Simple Summary
The study was aimed to investigate the frequency of accurate pathology report and sentinel lymph node biopsy for staging clinically node-negative >1 mm melanomas across European countries, which are standard care indicators having relevant consequences for survival. 4245 melanoma cases from in six European countries in 2009–2013 were analyzed by multivariable logistic regression in order to estimate the odds ratio of having such indicators performed. Model-based survival to estimate the five-year relative excess risks of death were computed. Results showed how much accurate pathology profiling and sentinel lymph node biopsy carried survival benefit. Narrowing down between-countries differences in adhesion to guidelines might achieve better outcomes.
Abstract
Objectives: Standard care for cutaneous melanoma includes an accurate pathology report (PR) and sentinel lymph node biopsy (SLNB) for staging clinically node-negative >1 mm melanomas. We aimed to investigate the frequency of these indicators across European countries, also assessing consequences for survival. Methods: We analyzed 4245 melanoma cases diagnosed in six European countries in 2009–2013. Multivariable logistic regression was used to estimate the Odds Ratio (OR) of receiving complete PR with eight items or SLNB and model-based survival to estimate the five-year relative excess risks of death (RER). Results: Overall, 12% patients received a complete PR (range 2.3%, Estonia—20.1%, Italy); SLNB was performed for 68.8% of those with cN0cM0 stage (range 54.4%, Spain—81.7%, Portugal). The adjusted OR of receiving a complete PR was lower than the mean in Estonia (OR 0.11 (0.06–0.18)) and higher in Italy (OR 6.39 (4.90–8.34)) and Portugal (OR 1.39 (1.02–1.89)); it was higher for patients operated on in specialized than general hospitals (OR 1.42 (1.08–1.42)). In the multivariate models adjusted for age, sex, country and clinical-pathological characteristics, the RER resulted in being higher than the reference for patients not receiving a complete PR with eight items (RER 1.72 (1.08–2.72)), or for those not undergoing SLNB (RER 1.76 (1.26–2.47)) Patients with non-metastatic node-negative thickness >1 mm melanoma who did not undergo SLNB had a higher risk of death (RER (RER 1.69 (1.02–2.80)) than those who did. Conclusions: Accurate pathology profiling and SLNB carried survival benefit. Narrowing down between-countries differences in adhesion to guidelines might achieve better outcomes.
1. Introduction
The quality and completeness of pathology reports (PR), with full descriptions of key parameters, is considered an indicator of standard care [1,2,3,4], as it is important to accurately profile and stage patients, guiding the selection of appropriate treatment and consequently improving the quality of care and outcomes. Despite recent updates in guidelines [5,6], the features currently considered of primary importance in melanoma care and outcomes largely correspond to those used in 2009–2013.
Sentinel Lymph Node Biopsy (SLNB) is recommended as a staging procedure for clinically node-negative cutaneous melanoma of Breslow thickness >1 mm or, on an individual basis, for thinner melanoma in patients with ulceration, high mitotic index (MI), or lympho-vascular invasion [1,7,8] However, the utility of SLNB in controlling distant metastases and improving survival is still debated [9,10].
Differences in the use of SLNB for melanoma evidenced by national population-based studies [11,12,13] can be partially attributable to factors such as the distribution of stage, anatomic location, age at diagnosis, comorbidities, clinicians’ expertise, the health system organization, or may also reflect the lack of solid evidence of benefit for this procedure [10].
The European High Resolution studies (http://www.hrstudies.it/, accessed on 4 July 2022) on samples of cancer cases archived in European population-based cancer registries (CRs) collect more clinical information than is routinely provided by population CRs, according to standardized protocols. Using these data, we aimed to investigate in a real-world context:
(i) the frequency of PR completeness and SLNB use across several European countries, in relation with patients’ and tumor characteristics.
(ii) the impact of PR completeness and SLNB on five-year survival, adjusted by clinic-pathological characteristics, demographic factors and comorbidity.
2. Methods
The High Resolution study protocol asked participating CRs to provide at least 300 malignant cutaneous melanoma adult (aged 15 years or more) cases, classified according to the International Classification of Diseases for Oncology, third revision (ICD-O-3) [14], with the morphology code 8720–8790 and topography code C44.0–44.9. Cases had to be diagnosed in 2009–2013 (latest years available), followed up at least to 31 December 2014 and had to include specified information from the clinical records of each case. Trained CR personnel accessed the clinical records and abstracted the relevant information envisaged by the study protocol.
Most CRs provided all the incidences of cases in one or more years of the study period. Registries covering large areas sampled cases from a defined incidence period using a randomized procedure. Supplementary Table S1 shows the criteria for sampling the cases. The process of identifying eligible cases for the analyses is shown in Figure 1.
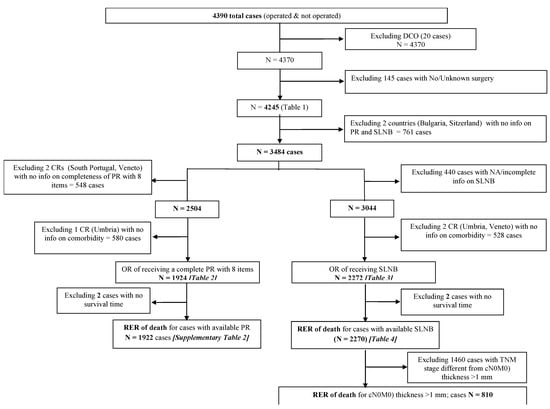
Figure 1.
Process of identifying eligible cases for the analyses.
We analyzed 4245 operated melanoma cases. Eight countries, with either national (Bulgaria, Estonia) or regional cancer registries (Italy 4 CRs; Portugal 2 CRs; Spain 3 CRs; Switzerland 1 CR), contributed data.
Age at diagnosis was classified as 15–54, 55–64, 65–74, and 75+ years. A score from 1 to 6 was assigned to each Charlson Comorbidity Index (CCI) item [15], and the total was calculated as the sum of the scores for the 19 items. The sum was then rated as 0 (no comorbidities), or ≥1 (one or more).
The anatomical site and morphology of the primary melanoma were coded according to ICD-O3. Topography codes were grouped as the head and neck (ICD-O-3 code C44.0–C44.4), trunk (C44.5), upper limb and shoulder (C44.6), lower limb and hip (C44.7), and unspecified and overlapping regions (C44.8–9). Melanoma morphology was grouped in six subgroups: nodular melanoma (ICD-O-3 code 8721); lentigo malignant melanoma (8741; 8742); superficial spreading melanoma (8743); acral lentiginous melanoma (8744); other types (8722; 8730; 8740; 8744; 8745; 8761; 8770; 8771; 8772); not otherwise specified (NOS) (8720; 8723).
Stage at diagnosis was classified according to the TNM classification, 7th edition [16] and grouped as categories I-IV, or unknown. Tumor thickness was categorized as ≤1 mm, 1.01 mm–2.00 mm, 2.01 mm–4.00 mm, >4.00 mm or unknown.
The Sentinel Lymph Node Biopsy (SLNB) was coded as done, not done and unknown. The following eight items in the PR were considered to be indicative of completeness: melanoma thickness (in millimeters), ulceration (present; absent; unknown), histological subtype, mitotic rate index (0 mitoses per mm2; ≥1 mitoses per mm2; not mentioned/not available in PR), growth phase (vertical; horizontal; mixed (vertical and horizontal); unknown), lymphocyte infiltration (absent; present, with or without brisk; unknown), tumor regression, and vascular or neural involvement.
A score from 1 to 6 was assigned to each Charlson Comorbidity Index (CCI) item, and the total was calculated as the sum of the scores for the 19 items. The sum was then rated as 0–1 points (no comorbiditiy), ≥1 points (presence of comorbidity) or unknown.
Statistical Analysis
Multivariable logistic regression was used to establish the roles of different covariates on the PR completeness (versus not complete) and SLNB (compared to not done). Countries’ odds ratios (OR with 95% confidence intervals (CI)) were based on the differences from the balanced grand mean; the common reference for the areas is therefore their grand mean [17].
Relative survival (RS) was calculated as the ratio of the observed survival and the expected survival in the general underlying population. We estimated expected survival by the Ederer II method [18] using CR population life tables stratified by sex, age and the year of diagnosis.
The Relative Excess rate of Risk of death (RER) 5 years after diagnosis, with 95% CI, was estimated with generalized linear models, using 5-year relative survival (RS) as the dependent variable and the other variables under study as covariates [19].
The Akaike Information Criterion (AIC) score was used to determine which models fitted the data best, in order to select the most appropriate variables [20].
Data was analyzed with Stata software, version 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.)
The study was approved by all participating CRs and by the Institutional Ethical Committee Board of the leading study Institution.
3. Results
We analyzed 4245 operated melanoma cases. Bulgaria contributed the fewest number of cases (6.9% of all cases); Italy and Spain had respectively 34.9 and 21.8% of all cases. Patients aged 15–54 years represented 38% of all cases and more than 40% of cases were older than 65 years (Table 1).

Table 1.
Distribution and clinical and pathological characteristics of 4245 operated cutaneous melanoma patients diagnosed in 2009–2013 in six European countries.
The trunk was the most frequent anatomic location in all countries except for Portugal. Superficial spreading melanoma was the most frequent subtype, followed by not-otherwise specified melanoma (NOS). In all the countries, surgery was done mostly in specialized (university or teaching) hospitals (overall 51.6%), or general hospitals (24.2%); only 9.4% were treated as outpatients, or the place of surgery was not specified (14.8%).
Considering the four countries where information on PR was available, overall, 15.2% patients received a complete PR with eight items (range 2.3%, Estonia—20.1%, Italy); in the same countries, SLNB was carried out for 42.6% of the total operated patients, more frequently in Portugal (57.9%), than in Estonia (45.2%), Spain (36.9%) and Italy (38.0%). In these countries, considering the total 795 clinically non-metastatic negative node (cN0M0) and Breslow thickness >1 mm melanoma patients, for whom the procedure is recommended, the overall figure was 68.8%, an average of 81.7% (Portugal), 69.1% (Italy), 65.7% (Estonia) and 54.4% (Spain).
Overall, more than half the patients (54.5%) had TNM I stage at diagnosis, and 3% had TNM IV, but there were notable differences in the stage distribution across countries, e.g., Bulgaria showed the lowest percentage of TNM I and the highest percentage of stage IV, Switzerland had the highest percentage of cases with unknown or incomplete TNM stage; information on comorbidities was available for Estonia, Italy, Portugal and Spain. In these countries, the majority (68.4%) of patients had no comorbidity at diagnosis, while 32% had at least one comorbidity.
Table 2 shows the number of cases receiving a complete pathological report (PR) with eight items, and the adjusted OR with 95% CI, in the four countries providing the relevant information.

Table 2.
Number of cases (N) receiving a complete pathological report (PR) with eight recommended histopathological items 1 and adjusted Odds Ratios (OR) with 95% Confidence Intervals (CI), for cutaneous melanoma patients diagnosed in 2009–2013 in four European countries.
This multivariable regression model, adjusted by country, age, sex, stage, place of treatment and CCI, evidenced that, with reference to the mean, the odds of PR completeness were significantly lower in Estonia (OR 0.11 (0.06–0.18)) and higher in Italy (OR 6.39 (4.90–8.34)) and Portugal (OR 1.39 (1.02–1.89)). The odds for PR completeness was higher for patients operated on in specialized rather than general hospitals and for patients with incomplete data on staging (OR 1.74 (1.06–2.87)) than for those with stage I; the lower than reference OR for stage IV (OR 0.22 (0.008–0.61)) was based on five cases only.
The multivariable analysis carried out to investigate whether PR completeness with eight items was associated with survival, showed that, after adjustment by country, age, sex, and stage at diagnosis, patients not receiving a complete PR with eight items had a higher RER of death than those receiving it (RER 2.38 (1.41–4.02)). Supplementary Table S2 shows the coefficients of each variable included in the model. Of note, a less complete PR, i.e., with four items, was not associated with RER.
Table 3 shows, for the 2272 cases with available information on SLNB, the results of the multivariable analysis carried out to estimate the odds of receiving SLNB, adjusted by country, age, TNM stage, place of treatment and comorbidity.

Table 3.
Number of cases (N) receiving Sentinel Lymph Node Biopsy and adjusted Odds Ratios (OR) with 95% Confidence Intervals (CI), for cutaneous melanoma patients diagnosed in 2009–2013 in four European countries.
For each covariate, the table shows the number of cases receiving SLNB and the adjusted OR of receiving this procedure, with 95% CI. With reference to the mean of the pooled countries, the adjusted OR for SLNB was significantly higher in Portugal (OR 1.85 (1.56–2.20)) and lower in Spain (OR 0.82 (0.70–0.95)) and was borderline lower in Italy; with reference to patients aged ≥75, all the younger age classes showed significantly higher ORs of receiving SLNB.
When compared to patients with stage I, those with stage II and III at diagnosis were significantly more likely to receive SLNB (OR 3.99 (3.15–5.10) and 7.18 (5.26–9.79), respectively); the OR was lower than the reference for patients whose stage at diagnosis data were incomplete (OR 0.38 (0.21–0.69)). The odds of receiving SLNB was higher for patients operated on in specialized centers rather than in general hospitals (OR 1.86 (1.50–2.30)).
The multivariable survival analysis (Table 4) including all the 2270 cases with available information on SLNB showed that, by adjusting by the clinic pathological covariates in the model, no significant RER differences across countries were evident.

Table 4.
Number of cases and adjusted 5-year Relative Excess rate of Risk of death (RER) with 95% Confidence Intervals (CI) for cutaneous melanoma patients diagnosed in 2009–2013 in four European countries.
Patients who did not undergo SLNB had a significantly higher risk of death (RER 1.61 (1.20–2.15)) than those for whom a biopsy was taken. Melanoma pathological features and stage at diagnosis were independent predictors of survival: with reference to thickness >4 mm, the RER of patients with thinner lesions resulted in being significantly lower; ulceration (RER 1.94 (1.38–2.74)) and high or not-mentioned MI (RER 4.22 (1.43–12.44), 3.32 (1.10–10.03)) were independently associated with a higher than reference risk of death. Patients with N0 stage at diagnosis had a lower RER than those with nodal metastases (RER 0.57 (0.40–0.81)) and those with distant metastases at diagnosis had a higher risk (RER 5.24 (2.54–10.83)) than those with no distant metastases.
A model with the same covariates, fitted on the 810 patients with non-metastatic clinically node-negative (cN0M0) thickness >1 mm melanoma, showed that patients who did not undergo SLNB had a higher risk of death (RER 1.69 (1.02–2.80)) than those who did (Figure 2).
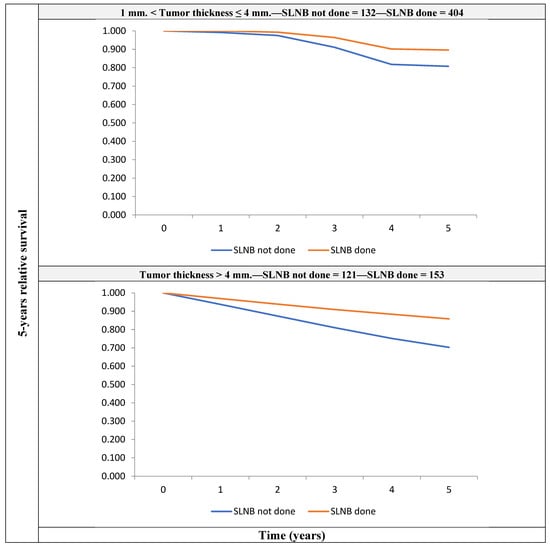
Figure 2.
5-year crude relative survival by tumor thickness, for patients with non-metastatic clinically node-negative (cN0M0) melanoma with thickness >1 mm, for those who underwent SLNB and those who did not receive it.
For patients with melanoma thickness between 1 mm and 4 mm, the figures were 89.6 (Confidence Interval—CI: 83.5–94.4) vs. 80.8 (CI: 65.0–98.7), respectively; for patients with thickness >4 mm, the figures were 85.8 (CI: 58.4–97.1) vs. 70.3 (CI: 37.6–93.6), respectively.
4. Discussion
We found remarkable differences between countries in their adhesion to clinical guidelines on the completeness of PR and SLNB execution for cutaneous melanoma diagnosed in 2009–2013 in Europe. In the multivariable analyses adjusting for potential confounders both these indicators were associated with a five-year risk of death, suggesting that adherence to clinical guidelines can improve disease outcomes.
PR for cutaneous melanoma documents features that are relevant not only in staging and clinical management but are a requisite for planning therapy—particularly with a view to personalized treatment. [1,2,3] Adequately documented PR can support favorable outcomes [21]. We did in fact find that melanoma patients with a well-documented PR had a lower mortality than those with an incomplete, or less complete PR.
The across country differences in the completeness of PR highlighted by our study are in line with results of other studies reporting variation in the compliance with guidelines—especially for differences across countries—as observed in the International Collaboration on Cancer Reporting panel (ICCR) [22] and other studies [23,24,25,26]. Geographic differences in the availability of pathological information may also depend on the availability and accuracy of the PR themselves. Therefore, adherence to international evidence-based protocols yielding more complete PRs is to be encouraged and remains a main goal in recent clinical procedures [21,24].
The higher odds of receiving complete PR and SLNB in specialized oncologic centers than in general hospitals points to the usefulness of centralizing the management of melanoma patients [27]. The lack of association of comorbidity with SLNB performance or PR completeness, once the place of treatment (and other factors) was adjusted for in the multivariable analysis, suggests that specialized oncologic facilities may provide better melanoma management than others, independently from the presence of comorbid conditions.
In our study, SLNB was performed for around 43% of total cases, with notable differences between countries, however, by restricting the analysis to patients with non-metastatic clinically node negative melanoma (cN0M0) with a tumor thickness > 1 mm, for whom the procedure is recommended, the percentage of biopsies rose to almost 70% (69.8%), a figure close to that reported in the US Surveillance Epidemiology and End Results (SEER) in a comparable study period [23]. Although the intercountry variability in the SLNB use attenuated when considering clinically node-negative cases only, the multivariable analysis confirmed the differences by country, as well as the lower odds of receiving SLNB for elderly than younger patients, a finding reported also by other studies [11,28].
Our results are consistent with those of other studies reporting slightly higher than European mean SLNB frequencies in Spain [11] and lower ones in Italy [29]; in both these countries, a remarkable within-country variation in SLNB use was highlighted. In the present study, the large proportion of early-stage melanomas in Spain (Table 1) might explain the overall low odds of SLNB, as well as an RER in line with the European average. Other nationwide registrations or dedicated quality registries on melanomas detected within-country differences: for instance, during 2005–13 in the Netherlands, SLNB was performed for 50% of non-distant metastatic melanoma patients on average, ranging from 22.5 to 56.5% across regions [12] The German national melanoma registry reported a higher percentage of SLNB (82%), but did not look into regional differences [13].
We noted that in our population-based real-world setting, SLNB was done not only for patients with intermediate thickness or thick clinically node-negative non distant metastatic melanoma, for whom the benefit of this procedure has been proven [8,30], but also for a proportion of patients with thinner or unknown-thickness lesions, or patients with uncertain nodal status. Underreporting and incompleteness of clinical documentation might have prevented a more precise definition of tumor stage for these patients. Also, it cannot be excluded that some of them presented clinical indications to SLNB that were not captured by our study.
The survival benefit carried by SLNB for intermediate-thickness and thick melanomas found by our study is in line with population- [28] and hospital-based studies [31]. The finding that in comparison with thick melanoma the RER carried by SLNB decreased also for thin melanoma is consistent with recent studies indicating SLNB should be considered for selected high-risk patients, defined by features such as a high MI, ulceration, lympho-vascular invasion, tumor-infiltrating lymphocytes, or regression [31,32,33].
Lymph node dissection could have improved the survival of cases with nodal metastases. In our study, considering patients with stage II-IV, the inclusion of nodal dissection execution in a multivariable analysis adjusted by all the above clinical-pathological factors did not evidence a statistical significant effect of this surgery on the risk of death (RER of patients undergoing lymph-node dissection 0.67, 95% CI 0.33–1.34).
Past population-based studies have evidenced higher survival for women than men [34] and for younger than older patients [35,36], as well as remarkable across-European-country survival inequalities [37]. In the present study, adjustment for stage at diagnosis and pathological features, such as MI, ulceration, thickness, explained the lower mortality of women and younger ages, as well as geographic differences.
The presence of comorbid conditions may cause a delay in diagnosis or contraindicate intensive treatments, thereby decreasing survival. In contrast with other studies, in our dataset comorbidity resulted in not being independently associated with the RER. In a recent population-based study the detrimental effect of comorbidity on melanoma survival was concentrated in patients with an advanced tumor stage at diagnosis [38]. Another study documented that melanoma patients with >2 CCI had a significantly higher risk of death than those with no comorbidity [39]. The lack of statistically significant associations of comorbidity with RER in our study could be attributable to the low number of cases with advanced stage at diagnosis, or with severe comorbidities. In fact, our study population was largely represented by early-stage melanoma (54% TNM stage I) and patients with no comorbidities (68% overall, with similar distribution by stage).
Comorbidity data were abstracted from each patient’s clinical record and their availability and completeness may vary by hospitals and clinician’s attitudes to documenting comorbid conditions in the clinical notes. However, we cannot exclude that, for a certain proportion of cases with comorbidity coded as “absent”, the relevant information was actually unknown.
In all countries, more than 50% of melanoma patients had tumor stage I at diagnosis; a notable exceptions was Bulgaria, where the most frequent stage category was represented by stage II, and the percentage of stage IV was the highest among the included countries. The more advanced tumor stage at diagnosis likely explains the lower-than-European average melanoma survival in this country that was previously reported [40]. The five-year relative survival of Bulgarian patients included in this study was 67% (95% CI 0,54–0.77). In contrast, the relatively low frequency of stage I melanoma in Switzerland (42%) is counterbalanced by the highest percentage of cases with unknown stage at diagnosis (45%) and was not associated with low survival: the 5-year relative survival of Swiss patients in this study was 98% (95 CI 0.129–0.999). Due to a lack of information we could not analyze the association of stage at diagnosis with SLNB or PR in these two countries.
The present study did not focus on treatment. However, patients were diagnosed prior to the use of the new anti BRAF/MEK drugs or immunotherapy as adjuvant treatment, starting in 2018. The use of interferon, the drug previously approved in adjuvant therapy, varied considerably in the different countries [40]. In our study, 90 patients received adjuvant treatment in addition to surgery, of whom 68 received interferon.
In the multivariable analysis adjusted by all clinical pathological factors considered in the study, the administration of adjuvant chemotherapy or target treatment resulted in being not associated with survival (RER of patients receiving adjuvant chemotherapy or target treatment 0.96, 95% CI 0.38–2.46).
A strength of our study was the use of all incidences of cases (or representative samples of them) during the study years in the participating CR areas, irrespective of the type of treatment and hospital, or its location within or outside the CR area. Hence, variations in the completeness and quality of data provided by the hospitals reflect actual variations in current clinical practices. Furthermore, the centralization of data for common checks and analyses ensured uniform methods of analysis and data comparability.
5. Conclusions
Despite the existence of clinical recommendations, we found notable across-country differences in Europe in the completeness of PR for cutaneous melanoma, as well as in the use of SLNB. The odds of receiving a complete PR or undergoing SLNB was higher for patients treated in specialized oncologic centers than in general hospitals. Multivariable analysis adjusting for potential confounders suggested that a complete pathological report and SLNB were associated with survival benefit. Narrowing down the differences between countries by adherence to guidelines is important for achieving more favorable outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14184379/s1, Table S1: Total number of cutaneous melanoma cases submitted for inclusion in the HR study by country and registry, with criteria for selection of cases; Table S2: Number of cases and adjusted 5-year Relative Excess rate of Risk of death (RER) with 95% Confidence Interval (CI) for cutaneous melanoma patients diagnosed in 2009-2013 in four European countries.
Author Contributions
M.S.: Conceptualization; Investigation; Writing—Original draft; Writing—Review & editing. M.C.M.: Data curation; Formal analysis; Methodology; Software; Writing—Original draft. A.M.: Conceptualization; Supervision; Validation; Writing—Review & editing. R.L.: Data curation; Formal analysis; Methodology; Software; Visualization; Writing—Original draft. M.J.B.: Data curation; Validation; Writing—Review & editing. E.A.: Data curation; Validation; Writing—Review & editing. M.G.: Data curation; Validation; Writing—Review & editing. K.I.: Data curation; Validatiraon; Writing—Review & editing. R.M.-G.: Data curation; Validation; Writing—Review & editing. J.R.-C.: Data curation; Validation; Writing—Review & editing. M.-J.S.P.: Data curation; Validation; Writing—Review & editing. R.T.: Data curation; Validation; Writing—Review & editing. M.R.: Data curation; Supervision; Validation; Writing—Review & editing. P.M.: Conceptualization; Formal analysis; Methodology; Software; Validation; Writing—Review & editing. The Melanoma HR Study Working Group: Conceptualization; Data curation; Supervision; Validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was (partially) funded by Italian Ministry of Health “Ricerca Corrente” funds.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards and approved by the Ethics Committee of Fondazione IRCCS Istituto Nazionale dei Tumori (N. INT 0197/14).
Informed Consent Statement
Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Data Availability Statement
Data are not publicly available. They can be obtained by the Melanoma HR Study Working Group after motivated request.
Acknowledgments
This work was carried out thanks to the invaluable contribution of Maria Chiara Magri who sadly passed away on the 28 January 2022. Judith Baggot reviewed the English language and Simone Bonfarnuzzo provided technical and administrative support. All the participating cancer registries collected and prepared the data as part of their essential role in cancer control. This work was carried out as part of the European High resolution project on prognosis and care of cancer patients. The Melanoma HR Study Working Group: Bulgaria: M. Yordanova, Z. Valerianova (Bulgarian CR); Estonia: K. Innos, M. Mägi (Estonia CR); Iceland: E. J. Ólafsdóttir, L. Tryggvadóttir (Iceland—National CR); Italy: P. Baili, S. Bonfarnuzzo, R. Lillini, M.C. Magri, G.Moretti, M. Sant (Fondazione IRCCS Istituto Nazionale dei Tumori, Milan); G. Carrozzi, C. Cirilli (Modena CR); G. Frasca, R. Tumino (Ragusa CR, ASP); F. Bianconi, F. Stracci (Umbria CR); S. Guzzinati, M. Zorzi, M. Rugge (Veneto CR); Portugal: L. Antunes, M. J. Bento (Northern Portugal CR); A. Mayer-da-Silva, A. Miranda (Southern Portugal CR); Spain: R. Marcos-Gragera, M. Puigdemont (Girona CR); M. Rodriguez-Barranco, M.-J. Sánchez-Pérez (Granada CR, EASP, CIBERESP, ibs.Granada); E. Ardanaz, M. Guevara, (Navarra CR, CIBERESP, IdiSNa); Switzerland: E. Fournier, E. Rapiti (Geneva CR, University of Geneva); UK-England: P. Minicozzi (London School of Hygiene and Tropical Medicine, London).
Conflicts of Interest
Kaire Innos reports her institution was supported by the Estonian Research Council (Eesti Teadusagentuur, grant no PRG722). Jordi Rubio-Casadevall was a speaker in Advisory Board in metastatic melanoma by Novartis 22 June 2020. For the remaining authors no conflict of interest or support was declared.
References
- Dummer, R.; Hauschild, A.; Lindenblatt, N.; Pentheroudakis, G.; Keilholz, U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v126–v132. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.Z.; Van Den Berg, K.S.; Colman, M.H.; McCarthy, S.W.; Thompson, J.F.; Scolyer, R.A. The advantage of using a synoptic pathology report format for cutaneous melanoma. Histopathology 2007, 52, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Scolyer, R.A.; Rawson, R.V.; Gershenwald, J.E.; Ferguson, P.M.; Prieto, V.G. Melanoma pathology reporting and staging. Mod. Pathol. 2020, 33, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Tumino, R.; Minicozzi, P.; Frasca, G.; Allemani, C.; Crocetti, E.; Ferretti, S.; Giacomin, A.; Natali, M.; Mangone, L.; Falcini, F.; et al. Population-based method for investigating adherence to international recommendations for pathology reporting of primary cutaneous melanoma: Results of a EUROCARE-5 high resolution study. Cancer Epidemiol. 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Jobson, D.; Roffey, B.; Arnold, C.; Azzi, A.; Button-Sloan, A.; Dawson, T.; Fernandez-Penas, P.; Fishburn, P.; Gyorki, D.E.; Hiscutt, E.L.; et al. Development of melanoma clinical quality indicators for the Australian melanoma clinical outcomes registry (MelCOR): A modified Delphi study. Australas. J. Dermatol. 2022, 63, 344–351. [Google Scholar] [CrossRef]
- McKay, D.R.; Nguyen, P.; Wang, A.; Hanna, T.P. A population-based study of administrative data linkage to measure melanoma surgical and pathology quality. PLoS ONE 2022, 17, e0263713. [Google Scholar] [CrossRef]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Elashoff, R.; Essner, R.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; et al. Sentinel-Node Biopsy or Nodal Observation in Melanoma. N. Engl. J. Med. 2006, 355, 1307–1317. [Google Scholar] [CrossRef]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; Puleo, C.A.; Coventry, B.J.; et al. Final Trial Report of Sentinel-Node Biopsy versus Nodal Observation in Melanoma. N. Engl. J. Med. 2014, 370, 599–609. [Google Scholar] [CrossRef]
- Madu, M.F.; Wouters, M.W.J.M.; van Akkooi, A.C.J. Sentinel node biopsy in melanoma: Current controversies addressed. Eur. J. Surg. Oncol. 2017, 43, 517–533. [Google Scholar] [CrossRef]
- Stadler, R.; Leiter, U.; Garbe, C. Lack of survival benefit in sentinel lymph node-positive melanoma with immediate complete lymphadenectomy—A review. J. Ger. Soc. Dermatol. 2019, 17, 7–13. [Google Scholar] [CrossRef]
- Guevara, M.; Rodríguez-Barranco, M.; Puigdemont, M.; Minicozzi, P.; Yanguas-Bayona, I.; Porras-Povedano, M.; Rubió-Casadevall, J.; Sánchez Pérez, M.J.; Marcos-Gragera, R.; Ardanaz, E. Disparities in the management of cutaneous malignant melanoma. A population-based high-resolution study. Eur. J. Cancer Care 2019, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Verstijnen, J.; Damude, S.; Hoekstra, H.J.; Kruijff, S.; ten Tije, A.J.; Louwman, W.J.; Bastiaannet, E.; Stuiver, M.M. Practice variation in Sentinel Lymph Node Biopsy for melanoma patients in different geographical regions in the Netherlands. Surg. Oncol. 2017, 26, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, E.; Windemuth-Kieselbach, C.; Eigentler, T.K.; Rompel, R.; Trefzer, U.; Nashan, D.; Rotterdam, S.; Ugurel, S.; Schadendorf, D. A first prospective population-based analysis investigating the actual practice of melanoma diagnosis, treatment and follow-up. Eur. J. Cancer 2011, 47, 1977–1989. [Google Scholar] [CrossRef] [PubMed]
- W.H.O. (WHO) International Classification of Diseases for Oncology (ICD-O), 3rd ed.; 1st revision; WHO Libr. Cat., III; World Health Organization: La Valletta, Malta, 2013. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C.H. (Eds.) TNM Classification of Malignant Tumours, 7th ed.; Wiley: Chichester, UK, 2010; p. 310. [Google Scholar]
- Menard, S. Applied Logistic Regression Analysis; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2002. [Google Scholar] [CrossRef]
- Ederer, F.; Axtell, L.M.; Cutler, S.J. The relative survival: A statistical methodology. J. Natl. Cancer Inst. Monogr. 1961, 6, 101–121. [Google Scholar]
- Dickman, P.W.; Coviello, E. Estimating and modeling relative survival. Stata J. 2015, 15, 186–215. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of maximum likelihood principle. In Second International Symposium on Information Theory; Petrov, B.N., Csáki, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Thompson, J.F.; Scolyer, R.A. Cooperation between surgical oncologists and pathologists: A key element of multidisciplinary care for patients with cancer. Pathology 2004, 36, 496–503. [Google Scholar] [CrossRef]
- Scolyer, R.A.; Judge, M.J.; Evans, A.; Frishberg, D.P.; Prieto, V.G.; Thompson, J.F.; Trotter, M.J.; Walsh, M.Y.; Walsh, N.M.; Ellis, D.W. International Collaboration on Cancer Reporting. Data set for pathology reporting of cutaneous invasive melanoma: Recommendations from the international collaboration on cancer reporting (ICCR). Am. J. Surg. Pathol. 2013, 37, 1797–1814. [Google Scholar] [CrossRef]
- Blakely, A.M.; Comissiong, D.S.; Vezeridis, M.P.; Miner, T.J. Suboptimal Compliance With National Comprehensive Cancer Network Melanoma Guidelines. Am. J. Clin. Oncol. 2018, 41, 754–759. [Google Scholar] [CrossRef]
- Thompson, B.; Austin, R.; Coory, M.; Aitken, J.F.; Walpole, E.; Francis, G.; Fritschi, L. Completeness of Histopathology Reporting of Melanoma in a High-Incidence Geographical Region. Dermatology 2009, 218, 7–14. [Google Scholar] [CrossRef]
- Haydu, L.E.; Holt, P.E.; Karim, R.Z.; Madronio, C.M.; Thompson, J.F.; Armstrong, B.K.; Scolyer, R.A. Quality of histopathological reporting on melanoma and influence of use of a synoptic template. Histopathology 2010, 56, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Maley, A.; Patrawala, S.; Stoff, B. Compliance with the College of American Pathologists Protocol for Melanoma in Synoptic and Non-Synoptic reports: A cross-sectional study. J. Am. Acad. Dermatol. 2016, 74, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Narang, J.; Hue, J.J.; Bingmer, K.; Hardacre, J.M.; Winter, J.M.; Ocuin, L.M.; Ammori, J.B.; Mangla, A.; Bordeaux, J.; Rothermel, L.D. Sentinel lymph node biopsy guideline concordance in melanoma: Analysis of the National Cancer Database. J. Surg. Oncol. 2021, 124, 669–678. [Google Scholar] [CrossRef]
- Murtha, T.D.; Han, G.; Han, D. Predictors for Use of Sentinel Node Biopsy and the Association with Improved Survival in Melanoma Patients Who Have Nodal Staging. Ann. Surg. Oncol. 2018, 25, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Sant, M.; Minicozzi, P.; Allemani, C.; Cirilli, C.; Federico, M.; Capocaccia, R.; Budroni, M.; Candela, P.; Crocetti, E.; Falcini, F.; et al. Regional inequalities in cancer care persist in Italy and can influence survival. Cancer Epidemiol. 2012, 36, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Ross, M.I. Sentinel-Lymph-Node Biopsy for Cutaneous Melanoma. N. Engl. J. Med. 2011, 364, 1738–1745. [Google Scholar] [CrossRef]
- Hayek, S.A.; Munoz, A.; Dove, J.T.; Hunsinger, M.; Arora, T.; Wild, J.; Shabahang, M.; Blansfield, J. Hospital-Based study of compliance with nCCN guidelines and predictive factors of sentinel lymph node biopsy in the setting of thin melanoma using the national cancer database. Am. Surg. 2018, 84, 672–679. [Google Scholar] [CrossRef]
- Sekula-Gibbs, S.A.; Shearer, M.A. Sentinel node biopsy should be offered in thin melanoma with mitotic rate greater than one. Dermatol. Surg. 2011, 37, 1080–1088. [Google Scholar] [CrossRef]
- Maurichi, A.; Miceli, R.; Patuzzo, R.; Barretta, F.; Gallino, G.; Mattavelli, I.; Barbieri, C.; Leva, A.; Cortinovis, U.; Tolomio, E.; et al. Analysis of sentinel node biopsy and clinicopathologic features as prognostic factors in patients with atypical melanocytic tumors. J. Natl. Compr. Cancer Netw. 2020, 18, 1327–1336. [Google Scholar] [CrossRef]
- Innos, K.; Padrik, P.; Valvere, V.; Aareleid, T. Sex differences in cancer survival in Estonia: A population-based study. BMC Cancer 2015, 15, 72. [Google Scholar] [CrossRef]
- Lasithiotakis, K.; Leiter, U.; Meier, F.; Eigentler, T.; Metzler, G.; Moehrle, M.; Breuninger, H.; Garbe, C. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer 2008, 112, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Lambert, P.C.; Rutherford, M.J. Understanding the impact of sex and stage differences on melanoma cancer patient survival: A SEER-based study. Br. J. Cancer 2021, 124, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, E.; Mallone, S.; Robsahm, T.E.; Gavin, A.; Agius, D.; Ardanaz, E.; Lopez, M.C.; Innos, K.; Minicozzi, P.; Borgognoni, L.; et al. EUROCARE-5 Working Group. Survival of patients with skin melanoma in Europe increases further: Results of the EUROCARE-5 study. Eur. J. Cancer 2015, 51, 2179–2190. [Google Scholar] [CrossRef]
- Grann, A.F.; Frøslev, T.; Olesen, A.B.; Schmidt, H.; Lash, T.L. The impact of comorbidity and stage on prognosis of Danish melanoma patients, 1987–2009: A registry-based cohort study. Br. J. Cancer 2013, 109, 265–271. [Google Scholar] [CrossRef]
- Chang, C.K.; Hsieh, Y.S.; Chen, P.N.; Chu, S.C.; Huang, J.Y.; Wang, Y.H.; Wei, J.C. A Cohort Study: Comorbidity and Stage Affected the Prognosis of Melanoma Patients in Taiwan. Front. Oncol. 2022, 12, 846760. [Google Scholar] [CrossRef]
- Garbe, C.; Eigentler, T.K.; Keilholz, U.; Hauschild, A.; Kirkwood, J.M. Systematic review of medical treatment in melanoma: Current status and future prospects. Oncologist 2011, 16, 5–24. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).