Simple Summary
Tenofovir disoproxil fumarate (TDF) and entecavir (ETV) are the preferred anti-viral agents used as first-line treatments for chronic hepatitis B. Despite many meta-analyses being conducted, it is still not clear whether TDF is more effective than ETV at reducing the risk of HCC due to the inconsistent statistical methodologies employed in previous observational studies. To reduce heterogeneity, we analysed only hospital cohort data studies with anti-viral naive patients. Additionally, unlike previous studies, we conducted subgroup analyses with enrolment criteria and socioeconomic factors that could not be corrected with statistical techniques. There is no difference between the two drugs in terms of reducing the risk of HCC in a pooled analysis of PS-matched patients. In the subgroup analysis, if there was interval of over three years from the start point of patient enrolment, we found that TDF was associated with significantly lower HCC risk. This result will provide new perspectives for future research.
Abstract
Tenofovir disoproxil fumarate (TDF) and entecavir (ETV) are the preferred anti-viral agents used as first-line treatments for chronic hepatitis B (CHB). However, the efficacy of these agents in reducing the incidence of hepatocellular carcinoma (HCC) remains unclear. We conducted this meta-analysis to assess the efficacy of anti-viral agent on preventing HCC in CHB. Two investigators independently searched all relevant studies that examined the efficacy of anti-viral agent for preventing HCC using MEDLINE, Embase, and Cochrane Library databases through August 2021. The extracted data were analysed using a random-effects meta-analysis model based on the inverse-variance method (DerSimonian–Laird) and expressed as hazard ratio (HR) and 95% confidence interval (95% CI). We included 19 retrospective studies in the analysis. Although there was substantial heterogeneity between the studies, the overall pooled HR indicated that TDF significantly lowered the risk of HCC (HR: 0.72, 95% CI: 0.58–0.90, I2 = 66.29%). However, the pooled analysis of propensity score (PS)-matched subpopulations showed no significant differences (HR, 0.83; 95% CI, 0.65–1.06; I2 = 52.30%) between TDF and ETV. In a subgroup analysis, an interval of over three years in the start point of patient enrolment and excluding alcoholic liver disease patients significantly lowered the HCC risk associated with TDF. In conclusion, TDF may be more effective than ETV at reducing HCC incidence in treatment-naive CHB patients, but this effect was not consistent in the PS-matched subpopulation that reduced heterogeneity. As a result of subgroup analysis, the conflicting findings of previous studies may result from heterogeneous inclusion criteria. Further studies with standardised protocols are needed to reduce the residual heterogeneity.
1. Introduction
Chronic hepatitis B (CHB) infection is one of the most common causes of chronic liver disease, affecting approximately 300 million patients worldwide. According to the World Health organisation, CHB caused an estimated 820,000 deaths from cirrhosis and hepatocellular carcinoma (HCC) in 2019 [1]. With the development of hepatitis B antiviral agents and the inhibition of hepatitis B virus (HBV) replication with long-term nucleos(t)ide analogue (NA) therapy, the overall survival of CHB patients has increased [2]. However, the risk of HCC persists [3]. In a real-world clinic, a lifetime prescription of medication for CHB is a critical issue and should be based on a high level of evidence. However, prescribing drugs that reduce the risk of HCC can contribute to reducing socioeconomic costs [4].
Among the available NA therapies, entecavir (ETV) and tenofovir disoproxil fumarate (TDF) are both recommended as first-line treatments for CHB [5,6]. Many conflicting studies have been published since Choi et al. reported a low risk of HCC in a TDF user group within a CHB hospital cohort and South Korea’s nationwide claim data [7]. However, previous studies have been highly heterogeneous in terms of baseline characteristics, follow-up duration, use of other NAs, and statistical methodology, making it difficult to make objective comparisons. Similarly, previous meta-analyses [8,9,10,11,12,13,14,15,16,17,18,19,20] have also failed to reach an agreement owing to the following limitations: pooled analysis of odds ratios with different follow-up durations [21], a mix of antiviral naïve and non-naïve patients, and pooled analysis of hazard ratios (HRs) using retrospective hospital cohort data and administrative databases or claim data at once. These inconsistent statistical methodologies of the previous studies were pointed out in a recent review article [22].
Therefore, whether TDF is more effective than ETV at reducing the risk of HCC remains inconclusive. This systematic review and meta-analysis aims to compensate for the limitations of previous studies and obtain new insights into the efficacies of TDF and ETV on incidence of HCC in CHB patients.
2. Materials and Methods
2.1. Data and Literature Source
Two investigators (Hyunwoo Oh and Hyo Young Lee, Department of Internal Medicine, Eulji University School of Medicine, Uijeongbu, Korea) independently searched the MEDLINE, EMBASE, and Cochrane Library databases using the following keywords: “tenofovir”, “entecavir”, and “hepatocellular carcinoma”. Additional references were obtained from the bibliographies of relevant articles published through 31 August 2021 (Table 1). There was 96.4% agreement between the reviewers regarding the eligibility of articles after full-text screening, corresponding to a substantial agreement (k = 0.867). Any disagreement or unresolved concern was independently reviewed by the corresponding author (Figure 1).

Table 1.
Main characteristics of the included studies (all studies were retrospective observational studies, and the number of decimal places is borrowed from the adopted articles).
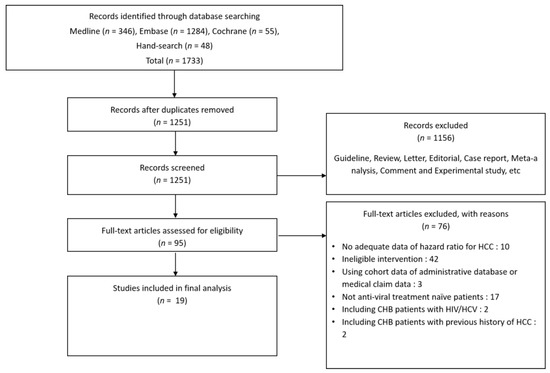
Figure 1.
Flow diagram showing the literature search (31 August 2021 record).
2.2. Inclusion and Exclusion Criteria
The inclusion criteria for the study selection were (1) antiviral-naive patients with CHB over 18 years of age; (2) human subject study design including randomised control trials (RCTs) and non-RCTs with two arms of either ETV or TDF monotherapy; and (3) suggesting the risk of HCC development with HR as a primary or secondary outcome.
Studies on (1) co-infection with other hepatotropic viruses (i.e., hepatitis C, D, or E virus) or human immunodeficiency virus; (2) unreported HCC incidence in either the TDF or ETV arm; (3) combination antiviral therapy or sequential therapy; and (4) observational retrospective cohort studies using administrative database or medical claim data were excluded from our analysis.
2.3. Data Extraction
Two investigators independently extracted data from each study using a predefined electronic spreadsheet to minimise random and bias errors. Any disagreement or unresolved concerns were independently reviewed by the corresponding author. If necessary, we contacted the co-author or corresponding author to rule out uncertainty (no mention of reference value being selected for multivariable Cox proportional hazards model: ETV or TDF [30,31]). As a result, all HRs were presented for the excess risk of each outcome among patients treated with TDF compared to ETV (extracted and calculated using ETV as a reference value).
2.4. Assessment of Methodological Quality
Two investigators independently evaluated the quality of the included studies using the Newcastle–Ottawa scale (NOS) for non-randomised studies (Table S2) [41]. Any disagreement or unresolved concerns were independently reviewed by the corresponding author.
2.5. Statistical Analysis
Extracted data were analysed with the inverse variance (IV) using the natural log of HRs as described by Parmar et al. [42] and the DerSimonian–Laird random-effects model for the meta-analysis. Heterogeneity across the enrolled studies was investigated using the Cochran Q test and Higgins I2 value. I2 values exceeding 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively. The level of significance for the test for heterogeneity was investigated using the chi-squared test [43]. Potential sources of heterogeneity were investigated using subgroup analyses with commonly applied enrolment criteria in the included studies and the start point of patient enrolment not reflected in enrolment criteria. To evaluate the source of heterogeneity, we examined the adopted variables for univariate and multivariate Cox regression analysis and propensity score matching (PSM) analysis (Tables S3 and S4). We also collected and compared the statistical techniques used in the studies, including methods for variable selection for Cox regression analysis, p-value cut-off for variable selection in the multivariate model, PSM method and calliper size, inverse probability treatment weighting (IPTW), competing for risk analysis, and multiple imputations for missing data. We used a funnel plot to visualise the publication bias. Using the arcsine Thompson’s (AS-Thompson’s) test, we evaluated the funnel plot asymmetry due to the high heterogeneity of the enrolled studies [44]. Statistical analyses were performed using R statistical software (version 3.6.3 (accessed on 29 February 2020)); R Foundation, Inc, Vienna, Austria.; (http://cran.r-project.org (accessed on 24 May 2022)) R package ‘meta’ and ‘metasens’.
The present systematic review of the literature was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement and checklist [45]. Patient consent and Institutional Review Board approval were not required because this was a systematic review of already published articles. This study is registered with the Open Science Framework (https://osf.io/ (accessed on 24 May 2022)), and its unique identifying number is: 10.17605/OSF.IO/964UA.
3. Results
Nineteen out of 1733 studies were included in the final meta-analysis. All included studies were observational retrospective cohort studies with 57,455 antiviral-naïve patients from hospital cohorts (Figure 1, Table S1). All studies were reported between 2017 and 2021, and 12 out of 19 studies were conducted in Korea (Table 1). The number of enrolled patients in major countries was 30,858 in Hong Kong, 18,684 in South Korea, 5565 in Taiwan, and 1819 in the U.S.A. The different studies had diverse inclusion and exclusion criteria (Table 2). The TDF and ETV treatment groups in these studies differed in terms of the time of treatment initiation (calendar year) and risk factors (host, hepatic, and viral). The studies used different variables for univariate and multivariable Cox regression analyses of the risk of HCC development and PSM analysis (Tables S3 and S4). In addition, the statistical methods used in the included articles were diverse (Table S5). All studies scored six to eight stars in the NOS, indicating satisfactory quality (Table S2).

Table 2.
Inclusion and exclusion criteria used in the included studies.
3.1. Pooled Analysis of Representative HRs Presented in Individual Papers
The pooled HR of 19 studies for HCC development with TDF over ETV monotherapy was 0.72 (95% confidence interval [CI], 0.58–0.90, p < 0.01) (Figure 2), indicating a significantly lower HR for HCC development in the TDF group than in the ETV group. However, the outcomes of the included studies showed substantial heterogeneity (I2 = 66.29%, p < 0.01) (Figure 2). The AS-Thompson test for publication bias found no significant asymmetry in the funnel plot (p > 0.1) (Figure S1).
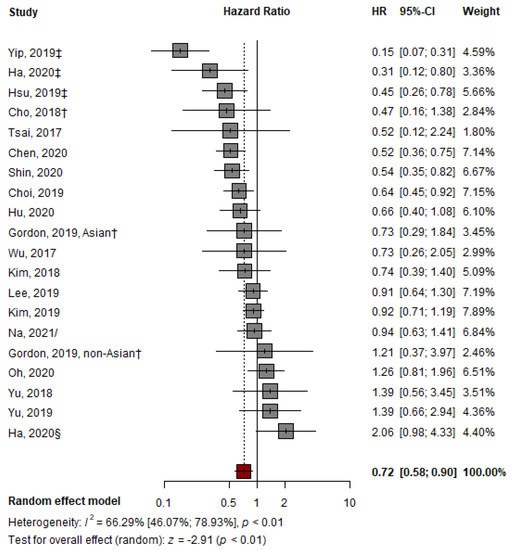
Figure 2.
Pooled analysis of representative HRs presented in individual papers comparing the effectiveness of TDF vs. ETV at reducing HCC development. HR: hazard ratio; † abstract; ‡ suggest outcomes from competing risk analysis; § Ha from CHA Bundang Medical Center, CHA University; / from unadjusted cohort at the time of CVR [7,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40].
3.2. Adjusted HR by Multivariable Analysis
Compared to the pooled HR of representative HRs from studies that presented adjusted HR, the adjusted pooled HR of 11 studies was 0.75 (TDF vs. ETV; 95% CI, 0.64–0.88; p < 0.01). This result indicates that the HR for HCC development in the TDF group was significantly lower than that in the ETV group. No significant heterogeneity was detected using the Q-test (I2 = 24%, p = 0.21) (Figure 3). The AS-Thompson test for publication bias found no significant asymmetry in the funnel plot (p > 0.1) (Figure S1). None of the variables were commonly adopted for multivariable analysis in all included studies (Table S3).
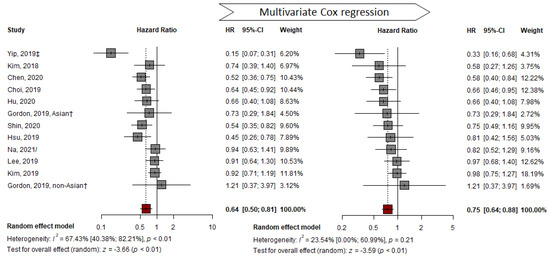
Figure 3.
Multivariable adjusted HR pooled analysis comparing the effectiveness of TDF vs. ETV at reducing HCC development. HR: hazard ratio; † abstract; ‡ suggest outcomes from competing risk analysis; / from unadjusted cohort at the time of CVR [7,23,24,25,26,27,29,33,38,39,40].
3.3. PS-Matched Population
In contrast to the pooled analysis of representative HRs and adjusted HRs, pooled analysis of the PS-matched sub-cohort with 10 studies showed no significant difference between the two groups (from HR: 0.65, 95% CI: 0.47–0.90, p < 0.05 to HR: 0.83, 95% CI: 0.65–1.06, p = 0.13). Substantial heterogeneity (I2 = 52%, p = 0.03) was detected in the outcomes (Figure 4). The number of subjects decreased from 49,706 to 20,151 after PSM (Table S6). No significant asymmetry in the funnel plot was observed using the AS-Thompson test for publication bias (p > 0.1) (Figure S1).
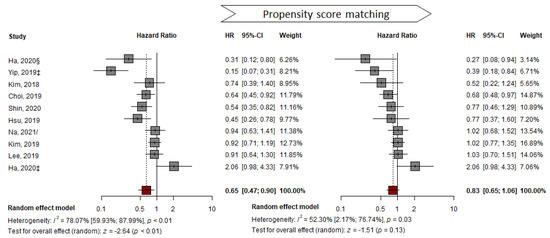
Figure 4.
Propensity-score matched HR pooled analysis comparing the effectiveness of TDF vs. ETV at reducing HCC development. ‡ suggest outcomes from competing risk analysis; § Ha from CHA Bundang Medical Center, CHA University; / from unadjusted cohort at the time of CVR [7,24,25,26,27,29,33,35,37,40].
3.4. Cirrhotic Subcohort
In CHB cirrhosis sub-cohorts, the pooled representative HRs showed a significantly lower risk of HCC development in the TDF group than in the ETV group (HR: 0.75, 95% CI: 0.58–0.96, p = 0.02). However, this was not consistent with the findings of the pooled analysis with adjusted HR (HR: 0.80, 95% CI: 0.64–1.00, p = 0.054) or the PS-matched population (HR: 0.95, 95% CI: 0.78–1.16, p = 0.632). Since fewer than 10 studies were included, the results should be interpreted with caution (Figure S2).
3.5. Subgroup Analysis
To determine the cause of heterogeneity, a subgroup analysis was conducted based on each study design and patient enrolment criterion. An interval of over three years in the start points of patient enrolment (or U.S. Food and Drug Administration (FDA) approval date of TDF and ETV) between the two groups resulted in a lower risk of HCC development in the TDF group than that in the ETV group (HR: 0.83, 95% CI: 0.62–1.12 vs. HR: 0.69, 95% CI: 0.51–0.92) (Table 3, Figure S3). Additionally, the exclusion of patients with significant alcoholic liver disease lowered the risk of developing HCC in the TDF group compared to the ETV group (HR: 0.58, 95% CI: 0.44–0.76, p < 0.01) (Table 3, Figure S4). All four studies that excluded patients with significant alcoholic liver disease were conducted in Taiwan.

Table 3.
Subgroup analysis comparing clinical outcomes based on the inclusion and exclusion criteria.
4. Discussion
The novelty of this study lies in the fact that we extracted and analysed data from only antiviral-naïve CHB patients. In a 12-year follow-up cohort study, Papatheodoridis et al. found a significant difference in the development of HCC in NA-naïve (67/1128; 5.9%) vs. NA-experienced (76/807 or 9.4%) patients (p = 0.004) [46]. Since we analysed only NA-naïve CHB patients, there was no concern about ETV resistance caused by previous drug exposure, thereby reducing the heterogeneity when comparing the effects of drugs.
In our study, the significance of pooled HR was negligible in the PS-matched population when compared with the representative HRs presented in individual papers with adjusted HRs. Therefore, it is important to determine the compounding factors that reduce heterogeneity in adjusted, PS-matched subpopulations and affect HCC development other than drug choice.
In the subgroup analysis, we observed significant differences in the clinical outcomes of the two groups due to differences in patient enrolment timing (Table 3). After the FDA approval of the two drugs (ETV 2005 and TDF 2008), there have been many modifications to the international treatment guidelines, and the indications for the application of NAs vary from country to country (Figure 5). Although the analysis methods are different, inconsistency in clinical outcomes could arise from disparities in the follow-up length, as discussed in a similar meta-analysis [8]. Additionally, a study by Chen et al. addresses the implications of this disparity [39]. Taiwan is a country with a National Health Insurance system, and TDF has been included in the benefits eligibility since 2011 (Table S7) [47]. Chen et al. found that TDF treatment was associated with a lower risk of HCC in the entire (n = 1560, HR: 0.585, 95% CI: 0.425–0.806, p < 0.001) and treatment-naïve (n = 1353, HR: 0.523, 95% CI: 0.363–0.752, p < 0.005) cohorts [39]. However, a subgroup analysis of patients (not restricted to naive patients only) enrolled after 2011 did not find a lower risk of HCC (n = 1162, HR: 1.987 95% CI: 1.392–2.837, p < 0.001). Before reimbursements for TDF treatments began, CHB patients with a relatively high risk of developing HCC and waiting to be reimbursed for antiviral treatments started to take ETV, which may account for the higher incidence of HCC in the ETV group.
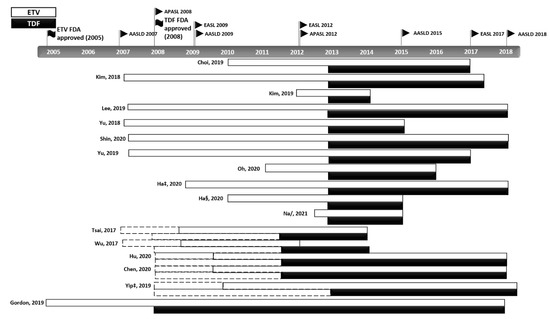
Figure 5.
Modifications to the international treatment guidelines for CHB. The various changes and updates to the CHB guidelines and reimbursement policies and differences in the start dates of patient enrolment are shown. Dotted line: drug prescription available; solid line: drug prescription with reimbursement benefits available. ‡ suggest outcomes from competing risk analysis; § Ha from CHA Bundang Medical Center, CHA University; / from unadjusted cohort at the time of CVR [7,23,24,25,26,27,28,29,30,31,32,35,36,37,38,39,40].
Similar to Taiwan, South Korea also has a National Health Insurance system. Twelve studies in this analysis (63%) included CHB patients from South Korea. In South Korea, when the TDF reimbursement benefits were available, the indications for its use were eased compared to those for ETV. As a result, the severity of the antiviral treated patient group decreased. A cohort study comparing the ETV and TDF groups by year of enrolment or reimbursement policy should be designed to demonstrate this. Oh et al. designed a study to reduce the influence of reimbursement policy, controlling the treatment start date so that the same criteria were applied. They found that TDF treatment was not associated with a lower risk of HCC (HR: 1.26, 95% CI: 0.81–1.97, p = 0.303). However, a limitation of their study was that the treatment starting date for the two groups did not match [36].
A subgroup analysis also showed a significantly lower risk of HCC development in the TDF group when patients with alcoholic liver disease were excluded from the study. All of these studies were conducted in Taiwan. Although not significant, Taiwanese studies included a relatively higher proportion of male CHB patients (>70%) than studies from other countries. If there is a difference even in patients with alcoholic liver disease with a relatively high risk of HCC due to high drinking and smoking rates, the characteristics of the female group may have contributed to it, given the preference for TDF in women of childbearing age, which may have caused a bias [48].
There was substantial heterogeneity in the enrolled studies. One of the causes for this is the differences in their inclusion and exclusion criteria. First, while some papers excluded CHB patients who developed HCC within six months, others excluded those who developed HCC within one year. It has been reported that the tumour volume doubling time (TVDT) of HCC is approximately 4–5 months [49], and regular HCC surveillance is typically implemented every six months. It is therefore difficult to rule out the possibility that HCC present at the start of the treatment was not included by excluding patients who developed HCC within six months. Second, patients with baseline HBV DNA levels of <2000 IU/mL were excluded in several studies [7,24,28,35]. Four such studies report contradictory statistical significance, possibly due to differences in their exclusion criteria. In Korea, which accounted for 63% of the studies in the present meta-analysis, if patients with baseline HBV DNA levels of <2000 IU/mL were excluded, decompensated cirrhosis patients would have been automatically excluded owing to the change in reimbursement policy since September 2015 (Table S7), resulting in a significant difference in HCC risk between the TDF and ETV groups.
Moreover, comorbidities in CHB patients could affect the development of HCC. Patients with chronic kidney disease (CKD) and osteoporosis might have been prioritised for ETV treatment due to safety issues, even when both drugs were available for prescription [6]. In the articles included in this study, before PS matching, the age and comorbidities, including hypertension, diabetes, and CKD, were higher in the ETV group, although not significant. In a recent case-control study using medical claims data from South Korea, the proportion of patients with CKD was higher among those with CHB than among matched controls (3.02% vs. 1.14%, p < 0.01) [50]. In a retrospective observational study, patients with stages 4 and 5 CKD showed a higher incidence of HCC, although the cohort included patients with chronic hepatitis C and hepatitis B and C co-infected patients [51].
Medication compliance was also an important covariate. Very few studies in the present meta-analysis adopted the cumulative defined daily dose (cDDD) as a covariate [25,37]. Medication compliance should be treated as an important compounding factor, as poor compliance affects the emergence of the ETV mutant, which is one of the risk factors for HCC development [52]. Unlike TDF, ETV is a pre-meal drug, and its pre-prandial administration is associated with non-adherence [53]. In the enrolled studies, the ETV group appeared to have a relatively low cDDD [25,37]. In a study by Choi et al. [7], the treatment modification rate was significantly higher among ETV users than among TDF users (182/1560; 11.7% vs. 2/1141; 0.2%). In South Korea, replacing or switching to other NAs is difficult because of reimbursement issues unless physicians prove drug resistance, insufficient treatment response, pregnancy, or serious side effects through documentation. Therefore, for the reasons listed above, it can be assumed that the treatment response in the ETV user group was poor.
Differences in methods of statistical analysis were also considered. Previous studies have used several techniques, such as PSM, IPTW, multiple imputations, and competing risk analysis. The Cox proportional hazards model can adopt a direction/forward/backward stepwise method. Depending on the study design, both direction methods are generally recommended; however, most enrolled studies did not mention which method was chosen (Table S5).
A limitation of retrospective cohort studies is that several unobservable confounding factors may be present. Even if residual heterogeneity is allowed, patients may experience deterioration of cirrhosis owing to lifestyle [54], alcohol consumption, or poor medication compliance. Moreover, it is well known that fatty liver disease [55], family history [56], concomitant medications [57,58], and exposure to aflatoxin B1 [59] affect HCC incidence. These above factors, causing inflammatory reactions, can stimulate the multistep process of hepatocarcinogenesis [60]. Therefore, predicting HCC development based on the baseline characteristics without considering events occurring during the observation period can lead to inaccuracy. In a recent study, Lee et al. suggested that the presence of cirrhosis at the time of HBeAg seroclearance could be a compounding factor for HCC development [61].
This study has considerable limitations. About 97% of patients were derived from Asia, and thus, the characteristics of patients in this study may be different from Caucasian cohorts. In a recent study comparing 9143 Korean and 719 Caucasian CHB patients, Jang et al. found higher HBeAg positivity among Koreans (49.1%) than Caucasians (20.3%). Nevertheless, the HBeAg-positive phase occurs early in the natural course, while the proportion of LC was also higher among Koreans (41.1%) than Caucasians (31.5%) [62]. Moreover, the majority of studies did not present virologic data, such as the genotype of HBV, which is known to have different geographical distributions [63]. Therefore, HBeAg status as well as various characteristics such as cirrhosis status and genotype of HBV should be taken into account when interpreting the results.
5. Conclusions
In conclusion, our analysis found that the incidence of HCC following TDF monotherapy was significantly lower than after ETV monotherapy with high heterogeneity. However, this difference was not seen with a pooled HR in a PS-matched sub-cohort that reduced the heterogeneity of the TDF and ETV user groups. There are many observable and unobservable confounding factors that can affect the heterogeneity of these studies. Even with several statistical techniques, such as PSM analysis, socioeconomic factors such as reimbursement policies may not be corrected. As a limitation of retrospective-cohort studies, there is not enough data to establish different efficacies of TDF and ETV on incidence of HCC in CHB patients. Therefore, further prospective studies with standardised protocols or individual patient data meta-analyses are needed to reduce the residual heterogeneity that may affect HCC development by mechanisms other than drug choice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14112617/s1, Figure S1: Analysis of publication bias, Figure S2: Pooled analysis of representative HRs presented in individual papers/multivariable-adjusted HR/propensity score-matched HR in the cirrhotic subcohort, Figure S3: Pooled HR from a subgroup analysis according to the starting point, Figure S4: Pooled HR from a subgroup analysis after excluding alcoholic liver disease, Table S1: Search strategies; Table S2: Newcastle-Ottawa scale for non-randomized studies (abstracts were excluded from assessment), Table S3: Adjusted variables for Cox regression analyses for risk of HCC development in the included articles, Table S4: Adopted variables for propensity score matching analysis to reduce selection bias and the effect of potential confounders in the included articles, Table S5: Statistical methods used in the included articles, Table S6: Characteristics after propensity score matching analysis in the included studies, Table S7: Reimbursement policies for antiviral therapies.
Author Contributions
Conceptualization, Y.J.K.; methodology, H.O.; formal analysis, H.O.; investigation, H.O. and H.Y.L.; data curation, H.O. and H.Y.L.; writing—original draft preparation, H.O. and H.Y.L.; writing—review and editing, H.O, H.Y.L., J.K. and Y.J.K.; visualization, H.O.; supervision, Y.J.K.; project administration, Y.J.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by an investigator-initiated grant (Grant number: SJ-IIT-23-11) from Samjin Pharmaceutical Co., Ltd. Korea, which was not involved in the study design, data collection and analysis, or decision to publish.
Institutional Review Board Statement
Ethical review and approval were waived for this study because this is a meta-analysis based on the previously published studies.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are included in the study and supplementary information.
Conflicts of Interest
Y.J.K.: Research grants from Samjin Pharmaceutical Co., Ltd., BTG, AstraZeneca, Roche, Bristol-Myers Squibb, MSD, and Gilead Sciences.
References
- World Health Organization. Hepatitis B. 27 July 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 18 April 2021).
- Hui, V.W.; Chan, S.L.; Wong, V.W.; Liang, L.Y.; Yip, T.C.; Lai, J.C.; Yuen, B.W.; Luk, H.W.; Tse, Y.K.; Lee, H.W.; et al. Increasing antiviral treatment uptake improves survival in patients with HBV-related HCC. JHEP Rep. 2020, 2, 100152. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.U.; Seo, Y.S.; Lee, H.A.; Kim, M.N.; Lee, E.J.; Shin, H.J.; Lee, Y.R.; Lee, H.W.; Park, J.Y.; Kim, D.Y.; et al. Hepatocellular Carcinoma Risk Steadily Persists over Time Despite Long-Term Antiviral Therapy for Hepatitis B: A Multicenter Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 832–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.L.; Kim, G.A.; Park, J.A.; Kang, H.R.; Lee, E.K.; Lim, Y.S. Cost-effectiveness of antiviral treatment in adult patients with immune-tolerant phase chronic hepatitis B. Gut 2020, 70, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef] [PubMed]
- EASL. 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Kim, H.J.; Lee, J.; Cho, S.; Ko, M.J.; Lim, Y.S. Risk of Hepatocellular Carcinoma in Patients Treated with Entecavir vs. Tenofovir for Chronic Hepatitis B: A Korean Nationwide Cohort Study. JAMA Oncol. 2019, 5, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Peng, Y.; Hao, F.B.; Wang, Y.Q.; Wang, C.R.; Zhong, G.C. No difference in hepatocellular carcinoma risk in chronic hepatitis B patients treated with tenofovir vs. entecavir: Evidence from an updated meta-analysis. Aging 2021, 13, 7147–7165. [Google Scholar] [CrossRef]
- Dave, S.; Park, S.; Murad, M.H.; Barnard, A.; Prokop, L.; Adams, L.A.; Singh, S.; Loomba, R. Comparative Effectiveness of Entecavir Versus Tenofovir for Preventing Hepatocellular Carcinoma in Patients with Chronic Hepatitis B: A Systematic Review and Meta-Analysis. Hepatology 2021, 73, 68–78. [Google Scholar] [CrossRef]
- Choi, W.M.; Choi, J.; Lim, Y.S. Effects of Tenofovir vs. Entecavir on Risk of Hepatocellular Carcinoma in Patients with Chronic HBV Infection: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 246–258.e9. [Google Scholar] [CrossRef]
- Xia, Z.; He, L.; Xiong, L.; Wen, T. The comparison of different antiviral therapies on the prognosis of hepatitis B virus-related hepatocellular carcinoma after curative treatments: A network meta-analysis. Medicine 2020, 99, e20877. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Dang, Z.; Yu, L.; Jiang, Y.; Wang, X.; Yan, Z. Nucleos(t)ide Analogues for Reducing Hepatocellular Carcinoma in Chronic Hepatitis B Patients: A Systematic Review and Meta-Analysis. Gut Liver 2020, 14, 232–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.-H.; Hsu, Y.-C.; Chen, T.-H.; Ji, F.; Chen, I.S.; Tsai, Y.-N.; Hai, H.; Thuy, L.T.T.; Hosaka, T.; Sezaki, H.; et al. Hepatocellular carcinoma incidence with tenofovir versus entecavir in chronic hepatitis B: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 1039–1052. [Google Scholar] [CrossRef]
- Teng, Y.X.; Li, M.J.; Xiang, B.D.; Zhong, J.H. Tenofovir may be superior to entecavir for preventing hepatocellular carcinoma and mortality in individuals chronically infected with HBV: A meta-analysis. Gut 2020, 69, 1900–1902. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, Y.; Hayden, J.C.; Ryan, P.M.; Rahmani, J.; Yu, G. Tenofovir Treatment Has Lower Risk of Hepatocellular Carcinoma than Entecavir Treatment in Patients with Chronic Hepatitis B: A Systematic Review and Meta-Analysis. Liver Cancer 2020, 9, 468–476. [Google Scholar] [CrossRef]
- Li, M.; Lv, T.; Wu, S.; Wei, W.; Wu, X.; Ou, X.; Ma, H.; Chow, S.C.; Kong, Y.; You, H.; et al. Tenofovir versus entecavir in lowering the risk of hepatocellular carcinoma development in patients with chronic hepatitis B: A critical systematic review and meta-analysis. Hepatol. Int. 2020, 14, 105–114. [Google Scholar] [CrossRef]
- Gu, L.; Yao, Q.; Shen, Z.; He, Y.; Ng, D.M.; Yang, T.; Chen, B.; Chen, P.; Mao, F.; Yu, Q. Comparison of tenofovir versus entecavir on reducing incidence of hepatocellular carcinoma in chronic hepatitis B patients: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2020, 35, 1467–1476. [Google Scholar] [CrossRef]
- Cheung, K.S.; Mak, L.Y.; Liu, S.H.; Cheng, H.M.; Seto, W.K.; Yuen, M.F.; Lai, C.L. Entecavir vs. Tenofovir in Hepatocellular Carcinoma Prevention in Chronic Hepatitis B Infection: A Systematic Review and Meta-Analysis. Clin. Transl. Gastroenterol. 2020, 11, e00236. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Yang, J.; Hu, K.; Huang, Y. The effectiveness of TDF versus ETV on incidence of HCC in CHB patients: A meta analysis. BMC Cancer 2019, 19, 511. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Cho, Y.; Park, S.M.; Kim, W. Differential Effectiveness of Tenofovir and Entecavir for Prophylaxis of Hepatocellular Carcinoma in Chronic Hepatitis B Patients Depending on Coexisting Cirrhosis and Prior Exposure to Antiviral Therapy: A Systematic Review and Meta-analysis. J. Clin. Gastroenterol. 2021, 55, e77–e86. [Google Scholar] [CrossRef]
- Kunz, L.M.; Normand, S.L.; Sedrakyan, A. Meta-analysis of rate ratios with differential follow-up by treatment arm: Inferring comparative effectiveness of medical devices. Stat. Med. 2015, 34, 2913–2925. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.M.; Yip, T.C.; Lim, Y.S.; Wong, G.L.; Kim, W.R. Methodological challenges of performing meta-analyses to compare the risk of hepatocellular carcinoma between chronic hepatitis B treatments. J. Hepatol. 2022, 76, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.C.; Zhou, Y.; Li, J.; Rupp, L.B.; Boscarino, J.A.; Daida, Y.G.; Schmidt, M.A.; Trudeau, S.; Lu, M. Effect of treatment of hepatitis B patients with tenofovir disoproxil or entecavir on risk of hepatocellular cancer death in a U.S. Cohort. J. Hepatol. 2019, 70, e147. [Google Scholar] [CrossRef]
- Kim, B.G.; Park, N.H.; Lee, S.B.; Lee, H.; Lee, B.U.; Park, J.H.; Jung, S.W.; Jeong, I.D.; Bang, S.J.; Shin, J.W. Mortality, liver transplantation and hepatic complications in patients with treatment-naïve chronic hepatitis B treated with entecavir vs. tenofovir. J. Viral Hepat. 2018, 25, 1565–1575. [Google Scholar] [CrossRef]
- Shin, J.W.; Jeong, J.; Jung, S.W.; Lee, S.B.; Park, B.R.; Kim, M.-J.; Park, E.J.; Park, N.H. Comparable Incidence of Hepatocellular Carcinoma in Chronic Hepatitis B Patients Treated with Entecavir or Tenofovir. Dig. Dis. Sci. 2020, 66, 1739–1750. [Google Scholar] [CrossRef]
- Kim, S.U.; Seo, Y.S.; Lee, H.A.; Kim, M.N.; Lee, Y.R.; Lee, H.W.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H.; et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naïve chronic hepatitis B in South Korea. J. Hepatol. 2019, 71, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kwon, J.H.; Lee, H.L.; Yoo, S.H.; Nam, H.C.; Sung, P.S.; Nam, S.W.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; et al. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naïve patients with chronic hepatitis B in Korea: A large-scale, propensity score analysis. Gut 2020, 69, 1301–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, M.C.; Chen, C.H.; Hu, T.H.; Lu, S.N.; Lee, C.M.; Wang, J.H.; Hung, C.H. Long-term outcomes of hepatitis B virus-related cirrhosis treated with nucleos(t)ide analogs. J. Formos. Med. Assoc. 2017, 116, 512–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, T.C.; Wong, V.W.; Chan, H.L.; Tse, Y.K.; Lui, G.C.; Wong, G.L. Tenofovir Is Associated with Lower Risk of Hepatocellular Carcinoma Than Entecavir in Patients with Chronic HBV Infection in China. Gastroenterology 2020, 158, 215–225.e216. [Google Scholar] [CrossRef]
- Yu, J.H.; Jin, Y.J.; Lee, J.W.; Lee, D.H. Remaining hepatocellular carcinoma risk in chronic hepatitis B patients receiving entecavir/tenofovir in South Korea. Hepatol. Res. 2018, 48, 862–871. [Google Scholar] [CrossRef]
- Yu, J.H.; Suh, Y.J.; Jin, Y.J.; Heo, N.Y.; Jang, J.W.; You, C.R.; An, H.Y.; Lee, J.W. Prediction model for hepatocellular carcinoma risk in treatment-naive chronic hepatitis B patients receiving entecavir/tenofovir. Eur. J. Gastroenterol. Hepatol. 2019, 31, 865–872. [Google Scholar] [CrossRef]
- Wu, I.T.; Hu, T.H.; Hung, C.H.; Lu, S.N.; Wang, J.H.; Lee, C.M.; Chen, C.H. Comparison of the efficacy and safety of entecavir and tenofovir in nucleos(t)ide analogue-naive chronic hepatitis B patients with high viraemia: A retrospective cohort study. Clin. Microbiol. Infect. 2017, 23, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.C.; Wong, G.L.; Chen, C.H.; Peng, C.Y.; Yeh, M.L.; Cheung, K.S.; Toyoda, H.; Huang, C.F.; Trinh, H.; Xie, Q.; et al. Tenofovir Versus Entecavir for Hepatocellular Carcinoma Prevention in an International Consortium of Chronic Hepatitis B. Am. J. Gastroenterol. 2020, 115, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.W.; Jang, J.W.; Chae, H.B.; Kim, S.B.; Song, I.H. Long-term clinical outcomes of chronic Hepatitis B patients treated with Entecavir vs. Tenofovir: A retrospective, obseravational, comparative study. In Hepatology; Posters (Abstracts 301–2389); Wiley: Hoboken, NJ, USA, 2018; Volume 68, pp. 184–1353. [Google Scholar]
- Ha, I.; Chung, J.W.; Jang, E.S.; Jeong, S.H.; Kim, J.W. Comparison of the on-treatment risks for hepatocellular carcinoma between entecavir and tenofovir: A propensity score matching analysis. J. Gastroenterol. Hepatol. 2020, 35, 1774–1781. [Google Scholar] [CrossRef]
- Oh, H.; Yoon, E.L.; Jun, D.W.; Ahn, S.B.; Lee, H.Y.; Jeong, J.Y.; Kim, H.S.; Jeong, S.W.; Kim, S.E.; Shim, J.J.; et al. No Difference in Incidence of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B Virus Infection Treated with Entecavir vs. Tenofovir. Clin. Gastroenterol. Hepatol. 2020, 18, 2793–2802.e2796. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Chon, Y.E.; Kim, M.N.; Lee, J.H.; Hwang, S.G. Hepatocellular carcinoma and death and transplantation in chronic hepatitis B treated with entecavir or tenofovir disoproxil fumarate. Sci. Rep. 2020, 10, 13537. [Google Scholar] [CrossRef]
- Hu, T.H.; Yueh-Hsia Chiu, S.; Tseng, P.L.; Chen, C.H.; Lu, S.N.; Wang, J.H.; Hung, C.H.; Kee, K.M.; Lin, M.T.; Chang, K.C.; et al. Five-year comparative risk of hepatocellular carcinoma development under entecavir or tenofovir treatment-naïve patients with chronic hepatitis B-related compensated cirrhosis in Taiwan. Aliment. Pharm. Ther. 2020, 52, 1695–1706. [Google Scholar]
- Chen, C.H.; Chen, C.Y.; Wang, J.H.; Lai, H.C.; Hung, C.H.; Lu, S.N.; Peng, C.Y. Comparison of incidence of hepatocellular carcinoma between chronic hepatitis B patients with cirrhosis treated with entecavir or tenofovir in Taiwan—A retrospective study. Am. J. Cancer Res. 2020, 10, 3882–3895. [Google Scholar]
- Na, J.E.; Sinn, D.H.; Lee, J.H.; Jang, H.J.; Baek, S.Y.; Kim, K.A.; Kang, W.S.; Gwak, G.Y.; Paik, Y.H.; Kim, Y.J.; et al. Efficacy of entecavir versus tenofovir in preventing hepatocellular carcinoma in patients with chronic hepatitis B with maintained virologic response. J. Viral Hepat. 2021, 28, 1392–1399. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [Green Version]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Cochrane: London, UK, 2021; Available online: www.training.cochrane.org/handbook (accessed on 10 October 2021).
- Rücker, G.; Schwarzer, G.; Carpenter, J. Arcsine test for publication bias in meta-analyses with binary outcomes. Stat. Med. 2008, 27, 746–763. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Papatheodoridis, G.V.; Dalekos, G.N.; Idilman, R.; Sypsa, V.; Van Boemmel, F.; Buti, M.; Calleja, J.L.; Goulis, J.; Manolakopoulos, S.; Loglio, A.; et al. Similar risk of hepatocellular carcinoma during long-term entecavir or tenofovir therapy in Caucasian patients with chronic hepatitis B. J. Hepatol. 2020, 73, 1037–1045. [Google Scholar] [CrossRef]
- Lim, S.G.; Amarapurkar, D.N.; Chan, H.L.; Crawford, D.H.; Gane, E.J.; Han, K.H.; Ahn, S.H.; Jafri, W.; Jia, J.; Kao, J.H.; et al. Reimbursement policies in the Asia-Pacific for chronic hepatitis B. Hepatol. Int. 2015, 9, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Q.; Duan, Z.; Dai, E.; Zhang, S.; Han, G.; Wang, Y.; Zhang, H.; Zou, H.; Zhu, B.; Zhao, W.; et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. N. Engl. J. Med. 2016, 374, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Nathani, P.; Gopal, P.; Rich, N.; Yopp, A.; Yokoo, T.; John, B.; Marrero, J.; Parikh, N.; Singal, A.G. Hepatocellular carcinoma tumour volume doubling time: A systematic review and meta-analysis. Gut 2021, 70, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Jun, D.W.; Lee, I.H.; Ahn, H.J.; Kim, B.O.; Jung, S.; Nguyen, M.H. Increasing comorbidities in a South Korea insured population-based cohort of patients with chronic hepatitis B. Aliment. Pharmacol. Ther. 2020, 52, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hsieh, S.Y.; Lin, J.L.; Liu, M.S.; Yen, T.H. Hepatocellular carcinoma in patients with chronic kidney disease. World J. Gastroenterol. 2013, 19, 2466–2472. [Google Scholar] [CrossRef]
- Miyauchi, T.; Kanda, T.; Shinozaki, M.; Kamezaki, H.; Wu, S.; Nakamoto, S.; Kato, K.; Arai, M.; Mikami, S.; Sugiura, N.; et al. Efficacy of lamivudine or entecavir against virological rebound after achieving HBV DNA negativity in chronic hepatitis B patients. Int. J. Med. Sci. 2013, 10, 647–652. [Google Scholar] [CrossRef] [Green Version]
- Koyanagi, K.; Kubota, T.; Kobayashi, D.; Kihara, T.; Yoshida, T.; Miisho, T.; Miura, T.; Sakamoto, Y.; Takaki, J.; Seo, T.; et al. Prescription Factors Associated with Medication Non-adherence in Japan Assessed from Leftover Drugs in the SETSUYAKU-BAG Campaign: Focus on Oral Antidiabetic Drugs. Front. Pharmacol. 2016, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Chun, H.S.; Park, S.; Lee, M.; Cho, Y.; Kim, H.S.; Choe, A.R.; Kim, H.Y.; Yoo, K.; Kim, T.H. Association of Physical Activity with the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B. Cancers 2021, 13, 3424. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, R.; Nault, J.C.; Roberts, L.R.; Zucman-Rossi, J. Genomic Medicine and Implications for Hepatocellular Carcinoma Prevention and Therapy. Gastroenterology 2019, 156, 492–509. [Google Scholar] [CrossRef]
- Park, C.H.; Jeong, S.H.; Yim, H.W.; Kim, J.D.; Bae, S.H.; Choi, J.Y.; Yoon, S.K. Family history influences the early onset of hepatocellular carcinoma. World J. Gastroenterol. 2012, 18, 2661–2667. [Google Scholar] [CrossRef] [PubMed]
- Hui, V.W.; Yip, T.C.; Wong, V.W.; Tse, Y.K.; Chan, H.L.; Lui, G.C.; Wong, G.L. Aspirin Reduces the Incidence of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B Receiving Oral Nucleos(t)ide Analog. Clin. Transl. Gastroenterol. 2021, 12, e00324. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.J.; Sinn, D.H.; Kim, S.; Woo, S.Y.; Cho, H.; Kang, W.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; et al. Statin Use and the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B. Hepatology 2020, 71, 2023–2032. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [Green Version]
- Leone, P.; Solimando, A.G.; Fasano, R.; Argentiero, A.; Malerba, E.; Buonavoglia, A.; Lupo, L.G.; De Re, V.; Silvestris, N.; Racanelli, V. The Evolving Role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma Treatment. Vaccines 2021, 9, 532. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, J.I.; Kim, S.; Kim, S.; Chang, H.Y.; Lee, K.S. Cumulative incidence of hepatocellular carcinoma and hepatitis B surface antigen Seroclearance after Nucleos(t) ide analogue-induced hepatitis B e antigen Seroclearance. BMC Gastroenterol. 2020, 20, 11360. [Google Scholar] [CrossRef]
- Jang, H.; Yoon, J.S.; Park, S.Y.; Lee, H.A.; Jang, M.-j.; Kim, S.U.; Sinn, D.H.; Seo, Y.S.; Kim, H.Y.; Kim, S.E.; et al. Impact of HBeAg on Hepatocellular Carcinoma Risk During Oral Antiviral Treatment in Patients with Chronic Hepatitis B. Clin. Gastroenterol. Hepatol. 2022, 20, 1343–1353. [Google Scholar] [CrossRef]
- Wong, G.L.; Chan, H.L.; Yiu, K.K.; Lai, J.W.; Chan, V.K.; Cheung, K.K.; Wong, E.W.; Wong, V.W. Meta-analysis: The association of hepatitis B virus genotypes and hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2013, 37, 517–526. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).