Simple Summary
Distal pancreatectomy has become the standard surgery for patients with pancreatic body–tail cancers. The optimal area for dissection remains controversial, and there is an emphasis on preserving as much tissue as possible while eradicating the cancer. This multicenter, retrospective study evaluated the frequency and patterns of lymph node metastasis based on tumor site among 235 patients with pancreatic body–tail cancers to determine the optimal lymph node dissection area for distal pancreatectomy. Patients with pancreatic body cancer tumors showed no metastasis to the splenic hilum lymph node. Spleen-preserving pancreatectomy might be feasible for Pb cancer.
Abstract
Distal pancreatectomy (DP) with lymphadenectomy is the standard surgery for pancreatic body–tail cancer. However, the optimal lymph node (LN) dissection area for DP remains controversial. Thus, we evaluated the frequency and patterns of LN metastasis based on the tumor site. In this multicenter retrospective study, we examined 235 patients who underwent DP for pancreatic cancer. Tumor sites were classified as confined to the pancreatic body (Pb) or pancreatic tail (Pt). The efficacy index (EI) was calculated by multiplying the frequency of metastasis to each LN station by the five-year survival rate of patients with metastasis to that station. LN metastasis occurred in 132/235 (56.2%) of the patients. Patients with Pb tumors showed no metastasis to the splenic hilum LN. Distal splenic artery LNs and anterosuperior/posterior common hepatic artery LNs did not benefit from dissection for Pb and Pt tumors, respectively. In multivariate analysis, splenic artery LN metastasis was identified as an independent predictor of poor overall survival in patients with pancreatic body–tail cancer. In conclusion, differences in metastatic LN sites were evident in pancreatic body–tail cancers confined to the Pb or Pt. Spleen-preserving pancreatectomy might be feasible for Pb cancer.
1. Introduction
Surgical resection for pancreatic ductal adenocarcinoma (PDAC) provides a chance for long-term survival; however, the survival after curative pancreatectomy is not long enough [1,2]. The treatment for PDAC is shifting away from surgery alone to include multidisciplinary treatments, such as neoadjuvant or adjuvant therapy, to improve prognosis [2,3]. From this perspective, early introduction of adjuvant treatment and improvement of postoperative tolerability are important to enhance the synergistic effect of surgical and nonoperative treatments, such as chemotherapy. In general, major surgery induces tissue damage followed by an extensive immunosuppressive response. Intraoperative blood transfusion further augments the predominately immunosuppressive response seen secondary to tissue damage [4]. Perioperative blood transfusion following pancreatectomy for PDAC is associated with poor short- and long-term outcomes [5]. Immune suppression following surgery has been implicated in increased short-term postoperative complications, especially infectious complications [4]. Therefore, treatment of PDAC may require surgery that does not induce a postoperative immunosuppressive response. Although organ-preserving surgery for PDAC has not yet been reported, it is expected to be a key factor in the surgical treatment of PDAC.
In general, the standard operation for PDAC is thought to be pancreatectomy with lymphadenectomy, which involves dissecting an area based on lymphatic flow from the primary tumor. The recommended extent of lymph node (LN) dissection for distal pancreatectomy (DP) has been defined in the General Rules for the Study of Pancreatic Cancer (7th edition) [6] and the consensus statement by the International Study Group on Pancreatic Surgery (ISGPS) [7]. However, unlike pancreatoduodenectomy, no clinical studies have considered the optimal lymphadenectomy for DP, which remains controversial [8,9,10,11,12].
We have shown the difference in favorable LN metastasis based on tumor site in a single institution [13]. We found that patients with pancreatic body cancer had no metastasis to splenic hilar LNs. In pancreatic body or tail cancers that are eligible for DP, metastasis to LN stations far from the primary tumor, including regional LN stations, is rare. The purpose of the present multicenter study was to evaluate the difference in the frequency of LNs metastasis based on the primary tumor site.
2. Materials and Methods
2.1. Patients’ Selection
This multicenter retrospective cohort study was conducted by the Hokkaido Pancreatic Cancer Study Group (HOPS). All patients who underwent DP for PDAC between January 2001 and July 2017 at tertiary referral hospitals in Hokkaido Prefecture were enrolled. We excluded patients with intraepithelial cancer (Tis), acinar cell carcinoma, and anaplastic carcinoma. The institutional review boards of Hokkaido University Hospital (018-0317) and each participating hospital approved the study protocol, and the requirement for informed consent was waived. Comprehensive informed consent was obtained from all participants before surgery using the patient information of this study.
2.2. Operation Procedure
DP and D2 dissections were performed as standard procedures. In cases where the tumor was located close to the celiac axis (CA), DP with celiac axis resection (DP-CAR) was performed [14,15]. The indications for DP-CAR were (1) tumors contacting with the common hepatic artery (CHA) and/or the CA, with a cancer-free margin of at least 5–7 mm ligated at its origin from the aorta, and (2) tumors close to the root of the splenic artery within 10 mm that don’t contact with either the CHA or CA [13].
2.3. The Definition of Tumor Location
The tumor site was categorized by preoperative multidetector-row computed tomography and/or ultrasonography (US). The distal pancreas is defined as the left border of the superior mesenteric vein (SMV) and portal vein (PV), while a line bisecting the distal pancreas was considered the border between the body and tail of the pancreas [16]. The pancreatic neck is included in the pancreatic body in this study, which is located anterior to the SMV/PV and to the left side of the gastroduodenal artery. The tumor sites were classified as Pb (pancreatic body), Pbt (both the body and tail of the pancreas), or Pt (pancreatic tail) (Figure 1). The pathological stage, number of LNs, and LN dissection criteria were determined according to the General Rules for the Study of Pancreatic Cancer (6th edition) [16]. The number of LNs was defined as follows: LN in the anterosuperior group along the CHA (#8a), LN in the posterior group along the CHA (#8p), LN in the splenic hilum (#10), LN around the proximal splenic artery (#11p), LN around the distal splenic artery (#11d), LN around the proximal superior mesenteric artery (#14p), LN in the para-aorta region (from the lower margin of the left renal vein to the upper margin of the inferior mesenteric artery) (#16b1), and LN along the inferior margin of the pancreas (#18) (Figure 1). Specimens were cut into 5 mm thickness /section on a sagittal plane, mounted on slides, and stained with hematoxylin and eosin. The location of the LN metastasis was confirmed by pathological reports with details of the metastatic sites.
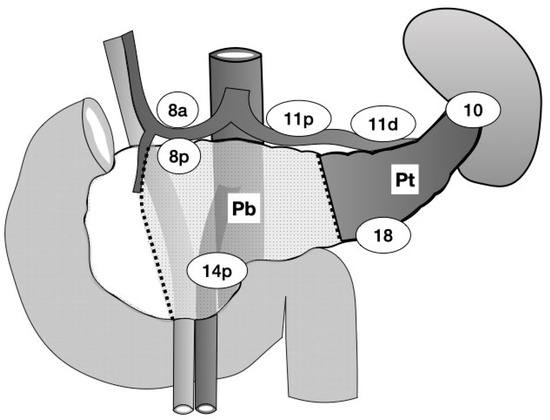
Figure 1.
The dotted line indicates the border of each location. The dark area indicates the pancreatic tail (Pt) tumor site. The light-dotted area indicates the site of the pancreatic body (Pb) tumor. Pbt tumors spread to both the Pb and Pt sites. The number in the circle indicates the name of lymph node station according to the Japanese Classification of Gastric Carcinomas.
2.4. The Efficacy Index Assessment for Each Nodal Station
We analyzed the relationship between the LN metastasis at each station and the prognosis using the efficacy index proposed by Sasako [17]. The efficacy index was a measure of survival benefit calculated by multiplying the frequency of metastasis to the station by the 5-year survival rate and 2-year disease-free survival rate of patients who metastasized to that station.
2.5. Multidisciplinary Treatment
Neoadjuvant treatment (NAT) has been administered through clinical studies since 2014, although there are differences in participation criteria among institutions. The use of adjuvant therapy depended on the treatment policy at each institution and on the patient’s postoperative condition. Adjuvant therapies, such as gemcitabine or S-1, were initiated in clinical studies in 2007, and S-1 was established as a standard adjuvant therapy in 2012.
2.6. Follow-Up and Recurrent Data
Postoperative follow-up investigations consisted of a physical examination, laboratory studies, and CT imaging at 3- to 4-month intervals for the first two years, at 6-month intervals for 3–5 years, and then at yearly intervals thereafter. Recurrent patterns and survival data were retrospectively collected in December 2019.
2.7. Statistical Analysis
Continuous variables are expressed as medians and interquartile ranges. Categorical variables are expressed as numbers and percentages. For comparisons of categorical data, the χ2 test or Fisher’s exact test was used. The Mann–Whitney U test was used to compare continuous data. The Wilcoxon test was used to compare the three groups. Overall patient survival (OS) was calculated from the date of surgery to the date of the last follow-up or the date of patient death. Disease-free survival (DFS) was calculated from the date of surgery to the date of the last follow-up or confirmation of recurrence. The Kaplan–Meier method was used to estimate OS and DFS, and survival differences were analyzed using the log-rank test based on the comparison of tumor site and pathological node category. The stepwise selection was performed based on Akaike’s information criterion (AIC), which included factors that were statistically significant in the univariate analysis (p < 0.1), and a multivariate analysis was performed using the Cox proportional hazard model. A p significance was set at p < 0.05. The JMP Pro 16.0 statistical software program (JMP Japan, Tokyo, Japan) was used for all analyses.
3. Results
3.1. Characteristics of the Entire Population
In total, 235 patients who underwent DP were enrolled. The distribution of patients is shown in Table 1. Fifty-six patients were enrolled from Hokkaido University, 59 from Sapporo Medical University, 101 from Teine-Keijinkai Hospital, and 19 from Kin-ikyo Chuo Hospital. The clinical and pathological characteristics of the patients were compared among the primary tumor sites. LN metastasis occurred in 132/235 (56.2%) patients, with 56 (51.4%), 29 (56.9%), and 47 (62.7%) tumors located in the Pb, Pbt, and Pt, respectively. NAT was administered to 43 (18.3%) patients. The patients who were administered the NAT treatment did not differ significantly between tumor sites.

Table 1.
Demographic and surgical characteristics of patients who underwent distal pancreatectomy for pancreatic cancer.
The operation time was longer in patients with Pb tumors than in those with Pt tumors (p = 0.042), but the Pt tumors were larger than the Pb tumors (p = 0.040). The rates of pPV and pOO positivity were higher for Pt tumors than they were for Pb tumors (p = 0.015 and p < 0.001, respectively). The number of harvested LNs was higher in patients with Pb tumors than in those with Pt tumors (p = 0.022).
Regarding the characteristics of the patients with Pbt tumors, the proportion of male patients was significantly higher compared with that in the other tumor types (p = 0.023). Patients with Pbt tumors underwent DP-CAR more frequently than those with Pb or Pt tumors (p < 0.01). Patients with Pbt tumors had a longer operation time, larger blood loss, higher incidence of CD IIIa or more, and longer postoperative hospital stays (p < 0.01, p < 0.01, p = 0.021, and p = 0.003, respectively) than did those with other tumor types. Regarding the pathological findings, the Pbt tumor was larger than the other tumor categories (p < 0.01). The rate of positive pPV was higher for Pbt tumors than for other tumors (p = 0.04).
3.2. The Pattern of LN Metastasis According to Tumor Site
Table 2 shows the frequency of LNs metastasis based on the tumor site. LN metastases to #11d, #11p, and #18 were found in >15% of all patients. In addition, >10% of the patients with Pb tumors had #8a/p LN metastasis, while those with Pt tumors had #10 LN metastasis. The efficacy index was calculated using the 5-year OS rate (Table 2) and 2-year DFS rate (Table S1).

Table 2.
Distribution of lymph node metastasis based on tumor site and efficacy index according to the 5-year survival rate.
Patients with Pb tumors had no metastases to the #10 LNs. Five-year survivors did not have Pb tumors with metastatic spread to #11d LNs. Regarding the efficacy index assessment, #8a/p, 11p, #14p, and #18 LNs benefited from LN dissection in Pb tumors, whereas in #11d LNs, the 5-year OS index was 0 points and the 2-year DFS index was very small (0.92). Therefore, metastasis to #11d LN considerably worsened the prognosis of patients with Pb tumors.
In Pt tumors, five-year survivors did not have metastatic spread to #8a/p LNs. Regarding the efficacy index assessment, both the 5-year OS index and 2-year DFS index were 0 points in #8a/p LNs. Further, #8a/p LNs did not benefit from LN dissection, even when including regional LNs because of the poor prognosis equivalent to distant metastasis as para-aortic #16b1 node metastasis.
3.3. Long-Term Outcomes
In the 235 patients, 18 clinicopathological variables related to OS were analyzed using the Cox proportional hazard model. In the univariate analyses, 10 of the 18 variables were statistically significant (Table 3). For multiple analyses, a stepwise selection was performed based on the AIC. When portal venous system invasion, invasion of other organs, and #11p and #18 LN metastasis were excluded, and eight factors were selected, the AIC value was minimized (1247.96). In a multivariate analysis, the following four factors were identified as independent poor prognostic factors: preoperative CA19-9 values >100 U/mL, tumor size >20 mm, #11d LN metastasis, and no adjuvant treatment (Table 3).

Table 3.
Univariate and multivariate analyses of the influence of various factors on overall survival.
DFS and OS are shown in Figure 2. The median DFS and OS were 725 and 1298 days, respectively. The five-year DFS and OS rates were 30.5% and 32.2%, respectively (Figure 2). Patients with any LNs metastases and those with #11d LNs metastasis had a poorer prognosis (p < 0.01, and p < 0.01, respectively) than those without (Figure 2). The median OS was 606 days in patients with #11d LNs metastasis.
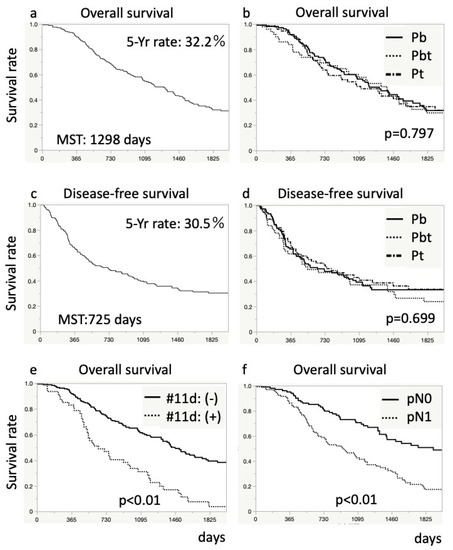
Figure 2.
Kaplan–Meier survival curves for patients after distal pancreatectomy. The upper graphs (a,b) show overall survival rate, and the middle graphs (c,d) show disease-free survival rate. The graphs (a,c) present survival rate for all patients. The graphs (b,d) show a comparison in survival rates based on the tumor site. The lower graphs (e,f) show an overall comparison of survival rates based on lymph node metastasis: (e), pathological node category; and (f), #11d lymph node metastasis. Differences in survival were analyzed using log-rank tests.
4. Discussion
The frequency of LN metastasis after DP for pancreatic body and tail cancers was summarized in this multicenter study. Our study showed that metastasis to #11 and #18 LNs, which are generally defined as the region of D1 LN dissection, occurred in more than 10% of the patients. In the analysis by tumor localization, no patient with Pb cancer was observed to have metastasis to #10 or #11d LNs, and those with Pt cancer did not have metastasis to #8a/p LNs, demonstrating a zero-efficacy index despite regional LNs. Patients with #11d LNs metastasis had a poor prognosis as an independent poor prognostic factor.
The poor prognostic factors in our study were similar to those of previous reports [1,2,18,19,20]; the preoperative CA19-9 value and pN were previously identified as a biological and pathological poor prognostic factor, respectively. In this study, #11d LN metastasis was an independent poor prognostic factor. Therefore, patients with #11d LN metastasis after DP would require more intense postoperative therapy. Adjuvant therapy was also identified as an independent prognostic factor in this study. However, caution must be used when interpreting these data considering the recent advancement of therapeutic agents after recurrence because many of the patients did not receive adjuvant therapy in the past.
This study was conducted at multiple institutions to validate the results of a previous study performed in a single-center study conducted at Hokkaido University [13]. The major difference between the previous study and the present study is the definition of the boundary between the pancreatic body and tail. The boundary in the previous study was set at the left border of the aorta as defined by the General Rules for the Study of Pancreatic Cancer (7th edition) [6]. On the other hand, in the present study, the boundary was set as a line bisecting the distal pancreas from the left border of the SMV/PV as defined by the General Rules for the Study of Pancreatic Cancer (6th edition) [16]. Although patient-dependent, the boundary of this study is a few centimeters closer to the splenic hilum than to the left border of the aorta. By setting the boundary between the pancreatic body and tail more toward the spleen, we would like to ensure that there is no LN metastasis at the splenic hilum, even when the tumor is located closer to the splenic hilum.
The most frequent sites of LN metastasis were in the D1 dissection area, relatively close to the tumor, as described in the General Rules for the Study of Pancreatic Cancer (6th edition) [16]. Although the recommended extent of dissection is the same for tumors in both the body and tail of the pancreas in the rules, our study demonstrated a large difference in LN metastasis sites between tumors confined to the pancreatic body and those confined to the pancreatic tail. In our analysis using the efficacy index proposed by Sasako [17], we found that #11d LNs for Pb cancer and #8 LNs for Pt cancer had an efficacy index of zero despite being a regional LN. Metastasis to these lymph nodes suggests a poor prognosis, equivalent to distant metastasis, such as para-aortic #16b1 node metastasis. Therefore, more intensive preoperative chemotherapy is required when those LN metastases are suspected radiologically or proved pathologically. In addition, no patients with Pb cancer had LN metastasis to the splenic hilum, which leads us to surmise that the splenic hilum LN (#10) may not need to be dissected. Although our previous study [13] and other studies in Japan, Korea, and France [21,22,23] have reported about the splenic hilum LN after distal pancreatectomy, this is the first multicenter report to describe the detailed frequency of lymph node metastasis with a clearly defined tumor localization.
As a surgical strategy for pancreatic body cancer, spleen-preserving DP (Warshaw procedure) as an organ-preserving surgery is also an option because there is no LN metastasis to the splenic hilum. The merits of spleen-preserving surgery include conservation of splenic function, such as avoidance of the long-term risk of post-splenectomy sepsis related to encapsulated bacteria [24]. The risk of overwhelming post-splenectomy infection (OPSI) is estimated to be 1 per 400–500 patient-years, and that of fatal OPSI is 1 per 800–1000 patient-years [25]. Aiolfi et al. [26] reported that splenectomy for gastric cancer is significantly associated with postoperative infectious complications and overall morbidity. The risk of thromboembolic complications and OPSI, a rare but potentially lethal complication, increases with splenectomy. However, the operation technique to preserve blood flow around the splenic hilum could be difficult due to variations in vessels, inflammation, or obesity.
DP-CAR is often performed in patients with locally advanced pancreatic cancers. Ischemic gastropathy is sometimes a problematic postoperative complication of DP-CAR [27,28]. This ischemic gastropathy has been reported to occur particularly in the posterior wall of the fornix [29]. Left gastric artery preservation or arterial and venous reconstruction is useful for ischemic gastropathy, combining intraoperative ICG to confirm the blood flow after reconstruction; however, issues, such as congestion or early arterial occlusion after reconstruction, remain. One possible cause of ischemic gastropathy is the division of the short gastric artery. It is assumed that the blood flow of the fornix is relatively weak, with only the supply from the capillaries flowing within the stomach. The spleen-preserving DP-CAR (Warshaw procedure) for locally advanced pancreatic body cancer may be a technique that can solve the disadvantages of DP-CAR by avoiding ischemia of the fornix to preserve the connecting tissue, including vessels around the splenic hiatus, such as the short gastric artery and vein and left gastroepiploic artery and vein.
This study has several limitations, including its retrospective nature. It is unclear whether the omission of LN dissection based on tumor localization is oncologically justified, and the feasibility of the spleen-preserving procedure requires further clarification. Thus, as a next step, additional validation needs to be performed in a prospective trial. The laboratory data representative of immune response and nutritional status should be included in the multivariate analysis of long-term outcomes, but the wide variation among centers and time periods made analysis difficult for the multicenter retrospective study. Even though the patient selection differed from our previous study [13], such as the definition of the boundary between the pancreatic body and tail, and the inclusion of NAT patients, 42 patients were in both this and the previous study. The efficacy index assessment could be overestimated in the lymph node station with low rate of metastasis.
5. Conclusions
Differences in the metastatic LN sites were evident in pancreatic body-tail cancer when tumors were confined to the left or right side of a line bisecting the distal pancreas from the left border of the SMV/PV. In pancreatic body cancer, no patient had #10 LN metastases, suggesting that spleen-preserving pancreatectomy is feasible for pancreatic body cancer. Finally, #11d LNs metastasis was an independent poor prognostic factor.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14184409/s1, Table S1: Distribution of lymph node metastasis based on tumor site and efficacy index according to the 2-year disease-free survival rate.
Author Contributions
Conceptualization, K.T., Y.K. and T.N.; methodology, K.T., Y.K. and T.N.; data curation, T.H., S.Y., K.U. and T.M.; writing—original draft preparation, K.T.; writing—review and editing, K.T.; supervision, Y.K., T.N., Y.A., T.A. and S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Hokkaido University Hospital (018-0317).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study. Comprehensive informed consent was obtained from all participants before surgery using the patient information of this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The members of Hokkaido Pancreatic Cancer Study Group (HOPS) were supported for scientific advice and providing data on the patients.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zülke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef]
- Motoi, F.; Kosuge, T.; Ueno, H.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol. 2019, 49, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Torrance, H.D.; Pearse, R.M.; O’Dwyer, M.J. Does major surgery induce immune suppression and increase the risk of postoperative infection? Curr. Opin. Anaesthesiol. 2016, 29, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Livingston, E.H.; Myers, B.; Hines, O.J. The Effect of Perioperative Blood Transfusion on Long-Term Survival Outcomes After Surgery for Pancreatic Ductal Adenocarcinoma: A Systematic Review. Pancreas 2021, 50, 648–656. [Google Scholar] [CrossRef]
- Japan Pancreas Society. Classification of Pancreatic Carcinoma, 4th ed.; Kanehara: Tokyo, Japan, 2016. [Google Scholar]
- Tol, J.A.M.G.; Gouma, D.J.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bockhorn, M.; Büchler, M.W.; et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014, 156, 591–600. [Google Scholar] [CrossRef]
- Pedrazzoli, S.; DiCarlo, V.; Dionigi, R.; Mosca, F.; Pederzoli, P.; Pasquali, C.; Klöppel, G.; Dhaene, K.; Michelassi, F. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: A multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann. Surg. 1998, 228, 508–517. [Google Scholar] [CrossRef]
- Yeo, C.J.; Cameron, J.L.; Lillemoe, K.D.; Sohn, T.A.; Campbell, K.A.; Sauter, P.K.; Coleman, J.; Abrams, R.A.; Hruban, R.H. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: Randomized controlled trial evaluating survival, morbidity, and mortality. Ann. Surg. 2002, 236, 355–368. [Google Scholar] [CrossRef]
- Farnell, M.B.; Pearson, R.K.; Sarr, M.G.; DiMagno, E.P.; Burgart, L.J.; Dahl, T.R.; Foster, N.; Sargent, D.J.; Pancreas Cancer Working Group. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery 2005, 138, 618–628, discussion 628–630. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kang, M.J.; Heo, J.S.; Choi, S.H.; Choi, D.W.; Park, S.J.; Han, S.S.; Yoon, D.S.; Yu, H.C.; Kang, K.J.; et al. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann. Surg. 2014, 259, 656–664. [Google Scholar] [CrossRef]
- Nimura, Y.; Nagino, M.; Takao, S.; Takada, T.; Miyazaki, K.; Kawarada, Y.; Miyagawa, S.; Yamaguchi, A.; Ishiyama, S.; Takeda, Y.; et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: Long-term results of a Japanese multicenter randomized controlled trial. J. Hepato-Biliary-Pancreat. Sci. 2012, 19, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nakamura, T.; Asano, T.; Nakanishi, Y.; Noji, T.; Tsuchikawa, T.; Okamura, K.; Shichinohe, T.; Hirano, S. Pancreatic body and tail cancer and favorable metastatic lymph node behavior on the left edge of the aorta. Pancreatology 2020, 20, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Hirano, S.; Noji, T.; Asano, T.; Okamura, K.; Tsuchikawa, T.; Murakami, S.; Kurashima, Y.; Ebihara, Y.; Nakanishi, Y.; et al. Distal pancreatectomy with en bloc celiac axis resection (modified appleby procedure) for locally advanced pancreatic body cancer: A single-center review of 80 consecutive patients. Ann. Surg. Oncol. 2016, 23 (Suppl. S5), 969–975. [Google Scholar] [CrossRef]
- Hirano, S.; Kondo, S.; Hara, T.; Ambo, Y.; Tanaka, E.; Shichinohe, T.; Suzuki, O.; Hazama, K. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: Long-term results. Ann. Surg. 2007, 246, 46–51. [Google Scholar] [CrossRef]
- Japan Pancreas Society. Classification of Pancreatic Carcinoma, 3rd ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 2011. [Google Scholar]
- Sasako, M.; McCulloch, P.; Kinoshita, T.; Maruyama, K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br. J. Surg. 1995, 82, 346–351. [Google Scholar] [CrossRef]
- Schwarz, R.E.; Smith, D.D. Extent of lymph node retrieval and pancreatic cancer survival: Information from a large US population database. Ann. Surg. Oncol. 2006, 13, 1189–1200. [Google Scholar] [CrossRef]
- Riediger, H.; Keck, T.; Wellner, U.; Hausen, A.Z.; Adam, U.; Hopt, U.T.; Makowiec, F. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J. Gastrointest. Surg. 2009, 13, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Hirano, S.; Nakamura, T.; Tanaka, E.; Shichinohe, T.; Tsuchikawa, T.; Kato, K.; Matsumoto, J.; Kondo, S. A new preoperative prognostic scoring system to predict prognosis in patients with locally advanced pancreatic body cancer who undergo distal pancreatectomy with en bloc celiac axis resection: A retrospective cohort study. Surgery 2014, 155, 457–467. [Google Scholar] [CrossRef]
- Imamura, T.; Yamamoto, Y.; Sugiura, T.; Okamura, Y.; Ito, T.; Ashida, R.; Ohgi, K.; Uesaka, K. Reconsidering the optimal regional lymph node station according to tumor location for pancreatic cancer. Ann. Surg. Oncol. 2021, 28, 1602–1611. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, C.M.; Satoi, S.; Sho, M.; Nakamura, Y.; Lee, W.J. Proposal for splenectomy-omitting radical distal pancreatectomy in well-selected left-sided pancreatic cancer: Multicenter survey study. J. Hepato-Biliary-Pancreat. Sci. 2013, 20, 375–381. [Google Scholar] [CrossRef]
- Collard, M.; Marchese, T.; Guedj, N.; Cauchy, F.; Chassaing, C.; Ronot, M.; Dokmak, S.; Soubrane, O.; Sauvanet, A. Is routine splenectomy justified for all left-sided pancreatic cancers? Histological reappraisal of splenic hilar lymphadenectomy. Ann. Surg. Oncol. 2019, 26, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, R.J.; Irving, A.D.; Cuschieri, A. Postsplenectomy sepsis and its mortality rate: Actual versus perceived risks. Br. J. Surg. 1991, 78, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Lutwick, L.I. Life threatening infections in the asplenic or hyposplenic individual. Curr. Clin. Top. Infect. Dis. 2002, 22, 78–96. [Google Scholar] [PubMed]
- Aiolfi, A.; Asti, E.; Siboni, S.; Bernardi, D.; Rausa, E.; Bonitta, G.; Bonavina, L. Impact of spleen-preserving total gastrectomy on postoperative infectious complications and 5-year overall survival: Systematic review and meta-analysis of contemporary randomized clinical trials. Curr. Oncol. 2019, 26, e202–e209. [Google Scholar] [CrossRef]
- Hirano, S.; Kondo, S.; Tanaka, E.; Shichinohe, T.; Tsuchikawa, T.; Kato, K.; Matsumoto, J. Postoperative bowel function and nutritional status following distal pancreatectomy with en-bloc celiac axis resection. Dig. Surg. 2010, 27, 212–216. [Google Scholar] [CrossRef][Green Version]
- Okada, K.I.; Kawai, M.; Hirono, S.; Miyazawa, M.; Kitahata, Y.; Ueno, M.; Hayami, S.; Shimokawa, T.; Yamaue, H. Ischemic gastropathy after distal pancreatectomy with en bloc celiac axis resection for pancreatic body cancer. Langenbeck’s Arch. Surg. 2018, 403, 561–571. [Google Scholar] [CrossRef]
- Oba, A.; Inoue, Y.; Sato, T.; Ono, Y.; Mise, Y.; Ito, H.; Ishizawa, T.; Takahashi, Y.; Saiura, A. Impact of indocyanine green-fluorescence imaging on distal pancreatectomy with celiac axis resection combined with reconstruction of the left gastric artery. HPB 2019, 21, 619–625. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).