Simple Summary
Although the introduction of programmed cell death 1 (PD-1) immune checkpoint inhibitors (ICIs) has significantly improved the overall survival (OS) in patients with metastatic melanoma, a substantial number of patients do not benefit from ICIs. Therefore, the predictive value of single nucleotide polymorphisms (SNPs) in genes related to the PD-1 axis was investigated in patients with metastatic melanoma and anti-PD-1 monotherapy. Germline variation in the gene encoding for PD-1, PDCD1 804C > T (rs2227981), was associated with poorer OS with a 3-year OS rate of 51.8%, as compared to 71% in wild type patients. In addition, PDCD1 804C > T carriers had significantly lower mRNA expression in several tissues and a decreased fraction of PD-1+ CD4+ T cells, indicating that PDCD1 804C > T may affect clinical benefit from ICIs by decreasing transcriptional initiation and PD-1 expression in T cells. These findings show that germline genetics may significantly impact immune responses after ICIs.
Abstract
A substantial number of melanoma patients do not benefit from therapy with anti-PD-1. Therefore, we investigated the predictive value of single nucleotide polymorphisms (SNPs) in genes related to the PD-1 axis in patients with metastatic melanoma. From 119 consecutive melanoma patients who were treated with pembrolizumab or nivolumab monotherapy, blood samples were genotyped for 11 SNPs in nine genes. Associations between SNPs and OS were tested using Cox regression analysis and internally validated by bootstrapping. For SNPs with a statistical significance, an expression quantitative trait loci (eQTL) analysis was performed. In a subset of patients, immunophenotyping was performed. Patients with a SNP in PDCD1 (804C > T; rs2227981) had a significantly poorer OS with a 3-year OS rate of 51.8%, as compared to 71% in wild type patients (hazard ratio [HR] 2.37; 95% CI: 1.11–5.04; p = 0.026). eQTL analysis showed that this SNP was associated with decreased gene expression. In addition, PDCD1 804C > T carriers had a reduced fraction of peripheral PD-1+CD4+ T cells. No other associations between SNPs and OS were found. PDCD1 804C > T is associated with poorer OS after anti-PD-1 monotherapy in patients with metastatic melanoma. This SNP may affect clinical benefit from ICIs by decreasing transcription initiation and expression of PD-1 in T cells.
1. Introduction
Melanoma is the most aggressive skin cancer, with a poor prognosis in patients with advanced disease [1]. More than 100,000 new cases occur in Europe each year, and the incidence continues to increase [1]. Spontaneous regression of melanoma is observed in about 15% of patients with primary melanoma [2]. In addition, spontaneous regression of primary melanoma may occur in advanced stage disease, as primary melanoma cannot be detected in approximately 5% of patients with metastatic melanoma [2]. This spontaneous regression is the result of antitumor immunity with lymphocytic and histiocytic infiltration in primary melanomas, which may eventually result in the disappearance of tumor cells or even normal bystander melanocytes [3,4].
Vitiligo is a common autoimmune disease with depigmentation of the skin that is caused by the destruction of melanocytes by a T cell-mediated autoimmune response. In patients with metastatic melanoma, vitiligo is associated with improved overall survival (OS) [5]. The combination of antitumor immunity (i.e., spontaneous regression) and autoimmunity (i.e., vitiligo) in patients with melanoma is considered an effective immune response against melanocytic cells [6].
As effective spontaneous antitumor immunity is rare, immune checkpoint inhibitors (ICIs) have been developed to reinforce the endogenous immunity. Cancer cells are known to impair antitumor immunity by affecting the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1) axes with the subsequent downregulation of the T cell effector function [7]. While the introduction of ICIs targeting CTLA-4 was the first step towards improvement of OS in patients with advanced melanoma, PD-1 inhibitors further improved 5-year survival up to 52% [8]. Comparable to observations in melanoma patients with spontaneous remission, immune-related adverse events (irAEs) are associated with prolonged OS in patients with advanced melanoma who are treated with ICIs [9].
Therefore, it is conceivable that factors which are associated with autoimmunity may predict benefit from ICIs. For several germline variants, or single nucleotide polymorphisms (SNPs), associations with different autoimmune diseases have been described. SNPs contribute to the 0.1% difference in the DNA sequence of the human genome [10]. Nonsynonymous SNPs may alter the amino acid sequence; while both synonymous and nonsynonymous SNPs can influence gene expression, messenger RNA stability, and translational efficiency [10,11]. Thereby altering activity, function or quantity of the encoded proteins. Although some SNPs are harmless, other SNPs can cause diseases. For example, an increased risk of autoimmune vitiligo is associated with a germline variant in HLAA, a gene encoding for the major histocompatibility complex (MHC) that binds to the T cell receptor and stimulates T cell activation [12].
As autoimmunity may reflect antitumor immunity, the primary objective of the current study was to investigate whether SNPs in genes related to the PD-1 axis and involved in autoimmunity are predictive for OS in patients with metastatic melanoma and anti-PD-1 monotherapy. The secondary objectives were to assess the association of those germline variants with progression-free survival (PFS) and best overall response (BOR). To better understand the results, translational analyses were also performed, including multi-tissue expression quantitative trait loci (eQTL) analysis and peripheral immunophenotyping by flow cytometry.
2. Materials and Methods
2.1. Study Design
In this single-center study at Erasmus University Medical Center (Rotterdam, The Netherlands), prospectively collected whole blood samples were analyzed to determine germline variations in patients with metastatic melanoma who were treated with anti-PD-1 monotherapy. Patients were included if they fulfilled the following inclusion criteria: anti-PD-1 monotherapy (i.e., pembrolizumab or nivolumab), a minimum follow-up of one year at data cut-off, availability of a whole blood sample, and signed informed consent for DNA analysis (Erasmus Medical Center ethics board, Rotterdam, The Netherlands, study number MEC 02-1002). A subgroup of patients signed an additional informed consent for immunomonitoring (MULTOMAB study; Erasmus Medical Center ethics board, Rotterdam, The Netherlands, study number MEC 16-011).
2.2. Collection of Clinical Data
At the initiation of monotherapy with anti-PD-1, the following patient and disease characteristics were collected: age, gender, BRAF V600E/K mutation status, prior systemic therapy, prior radiotherapy, Eastern Cooperative Oncology Group (ECOG) performance status, serum lactate dehydrogenase (LDH; U/L), and presence of central nervous system (CNS) metastases. The cut-off for follow-up data was set at 9 December 2019.
OS was defined from the start of treatment to death by any cause. Patients were censored at the date on which they were last known to be alive. PFS was defined as the time from the start of treatment to the first documented progressive disease (PD) according to Response Evaluation Criteria in Solid Tumours version 1.1 (RECIST v1.1) [13] or death by any cause. The therapy response was determined according to RECIST v1.1, i.e., complete response (CR), partial response (PR), stable disease (SD), or PD. For BOR, confirmation of PR and CR was not required, and a minimum duration of 90 days was required for SD.
2.3. Selection of SNPs
SNPs in genes related to the PD-1 axis were selected based on their previous reported associations with autoimmunity in the literature. On 15 June 2018, a literature search was performed for the combination of polymorphisms, autoimmunity and genes that were related to T cell receptor (TCR) signaling (Kyoto Encyclopedia of Genes and Genomes [KEGG] TCR pathway) or the PD-1 pathway according to the QIAGEN Ingenuity Pathway Analysis (QIAGEN, Redwood City, CA, USA). SNPs were prioritized according to their strong associations with susceptibility to autoimmune diseases (generally reflected by the odds ratio (OR)) or their validation in independent populations. Common SNPs with a minor allele frequency (MAF) above 5% were included. Synonymous SNPs were excluded, except for SNPs with a predicted variant effect (i.e., intronic variation in the promotor region affecting mRNA transcription; estimated by the variant effect predictor tool; https://ensembl.org, accessed on 24 September 2018). Finally, eleven separate SNPs in nine genes were selected for the current analyses (Table 1). An overview of the search strategy is depicted in Figure S1.

Table 1.
Literature overview of selected single nucleotide polymorphisms (SNPs) and their associations with autoimmunity.
2.4. DNA Isolation and Genotyping
DNA was isolated from 400 μL of the whole blood specimens on the MagNaPure Compact Instrument (Roche Diagnostics GmbH, Mannheim, Germany) using the Nucleic Acid Isolation Kit I (Roche Diagnostics GmbH, Mannheim, Germany). Predesigned drug metabolism enzymes (DME) TaqMan allelic discrimination assays (Table 2) were used to genotype the selected SNPs on the Life Technologies TaqMan 7500 system (Applied Biosystems, Life Technologies Europe BV, Bleiswijk, The Netherlands). Each assay consisted of two allele-specific minor groove binding probes (MGB) labeled with 2′-chloro-7′phenyl-1,4-dichloro-6-carboxy-fluorescein (VIC) and 6-caboxyfluorescein (FAM) fluorescent dyes. To conduct qPCR, 20 ng of genomic DNA was analyzed using the assays and a TaqMan GTX press Master Mix (Applied Biosystems, Life Technologies Europe BV, Bleiswijk, The Netherlands). qPCR consisted of 40 cycles of denaturation (95 °C for 20 s), followed by annealing (92 °C for 3 s) and extension (60 °C for 30 s). Using the TaqMan 7500 software v2.3 for allelic discrimination (Applied Biosystems, Life Technologies Europe BV, Bleiswijk, The Netherlands), the genotypes were determined by measuring the allele-specific fluorescence.

Table 2.
Investigated SNPs.
2.5. Multi-Tissue Expression Quantitative Trait Loci (eQTL) Analysis
For germline variations with statistical significance in multivariate analyses, expression quantitative trait loci (eQTL) analysis was performed to determine the effect of genetic variations on gene expression in normal tissue. Global RNA expression was analyzed for the minor and major allele of the germline variant using the Genotype-Tissue Expression (GTEx) database (https://gtexportal.org, accessed on 17 May 2020) [23]. This open-source GTEx project allows one to study variation in gene expression levels of diverse tissues of the human body. The minor and major allele were tested against gene expression using linear regression analysis. The normalized effect size (NES) is defined as the slope of the linear regression and computed as the effect of the minor allele.
2.6. Flow Cytometry
From patients who signed an additional informed consent, peripheral blood mononuclear cells (PBMCs) were isolated at baseline (prior to the first administration) and during anti-PD-1 monotherapy (prior to the second and third administration). As previously described [24], flow cytometry was performed and frequencies of CD4+ and CD8+ T cells expressing PD-1 were determined. In addition, frequencies of regulatory T cells (Tregs) expressing PD-1 were measured.
2.7. Statistical Analysis
Data were presented as the prevalence (percentage) or median (range). The distribution of the genotypes was tested for Hardy–Weinberg equilibrium (HWE) using the Chi-square test. Two genetic models (i.e., the dominant and recessive model) were applied for further analyses [25]. To study associations between clinical baseline characteristics or SNPs with survival (OS and PFS), Cox proportional hazards regression analysis was used to calculate the HR with 95% confidence intervals (CIs). Variables with p ≤ 0.10 in univariable analyses were included in multivariable analyses where backward selection was applied with a threshold of p < 0.05. The proportional hazards assumption was determined for all Cox analyses by means of the Schoenfeld residuals. For SNPs with p < 0.10 in univariate analyses, Kaplan–Meier curves were applied for PFS and OS.
To identify patients with clinical benefit according to BOR, patients were categorized into “clinical benefit” (i.e., CR, PR, and SD) and “no clinical benefit” (i.e., PD). The genetic models were tested against BOR using logistic regression. The odds ratio (OR) and 95% CIs were calculated.
All associations with p < 0.10 were internally validated by bootstrapping [26]. For this purpose, one thousand bootstrap samples were generated (with replacements) and bias-corrected 95% CIs were calculated for the ORs and HRs.
The Mann–Whitney U test was used to test whether frequencies of CD4+ and CD8+ T cells with PD-1 expression were different between wild types and homozygous + heterozygous variants.
Correlation analyses of clinical baseline characteristics and SNPs were performed by either the rank-biserial (for ordinal variables) or Phi correlation test (for continuous variables) using IBM SPSS Statistics (version 24.0.0.1, Chicago, IL, USA). All other statistical analyses were performed using STATA (version 15.1, StataCorp LP, Lakeway Drive College Station, TX, USA).
3. Results
3.1. Clinical Characteristics
In total, 119 patients with metastatic melanoma and anti-PD-1 monotherapy were included. Baseline characteristics are shown in Table 3. Sixty-three patients (53%) were treated with twice-weekly nivolumab, (weight-based 3 mg/kg) and 56 patients (47%) were treated with thrice-weekly pembrolizumab (weight-based 2 mg/kg). Prior to the approval of anti-PD-1, one patient had been treated with pembrolizumab in the Keynote-001 trial (NCT01295827) [27], and one patient had been treated with pembrolizumab in a compassionate use program. At baseline, 32 patients (27%) had an ECOG performance status ≥1 and the median level of serum LDH was 211 U/L (range 118–1523 U/L). In the majority of patients (N = 89), anti-PD-1 monotherapy was administered as first-line treatment, whereas 30 patients (25%) had received at least one prior treatment line, including BRAF-MEK-inhibitors (N = 14), ipilimumab (N = 7), a sequential combination of both (N = 4), or talimogene laherparepvec (TVEC) (N = 5).

Table 3.
Baseline patients’ characteristics.
The median follow-up was 2.6 years (range 0.2–4.2 years). At data cut-off, 75 patients (63%) were still alive. The median OS was not reached and the median PFS was 19.6 months (range 9.0–30.1 months). In 117 patients, response evaluation was available for further analyses. Two patients were excluded for response evaluation due to insufficient baseline scans for radiological evaluation. After the initiation of anti-PD-1 monotherapy, 86 (73.5%) patients had clinical benefit, with 26 (22%), 43 (37%), and 17 (15%) patients having a CR, PR, and SD, respectively. Thirty-one patients (26%) had PD. During follow-up, 67 out of 117 patients (57%) eventually had PD according RECIST v.1.1.
3.2. Germline Variation Associated with OS, PFS and BOR
All investigated SNPs were in HWE (Table 2).
Among clinical baseline characteristics, age (≥65 vs. <65 years), ECOG performance status (≥1 vs. 0), and LDH levels (per U/L) were significantly associated with OS (HR 1.695, 95%CI 0.910–3.94; HR 3.625, 95% CI 2.037–6.651; and HR 1.004, 95%CI 1.000–1.005, respectively). In univariable analyses, germline variation in GZMB and PDCD1 showed a trend towards associations with OS according to the dominant model (HR 1.811, 95%CI 0.945–3.364, p = 0.060 and HR 2.027, 95%CI 0.998–4.118, p = 0.051, respectively). After correction for baseline factors, patients with the germline variant PDCD1 804C > T still had a significantly worse OS with a 3-year survival rate of 51.8%, as compared to 71.0% in wild type patients (OR 2.366; 95% CI 1.111–5.036; p = 0.026). These results were confirmed by internal validation (bias corrected 95%CI 1.039–6.246). As GZMB c.128C > A was not significant in the multivariable analysis, the SNP was excluded from the multivariable model. No violations of the proportional hazards assumption for Cox analyses were found. Univariable and multivariable analyses for OS are shown in Table 4. Figure S2A,B show the OS curves of patients with metastatic melanoma according to germline variation in PDCD1 (804C > T) and GZMB (c.128C > A), respectively.

Table 4.
Univariable and multivariable associations of clinical baseline characteristics and SNPs with OS.
Based on univariable analysis for PFS (p < 0.10), the following clinical baseline characteristics could be included in multivariate PFS analysis: ECOG performance (≥1 vs. 0), prior treatment (yes vs. no), BRAF mutation status, and LDH level (per U/L). In univariable analysis for PFS, GZMB c.128C > A fulfilled criteria for multivariable analyses according to the dominant model (HR 1.633, 95%CI 0.999–2.670, p = 0.051; Table S1). After correction for clinical baseline characteristics, germline variation GZMB c.128C > A was not associated with PFS (p = 0.159). No violations of the proportional hazards assumption for Cox analyses were found. Figure S2C,D show the PFS curves according to germline variation in PDCD1 (804C > T) and GZMB (c.128C > A), respectively.
There were no significant associations between the SNPS and BOR (Table S2).
Clinical baseline characteristics and SNPs with p < 0.10 were tested for their correlation. No significant correlations between SNPs and clinical baseline characteristics were found. Prior treatment was significantly correlated with poorer ECOG performance status (correlation coefficient 0.203, p = 0.030) and a BRAF mutation was correlated with age (correlation coefficient -0.340, p < 0.001) (Table S3).
3.3. PDCD1 804C > T and mRNA Expression in Human Tissue
In multivariable analysis, germline variation in PDCD1 (804C > T) remained significantly associated with OS. Moreover, the upstream variant PDCD1 804C > T was estimated to have regulatory implications (by the variant effect predictor tool). Next, an eQTL analysis was conducted to determine the impact of this SNP on mRNA expression of PDCD1 across tissue. This analysis showed that germline variation in PDCD1 (804C > T) is significantly associated with lower gene expression of PDCD1 in human tissue, in particular in subcutaneous adipose tissue (Figure S3).
3.4. PDCD1 804C > T and PD-1 Expression in Circulating T Cells
In 47 patients, the impact of germline variation PDCD1 804C > T on the PD-1 expression in circulating immune cell populations could be investigated in blood obtained prior to and after anti-PD-1 monotherapy. There was a consistent tendency that patients with heterozygous or homozygous variation of PDCD1 804C > T had a lower fraction of PD-1+ CD4+ T cells, which was observed in PBMCs prior to and during anti-PD-1 monotherapy (Figure 1A–C). In contrast, this tendency was not observed in CD8+ T cells and regulatory T cells (Figure 1D–F and Figure S4, respectively).
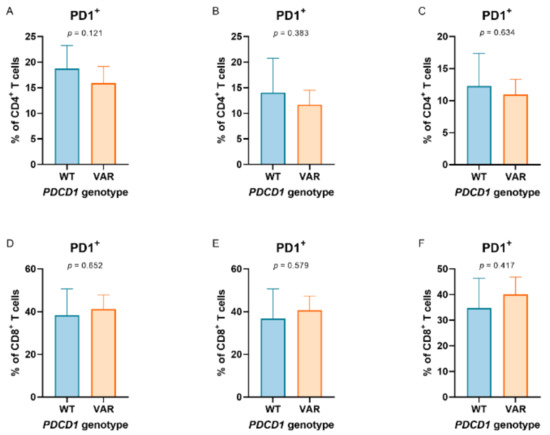
Figure 1.
Programmed cell death 1 (PD-1) expression in peripheral T cell populations according to PDCD1 804C > T genotypes. PD-1 expression in peripheral (A–C) CD4+ and (D–F) CD8+ T cells according to PDCD1 804C > T genotypes (comparing wild types (WT) and heterozygous variants + homozygous variants (VAR)). Peripheral T cells were collected prior to (baseline, graphs on the left) and during anti-PD-1 monotherapy (mid: prior to the second administration of anti-PD-1 monotherapy; right: prior to the third administration of anti-PD-1 monotherapy) in patients with metastatic melanoma (N = 47).
4. Discussion
The current study demonstrates that patients with metastatic melanoma and a SNP in PDCD1 (804C > T; rs2227981) have a significantly poorer OS after treatment with anti-PD-1 monotherapy, resulting in a 3-year survival rate of 51.8%, as compared to 71.0% for wild type patients. These results were internally validated and corrected for prognostic factors, including ECOG performance status and LDH. In addition, the SNP was associated with the decreased gene expression of PDCD1 in several tissues, in particular in subcutaneous adipose tissue. Besides, there was a consistent tendency that patients with heterozygous or homozygous variation of PDCD1 804C > T had a lower fraction of PD-1+ CD4+ T cells.
The PD-1 receptor is encoded by PDCD1 and is primarily expressed on CD4+ and CD8+ T cells [28]. The interaction of PD-1 on T cells to its ligands 1 or 2 (PD-L1 or PD-L2) suppresses T cell proliferation and function. As the SNP in PDCD1 (804C > T) is located in the promoter region of the gene, it may affect transcriptional initiation, resulting in a decreased expression of PD-1. In the current study, we have demonstrated this decreased PD-1 expression in both tissue and peripheral blood, thereby explaining the poorer OS in patients with the PDCD1 804C > T SNP. First, the locus was found to control PDCD1 transcription in tissues and the SNP (804C > T) was predicted to negatively impact transcription. Second, eQTL analysis showed that the SNP in PDCD1 (804C > T) was associated with a significantly lower mRNA expression in several human tissues, in particular in subcutaneous adipose tissue. This strong relation between a SNP in PDCD1 (804C > T) and PDCD1 expression in subcutaneous adipose tissue may be explained by the high fraction of CD4+ T cells with PD-1 expression in subcutaneous adipose tissue [29]. Third, peripheral immunophenotyping showed a trend towards a decreased fraction of PD-1+ CD4+ T cells in patients with the PDCD1 804C > T SNP. Likewise, patients with type 1 diabetes and the PDCD1 804C > T SNP have a significantly lower fraction of PD-1 expression in peripheral CD4+ T cells [30], which supports the observed trend. Together, these three findings suggest that the PDCD1 804C > T SNP affects transcriptional initiation and consequently results in a lower expression of PD-1 in CD4+ T cells.
In addition, patients with the PDCD1 804C > T SNP had an almost 20% lower 3-year survival rate as compared to wild type patients. This may be caused by a decreased PD-1 expression in T cells. Previously, PD-1 expression in tumor infiltrating lymphocytes has been associated with survival in several tumor types [31,32]. For example, patients with positive PD-1 immunostaining in melanoma lymph node metastases have improved melanoma-specific survival compared to patients without PD-1 expression [33].
Although PDCD1 804C > T was associated with poorer OS after anti-PD-1 monotherapy in our study, we did not observe significant associations between PDCD1 804C > T and PFS or BOR. However, ICI-induced tumor response is not always predictive of OS, as improved OS has been reported in patients with metastatic melanoma after continuation of anti-PD-1 beyond disease progression [34]. Besides, the use of RECIST v1.1 in this study may explain the discrepancy. While RECIST v1.1 is still used in routine clinical practice, tumor response to anti-PD-1 therapy can be underestimated in 15% of patients [35].
As observed previously in patients with non-small cell lung cancer (NSCLC) [36], patients with heterozygous or homozygous variation of GZMB c.128C > A have significantly worse PFS and OS after anti-PD-1 monotherapy in univariable analysis. After correction for baseline characteristics, however, these associations did not retain significance. GZMB encodes for the similar named protease granzyme B, that is released by effector T cells to induce apoptosis of tumor cells [37], and the c.128C > A SNP is hypothesized to define an isoform of granzyme B that is incapable of apoptosis in tumor cell lines [38]. As we found no significant association in this cohort between GZMB c.128C>A and survival in multivariable analysis, the clinical relevance of this SNP seems limited and needs to be verified in larger cohorts.
As SNPs in genes related to the PD-1 axis are associated with autoimmune diseases [12,14,15,16,17,18,19,20,21,22] and irAEs are correlated with treatment outcome of ICIs [39,40], it is conceivable that these SNPs are also associated with irAEs. In the current study, we did not evaluate irAEs, as the retrospective design would have resulted in underreporting of irAEs, in particular of low grade irAEs such as vitiligo. Although a previous retrospective study in patients with NSCLC did not show any association between PDCD1 804C > T and irAEs [41], prospective studies are needed to investigate the role of autoimmune disease-related SNPs in the development of irAEs.
Due to the retrospective data collection in this study, brain scans were not available for all patients at baseline. As a result, multivariable analyses could not be corrected for CNS metastases, a well-known prognostic factor in metastatic melanoma. Other limitations of the current study include the limited sample size and the lack of an external validation cohort, which was overcome by internal validation. Nevertheless, the large difference in 3-year OS rates between carriers and non-carriers of the 804C > T SNP in PDCD1 indicates that germline mutations in genes related to the PD-1 axis may have a significant impact on the outcome of patients with cancer after ICIs. Genome-wide association studies (GWAS) may contribute to the further identification of germline variants, affecting the delicate balance between autoimmunity and antitumor immunity after ICIs.
5. Conclusions
The present study demonstrates that a common upstream germline variant of PDCD1 (804C > T; rs2227981) is associated with worse OS in patients with metastatic melanoma after treatment with PD-1 ICIs. This SNP may affect clinical benefit from ICIs by decreasing transcription initiation and expression of PD-1 in T cells. These observations highlight the clinical need to understand how germline genetics affects immune responses during immunotherapy, and suggest the feasibility for future GWAS in immuno-oncology.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/6/1370/s1, Figure S1: Search strategy for the selection of single-nucleotide polymorphisms (SNPs), Figure S2: Kaplan-Meier plots showing the overall survival (OS) and progression free survival (PFS) of patients included in this study (N = 119) according to PDCD1 804C > T, Figure S3: Multi-tissue expression quantitative trait loci (eQTL) analysis, showing separate eQTL statistics for germline variation PDCD1 804C > T, Figure S4: PD-1 expression in peripheral regulatory T cells according to PDCD1 804C > T genotypes (comparing wild type [WT], heterozygous variant [HET] and homozygous variant [HVAR]), Table S1: Univariable associations of clinical baseline characteristics and SNPs with PFS, Table S2: Univariable associations of clinical baseline characteristics and SNPs with BOR, Table S3: Correlation matrix.
Author Contributions
Conceptualization: M.d.W., D.P.H., E.O.-d.H., S.B., S.E.B., M.v.B., R.D., J.G.J.V.A. and A.A.M.v.d.V.; resources: M.d.W., D.P.H., A.L., M.v.B., J.G.J.V.A., R.H.N.v.S., R.H.J.M. and A.A.M.v.d.V.; data curation: M.d.W., D.P.H., E.O.-d.H., S.E.B., M.v.B., R.H.N.v.S., R.H.J.M. and A.A.M.v.d.V.; formal analysis: M.d.W., D.P.H., E.O.-d.H., A.L., S.E.B., M.v.B. and A.A.M.v.d.V.; supervision: M.d.W., D.P.H., E.O.-d.H., S.B., R.D., J.G.J.V.A., R.H.N.v.S., R.H.J.M. and A.A.M.v.d.V.; validation: M.d.W., D.P.H., E.O.-d.H. and A.A.M.v.d.V.; investigation: M.d.W., D.P.H., A.L., S.B., S.E.B., M.v.B., J.G.J.V.A., R.H.N.v.S., R.H.J.M. and A.A.M.v.d.V.; visualization: M.d.W., D.P.H. and A.A.M.v.d.V.; methodology: M.d.W., D.P.H., E.O.-d.H., S.B., S.E.B., J.G.J.V.A., R.H.N.v.S., R.H.J.M. and A.A.M.v.d.V.; writing—original draft: M.d.W., D.P.H. and A.A.M.v.d.V.; project administration: M.d.W., D.P.H., R.H.J.M. and A.A.M.v.d.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Erasmus Medical Center, Rotterdam, The Netherlands (study number MEC 02-1002 and study number MEC 16-011).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in this article and Supplementary Materials.
Conflicts of Interest
Astrid van der Veldt has received consultancy fees (paid to the institution) from at BMS, MSD, Merck, Ipsen, Eisai, Sanofi, Pierre Fabre, Pfizer, Novartis and Roche. Ron Mathijssen has received investigator-initiated research grants from Astellas, Bayer, Boehringer-Ingelheim, Cristal Therapeutics, Pamgene, Pfizer, Novartis, Roche and Servier. Joachim Aerts has received personal fees from MSD, BMS, Boehringer-Ingelheim, Amphera, Eli-Lilly, Takeda, Bayer, Roche and Astra Zeneca. Reno Debets has received research grants from Merck, and travel expenses and consultancy fees from Genticel and Bluebird bio. All remaining authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, L. Spontaneous regression of malignant melanoma: A review of the literature on incidence, clinical features, and possible mechanisms. Natl. Cancer Inst. Monogr. 1976, 44, 67–76. [Google Scholar] [PubMed]
- Ronan, S.G.; Eng, A.M.; Briele, H.A.; Shioura, N.N.; Das Gupta, T.K. Thin malignant melanomas with regression and metastases. Arch. Dermatol. 1987, 123, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- McGovern, V.J.; Shaw, H.M.; Milton, G.W. Prognosis in patients with thin malignant melanoma: Influence of regression. Histopathology 1983, 7, 673–680. [Google Scholar] [CrossRef]
- Teulings, H.E.; Limpens, J.; Jansen, S.N.; Zwinderman, A.H.; Reitsma, J.B.; Spuls, P.I.; Luiten, R.M. Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta-analysis. J. Clin. Oncol. 2015, 33, 773–781. [Google Scholar] [CrossRef]
- Motofei, I.G. Melanoma and autoimmunity: Spontaneous regressions as a possible model for new therapeutic approaches. Melanoma Res. 2019, 29, 231–236. [Google Scholar] [CrossRef]
- Chen, D.S.; Irving, B.A.; Hodi, F.S. Molecular pathways: Next-generation immunotherapy—Inhibiting programmed death-ligand 1 and programmed death-1. Clin. Cancer Res. 2012, 18, 6580–6587. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Verheijden, R.J.; May, A.M.; Blank, C.U.; Aarts, M.J.B.; van den Berkmortel, F.; van den Eertwegh, A.J.M.; de Groot, J.W.B.; Boers-Sonderen, M.J.; van der Hoeven, J.J.M.; Hospers, G.A.; et al. Association of anti-tnf with decreased survival in steroid refractory ipilimumab and anti-pd1-treated patients in the dutch melanoma treatment registry. Clin. Cancer Res. 2020, 26, 2268–2274. [Google Scholar] [CrossRef]
- Shastry, B.S. Snp alleles in human disease and evolution. J. Hum. Genet. 2002, 47, 561–566. [Google Scholar] [CrossRef]
- Shastry, B.S. Snps: Impact on gene function and phenotype. Methods Mol. Biol. 2009, 578, 3–22. [Google Scholar] [PubMed]
- Jin, Y.; Andersen, G.; Yorgov, D.; Ferrara, T.M.; Ben, S.; Brownson, K.M.; Holland, P.J.; Birlea, S.A.; Siebert, J.; Hartmann, A.; et al. Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat. Genet. 2016, 48, 1418–1424. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.C. Association between interferon-gamma +874 t/a polymorphism and susceptibility to autoimmune diseases: A meta-analysis. Lupus 2016, 25, 710–718. [Google Scholar] [CrossRef]
- Kim, K.; Cho, S.K.; Sestak, A.; Namjou, B.; Kang, C.; Bae, S.C. Interferon-gamma gene polymorphisms associated with susceptibility to systemic lupus erythematosus. Ann. Rheum. Dis. 2010, 69, 1247–1250. [Google Scholar] [CrossRef]
- Ramos, P.S.; Criswell, L.A.; Moser, K.L.; Comeau, M.E.; Williams, A.H.; Pajewski, N.M.; Chung, S.A.; Graham, R.R.; Zidovetzki, R.; Kelly, J.A.; et al. A comprehensive analysis of shared loci between systemic lupus erythematosus (sle) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet. 2011, 7, e1002406. [Google Scholar] [CrossRef]
- Kurreeman, F.A.; Daha, N.A.; Chang, M.; Catanese, J.J.; Begovich, A.B.; Huizinga, T.W.; Toes, R.E. Association of il2ra and il2rb with rheumatoid arthritis: A replication study in a dutch population. Ann. Rheum. Dis. 2009, 68, 1789–1790. [Google Scholar] [CrossRef]
- Wang, X.X.; Chen, T. Meta-analysis of the association of il2ra polymorphisms rs2104286 and rs12722489 with multiple sclerosis risk. Immunol. Investig. 2018, 47, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.; Thomson, W.; Ke, X.; Eyre, S.; Hinks, A.; Bowes, J.; Plant, D.; Gibbons, L.J.; Wellcome Trust Case Control Consortium; YEAR Consortium; et al. Rheumatoid arthritis susceptibility loci at chromosomes 10p15, 12q13 and 22q13. Nat. Genet. 2008, 40, 1156–1159. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.C.; Kim, J.H.; Song, G.G. Meta-analysis of genetic polymorphisms in programmed cell death 1. Associations with rheumatoid arthritis, ankylosing spondylitis, and type 1 diabetes susceptibility. Z. Rheumatol. 2015, 74, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Narumi, Y.; Isomoto, H.; Shiota, M.; Sato, K.; Kondo, S.; Machida, H.; Yanagihara, K.; Mizuta, Y.; Kohno, S.; Tsukamoto, K. Polymorphisms of ptpn11 coding shp-2 as biomarkers for ulcerative colitis susceptibility in the japanese population. J. Clin. Immunol. 2009, 29, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, D.; Fourati, H.; Amouri, A.; Marques, I.; Abida, O.; Haddouk, S.; Ben Ayed, M.; Tahri, N.; Penha-Goncalves, C.; Masmoudi, H. Association of zap70 and ptpn6, but not bank1 or clec2d, with inflammatory bowel disease in the tunisian population. Genet. Test. Mol. Biomark. 2013, 17, 321–326. [Google Scholar] [CrossRef] [PubMed]
- GTEx Consortium. Erratum: Genetic effects on gene expression across human tissues. Nature 2018, 553, 530. [Google Scholar] [CrossRef]
- Kunert, A.; Basak, E.A.; Hurkmans, D.P.; Balcioglu, H.E.; Klaver, Y.; van Brakel, M.; Oostvogels, A.A.M.; Lamers, C.H.J.; Bins, S.; Koolen, S.L.W.; et al. Cd45ra(+)ccr7(-) cd8 t cells lacking co-stimulatory receptors demonstrate enhanced frequency in peripheral blood of nsclc patients responding to nivolumab. J. Immunother. Cancer 2019, 7, 149. [Google Scholar] [CrossRef]
- Lewis, C.M. Genetic association studies: Design, analysis and interpretation. Brief. Bioinform. 2002, 3, 146–153. [Google Scholar] [CrossRef]
- Efron, B. Bootstrap methods: Another look at the jackknife. Ann. Stat. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Leighl, N.B.; Hellmann, M.D.; Hui, R.; Carcereny, E.; Felip, E.; Ahn, M.J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (keynote-001): 3-year results from an open-label, phase 1 study. Lancet Respir. Med. 2019, 7, 347–357. [Google Scholar] [CrossRef]
- Agata, Y.; Kawasaki, A.; Nishimura, H.; Ishida, Y.; Tsubata, T.; Yagita, H.; Honjo, T. Expression of the pd-1 antigen on the surface of stimulated mouse t and b lymphocytes. Int. Immunol. 1996, 8, 765–772. [Google Scholar] [CrossRef]
- Damouche, A.; Pourcher, G.; Pourcher, V.; Benoist, S.; Busson, E.; Lataillade, J.J.; Le Van, M.; Lazure, T.; Adam, J.; Favier, B.; et al. High proportion of pd-1-expressing cd4(+) t cells in adipose tissue constitutes an immunomodulatory microenvironment that may support hiv persistence. Eur. J. Immunol. 2017, 47, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, R.; Haseda, F.; Tsutsumi, C.; Hiromine, Y.; Noso, S.; Kawabata, Y.; Mitsui, S.; Terasaki, J.; Ikegami, H.; Imagawa, A.; et al. Low programmed cell death-1 (pd-1) expression in peripheral cd4(+) t cells in japanese patients with autoimmune type 1 diabetes. Clin. Exp. Immunol. 2015, 180, 452–457. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Dai, W.; Cai, G.; Xu, Y.; Li, X.; Li, Q.; Cai, S. Prognostic impact of programed cell death-1 (pd-1) and pd-ligand 1 (pd-l1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol. Cancer 2016, 15, 55. [Google Scholar] [CrossRef]
- Pollari, M.; Brück, O.; Pellinen, T.; Vähämurto, P.; Karjalainen-Lindsberg, M.L.; Mannisto, S.; Kallioniemi, O.; Kellokumpu-Lehtinen, P.L.; Mustjoki, S.; Leivonen, S.K.; et al. Pd-l1(+) tumor-associated macrophages and pd-1(+) tumor-infiltrating lymphocytes predict survival in primary testicular lymphoma. Haematologica 2018, 103, 1908–1914. [Google Scholar] [CrossRef]
- Alessi, C.; Scapulatempo Neto, C.; Viana, C.R.; Vazquez, V.L. Pd-1/pd-l1 and vegf-a/vegf-c expression in lymph node microenvironment and association with melanoma metastasis and survival. Melanoma Res. 2017, 27, 565–572. [Google Scholar] [CrossRef]
- Beaver, J.A.; Hazarika, M.; Mulkey, F.; Mushti, S.; Chen, H.; He, K.; Sridhara, R.; Goldberg, K.B.; Chuk, M.K.; Chi, D.C.; et al. Patients with melanoma treated with an anti-pd-1 antibody beyond recist progression: A US Food and Drug Administration pooled analysis. Lancet Oncol. 2018, 19, 229–239. [Google Scholar] [CrossRef]
- Hodi, F.S.; Hwu, W.J.; Kefford, R.; Weber, J.S.; Daud, A.; Hamid, O.; Patnaik, A.; Ribas, A.; Robert, C.; Gangadhar, T.C.; et al. Evaluation of immune-related response criteria and recist v1.1 in patients with advanced melanoma treated with pembrolizumab. J. Clin. Oncol. 2016, 34, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Hurkmans, D.P.; Basak, E.A.; Schepers, N.; Oomen-De Hoop, E.; Van der Leest, C.H.; El Bouazzaoui, S.; Bins, S.; Koolen, S.L.W.; Sleijfer, S.; Van der Veldt, A.A.M.; et al. Granzyme b is correlated with clinical outcome after pd-1 blockade in patients with stage iv non-small-cell lung cancer. J. Immunother. Cancer 2020, 8, e000586. [Google Scholar] [CrossRef]
- Vignali, D.A.; Collison, L.W.; Workman, C.J. How regulatory t cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, D.; Cartron, P.F.; Tuffery, P.; Dudoit, Y.; Samri, A.; Autran, B.; Vallette, F.M.; Debre, P.; Theodorou, I. A triple-mutated allele of granzyme b incapable of inducing apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 2562–2567. [Google Scholar] [CrossRef] [PubMed]
- Indini, A.; Di Guardo, L.; Cimminiello, C.; Prisciandaro, M.; Randon, G.; De Braud, F.; Del Vecchio, M. Immune-related adverse events correlate with improved survival in patients undergoing anti-pd1 immunotherapy for metastatic melanoma. J. Cancer Res. Clin. Oncol. 2019, 145, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Basak, E.A.; van der Meer, J.W.M.; Hurkmans, D.P.; Schreurs, M.W.J.; Oomen-de Hoop, E.; van der Veldt, A.A.M.; Bins, S.; Joosse, A.; Koolen, S.L.W.; Debets, R.; et al. Overt thyroid dysfunction and anti-thyroid antibodies predict response to anti-pd-1 immunotherapy in cancer patients. Thyroid 2020, 30, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Bins, S.; Basak, E.A.; El Bouazzaoui, S.; Koolen, S.L.W.; Oomen-de Hoop, E.; van der Leest, C.H.; van der Veldt, A.A.M.; Sleijfer, S.; Debets, R.; van Schaik, R.H.N.; et al. Association between single-nucleotide polymorphisms and adverse events in nivolumab-treated non-small cell lung cancer patients. Br. J. Cancer 2018, 118, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).