J.E.; Saller, J.J.; Saltos, A.N.; et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: A phase 1 trial. Nat. Med. 2021, 27, 1410–1418. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; Pruneri, G.; D’Alfonso, T.M.; Demaria, S.; Castaneda, C.; et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 2018, 52, 16–25. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Fountzilas, G.; Skarlos, D.; Dafni, U.; Gogas, H.; Briasoulis, E.; Pectasides, D.; Papadimitriou, C.; Markopoulos, C.; Polychronis, A.; Kalofonos, H.P.; et al. Postoperative dose-dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with high-risk operable breast cancer: A randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Ann. Oncol. 2005, 16, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Gogas, H.; Dafni, U.; Karina, M.; Papadimitriou, C.; Batistatou, A.; Bobos, M.; Kalofonos, H.P.; Eleftheraki, A.G.; Timotheadou, E.; Bafaloukos, D.; et al. Postoperative dose-dense sequential versus concomitant administration of epirubicin and paclitaxel in patients with node-positive breast cancer: 5-year results of the Hellenic Cooperative Oncology Group HE 10/00 phase III Trial. Breast Cancer Res. Treat 2012, 132, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, G.; Dafni, U.; Papadimitriou, C.; Timotheadou, E.; Gogas, H.; Eleftheraki, A.G.; Xanthakis, I.; Christodoulou, C.; Koutras, A.; Papandreou, C.N.; et al. Dose-dense sequential adjuvant chemotherapy followed, as indicated, by trastuzumab for one year in patients with early breast cancer: First report at 5-year median follow-up of a Hellenic Cooperative Oncology Group randomized phase III trial. BMC Cancer 2014, 14, 515. [Google Scholar] [CrossRef]
- Sparano, J.A.; Wang, M.; Martino, S.; Jones, V.; Perez, E.A.; Saphner, T.; Wolff, A.C.; Sledge, G.W., Jr.; Wood, W.C.; Davidson, N.E. Weekly paclitaxel in the adjuvant treatment of breast cancer. N. Engl. J. Med. 2008, 358, 1663–1671. [Google Scholar] [CrossRef]
- Martín, M.; Rodríguez-Lescure, A.; Ruiz, A.; Alba, E.; Calvo, L.; Ruiz-Borrego, M.; Munárriz, B.; Rodríguez, C.A.; Crespo, C.; de Alava, E.; et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by Paclitaxel for early breast cancer. J. Natl. Cancer Inst. 2008, 100, 805–814. [Google Scholar] [CrossRef]
- Citron, M.L.; Berry, D.A.; Cirrincione, C.; Hudis, C.; Winer, E.P.; Gradishar, W.J.; Davidson, N.E.; Martino, S.; Livingston, R.; Ingle, J.N.; et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J. Clin. Oncol. 2003, 21, 1431–1439. [Google Scholar] [CrossRef]
- Bayraktar, S.; Arun, B. Dose-dense chemotherapy for breast cancer. Breast J. 2012, 18, 261–266. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, D.; Wolff, A.C. The adjuvant treatment of HER2-positive breast cancer. Curr. Treat. Options Oncol. 2012, 13, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Moebus, V.; Jackisch, C.; Lueck, H.J.; du Bois, A.; Thomssen, C.; Kurbacher, C.; Kuhn, W.; Nitz, U.; Schneeweiss, A.; Huober, J.; et al. Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: Mature results of an AGO phase III study. J. Clin. Oncol. 2010, 28, 2874–2880. [Google Scholar] [CrossRef]
- Mamounas, E.P.; Bryant, J.; Lembersky, B.; Fehrenbacher, L.; Sedlacek, S.M.; Fisher, B.; Wickerham, D.L.; Yothers, G.; Soran, A.; Wolmark, N. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J. Clin. Oncol. 2005, 23, 3686–3696. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, L.; Ben-Aharon, I.; Vidal, L.; Gafter-Gvili, A.; Leibovici, L.; Stemmer, S.M. Dose-dense chemotherapy in nonmetastatic breast cancer: A systematic review and meta-analysis of randomized controlled trials. J. Natl. Cancer Inst. 2010, 102, 1845–1854. [Google Scholar] [CrossRef]
- Catacchio, I.; Silvestris, N.; Scarpi, E.; Schirosi, L.; Scattone, A.; Mangia, A. Intratumoral, rather than stromal, CD8+ T cells could be a potential negative prognostic marker in invasive breast cancer patients. Transl. Oncol. 2019, 12, 585–595. [Google Scholar] [CrossRef]
- Oshi, M.; Asaoka, M.; Tokumaru, Y.; Yan, L.; Matsuyama, R.; Ishikawa, T.; Endo, I.; Takabe, K. CD8 T Cell Score as a Prognostic Biomarker for Triple Negative Breast Cancer. Int. J. Mol. Sci. 2020, 21, 6968. [Google Scholar] [CrossRef]
- Koletsa, T.; Kotoula, V.; Koliou, G.A.; Manousou, K.; Chrisafi, S.; Zagouri, F.; Sotiropoulou, M.; Pentheroudakis, G.; Papoudou-Bai, A.; Christodoulou, C.; et al. Prognostic impact of stromal and intratumoral CD3, CD8 and FOXP3 in adjuvantly treated breast cancer: Do they add information over stromal tumor-infiltrating lymphocyte density? Cancer Immunol. Immunother. 2020, 69, 1549–1564. [Google Scholar] [CrossRef]
- Bos, R.; Marquardt, K.L.; Cheung, J.; Sherman, L.A. Functional differences between low- and high-affinity CD8(+) T cells in the tumor environment. Oncoimmunology 2012, 1, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; Sherman, L.A. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010, 70, 8368–8377. [Google Scholar] [CrossRef]
- Lin, G.; Fan, X.; Zhu, W.; Huang, C.; Zhuang, W.; Xu, H.; Lin, X.; Hu, D.; Huang, Y.; Jiang, K.; et al. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocyte in surgically resectable non-small cell lung cancer. Oncotarget 2017, 8, 83986–83994. [Google Scholar] [CrossRef]
- Yu, P.; Fu, Y.X. Tumor-infiltrating T lymphocytes: Friends or foes? Lab. Investig. 2006, 86, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Burugu, S.; Asleh-Aburaya, K.; Nielsen, T.O. Immune infiltrates in the breast cancer microenvironment: Detection, characterization and clinical implication. Breast Cancer 2017, 24, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Bense, R.D.; Sotiriou, C.; Piccart-Gebhart, M.J.; Haanen, J.; van Vugt, M.; de Vries, E.G.E.; Schroder, C.P.; Fehrmann, R.S.N. Relevance of Tumor-Infiltrating Immune Cell Composition and Functionality for Disease Outcome in Breast Cancer. J. Natl. Cancer Inst. 2017, 109, djw192. [Google Scholar] [CrossRef]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef]
- Gooden, M.J.; de Bock, G.H.; Leffers, N.; Daemen, T.; Nijman, H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br. J. Cancer 2011, 105, 93–103. [Google Scholar] [CrossRef]
- Rathore, A.S.; Kumar, S.; Konwar, R.; Makker, A.; Negi, M.P.; Goel, M.M. CD3+, CD4+ & CD8+ tumour infiltrating lymphocytes (TILs) are predictors of favourable survival outcome in infiltrating ductal carcinoma of breast. Indian J. Med. Res. 2014, 140, 361–369. [Google Scholar]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8(+) T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef]
- Savas, P.; Virassamy, B.; Ye, C.; Salim, A.; Mintoff, C.P.; Caramia, F.; Salgado, R.; Byrne, D.J.; Teo, Z.L.; Dushyanthen, S.; et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018, 24, 986–993. [Google Scholar] [CrossRef]
- De Laurentiis, M.; Cianniello, D.; Caputo, R.; Stanzione, B.; Arpino, G.; Cinieri, S.; Lorusso, V.; De Placido, S. Treatment of triple negative breast cancer (TNBC): Current options and future perspectives. Cancer Treat. Rev. 2010, 36 (Suppl. 3), S80–S86. [Google Scholar] [CrossRef]
- Thagaard, J.; Stovgaard, E.S.; Vognsen, L.G.; Hauberg, S.; Dahl, A.; Ebstrup, T.; Dore, J.; Vincentz, R.E.; Jepsen, R.K.; Roslind, A.; et al. Automated Quantification of sTIL Density with H&E-Based Digital Image Analysis Has Prognostic Potential in Triple-Negative Breast Cancers. Cancers 2021, 13, 3050. [Google Scholar] [CrossRef]
- Kotoula, V.; Chatzopoulos, K.; Lakis, S.; Alexopoulou, Z.; Timotheadou, E.; Zagouri, F.; Pentheroudakis, G.; Gogas, H.; Galani, E.; Efstratiou, I.; et al. Tumors with high-density tumor infiltrating lymphocytes constitute a favorable entity in breast cancer: A pooled analysis of four prospective adjuvant trials. Oncotarget 2016, 7, 5074–5087. [Google Scholar] [CrossRef]
- Fountzilas, G.; Dafni, U.; Gogas, H.; Linardou, H.; Kalofonos, H.P.; Briasoulis, E.; Pectasides, D.; Samantas, E.; Bafaloukos, D.; Stathopoulos, G.P.; et al. Postoperative dose-dense sequential chemotherapy with epirubicin, paclitaxel and CMF in patients with high-risk breast cancer: Safety analysis of the Hellenic Cooperative Oncology Group randomized phase III trial HE 10/00. Ann. Oncol. 2008, 19, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Glick, J.H.; Gelber, R.D.; Coates, A.S.; Thürlimann, B.; Senn, H.J. Meeting highlights: International expert consensus on the primary therapy of early breast cancer 2005. Ann. Oncol. 2005, 16, 1569–1583. [Google Scholar] [CrossRef]
- Zagouri, F.; Koliou, G.A.; Dimitrakopoulos, F.; Papadimitriou, C.; Binas, I.; Koutras, A.; Papakostas, P.; Markopoulos, C.; Venizelos, V.; Xepapadakis, G.; et al. Dose-dense sequential adjuvant chemotherapy in the trastuzumab era: Final long-term results of the Hellenic Cooperative Oncology Group Phase III HE10/05 Trial. Br. J. Cancer 2022, 127, 695–703. [Google Scholar] [CrossRef]
- Fountzilas, G.; Dafni, U.; Bobos, M.; Batistatou, A.; Kotoula, V.; Trihia, H.; Malamou-Mitsi, V.; Miliaras, S.; Chrisafi, S.; Papadopoulos, S.; et al. Differential response of immunohistochemically defined breast cancer subtypes to anthracycline-based adjuvant chemotherapy with or without paclitaxel. PLoS ONE 2012, 7, e37946. [Google Scholar] [CrossRef][Green Version]
- Cuzick, J.; Dowsett, M.; Pineda, S.; Wale, C.; Salter, J.; Quinn, E.; Zabaglo, L.; Mallon, E.; Green, A.R.; Ellis, I.O.; et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J. Clin. Oncol. 2011, 29, 4273–4278. [Google Scholar] [CrossRef] [PubMed]
- Bustreo, S.; Osella-Abate, S.; Cassoni, P.; Donadio, M.; Airoldi, M.; Pedani, F.; Papotti, M.; Sapino, A.; Castellano, I. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: A large case series study with a long-term follow-up. Br. Cancer Res. Treat. 2016, 157, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Bravaccini, S.; Bronte, G.; Scarpi, E.; Ravaioli, S.; Maltoni, R.; Mangia, A.; Tumedei, M.M.; Puccetti, M.; Serra, P.; Gianni, L.; et al. The impact of progesterone receptor expression on prognosis of patients with rapidly proliferating, hormone receptor-positive early breast cancer: A post hoc analysis of the IBIS 3 trial. Ther. Adv. Med. Oncol. 2020, 12, 1758835919888999. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef]
- Cheang, M.C.; Chia, S.K.; Voduc, D.; Gao, D.; Leung, S.; Snider, J.; Watson, M.; Davies, S.; Bernard, P.S.; Parker, J.S.; et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J. Natl. Cancer Inst. 2009, 101, 736–750. [Google Scholar] [CrossRef]
- Press, M.F.; Sauter, G.; Buyse, M.; Bernstein, L.; Guzman, R.; Santiago, A.; Villalobos, I.E.; Eiermann, W.; Pienkowski, T.; Martin, M.; et al. Alteration of topoisomerase II-alpha gene in human breast cancer: Association with responsiveness to anthracycline-based chemotherapy. J. Clin. Oncol. 2011, 29, 859–867. [Google Scholar] [CrossRef]
- Vanden Bempt, I.; Van Loo, P.; Drijkoningen, M.; Neven, P.; Smeets, A.; Christiaens, M.R.; Paridaens, R.; De Wolf-Peeters, C. Polysomy 17 in breast cancer: Clinicopathologic significance and impact on HER-2 testing. J. Clin. Oncol. 2008, 26, 4869–4874. [Google Scholar] [CrossRef] [PubMed]
- Knoop, A.S.; Knudsen, H.; Balslev, E.; Rasmussen, B.B.; Overgaard, J.; Nielsen, K.V.; Schonau, A.; Gunnarsdóttir, K.; Olsen, K.E.; Mouridsen, H.; et al. retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group. J. Clin. Oncol. 2005, 23, 7483–7490. [Google Scholar] [CrossRef] [PubMed]
- Beguinot, M.; Dauplat, M.M.; Kwiatkowski, F.; Lebouedec, G.; Tixier, L.; Pomel, C.; Penault-Llorca, F.; Radosevic-Robin, N. Analysis of tumour-infiltrating lymphocytes reveals two new biologically different subgroups of breast ductal carcinoma in situ. BMC Cancer 2018, 18, 129. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).