Simple Summary
Nipple aspirate fluid (NAF) is a promising source of markers for detection of breast cancer. NAF can be acquired via the nipple by aspiration using a suction device, which is well tolerated by women. Future possible applications of biomarkers for breast cancer derived from NAF could be (1) as a detection tool to identify the initiation of the cancer development process, (2) as an additional tool next to imaging (mammography and breast magnetic resonance imaging) or (3) as a replacement tool for when imaging is not advisable for women, such as during pregnancy and breastfeeding. With this paper, we present a narrative review and perspectives of NAF research at a glance.
Abstract
Nipple aspirate fluid (NAF) is an intraductal mammary fluid that, because of its close proximity to and origin from the tissue from which breast cancer originates, is a promising source of biomarkers for early breast cancer detection. NAF can be non-invasively acquired via the nipple by aspiration using a suction device; using oxytocin nasal spray helps increase yield and tolerability. The aspiration procedure is generally experienced as more tolerable than the currently used breast imaging techniques mammography and breast magnetic resonance imaging. Future applications of NAF-derived biomarkers include their use as a tool in the detection of breast carcinogenesis at its earliest stage (before a tumor mass can be seen by imaging), or as a supporting diagnostic tool for imaging, such as when imaging is less reliable (to rule out false positives from imaging) or when imaging is not advisable (such as during pregnancy and breastfeeding). Ongoing clinical studies using NAF samples will likely shed light on NAF’s content and clinical potential. Here, we present a narrative review and perspectives of NAF research at a glance.
1. What Is Nipple Aspirate Fluid?
NAF can be defined as the physiological fluid that is present in the breast ductal system (Figure 1). As indicated by the name, NAF can be acquired via the nipple by aspiration using a suction device, and, as such, non-invasively. This collection procedure differs from ductal lavage, a more invasive procedure in which a microcatheter is used to cannulate and flush the ducts [1]. Of relevance too is the difference in definition between NAF and pathological nipple discharge (PND). PND is defined as spontaneous, unilateral nipple discharge that can be an indication of benign (and rarely malignant) breast disease [2,3]. In contrast, NAF, which is fluid physiologically present in the breast ducts, in normal circumstances does not spontaneously leave the breast.
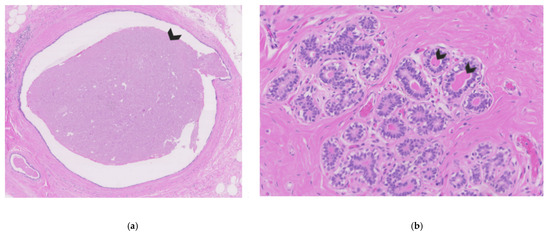
Figure 1.
Breast tissue of a non-lactating breast. (a) Cross-section of breast ducts filled with nipple fluid on the inside (see arrow). (b) Cross-section of breast lobules with acini filled with eosinophilic nipple fluid on the inside (arrows indicate two examples).
2. How Is NAF Produced?
The backbone of the breast is the lobular–ductal system which branches into the breast from the nipple as has elegantly been shown in studies using contrast-injection in the nipple followed by mammography (galactography) [4] and in three-dimensional computer reconstructions [5,6]. The lobular–ductal system contains two main cell types, the luminal cells and the myoepithelial (basal) cells (Figure 2), and is surrounded by fatty tissue and extracellular matrix [7,8].
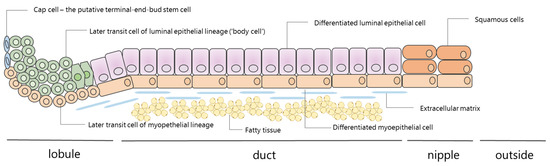
Figure 2.
The mammary lobular–ductal system: cellular composition of the lobule, duct, and nipple (half linear representation). The breast contains around 5–12 mammary lobulo–ductal systems that start at the terminal end-bud and reach the nipple. Several lobulo–ductal structures can come together, leading to branching of this system into common ducts that end in the nipple. The mammary duct is composed of an outer layer of myoepithelial cells and an inner layer of luminal epithelial cells. At the end, near the nipple, there is a transition to squamous cells. Nipple aspirate fluid is probably produced by the inner luminal layer. The cap cells can take on either a myoepithelial lineage or a luminal epithelial lineage and therefore are thought to be multipotent stem cells. This scheme is adapted from Smalley and Ashworth [29] and Jakub et al. [30].
NAF is believed to be produced by the luminal epithelial cells (Figure 2), which are also responsible for breast milk production during lactation [9]. More specifically, the components present in NAF are thought to be synthesized within the breast epithelium and directly released into the ducts, and/or to be transferred from the circulation into the breast fluid [10]. The first theory is based on studies that have shown that components like estrogen, which can be produced in breast epithelial cells and stromal breast cells, have a higher concentration in NAF compared to serum [11]. The latter theory rests on studies that have found exogenously derived substances in ductal fluid, like nicotine in NAF of smokers [12,13,14,15]. The physiology of NAF also includes a reabsorption mechanism to blood or lymphatics. This has been shown in studies in which intraductal injections of India ink moved through the ductal walls to the lymphatics [14] and also by the fact that intraductal injections of cisplatin in mice led to systemic side effects [16].
NAF is in theory present in every breast. The success percentages of obtaining NAF are very high (77–95%) [17,18,19,20]. Unsuccessful aspiration can be explained by factors like the collection procedure, the number and duration of attempts, use of oxytocin to release NAF and whether the existing NAF is able to reach the nipple. Next to the procedure itself, it is believed that characteristics from women, like younger age [21,22], history of breastfeeding [21,23], not taking oral contraception [24], and soy consumption [25] could be good predictors for successful NAF collection; however, other studies do not confirm these data [23,26,27,28]. The presence of nipple fluid can microscopically be seen in breast tissues, as shown in Figure 1.
3. How Is NAF Collected?
NAF can be obtained by nipple fluid aspiration (NFA) with a (manual or electrical) suction device that creates negative pressure around the nipple. The manual suction device is comparable to a breast pump and consists of a cup that is placed on the breast around the nipple, a plastic tube, and a syringe, which are connected (Figure 3). The negative pressure is created manually by drawing the plunger of the syringe (that is at the one end of the plastic tube) back and forth. In our experience, this movement can be facilitated by the use of a handle that fits the plunger of the syringe, which makes this more ergonomic (see Figure 3c). The aspiration cup used can be a Sartorius cup or a commercially available breast pump (e.g., FirstCyte aspirator; Cytyc Corporation, Marlborough, MA [24,31,32]).
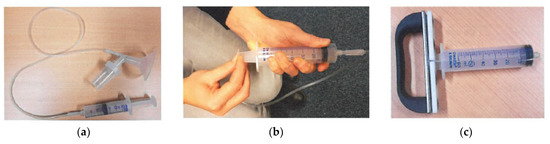
Figure 3.
Pictures of materials used for manual nipple fluid aspiration procedure. (a) Interconnection with a plastic tube between the cup that is placed on the breast around the nipple and a syringe; (b) figure of the research nurse showing how to grasp the plunger of the syringe to create vacuum. (c) Ergonomic handle that we developed to attach to the plunger of the syringe.
NFA is a non-invasive procedure that can be performed repeatedly and that allows obtaining a liquid biopsy from both breasts. Common steps of the procedure include the use of a scrubbing gel followed by cleansing the nipple surface with alcohol to remove keratin plugs clogging the orifices of the nipple ducts. Several pre-procedural and per-procedural additions have been proposed to improve the NFA success rate, such the application of warm compresses or heat pads to the breast [28], breast massage before and/or during the procedure [33,34,35], or repeated suction attempts [36,37,38]. Other approaches such as the application of an anaesthetic cream on the nipple and the intranasal administration of oxytocin have been suggested to diminish vacuum-induced pain and increase NAF collection success rate, respectively [17,38,39,40]. NFA requires a short training and can be mastered after 2 to 5 try-outs. Most studies collect NAF in the outpatient clinic setting, with the patient sitting in the upright position, but there are also a few studies that describe the collection of NAF from breast cancer patients directly prior to surgery under general anaesthesia [41,42].
4. Discomfort Associated with NFA
Tolerability of the NFA procedure has only been reported by a few studies. On a scale from 0 to 10, the discomfort of NFA has been rated with a mean score of 0.6 to 2.4 [17,26,39,40] in healthy female volunteers and women at high risk for breast cancer (Table 1). These scores are comparable to the scores given to a pap smear for cervical cancer screening and are generally lower than mammography, breast magnetic resonance imaging (MRI), physical breast examination and breastfeeding (see Table 1 for reported means). It is very encouraging to see that healthy women and women at high risk for breast cancer experience low discomfort with NFA, given that these are the women in whom a NAF test would especially be applicable.

Table 1.
Reported mean discomfort rates (on a scale from 0–10) for NFA (nipple fluid aspiration) compared to pap smear, mammography, breast magnetic resonance imaging (MRI), breastfeeding and breast examination. Abbreviations: N.R., not reported; H, healthy female volunteers; HR: women at high risk of developing breast cancer.
5. Why Investigate NAF?
It is assumed that biomarkers in body fluids that are closer to the tumor or its target organ have more potential as a disease biomarker because they represent local pathological processes better than the more distant fluids such as serum and plasma that also receive cellular debris and other components from other body parts. Many biomarker classes have been investigated in NAF, such as hormones (e.g., estrogen, testosterone), tumor markers (e.g., carcinoembryonic antigen and prostate-specific antigen (PSA)) and biochemical components (e.g., aluminum). Moreover, NAF contains molecular markers, such as DNA [43,44], RNA, microRNA [45] and proteins [46,47]. These findings have been summarized in two reviews from Edward Sauter and Ferdinando Mannello et al. [46,47]. In Table 2, we provide an overview of the molecules and molecular changes found in NAF and the corresponding techniques used. Regarding cellular composition, NAF samples are known to be quite acellular or paucicellular, with reports of the presence of foam, white, red blood and/or (atypical) epithelial cells [46,48,49,50]. Given that breast tumors develop from ductal and/or lobular epithelium, investigating NAF as biofluid secreted by breast cells provides the opportunity to assess biomarkers directly originating from the breast. Moreover, the NAF procedure provides an intra-patient control to compare an affected breast with the other healthy breast of the same woman. As such, NAF is a potentially very valuable source of biomarkers that can serve as a diagnostic tool for breast disease, including breast cancer.

Table 2.
Summary of molecules or molecular changes found in NAF and used techniques, their advantages, and limitations. This information was retrieved from the reviews of Sauter [46] and Mannello et al. [47]. Abbreviations: N.A.: not applicable.
6. How Could NAF Be of Added Value as an Early Breast Cancer Detection Tool?
With current image-based breast cancer screening tools, breast cancer can only be detected once it has formed a mass. Liquid biopsy approaches such as NAF could be of added value by detecting breast cancer at an earlier or even pre-invasive stage.
Three recent studies have suggested how NAF (or other liquid biopsies) could be integrated in an early detection pathway for breast cancer [1,70,71]. Shaheed et al. suggests using NAF collection as a first step in screening for breast cancer, followed by sample analysis by mass spectroscopy [1]. Another suggestion by Shaheed et al. is the use of an easy-to-interpret NAF self-collection kit [1]. These elements are also included in the recent publication of Jiwa et al., that describes a possible workflow for management of breast screening that integrates NAF [71]. A more specific potential application of NAF could be, as Zubor et al. suggests, the use of liquid biopsies as an adjunct to supplemental MRI for women with dense breasts or (additional) breast cancer related risk factors [70].
These proposed roles of NAF or other liquid biopsies for early detection of breast cancer, together with our views, are summarized as shown in Figure 4. In general, there are three possible roles for a new biomarker test in an already existing detection pathway, namely as a triage, add-on or replacement test [72]. A triage test is used before the existing imaging method; only women with an abnormal biomarker test result would continue in the detection pathway. Such a triage test may be less accurate but should provide advantages such as simplicity and low costs. An add-on test could be positioned after or together with imaging to identify false-positives or false-negatives and hence, avoid unnecessary breast biopsies and missing tumors, respectively. The third role, namely a replacement test, could be of value if there are actionable consequences to halt further tumor development or when imaging as a screening or diagnostic tool is not advisable, such as during pregnancy and breastfeeding. In the end, the true application of a NAF test will be dependent on the accuracy of the biomarkers for disease detection. In addition, it will be dependent on the characteristics of the cohorts used in clinical studies to test the NAF samples. Last but not least, involvement of medical decision-making experts and patient advocate groups are of utmost importance to discuss the translational applications.
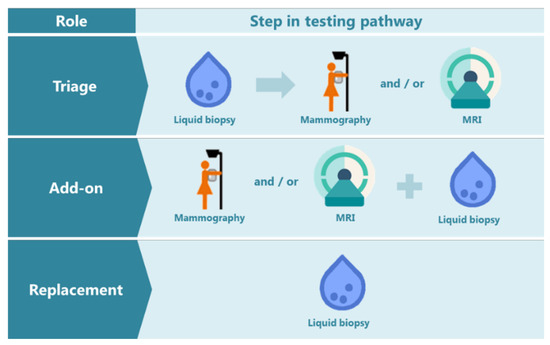
Figure 4.
The possible roles of a liquid biopsy test in the breast cancer detection pathway: triage, add-on, or replacement test. MRI: Magnetic resonance imaging. The mammography schematic icon is adapted from the Dutch information folder for participation in the National Programme for Breast Cancer Screening [73].
7. Challenges in Using NAF as a Biomarker Source
Even though NAF holds promise as a biomarker source for detection of breast cancer, some challenges emerge when handling NAF. An important limitation is the small volume (ranging from 1 to 500 µL [74]). Furthermore, viscosity and different colors have been shown to affect biomarker analyses [10,75]. In our experience, volume and viscosity are an issue when using fluorescence-activated cell sorting, spectrophotometry and microfluidics. Ways to render viscous, heterogeneous NAF specimens to a homogenous state, could be the use of mechanical liquefaction, such as vortexing with the aid of glass beads or sonication and/or chemical liquefaction with the use of mucolytic buffers and centrifugation through a QIAshredder homogenizer prior to nucleic acid extraction [76]. Since pipetting is also hampered by viscous samples, a requirement would be to have automatic positive displacement pipettes to ensure accuracy and reproducibility. Still, current relevant biomarker techniques like real time quantitative reverse transcription PCR (RT-qPCR) [45,55] and mass spectrometry [15,47] have been shown to be feasible for NAF samples.
Another limitation of NAF is that it is unclear from which of the 5–12 ducts within the breast the NAF droplets originates, which comes with the risk of not acquiring NAF from the duct where carcinogenesis takes place. Moreover, it could be that larger tumors may occlude the ducts distally and hence, obstruct NAF flow for collection; from our experience, NFA success percentages from the affected and unaffected breast in women with breast cancer are comparable (data not shown). Still, if NAF is to be used in the context of early breast cancer detection by making use of its biomarkers to signal carcinogenesis before an obstructing tumor mass has been formed, this should not be a matter of concern.
8. Historical Overview of Studies Focusing on NAF
NAF was first described by Papanicolaou et al. [77] and Fleming [78] in the mid-1950s. In 1977, Sartorius et al. developed a “Sartorius” suction cup for NFA which consisted of a small plastic cup attached to a 10-mL syringe by a short plastic tube [79]. Sartorius and his colleagues pioneered the epidemiological research using NAF samples by building up a cohort of over 3000 women of whom they attempted NFA [24]. Data from these cohorts was used for several publications, especially by himself, Eileen King, Nicholas Petrakis and Margaret Wrensch. So far, over 200 publications about NAF have been written. In order to give a historical overview of research related to NAF, we shortly describe the work of selected researchers that have especially dedicated themselves to NAF, namely Otto Sartorius, Nicholas Petrakis, Ferdinando Mannello, Edward Sauter, and Paul van Diest and Elsken van der Wall. The search strategy that led to this selection comprised a PubMed search for all articles that included the terms “nipple aspirate fluid” or “nipple aspiration”, together with snowballing. This was followed by a selection of articles by authors that had published more than four articles as first or last author, not including reviews. When co-authorship occurred often, selection was performed for the most senior author. A timeline with their list of publications is represented in Figures S1–S4.
The work of Petrakis et al. (Figure S1) about NAF was first published in 1975 and comprised investigations on success percentage of NFA, cytology analysis of NAF, and the association of both with breast cancer risk factors. The initial work was mainly focused on the success percentages of acquired NAF and relation to age, ethnicity, menopausal status, and cerumen type. A higher success rate of NAF yield was seen in Caucasians, women younger than 50 years old and with wet type cerumen [80,81], which was later confirmed in another study [82]. Additional variables with a positive influence on success rate of NFA were a higher fat consumption [83], history of parity and of breastfeeding [84]. Another scope of research was the relation between cells found in NAF and the risk of developing breast cancer [85,86]. In one publication it was shown that, when adding cellular atypia found in NAF, a minimal improvement of the Gail model for risk prediction was obtained [87]. In another publication, they described that atypical cells could be found in healthy women, women with benign breast disease and women with breast cancer, with increasing percentages per group (15%, 32%, and 64%, respectively) [88]. Later, for the first time, the association was made between cellular atypia in NAF and high mammographic density (>50% density) [50]. Moreover, foam cells were for the first time characterized in NAF [89]. An interventional study investigating the effect of the intake of soy protein showed that this led to an increased NAF secretion and the appearance of hyperplastic epithelial cells in NAF of premenopausal women [25]. Lastly, they analyzed the biochemical content of NAF for cholesterol (as it could be a potential carcinogen) [90] and lactose (as it could be considered a measure of secretory activity) [91]. In 1993, Petrakis gave a distinguished award lecture summarizing his work on NAF until then [92].
The work of Mannello et al. (Figure S2) started with publications in the beginning of the 21st century and mostly focused on the biochemical constituents of nipple aspirate such as P-cadherin [93], proteins involved in the of lipid peroxidation and oxidative stress [94,95,96], C-reactive protein [97], and aluminum [98,99,100]. Methodologically, for these investigations, Mannello et al. usually compared NAF from cohorts of healthy women with cohorts of women with breast cancer; comparing biomarker concentrations between NAF, plasma, and serum. Most of his studies were observational, but he also performed an interventional study by investigating the levels of urokinase-type plasminogen activator (uPa) in NAF after oral administration of colecoxib, a non-steroidal anti-inflammatory drug [101]. He also wrote four reviews summarizing current knowledge about proteins, hormones and aluminum detected in NAF of breast cancer patients compared to healthy controls [47,102,103,104]. His latest publication was a comment reflecting on the potential of investigating biomarkers in NAF [105].
Another researcher with NAF as a clear line of investigation is Sauter, who has compiled an impressive number of publications on NAF over almost 30 years (Figure S3). Sauter focused on the levels of markers in NAF, especially prostate-specific antigen (PSA) (PSA is produced by breast tissue) [67,106,107], and PSA’s association with hormones such as progesterone [108] and testosterone [64] and insulin-like growth factor binding protein-3 [109]. Specifically, PSA levels in NAF were inversely associated with the presence of breast cancer, suggesting a possible role for PSA to help establish breast cancer risk [67]. Moreover, the levels of PSA were investigated in breast cancer patients, and shown to decrease with advanced breast cancer stages, and to be able to better predict disease in pre-menopausal women compared to post-menopausal women [69]. Next to his focus on the biochemical composition of NAF [44,56,59,60,62,93,95,96,107,110,111,112,113,114,115,116], he also investigated the cellular components of NAF. In one study he established that there was no cellular variation in NAF along the menstrual cycle of 15 healthy women by evaluating weekly acquired NAF samples [117]. He also performed interventional studies where it was investigated whether celecoxib had effect on prostaglandin E2 concentrations in NAF from women at increased risk of breast cancer [118]. His latest publication is an editorial about a publication of our group [119].
The work of our group (Figure S4) on NAF started in 2007 with publications focused on the use of oxytocin nasal spray prior to the NFA procedure, which proved to yield a higher success rate, to be safe and to be well accepted by the participating women [17,39,40]. This was followed by a publication on methylation levels of tumor suppressor genes in NAF samples from women with breast cancer compared to healthy women. This study showed that cancerous nipple fluid contained increased levels of methylation biomarkers compared to healthy nipple fluid, albeit with an area under the curve that was lower than current imaging methods [120,121]. We further focused on NAF microRNA analysis, based on the fact that these biomarkers can be detected in several liquid biopsies and can make up a signature for oncological disease. We have proven that microRNAs (miRNAs)are measurable in NAF by RT-qPCR [45] and are currently investigating the feasibility of small RNA sequencing for miRNA detection in NAF samples. Now that our group has piled up a large sample biobank with blood and NAF from healthy women, women with breast cancer and serial samples from women at increased risk of developing breast cancer, more publications will soon follow [122,123,124]. These data will reveal whether early detection before mammography can be reliably achieved in NAF by means of miRNAs. The practical aspects of building up these cohorts have been described in a recent publication [125], followed by a publication reporting the technical aspects of analyzing biomarkers in NAF samples of different appearance [75]. We are currently investigating the role of miRNAs in the development of high breast mammographic density and the consequently increased risk of breast cancer, by using NAF as a surrogate for the breast microenvironment.
The latter three publications comprise the most recent publications about NAF, together with publications from George et al. [126] and Jiwa et al. [48,71,127]. The first described a proteomic evaluation of NAF [126] whereas the latter described in a systematic review that the diagnostic accuracy of nipple smear cytology is limited by poor sensitivity [48,127] and, in a questionnaire-based study, that there is a great readiness of women to undergo NFA [71]. Based on ongoing clinical studies using NAF samples (see Table 3), future data on NAF’s content and clinical potential will be generated and reported. The state of the art of NAF research is summarized in Box 1 and a list of past reviews about NAF is shown in Table 4.

Table 3.
List of registered trials on nipple aspiration fluid identified by searches in https://clinicaltrials.gov/, accessed on 25 December 2021. Trialregister.nl, ISRCTN and PubMed. The following syntax was used for a search on Pubmed: (“protocol”[Title]) AND (nipple aspirate fluid OR nipple aspirate*)).

Table 4.
List of reviews about nipple aspirate fluid or where nipple aspirate fluid is mentioned amongst other approaches in the manuscript. Abbreviations: R, review; SR, systematic review; W, workshop.
Box 1. Summary of the state of the art of NAF research: what we know, advantages, hurdles to be aware of, and recommendations and needs in NAF studies.
Box 1|Summary
What we know
- -
- Nipple aspirate fluid (NAF) is a physiological fluid that is produced by the luminal layer of the breast lobules and ducts
- -
- Nipple fluid aspiration (NFA) is well tolerated by women
- -
- NAF can be acquired in the majority of women
- -
- Many biomarkers can be found in NAF, such as DNA, RNA, microRNA and proteins
- -
- Cytology assessment in NAF has low diagnostic accuracy
Advantages
- -
- NAF originates from the location where breast cancer arises
- -
- NAF can be acquired repeatedly, easily and non-invasively
- -
- Bilateral NAF samples allow intra-patient control analyses
Hurdles to be aware of
- -
- NAF samples are of low volume, can be viscous, have low cellularity and have different colors, which may affect biomarker analysis
- -
- It is unclear which duct NAF derives from
Recommendations and needs in NAF studies
- -
- For the NFA procedure, use oxytocin nose spray to increase success rate and tolerability for the woman, and use an ergonomic handle for the research nurse performing the procedure
- -
- Combine nipple fluid research with imaging results and include anthropomorphic measures and risk factors for breast cancer
- -
- Development of technologies that are feasible for detection and interpretation of biomarkers in samples that are viscous and of low volume
- -
- To reduce sample viscosity, use mechanical and/or chemical liquefaction in sample processing steps
- -
- Report NAF biomarker results in diagnostic accuracy values in order to be able to interpret their translational role
- -
- Involve medical decision making experts and patient advocate groups to discuss the potential use of liquid biopsies in early detection
- -
- Develop a self-test for NFA collection and interpretation
9. Conclusions
NAF is a physiological fluid that rests in the ductal tree of the breast and can easily be obtained by non-invasive aspiration. It is an established source of biomarkers that deserves to be investigated for its potential application in breast cancer management such as in screening or as a confirmatory additive tool in imaging diagnostics. As research on the role of NAF is still actively being performed by many research groups, including our own, more data about NAF and its potential clinical value is expected to be reported in the near future.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers14010159/s1, Figures S1–S4. Timeline of published articles of Petrakis (S1), Mannello (S2), Sauter (S3), and Van Diest/Van der Wall (S4).
Author Contributions
Conceptualization, S.I.S.P.; methodology, S.I.S.P.; writing—original draft preparation, S.I.S.P.; writing—review and editing, C.B.M., K.P.M.S., E.v.d.W. and P.J.v.D.; visualization, S.I.S.P.; supervision, C.B.M., K.P.M.S., E.v.d.W. and P.J.v.D.; project administration, S.I.S.P.; funding acquisition, E.v.d.W. and P.J.v.D. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shaheed, S.U.; Tait, C.; Kyriacou, K.; Linforth, R.; Salhab, M.; Sutton, C. Evaluation of Nipple Aspirate Fluid as a Diagnostic Tool for Early Detection of Breast Cancer. Clin. Proteom. 2018, 15, 3. [Google Scholar] [CrossRef]
- Waaijer, L.; van Diest, P.; van der Pol, C.C.; Verolme, B.; Hennink, A.; Witkamp, A.J. Ductoscopy for Pathologic Nipple Discharge. Ned. Tijdschr. Voor Geneeskd. 2013, 157, A6358. [Google Scholar]
- Dixon, J.M.; Mansel, R.E. Abc of Breast Diseases. Symptoms Assessment and Guidelines for Referral. BMJ 1994, 309, 722–726. [Google Scholar] [CrossRef][Green Version]
- Sheiman, L.S.; Levesque, P.H. The In’s and Out’s of Ductography: A Comprehensive Review. Curr. Probl. Diagn. Radiol. 2016, 45, 61–70. [Google Scholar] [CrossRef]
- Ohtake, T.; Kimijima, I.; Fukushima, T.; Yasuda, M.; Sekikawa, K.; Takenoshita, S.; Abe, R. Computer-Assisted Complete Three-Dimensional Reconstruction of the Mammary Ductal/Lobular Systems: Implications of Ductal Anastomoses for Breast-Conserving Surgery. Cancer 2001, 91, 2263–2272. [Google Scholar] [CrossRef]
- Going, J.J.; Moffat, D.F. Escaping from Flatland: Clinical and Biological Aspects of Human Mammary Duct Anatomy in Three Dimensions. J. Pathol. 2004, 203, 538–544. [Google Scholar] [CrossRef]
- Jesinger, R.A. Breast Anatomy for the Interventionalist. Tech. Vasc. Interv. Radiol. 2014, 17, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Paine, I.S.; Lewis, M.T. The Terminal End Bud: The Little Engine That Could. J. Mammary Gland. Biol. Neoplasia 2017, 22, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Macias, H.; Hinck, L. Mammary Gland Development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef] [PubMed]
- King, B.L.; Love, S.M. The Intraductal Approach to the Breast: Raison D’etre. Breast Cancer Res. 2006, 8, 206. [Google Scholar] [CrossRef]
- Chatterton, R.T., Jr.; Geiger, A.S.; Mateo, E.T.; Helenowski, I.B.; Gann, P.H. Comparison of Hormone Levels in Nipple Aspirate Fluid of Pre- and Postmenopausal Women: Effect of Oral Contraceptives and Hormone Replacement. J. Clin. Endocrinol. Metab. 2005, 90, 1686–1691. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sartorius, O.W. The Biochemistry of Breast Cyst Fluids and Duct Secretions. Breast Cancer Res. Treat. 1995, 35, 255–266. [Google Scholar] [CrossRef]
- Petrakis, N.L. Breast Secretory Activity in Nonlactating Women, Postpartum Breast Involution, and the Epidemiology of Breast Cancer. Natl. Cancer Inst. Monogr. 1977, 47, 161–164. [Google Scholar]
- Petrakis, N.L. Physiologic, Biochemical, and Cytologic Aspects of Nipple Aspirate Fluid. Breast Cancer Res. Treat. 1986, 8, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Zangar, R.C.; Varnum, S.M.; Covington, C.Y.; Smith, R.D. A Rational Approach for Discovering and Validating Cancer Markers in Very Small Samples Using Mass Spectrometry and Elisa Microarrays. Dis Markers. 2004, 20, 135–148. [Google Scholar] [CrossRef] [PubMed]
- de Groot, J.S.; van Diest, P.J.; van Amersfoort, M.; Vlug, E.J.; Pan, X.; Ter Hoeve, N.D.; Rosing, H.; Beijnen, J.H.; Youssef, S.A.; de Bruin, A.; et al. Intraductal Cisplatin Treatment in a Brca-Associated Breast Cancer Mouse Model Attenuates Tumor Development but Leads to Systemic Tumors in Aged Female Mice. Oncotarget 2017, 8, 60750–60763. [Google Scholar] [CrossRef][Green Version]
- Suijkerbuijk, K.P.; van der Wall, E.; Meijrink, H.; Pan, X.; Borel Rinkes, I.H.; Ausems, M.G.; van Diest, P.J. Successful Oxytocin-Assisted Nipple Aspiration in Women at Increased Risk for Breast Cancer. Fam. Cancer 2010, 9, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, L.; Areias, V.R.; Pinto, R.C.; Matos Cda, S.; Rosa, M.F.; De Azevedo, C.M.; Alves, G. Protein Identification from Dried Nipple Aspirate Fluid on Guthrie Cards Using Mass Spectrometry. Mol. Med. Rep. 2015, 12, 159–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qin, W.; Gui, G.; Zhang, K.; Twelves, D.; Kliethermes, B.; Sauter, E.R. Proteins and Carbohydrates in Nipple Aspirate Fluid Predict the Presence of Atypia and Cancer in Women Requiring Diagnostic Breast Biopsy. BMC Cancer 2012, 12, 52. [Google Scholar] [CrossRef]
- Mannello, F.; Tonti, G.A.; Pederzoli, A.; Simone, P.; Smaniotto, A.; Medda, V. Detection of Superoxide Dismutase-1 in Nipple Aspirate Fluids: A Reactive Oxygen Species-Regulating Enzyme in the Breast Cancer Microenvironment. Clin. Breast Cancer 2010, 10, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Wrensch, M.R.; Petrakis, N.L.; Gruenke, L.D.; Ernster, V.L.; Miike, R.; King, E.B.; Hauck, W.W. Factors Associated with Obtaining Nipple Aspirate Fluid: Analysis of 1428 Women and Literature Review. Breast Cancer Res. Treat. 1990, 15, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.S.; Pang, D.; Wang, F.; Xue, Y.W.; Gao, D.N.; Li, H.; Li, K.; Wang, B.Y.; Wang, D.; Li, H.Y. Nipple Aspirate Fluid Collection, Related Factors and Relationship between Carcinoembryonic Antigen in Nipple Aspirate Fluid and Breast Diseases in Women in Harbin, Prc. Cancer Epidemiol. Prev. Biomark. 2009, 18, 732–738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, P.; Klemp, J.R.; Simonsen, M.; Welsko, C.M.; Zalles, C.M.; Kimler, B.F.; Fabian, C.J. Failure of High Risk Women to Produce Nipple Aspirate Fluid Does Not Exclude Detection of Cytologic Atypia in Random Periareolar Fine Needle Aspiration Specimens. Breast Cancer Res. Treat. 2004, 87, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Baltzell, K.A.; Wrensch, M.; Sison, J.D. A Descriptive Study of Variables Associated with Obtaining Nipple Aspirate Fluid in a Cohort of Non-Lactating Women. BMC Womens Health 2006, 6, 15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petrakis, N.L.; Barnes, S.; King, E.B.; Lowenstein, J.; Wiencke, J.; Lee, M.M.; Miike, R.; Kirk, M.; Coward, L. Stimulatory Influence of Soy Protein Isolate on Breast Secretion in Pre- and Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 1996, 5, 785–794. [Google Scholar]
- Klein, P.; Glaser, E.; Grogan, L.; Keane, M.; Lipkowitz, S.; Soballe, P.; Brooks, L.; Jenkins, J.; Steinberg, S.M.; DeMarini, D.M.; et al. Biomarker Assays in Nipple Aspirate Fluid. Breast J. 2001, 7, 378–387. [Google Scholar] [CrossRef]
- Djuric, Z.; Visscher, D.W.; Heilbrun, L.K.; Chen, G.; Atkins, M.; Covington, C.Y. Influence of Lactation History on Breast Nipple Aspirate Fluid Yields and Fluid Composition. Breast J. 2005, 11, 92–99. [Google Scholar] [CrossRef]
- Maskarinec, G.; Morimoto, Y.; Conroy, S.M.; Pagano, I.S.; Franke, A.A. The Volume of Nipple Aspirate Fluid Is Not Affected by 6 Months of Treatment with Soy Foods in Premenopausal Women. J. Nutr. 2011, 141, 626–630. [Google Scholar] [CrossRef]
- Smalley, M.; Ashworth, A. Stem Cells and Breast Cancer: A Field in Transit. Nat. Rev. Cancer. 2003, 3, 832–844. [Google Scholar] [CrossRef]
- Jakub, J.W.; Peled, A.W.; Gray, R.J.; Greenup, R.A.; Kiluk, J.V.; Sacchini, V.; McLaughlin, S.A.; Tchou, J.C.; Vierkant, R.A.; Degnim, A.C.; et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population with Brca Mutations: A Multi-Institutional Study. JAMA Surg. 2018, 153, 123–129. [Google Scholar] [CrossRef]
- Maskarinec, G.; Ollberding, N.J.; Conroy, S.M.; Morimoto, Y.; Pagano, I.S.; Franke, A.A.; Gentzschein, E.; Stanczyk, F.Z. Estrogen Levels in Nipple Aspirate Fluid and Serum During a Randomized Soy Trial. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1815–1821. [Google Scholar] [CrossRef]
- Dua, R.S.; Isacke, C.M.; Gui, G.P. The Intraductal Approach to Breast Cancer Biomarker Discovery. J. Clin. Oncol. 2006, 24, 1209–1216. [Google Scholar] [CrossRef]
- Menekse, E.; McKolanis, J.; Finn, O.J.; McAuliffe, P.F.; Johnson, R.; Soran, A. Anti-Muc1 Antibody in Nipple Aspirate Fluids Correlates with Tumor Aggressiveness in Breast Cancer: A Feasibility Study. Dis. Markers 2015, 2015, 179689. [Google Scholar] [CrossRef]
- Chatterton, R.T.; Muzzio, M.; Heinz, R.; Gann, P.H.; Khan, S.A. Methodological Considerations in Estrogen Assays of Breast Fluid and Breast Tissue. Steroids 2015, 99 Pt A, 103–107. [Google Scholar] [CrossRef]
- Tredwell, G.D.; Miller, J.A.; Chow, H.H.; Thompson, P.A.; Keun, H.C. Metabolomic Characterization of Nipple Aspirate Fluid by (1)H Nmr Spectroscopy and Gc-Ms. J. Proteome Res. 2014, 13, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Hebshi, S.; Custer, L.; Franke, A.A. The Relation of Soy Intake and Isoflavone Levels in Nipple Aspirate Fluid. Eur. J. Cancer Prev. 2008, 17, 67–70. [Google Scholar] [CrossRef] [PubMed]
- King, E.B.; Chew, K.L.; Hom, J.D.; Miike, R.; Wrensch, M.R.; Petrakis, N.L. Multiple Sampling for Increasing the Diagnostic Sensitivity of Nipple Aspirate Fluid for Atypical Cytology. Acta Cytol. 2004, 48, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shao, Z.M.; Beatty, P.; Sartippour, M.; Wang, H.J.; Elashoff, R.; Chang, H.; Brooks, M.N. The Use of Oxytocin in Nipple Fluid Aspiration. Breast J. 2003, 9, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Suijkerbuijk, K.P.; van der Wall, E.; van Diest, P.J. Oxytocin: Bringing Magic into Nipple Aspiration. Ann. Oncol. 2007, 18, 1743–1744. [Google Scholar] [CrossRef] [PubMed]
- de Groot, J.S.; Moelans, C.B.; Elias, S.G.; Hennink, A.; Verolme, B.; Suijkerbuijk, K.P.; Jager, A.; Seynaeve, C.; Bos, P.; Witkamp, A.J.; et al. Repeated Nipple Fluid Aspiration: Compliance and Feasibility Results from a Prospective Multicenter Study. PLoS ONE 2015, 10, e0127895. [Google Scholar] [CrossRef][Green Version]
- Shaheed, S.U.; Tait, C.; Kyriacou, K.; Mullarkey, J.; Burrill, W.; Patterson, L.H.; Linforth, R.; Salhab, M.; Sutton, C.W. Nipple Aspirate Fluid-a Liquid Biopsy for Diagnosing Breast Health. Proteom. Clin. Appl. 2017, 11, 1700015. [Google Scholar] [CrossRef]
- Phillips, H.A.; Howard, G.C.; Miller, W.R. Pilot Studies on the P53 Gene in Nipple Aspirate Fluid from Patients with Breast Cancer. Breast Cancer Res. Treat. 2000, 61, 139–143. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Ehya, H.; Lininger, J.; Sauter, E. Microsatellite Changes in Nipple Aspirate Fluid and Breast Tissue from Women with Breast Carcinoma or Its Precursors. Clin. Cancer Res. 2003, 9, 3029–3033. [Google Scholar] [PubMed]
- Krassenstein, R.; Sauter, E.; Dulaimi, E.; Battagli, C.; Ehya, H.; Klein-Szanto, A.; Cairns, P. Detection of Breast Cancer in Nipple Aspirate Fluid by Cpg Island Hypermethylation. Clin. Cancer Res. 2004, 10 Pt 1, 28–32. [Google Scholar] [CrossRef]
- Patuleia, S.I.S.; van Gils, C.H.; Oneto Cao, A.M.; Bakker, M.F.; van Diest, P.J.; van der Wall, E.; Moelans, C.B. The Physiological Microrna Landscape in Nipple Aspirate Fluid: Differences and Similarities with Breast Tissue, Breast Milk, Plasma and Serum. Int. J. Mol. Sci. 2020, 21, 8466. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.R. Analysis of Nipple Aspirate Fluid for Diagnosis of Breast Cancer: An Alternative to Invasive Biopsy. Expert Rev. Mol. Diagn. 2005, 5, 873–881. [Google Scholar] [CrossRef]
- Mannello, F.; Medda, V.; Tonti, G.A. Protein Profile Analysis of the Breast Microenvironment to Differentiate Healthy Women from Breast Cancer Patients. Expert Rev. Proteomics. 2009, 6, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Jiwa, N.; Gandhewar, R.; Chauhan, H.; Ashrafian, H.; Kumar, S.; Wright, C.; Takats, Z.; Leff, D.R. Diagnostic Accuracy of Nipple Aspirate Fluid Cytology in Asymptomatic Patients: A Meta-Analysis and Systematic Review of the Literature. Ann. Surg. Oncol. 2021, 28, 3751–3760. [Google Scholar] [CrossRef]
- Dabbs, D.J. Mammary Ductal Foam Cells: Macrophage Immunophenotype. Hum. Pathol. 1993, 24, 977–981. [Google Scholar] [CrossRef]
- Lee, M.M.; Petrakis, N.L.; Wrensch, M.R.; King, E.B.; Miike, R.; Sickles, E. Association of Abnormal Nipple Aspirate Cytology and Mammographic Pattern and Density. Cancer Epidemiol. Biomark. Prev. 1994, 3, 33–36. [Google Scholar]
- Scott, W.N.; Miller, W.R. The Mutagenic Activity of Human Breast Secretions. J. Cancer Res. Clin. Oncol. 1990, 116, 499–502. [Google Scholar] [CrossRef]
- Petrakis, N.L.; Maack, C.A.; Lee, R.E.; Lyon, M. Mutagenic Activity in Nipple Aspirates of Human Breast Fluid. Cancer Res. 1980, 40, 188–189. [Google Scholar] [PubMed]
- Isaacs, C.; Cavalli, L.R.; Cohen, Y.; Pennanen, M.; Shankar, L.K.; Freedman, M.; Singh, B.; Liu, M.; Gallagher, A.; Rone, J.D.; et al. Detection of Loh and Mitochondrial DNA Alterations in Ductal Lavage and Nipple Aspirate Fluids from Hngh-Risk Patients. Breast Cancer Res. Treat. 2004, 84, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Jakupciak, J.P.; Maggrah, A.; Maragh, S.; Maki, J.; Reguly, B.; Maki, K.; Wittock, R.; Robinson, K.; Wagner, P.D.; Thayer, R.E.; et al. Facile Whole Mitochondrial Genome Resequencing from Nipple Aspirate Fluid Using Mitochip V2.0. BMC Cancer 2008, 8, 95. [Google Scholar] [CrossRef]
- Moelans, C.B.; Patuleia, S.I.S.; van Gils, C.H.; van der Wall, E.; van Diest, P.J. Application of Nipple Aspirate Fluid Mirna Profiles for Early Breast Cancer Detection and Management. Int. J. Mol. Sci. 2019, 20, 5814. [Google Scholar] [CrossRef]
- Sauter, E.R.; Zhu, W.; Fan, X.J.; Wassell, R.P.; Chervoneva, I.; Du Bois, G.C. Proteomic Analysis of Nipple Aspirate Fluid to Detect Biologic Markers of Breast Cancer. Br. J. Cancer 2002, 86, 1440–1443. [Google Scholar] [CrossRef]
- Paweletz, C.P.; Trock, B.; Pennanen, M.; Tsangaris, T.; Magnant, C.; Liotta, L.A.; Petricoin, E.F., 3rd. Proteomic Patterns of Nipple Aspirate Fluids Obtained by Seldi-Tof: Potential for New Biomarkers to Aid in the Diagnosis of Breast Cancer. Dis. Markers 2001, 17, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Coombes, K.R.; Fritsche, H.A., Jr.; Clarke, C.; Chen, J.N.; Baggerly, K.A.; Morris, J.S.; Xiao, L.C.; Hung, M.C.; Kuerer, H.M. Quality Control and Peak Finding for Proteomics Data Collected from Nipple Aspirate Fluid by Surface-Enhanced Laser Desorption and Ionization. Clin. Chem. 2003, 49, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.R.; Davis, W.; Qin, W.; Scanlon, S.; Mooney, B.; Bromert, K.; Folk, W.R. Identification of a Beta-Casein-Like Peptide in Breast Nipple Aspirate Fluid That Is Associated with Breast Cancer. Biomark Med. 2009, 3, 577–588. [Google Scholar] [CrossRef]
- Alexander, H.; Stegner, A.L.; Wagner-Mann, C.; Du Bois, G.C.; Alexander, S.; Sauter, E.R. Proteomic Analysis to Identify Breast Cancer Biomarkers in Nipple Aspirate Fluid. Clin. Cancer Res. 2004, 10, 7500–7510. [Google Scholar] [CrossRef]
- Varnum, S.M.; Covington, C.C.; Woodbury, R.L.; Petritis, K.; Kangas, L.J.; Abdullah, M.S.; Pounds, J.G.; Smith, R.D.; Zangar, R.C. Proteomic Characterization of Nipple Aspirate Fluid: Identification of Potential Biomarkers of Breast Cancer. Breast Cancer Res. Treat. 2003, 80, 87–97. [Google Scholar] [CrossRef]
- Hsiung, R.; Zhu, W.; Klein, G.; Qin, W.; Rosenberg, A.; Park, P.; Rosato, E.; Sauter, E. High Basic Fibroblast Growth Factor Levels in Nipple Aspirate Fluid Are Correlated with Breast Cancer. Cancer J. 2002, 8, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, R.T., Jr.; Geiger, A.S.; Khan, S.A.; Helenowski, I.B.; Jovanovic, B.D.; Gann, P.H. Variation in Estradiol, Estradiol Precursors, and Estrogen-Related Products in Nipple Aspirate Fluid from Normal Premenopausal Women. Cancer Epidemiol. Biomark. Prev. 2004, 13, 928–935. [Google Scholar]
- Sauter, E.R.; Tichansky, D.S.; Chervoneva, I.; Diamandis, E.P. Circulating Testosterone and Prostate-Specific Antigen in Nipple Aspirate Fluid and Tissue Are Associated with Breast Cancer. Environ. Health Perspect. 2002, 110, 241–246. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.L.; Chang, H.; Barsky, S.H.; Nguyen, M. Breast-Cancer Diagnosis with Nipple Fluid Bfgf. Lancet 2000, 356, 567. [Google Scholar] [CrossRef]
- Sauter, E.R.; Garofalo, C.; Hewett, J.; Hewett, J.E.; Morelli, C.; Surmacz, E. Leptin Expression in Breast Nipple Aspirate Fluid (Naf) and Serum Is Influenced by Body Mass Index (Bmi) but Not by the Presence of Breast Cancer. Horm. Metab. Res. 2004, 36, 336–340. [Google Scholar] [PubMed]
- Sauter, E.R.; Daly, M.; Linahan, K.; Ehya, H.; Engstrom, P.F.; Bonney, G.; Ross, E.A.; Yu, H.; Diamandis, E. Prostate-Specific Antigen Levels in Nipple Aspirate Fluid Correlate with Breast Cancer Risk. Cancer Epidemiol. Biomark. Prev. 1996, 5, 967–970. [Google Scholar]
- Zhao, Y.; Verselis, S.J.; Klar, N.; Sadowsky, N.L.; Kaelin, C.M.; Smith, B.; Foretova, L.; Li, F.P. Nipple Fluid Carcinoembryonic Antigen and Prostate-Specific Antigen in Cancer-Bearing and Tumor-Free Breasts. J. Clin. Oncol. 2001, 19, 1462–1467. [Google Scholar] [CrossRef]
- Sauter, E.R.; Klein, G.; Wagner-Mann, C.; Diamandis, E.P. Prostate-Specific Antigen Expression in Nipple Aspirate Fluid Is Associated with Advanced Breast Cancer. Cancer Detect. Prev. 2004, 28, 27–31. [Google Scholar] [CrossRef]
- Zubor, P.; Kubatka, P.; Kajo, K.; Dankova, Z.; Polacek, H.; Bielik, T.; Kudela, E.; Samec, M.; Liskova, A.; Vlcakova, D.; et al. Why the Gold Standard Approach by Mammography Demands Extension by Multiomics? Application of Liquid Biopsy Mirna Profiles to Breast Cancer Disease Management. Int. J. Mol. Sci. 2019, 20, 2878. [Google Scholar] [CrossRef]
- Jiwa, N.; Takats, Z.; Leff, D.R.; Sutton, C. Breast Health Screening: A Uk-Wide Questionnaire. BMJ Nutr. Prev. Health 2021, 4, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, M.M.; Deeks, J.J.; Takwoingi, Y.; Macaskill, P. Cochrane Diagnostic Test Accuracy Reviews. Syst. Rev. 2013, 2, 82. [Google Scholar] [CrossRef] [PubMed]
- Uitnodigingsfolder ‘Bevolkingsonderzoek Borstkanker’. Available online: https://www.rivm.nl/documenten/folder-bevolkingsonderzoek-borstkanker-nl (accessed on 12 November 2021).
- Mannello, F.; Tonti, G.A.; Canestrari, F. Nutrients and Nipple Aspirate Fluid Composition: The Breast Microenvironment Regulates Protein Expression and Cancer Aetiology. Genes Nutr. 2008, 3, 77–85. [Google Scholar] [CrossRef][Green Version]
- Patuleia, S.I.S.; van der Wall, E.; van Gils, C.H.; Bakker, M.F.; Jager, A.; Voorhorst-Ogink, M.M.; van Diest, P.J.; Moelans, C.B. The Changing Microrna Landscape by Color and Cloudiness: A Cautionary Tale for Nipple Aspirate Fluid Biomarker Analysis. Cell. Oncol. 2021, 44, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Qiashredder Homogenizer. Available online: https://www.qiagen.com/us/products/instruments-and-automation/accessories/qiashredder/ (accessed on 16 December 2021).
- Papanicolaou, G.N.; Bader, G.M.; Holmquist, D.G.; Falk, E.A. Cytologic Evaluation of Breast Secretions. Ann. N. Y. Acad. Sci. 1956, 63, 1409–1421. [Google Scholar] [CrossRef]
- Fleming, R.M. Cytological Studies in Lesions of the Breast: Findings in Nipple Secretions and Aspirates from Tumors. South Med. J. 1955, 48, 74–78. [Google Scholar] [CrossRef]
- Sartorius, O.W.; Smith, H.S.; Morris, P.; Benedict, D.; Friesen, L. Cytologic Evaluation of Breast Fluid in the Detection of Breast Disease. J. Natl. Cancer Inst. 1977, 59, 1073–1080. [Google Scholar] [CrossRef]
- Petrakis, N.L.; Mason, L.; Lee, R.; Sugimoto, B.; Pawson, S.; Catchpool, F. Association of Race, Age, Menopausal Status, and Cerumen Type with Breast Fluid Secretion in Nonlactating Women, as Determined by Nepple Aspiration. J. Natl. Cancer Inst. 1975, 54, 829–834. [Google Scholar]
- Petrakis, N.L.; Ernster, V.L.; Sacks, S.T.; King, E.B.; Schweitzer, R.J.; Hunt, T.K.; King, M.C. Epidemiology of Breast Fluid Secretion: Association with Breast Cancer Risk Factors and Cerumen Type. J. Natl. Cancer Inst. 1981, 67, 277–284. [Google Scholar] [PubMed]
- Petrakis, N.L.; King, E.B.; Lee, M.; Miike, R. Cerumen Phenotype and Proliferative Epithelium in Breast Fluids of U.S.-Born Vs. Immigrant Asian Women: A Possible Genetic-Environmental Interaction. Breast Cancer Res. Treat. 1990, 16, 279–285. [Google Scholar] [CrossRef]
- Lee, M.M.; Wrensch, M.R.; Miike, R.; Petrakis, N.L. The Association of Dietary Fat with Ability to Obtain Breast Fluid by Nipple Aspiration. Cancer Epidemiol. Biomark. Prev. 1992, 1, 277–280. [Google Scholar]
- Petrakis, N.L.; Lee, M.M.; Wrensch, M.R.; Ernster, V.L.; Miike, R.; Koo, L.C.; Ho, J.C. Birthplace and Yield of Nipple Aspirate Fluid in Chinese Women. Cancer Epidemiol. Biomark. Prev. 1998, 7, 835–839. [Google Scholar]
- Petrakis, N.L.; Ernster, V.L.; King, E.B.; Sacks, S.T. Epithelial Dysplasia in Nipple Aspirates of Breast Fluid: Association with Family History and Other Breast Cancer Risk Factors. J. Natl. Cancer Inst. 1982, 68, 9–13. [Google Scholar] [PubMed]
- King, E.B.; Chew, K.L.; Petrakis, N.L.; Ernster, V.L. Nipple Aspirate Cytology for the Study of Breast Cancer Precursors. J. Natl. Cancer Inst. 1983, 71, 1115–1121. [Google Scholar] [PubMed]
- Tice, J.A.; Miike, R.; Adduci, K.; Petrakis, N.L.; King, E.; Wrensch, M.R. Nipple Aspirate Fluid Cytology and the Gail Model for Breast Cancer Risk Assessment in a Screening Population. Cancer Epidemiol. Biomark. Prev. 2005, 14, 324–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petrakis, N.L. Genetic-Environmental Interactions in Relation to Low Dose Studies: A Possible Model from Breast Cancer. Environ. Health Perspect. 1981, 42, 97–102. [Google Scholar] [CrossRef][Green Version]
- King, E.B.; Kromhout, L.K.; Chew, K.L.; Mayall, B.H.; Petrakis, N.L.; Jensen, R.H.; Young, I.T. Analytic Studies of Foam Cells from Breast Cancer Precursors. Cytometry 1984, 5, 124–130. [Google Scholar] [CrossRef]
- Gruenke, L.D.; Wrensch, M.R.; Petrakis, N.L.; Miike, R.; Ernster, V.L.; Craig, J.C. Breast Fluid Cholesterol and Cholesterol Epoxides: Relationship to Breast Cancer Risk Factors and Other Characteristics. Cancer Res. 1987, 47, 5483–5487. [Google Scholar]
- Petrakis, N.L.; Lim, M.L.; Miike, R.; Lee, R.E.; Morris, M.; Lee, L.; Mason, L. Nipple Aspirate Fluids in Adult Nonlactating Women--Lactose Content, Cationic Na+, K+, Na+/K+ Ratio, and Coloration. Breast Cancer Res. Treat. 1989, 13, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, N.L. Aspo Distinguished Achievement Award Lecture. Studies on the Epidemiology and Natural History of Benign Breast Disease and Breast Cancer Using Nipple Aspirate Fluid. Cancer Epidemiol. Biomark. Prev. 1993, 2, 3–10. [Google Scholar]
- Mannello, F.; Tonti, G.A.; Medda, V.; Pederzoli, A.; Sauter, E.R. Increased Shedding of Soluble Fragments of P-Cadherin in Nipple Aspirate Fluids from Women with Breast Cancer. Cancer Sci. 2008, 99, 2160–2169. [Google Scholar] [CrossRef]
- Mannello, F.; Tonti, G.A.; Medda, V. Protein Oxidation in Breast Microenvironment: Nipple Aspirate Fluid Collected from Breast Cancer Women Contains Increased Protein Carbonyl Concentration. Cell. Oncol. 2009, 31, 383–392. [Google Scholar] [CrossRef]
- Mannello, F.; Qin, W.; Zhu, W.; Fabbri, L.; Tonti, G.A.; Sauter, E.R. Nipple Aspirate Fluids from Women with Breast Cancer Contain Increased Levels of Group Iia Secretory Phospholipase A2. Breast Cancer Res. Treat. 2008, 111, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Tonti, G.A.; Pagliarani, S.; Benedetti, S.; Canestrari, F.; Zhu, W.; Qin, W.; Sauter, E.R. The 8-Epimer of Prostaglandin F(2alpha), a Marker of Lipid Peroxidation and Oxidative Stress, Is Decreased in the Nipple Aspirate Fluid of Women with Breast Cancer. Int. J. Cancer 2007, 120, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Tonti, G.A.; Simone, P.; Ligi, D.; Medda, V. Iron-Binding Proteins and C-Reactive Protein in Nipple Aspirate Fluids: Role of Iron-Driven Inflammation in Breast Cancer Microenvironment? Am. J. Transl. Res. 2010, 3, 100–113. [Google Scholar]
- Mannello, F.; Ligi, D.; Canale, M. Aluminium, Carbonyls and Cytokines in Human Nipple Aspirate Fluids: Possible Relationship between Inflammation, Oxidative Stress and Breast Cancer Microenvironment. J. Inorg. Biochem. 2013, 128, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Tonti, G.A.; Medda, V.; Simone, P.; Darbre, P.D. Analysis of Aluminium Content and Iron Homeostasis in Nipple Aspirate Fluids from Healthy Women and Breast Cancer-Affected Patients. J. Appl. Toxicol. 2011, 31, 262–269. [Google Scholar] [CrossRef]
- Darbre, P.D.; Pugazhendhi, D.; Mannello, F. Aluminium and Human Breast Diseases. J. Inorg. Biochem. 2011, 105, 1484–1488. [Google Scholar] [CrossRef]
- Qin, W.; Zhu, W.; Hewett, J.E.; Rottinghaus, G.; Chen, Y.C.; Flynn, J.T.; Kliethermes, B.; Mannello, F.; Sauter, E.R. Upa Is Upregulated by High Dose Celecoxib in Women at Increased Risk of Developing Breast Cancer. BMC Cancer 2008, 8, 298. [Google Scholar] [CrossRef]
- Darbre, P.D.; Mannello, F.; Exley, C. Aluminium and Breast Cancer: Sources of Exposure, Tissue Measurements and Mechanisms of Toxicological Actions on Breast Biology. J. Inorg. Biochem. 2013, 128, 257–261. [Google Scholar] [CrossRef]
- Mannello, F.; Ligi, D. Resolving Breast Cancer Heterogeneity by Searching Reliable Protein Cancer Biomarkers in the Breast Fluid Secretome. BMC Cancer 2013, 13, 344. [Google Scholar] [CrossRef]
- Mannello, F.; Medda, V.; Smaniotto, A.; Tonti, G.A. Intracrinology of Breast Microenvironment: Hormonal Status in Nipple Aspirate Fluid and Its Relationship to Breast Cancer. Expert Rev. Endocrinol. Metab. 2009, 4, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F. New Horizon for Breast Cancer Biomarker Discoveries: What Might the Liquid Biopsy of Nipple Aspirate Fluid Hold? Proteom. Clin. Appl. 2017, 11, 1700060. [Google Scholar] [CrossRef]
- Sauter, E.R.; Diamandis, E.P. Prostate-Specific Antigen Levels in Nipple Aspirate Fluid. J. Clin. Oncol. 2001, 19, 3160. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.R.; Lininger, J.; Magklara, A.; Hewett, J.E.; Diamandis, E.P. Association of Kallikrein Expression in Nipple Aspirate Fluid with Breast Cancer Risk. Int. J. Cancer 2004, 108, 588–591. [Google Scholar] [CrossRef]
- Sauter, E.R.; Babb, J.; Daly, M.; Engstrom, P.F.; Ehya, H.; Malick, J.; Diamandis, E. Prostate-Specific Antigen Production in the Female Breast: Association with Progesterone. Cancer Epidemiol. Biomark. Prev. 1998, 7, 315–320. [Google Scholar]
- Sauter, E.R.; Chervoneva, I.; Diamandis, A.; Khosravi, J.M.; Litwin, S.; Diamandis, E.P. Prostate-Specific Antigen and Insulin-Like Growth Factor Binding Protein-3 in Nipple Aspirate Fluid Are Associated with Breast Cancer. Cancer Detect. Prev. 2002, 26, 149–157. [Google Scholar] [CrossRef]
- Pavlou, M.P.; Kulasingam, V.; Sauter, E.R.; Kliethermes, B.; Diamandis, E.P. Nipple Aspirate Fluid Proteome of Healthy Females and Patients with Breast Cancer. Clin. Chem. 2010, 56, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhu, W.; Schlatter, L.; Miick, R.; Loy, T.S.; Atasoy, U.; Hewett, J.E.; Sauter, E.R. Increased Expression of the Inflammatory Protein Ykl-40 in Precancers of the Breast. Int. J. Cancer 2007, 121, 1536–1542. [Google Scholar] [CrossRef]
- Sauter, E.R.; Wagner-Mann, C.; Ehya, H.; Klein-Szanto, A. Biologic Markers of Breast Cancer in Nipple Aspirate Fluid and Nipple Discharge Are Associated with Clinical Findings. Cancer Detect. Prev. 2007, 31, 50–58. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.R.; Sauter, E.R.; Quinn, T.P.; Deutscher, S.L. Thomsen-Friedenreich and Tn Antigens in Nipple Fluid: Carbohydrate Biomarkers for Breast Cancer Detection. Clin. Cancer Res. 2005, 11 Pt 1, 6868–6871. [Google Scholar] [CrossRef]
- Sauter, E.R.; Shan, S.; Hewett, J.E.; Speckman, P.; Du Bois, G.C. Proteomic Analysis of Nipple Aspirate Fluid Using Seldi-Tof-Ms. Int. J. Cancer 2005, 114, 791–796. [Google Scholar] [CrossRef]
- Qin, W.; Zhu, W.; Wagner-Mann, C.; Sauter, E.R. Nipple Aspirate Fluid Expression of Urokinase-Type Plasminogen Activator, Plasminogen Activator Inhibitor-1, and Urokinase-Type Plasminogen Activator Receptor Predicts Breast Cancer Diagnosis and Advanced Disease. Ann. Surg. Oncol. 2003, 10, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhu, W.; Wagner-Mann, C.; Folk, W.; Sauter, E.R. Association of Upa, Pat-1, and Upar in Nipple Aspirate Fluid (Naf) with Breast Cancer. Cancer J. 2003, 9, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Trott, P.A.; Morris, L.; Coleman, N.; Sauter, E.; Eeles, R.A. Cellular Characteristics of Nipple Aspiration Fluid During the Menstrual Cycle in Healthy Premenopausal Women. Cytopathology 2001, 12, 184–196. [Google Scholar] [CrossRef]
- Qin, W.; Holick, M.F.; Sorensen, W.; Walker, C.R.; Sauter, E.R. Vitamin D3 Treatment Influences Pge2 and Tgfbeta in Normal and Increased Breast Cancer Risk Women. Anticancer Res. 2016, 36, 5347–5353. [Google Scholar] [CrossRef]
- Sauter, E.R. Using Organ Specific and Circulatory Biofluids to Screen Individuals at High Risk for Breast Cancer Presents Unique Challenges and Opportunities. Cancer Epidemiol. Biomark. Prev. 2021, 30, 429–431. [Google Scholar] [CrossRef]
- de Groot, J.S.; Moelans, C.B.; Elias, S.G.; Jo Fackler, M.; van Domselaar, R.; Suijkerbuijk, K.P.; Witkamp, A.J.; Sukumar, S.; van Diest, P.J.; van der Wall, E. DNA Promoter Hypermethylation in Nipple Fluid: A Potential Tool for Early Breast Cancer Detection. Oncotarget 2016, 7, 24778–24791. [Google Scholar] [CrossRef][Green Version]
- de Groot, J.S.; Pan, X.; Meeldijk, J.; van der Wall, E.; van Diest, P.J.; Moelans, C.B. Validation of DNA Promoter Hypermethylation Biomarkers in Breast Cancer--a Short Report. Cell. Oncol. 2014, 37, 297–303. [Google Scholar] [CrossRef]
- Early Detection of Hereditary Breast Cancer by Monitoring Microrna Expression in Nipple Aspirate Fluid. Available online: https://www.trialregister.nl/trial/8661 (accessed on 3 September 2021).
- Breast Cancer Biomarkers in Nipple Aspirate Fluid and Blood in Healthy Women. Available online: https://www.trialregister.nl/trial/8987 (accessed on 3 September 2021).
- The Ornament Study: A Multicenter, Cross Sectional, Study to Assess Microrna Expression in Nipple Aspirated Fluid, Blood and Tumor Material in Women with Primary Breast Cancer Compared with Healthy Controls. Available online: https://www.trialregister.nl/trial/6031 (accessed on 3 September 2021).
- Patuleia, S.I.S.; Hagenaars, S.C.; Moelans, C.B.; Ausems Mgem van Gils, C.H.; Tollenaar Raem van Diest, P.J.; Mesker, W.E.; van der Wall, E. Lessons Learned from Setting up a Prospective, Longitudinal, Multicenter Study with Women at High Risk for Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2021, 30, 441–449. [Google Scholar] [CrossRef] [PubMed]
- George, A.L.; Shaheed, S.U.; Sutton, C.W. High-Throughput Proteomic Profiling of Nipple Aspirate Fluid from Breast Cancer Patients Compared with Non-Cancer Controls: A Step Closer to Clinical Feasibility. J. Clin. Med. 2021, 10, 2243. [Google Scholar] [CrossRef]
- Jiwa, N.; Takats, Z.; Leff, D.R. Aso Author Reflections: Diagnostic Accuracy of Nipple Aspirate Fluid Cytology in Asymptomatic Patients and Its Predictive Validity on Future Risk of Breast Cancer: A Meta-Analysis and Systematic Review of the Literature. Ann. Surg. Oncol. 2021, 28, 3761–3762. [Google Scholar] [CrossRef]
- Nipple Aspirate Fluid in Detecting Breast Cancer. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03715959?term=NAF%2C+nipple+aspirate+fluid&recrs=ab&draw=2&rank=1 (accessed on 3 September 2021).
- Physical Activity and Dietary Counseling and Supervised Group Exercise for First-Time Pregnant Women—A Feasibility Study of a Controlled Trial. Available online: https://www.isrctn.com/ISRCTN21512277?q=nipple%20aspirate%20fluid&filters=&sort=&offset=1&totalResults=1&page=1&pageSize=10&searchType=basic-search (accessed on 3 September 2021).
- Martinez, J.A.; Chalasani, P.; Thomson, C.A.; Roe, D.; Altbach, M.; Galons, J.P.; Stopeck, A.; Thompson, P.A.; Villa-Guillen, D.E.; Chow, H.H. Phase Ii Study of Metformin for Reduction of Obesity-Associated Breast Cancer Risk: A Randomized Controlled Trial Protocol. BMC Cancer 2016, 16, 500. [Google Scholar] [CrossRef]
- Kelsey, J.L.; Bernstein, L. Epidemiology and Prevention of Breast Cancer. Annu. Rev. Public Health 1996, 17, 47–67. [Google Scholar] [CrossRef]
- Phillips, H.A.; Howard, G.C.; Miller, W.R. Nipple Aspirate Fluid in Relation to Breast Cancer. Breast 1999, 8, 169–174. [Google Scholar] [CrossRef]
- Klein, P.M.; Lawrence, J.A. Lavage and Nipple Aspiration of Breast Ductal Fluids: A Source of Biomarkers for Environmental Mutagenesis. Environ. Mol. Mutagen. 2002, 39, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.J.; Kimler, B.F. Breast Cancer Chemoprevention: Current Challenges and a Look toward the Future. Clin. Breast Cancer 2002, 3, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Dooley, W.C. Ductal Lavage, Nipple Aspiration, and Ductoscopy for Breast Cancer Diagnosis. Curr. Oncol. Rep. 2003, 5, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A. The Role of Ductal Lavage in the Management of Women at High Risk for Breast Carcinoma. Curr. Treat. Options Oncol. 2004, 5, 145–151. [Google Scholar] [CrossRef]
- Kenney, P.J.; Ellison, M.C. Ductal Lavage in the Screening of High-Risk Women. Curr. Oncol. Rep. 2004, 6, 69–73. [Google Scholar] [CrossRef]
- Khan, S.A.; Bhandare, D.; Chatterton, R.T., Jr. The Local Hormonal Environment and Related Biomarkers in the Normal Breast. Endocr.-Relat. Cancer 2005, 12, 497–510. [Google Scholar] [CrossRef]
- King, B.L.; Love, S.M.; Rochman, S.; Kim, J.A. The Fourth International Symposium on the Intraductal Approach to Breast Cancer, Santa Barbara, California, 10–13 March 2005. Breast Cancer Res. 2005, 7, 198–204. [Google Scholar] [CrossRef]
- Coombes, K.R.; Tsavachidis, S.; Morris, J.S.; Baggerly, K.A.; Hung, M.C.; Kuerer, H.M. Improved Peak Detection and Quantification of Mass Spectrometry Data Acquired from Surface-Enhanced Laser Desorption and Ionization by Denoising Spectra with the Undecimated Discrete Wavelet Transform. Proteomics 2005, 5, 4107–4117. [Google Scholar] [CrossRef]
- Fabian, C.J.; Kimler, B.F.; Mayo, M.S.; Khan, S.A. Breast-Tissue Sampling for Risk Assessment and Prevention. Endocr.-Relat. Cancer 2005, 12, 185–213. [Google Scholar] [CrossRef]
- Escobar, P.F.; Crowe, J.P.; Matsunaga, T.; Mokbel, K. The Clinical Applications of Mammary Ductoscopy. Am. J. Surg. 2006, 191, 211–215. [Google Scholar] [CrossRef]
- Hu, S.; Loo, J.A.; Wong, D.T. Human Body Fluid Proteome Analysis. Proteomics 2006, 6, 6326–6353. [Google Scholar] [CrossRef]
- Lang, J.E.; Kuerer, H.M. Breast Ductal Secretions: Clinical Features, Potential Uses, and Possible Applications. Cancer Control. 2007, 14, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Ruhlen, R.L.; Sauter, E.R. Proteomics of Nipple Aspirate Fluid, Breast Cyst Fluid, Milk, and Colostrum. Proteom. Clin. Appl. 2007, 1, 845–852. [Google Scholar] [CrossRef]
- Ruhlen, R.L.; Sauter, E.R. Proteomic Analysis of Breast Tissue and Nipple Aspirate Fluid for Breast Cancer Detection. Biomark Med. 2007, 1, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.; Meleady, P.; Dowling, P.; Clynes, M. Proteomic Approaches for Serum Biomarker Discovery in Cancer. Anticancer Res. 2007, 27, 1247–1255. [Google Scholar] [PubMed]
- LaKind, J.S.; Wilkins, A.A.; Bates, M.N. Human Breast Biomonitoring and Environmental Chemicals: Use of Breast Tissues and Fluids in Breast Cancer Etiologic Research. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 525–540. [Google Scholar] [CrossRef]
- Suijkerbuijk, K.P.; van der Wall, E.; Vooijs, M.; van Diest, P.J. Molecular Analysis of Nipple Fluid for Breast Cancer Screening. Pathobiology 2008, 75, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F. Analysis of the Intraductal Microenvironment for the Early Diagnosis of Breast Cancer: Identification of Biomarkers in Nipple-Aspirate Fluids. Expert Opin. Med. Diagn. 2008, 2, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Debald, M.; Wolfgarten, M.; Walgenbach-Brunagel, G.; Kuhn, W.; Braun, M. Non-Invasive Proteomics-Thinking About Personalized Breast Cancer Screening and Treatment. EPMA J. 2010, 1, 413–420. [Google Scholar] [CrossRef][Green Version]
- Green, V.L. Breast Cancer Risk Assessment, Prevention, and the Future. Obstet Gynecol Clin North Am. 2013, 40, 525–549. [Google Scholar] [CrossRef]
- Maskarinec, G. The Human Mammary Gland as a Target for Isoflavones: How Does the Relation Vary in Individuals with Different Ethnicity? Planta Med. 2013, 79, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Schaaij-Visser, T.B.; de Wit, M.; Lam, S.W.; Jimenez, C.R. The Cancer Secretome, Current Status and Opportunities in the Lung, Breast and Colorectal Cancer Context. Biochim Biophys Acta. 2013, 1834, 2242–2258. [Google Scholar] [CrossRef]
- Masood, S. Development of a Novel Approach for Breast Cancer Prediction and Early Detection Using Minimally Invasive Procedures and Molecular Analysis: How Cytomorphology Became a Breast Cancer Risk Predictor. Breast J. 2015, 21, 82–96. [Google Scholar] [CrossRef]
- Hornberger, J.; Chen, S.C.; Li, Q.; Kakad, P.; Quay, S.C. Proliferative Epithelial Disease Identified in Nipple Aspirate Fluid and Risk of Developing Breast Cancer: A Systematic Review. Curr. Med. Res. Opin. 2015, 31, 253–262. [Google Scholar] [CrossRef][Green Version]
- Parida, S.; Sharma, D. The Power of Small Changes: Comprehensive Analyses of Microbial Dysbiosis in Breast Cancer. Biochim Biophys Acta Rev Cancer. 2019, 1871, 392–405. [Google Scholar] [CrossRef]
- Li, J.; Guan, X.; Fan, Z.; Ching, L.M.; Li, Y.; Wang, X.; Cao, W.M.; Liu, D.X. Non-Invasive Biomarkers for Early Detection of Breast Cancer. Cancers 2020, 12, 2767. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).