Simple Summary
The epithelial-mesenchymal transition (EMT) is an important hallmark in drug resistance and cancer malignancy in cancer stem cells (CSCs). In this study, gene expression in diffuse- and intestinal-type gastric cancer (GC) was investigated to reveal the precise mechanism of EMT. The pathways of cell cycle regulation and DNA damage response were found to be altered in diffuse- and intestinal-type GC. The findings of this study may provide broader insights into CSCs, with new possibilities of the involvement of the cell cycle in EMT.
Abstract
Dynamic regulation in molecular networks including cell cycle regulation and DNA damage response play an important role in cancer. To reveal the feature of cancer malignancy, gene expression and network regulation were profiled in diffuse- and intestinal-type gastric cancer (GC). The results of the network analysis with Ingenuity Pathway Analysis (IPA) showed that the activation states of several canonical pathways related to cell cycle regulation were altered. The G1/S checkpoint regulation pathway was activated in diffuse-type GC compared to intestinal-type GC, while canonical pathways of the cell cycle control of chromosomal replication, and the cyclin and cell cycle regulation, were activated in intestinal-type GC compared to diffuse-type GC. A canonical pathway on the role of BRCA1 in the DNA damage response was activated in intestinal-type GC compared to diffuse-type GC, where gene expression of BRCA1, which is related to G1/S phase transition, was upregulated in intestinal-type GC compared to diffuse-type GC. Several microRNAs (miRNAs), such as mir-10, mir-17, mir-19, mir-194, mir-224, mir-25, mir-34, mir-451 and mir-605, were identified to have direct relationships in the G1/S cell cycle checkpoint regulation pathway. Additionally, cell cycle regulation may be altered in epithelial-mesenchymal transition (EMT) conditions. The alterations in the activation states of the pathways related to cell cycle regulation in diffuse- and intestinal-type GC highlighted the significance of cell cycle regulation in EMT.
1. Introduction
In cancer stem cells (CSCs) in general, epithelial-mesenchymal transition (EMT) networks play an important role in acquiring drug resistance and malignant cancer feature [1]. Alterations in the gene expression of molecular network pathways result in phenotypic changes. Diffuse-type gastric cancer (GC) has many more mesenchymal characteristics, which is an important feature of EMT, compared with intestinal-type GC. Accordingly, the gene expression profiles have been analyzed for diffuse- and intestinal-type GC to reveal the network pathways in EMT and CSCs [2]. Our previous findings identified several molecular networks and the related microRNAs (miRNAs) in intestinal- and diffuse-type GC [2,3]. A few recent studies have revealed the regulation of non-coding RNAs, including miRNAs, in drug resistance and EMT in cancer [4,5,6]. It has also been indicated that the knockdown of circular NOP10, a circular RNA that promotes tumor metastasis and EMT, decreased the numbers of cells in the S phase and increased the number of cells in the G2/M phase [6].
The gene expression signature revealed that the ratio of CDH2 (N-cadherin) to CDH1 (E-cadherin) distinguishes diffuse- and intestinal-type GC [7]. The immunohistochemical expression of E-cadherin clearly discriminated diffuse- and intestinal-type GC [8]. The decreased expression of E-cadherin leads to the sparse phenotype of the cells, which represents the phenotype of EMT. The EMT programs induce CSC stemness, conferring therapeutic resistance [9]. Gastric cancer subtypes can be classified into diffuse (genomically stable; GS), intestinal (chromosomally instable; CIN), microsatellite instability-high, and Epstein-Barr virus-positive subtypes [10]. Since defects in the DNA damage response lead to genome instability and cancer progression, poly(ADP-ribose) polymerase (PARP) inhibitors have the potential to treat GC by blocking the single-strand break repair, followed by tumor cell death and synthetic lethality in homologous recombination-deficient tumor cells [11]. Immune checkpoint inhibition therapy has some limited application in GC, since anti-PD1 monoclonal antibodies, such as pembrolizumab and nivolumab, have significant responses only in minor populations of GC, such as microsatellite instability-high or Epstein-Barr virus-positive subtypes [12]. Gene expression and pathway analyses of CTNNB1 (β-catenin) identified an important role of β-catenin signaling in the regulation of stem cell pluripotency and cancer signaling [13]. Although cell proliferation and cell cycle regulation are essential for identifying potential therapeutic targets in cancer, the precise mechanism of cell cycle regulation and EMT has not been fully revealed. This article focuses on the roles of cell cycle regulation pathways in diffuse- and intestinal-type GC, as cell cycle regulation may play a critical role in intestinal- and diffuse-type GC. Consequently, the mechanism of cancer drug resistance through the involvement of the cell cycle in EMT and CSCs is highlighted, which might reveal future therapeutic potential.
2. Materials and Methods
2.1. Data Analysis of Diffuse- and Intestinal-Type GC
The RNA sequencing data of diffuse- and intestinal-type GC are publicly available in The Cancer Genome Atlas (TCGA) of the cBioPortal for Cancer Genomics database [10,14,15] at the National Cancer Institute (NCI) Genomic Data Commons (GDC) data portal [10,16]. Publicly available data on stomach adenocarcinoma in the TCGA [15] were compared between diffuse-type GC, which is genomically stable (n = 50), and intestinal-GC, which has a feature of chromosomal instability (n = 223), in TCGA Research Network publications, as previously described [2,10].
2.2. Network Analysis
Data on intestinal- and diffuse-type GC from the TCGA cBioPortal for Cancer Genomics were uploaded and analyzed through the use of Ingenuity Pathway Analysis (IPA) (QIAGEN Inc., Hilden, Germany) [17].
2.3. Data Visualization
The results of network analyses of the gene expression data on diffuse- and intestinal-type GC were visualized with Tableau software (https://www.tableau.com (accessed on 18 November 2021)).
2.4. Statistical Analysis
The RNA sequencing data on diffuse- and intestinal-type GC were analyzed in Student’s t-test. The z-scores of intestinal- and diffuse-type GC samples were compared, and the difference was considered to be significant at p < 0.00001, as previously described [2]. Activation z-score in each pathway was calculated in IPA to show the level of the activation.
3. Results
3.1. Canonical Pathways in Diffuse- and Intestinal-Type GC
Canonical pathways in diffuse- and intestinal-type GC are shown with activation z-score in Figure 1 and Table 1. Genes significantly altered in diffuse- and intestinal-type GC (2815 IDs) were analyzed by a network analysis, which identified 47 canonical pathways with absolute z-scores > 1 in the diffuse- and intestinal-type GC (Table 1). These canonical pathways included Cell cycle control of chromosomal replication, Cyclins and cell cycle regulation, and Role of BRCA1 in DNA damage response, which were activated in intestinal-type GC compared to diffuse-type GC, and Cell cycle: G1/S cell cycle checkpoint regulation, and Cell cycle: G2/M DNA damage checkpoint regulation, which were activated in diffuse-type GC compared to intestinal-type GC.
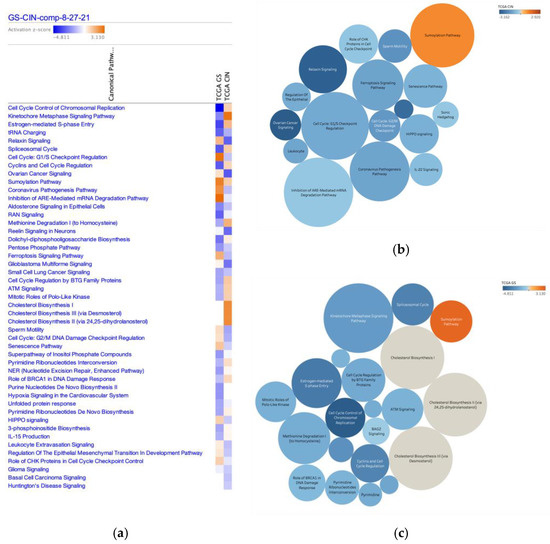
Figure 1.
Canonical pathways altered in diffuse- and intestinal-type GC. (a) Canonical pathways with an absolute z-score of >1 for diffuse- and intestinal-type GC are shown (TCGA GS; diffuse-type GC, TCGA CIN; intestinal-type GC) (b). The size of each circle shows the activation score in diffuse-type GC. Color indicates the activation score in intestinal-type GC. (c) The size of each circle indicates the activation z-score for intestinal-type GC. Color indicates the activation z-score of intestinal-type GC.

Table 1.
Canonical pathways altered in diffuse- and intestinal-type GC. The pathways are sorted in the order of the activation z-scores.
3.2. Cell Cycle-Related Canonical Pathways in Diffuse- and Intestinal-Type GC
3.2.1. Cell Cycle Control of Chromosomal Replication was Activated in Intestinal-Type GC
The molecule activity predictor in IPA-predicted activation of the cell cycle control of chromosomal replication pathway in intestinal-type GC (Figure 2). During cell cycle progression, DNA topoisomerase II activity is controlled [18]. Analysis of the direct relationships of RNA–RNA interactions of microRNA targeting the cell cycle control of chromosomal replication pathway revealed the relationships between miRNAs and the targeted molecules (Table 2).
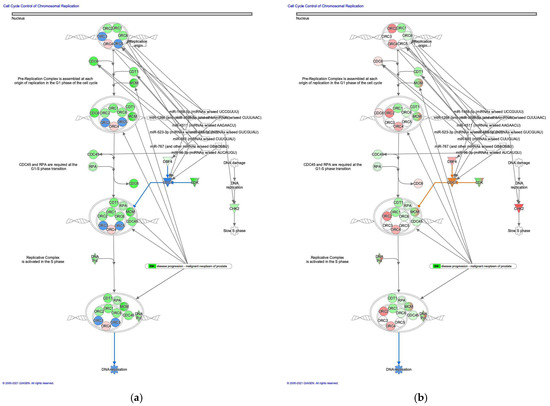
Figure 2.
Cell cycle control of chromosomal replication was activated in intestinal-type GC. (a) Gene expression and pathway activity predictions in diffuse-type GC are shown. (b) Gene expression and pathway activity predictions in intestinal-type GC are shown. The genes whose expression was altered in diffuse- and intestinal-type GC are shown in pink (upregulated) or green (downregulated). Predicted activation and inhibition are shown in orange and blue, respectively.

Table 2.
Direct relationships of miRNAs and targeted molecules in cell cycle control of chromosomal replication.
3.2.2. The G1/S Cell Cycle Checkpoint Regulation Pathway was Activated in Diffuse-Type GC
The molecule activity predictor in IPA predicted the activation of the G1/S cell cycle checkpoint regulation pathway in diffuse-type GC (Figure 3). In the G1/S cell cycle checkpoint regulation pathway, DNA damage induces p53, which is expected to be activated in diffuse-type GC. Analysis of the direct relationships of RNA–RNA interactions in the G1/S cell cycle checkpoint regulation pathway revealed non-coding RNAs, including nine miRNAs and a biologic drug (MYC-targeting siRNA DCR-MYC) (Table 3).
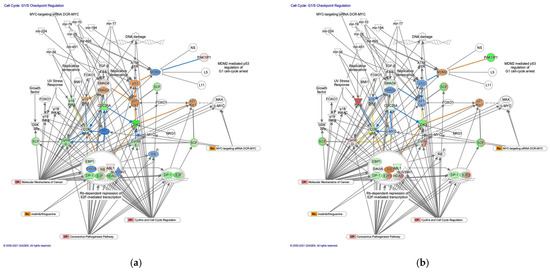
Figure 3.
The G1/S cell cycle checkpoint regulation pathway was activated in diffuse-type GC. (a) Gene expression and pathway activity predictions in diffuse-type GC are shown. (b) Gene expression and pathway activity predictions in intestinal-type GC are shown. The genes whose expression was altered in diffuse- and intestinal-type GC are shown in pink (upregulated) or green (downregulated). Predicted activation and inhibition are shown in orange and blue, respectively.

Table 3.
Non-coding RNAs which have direct relationships in the G1/S cell cycle checkpoint regulation pathway.
3.2.3. The Cyclin and Cell Cycle Regulation Pathway was Activated in Intestinal-Type GC
Molecule activity predictor in IPA predicted activation of the cyclins and cell cycle regulation pathway in intestinal-type GC (Figure 4). A study has reported that prolyl 4-hydroxylase subunit alpha 1, which regulates cyclin-dependent kinases (CDKs), cyclins, and CDK inhibitors, was associated with EMT, tumor cell invasion and metastasis of lung adenocarcinoma [19]. An analysis of the direct relationships of RNA–RNA interactions of microRNA targeting in the cyclins and cell cycle regulation pathway revealed the relationships between miRNAs and the targeted molecules (Table 4).
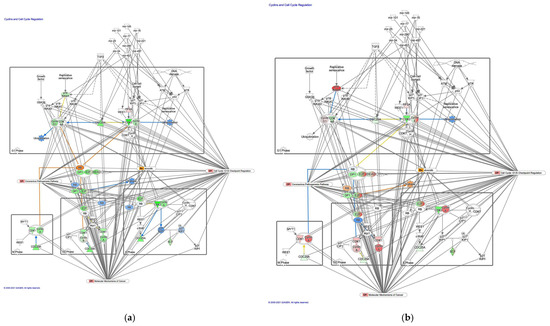
Figure 4.
The cyclin and cell cycle regulation pathway was activated in intestinal-type GC. (a) Gene expression and pathway activity predictions in diffuse-type GC are shown. (b) Gene expression and pathway activity predictions in intestinal-type GC are shown. The genes whose expression was altered in diffuse- and intestinal-type GC are shown in pink (upregulated) or green (downregulated). Predicted activation and inhibition are shown in orange and blue, respectively.

Table 4.
Direct relationships of miRNAs and targeted molecules in cyclin and cell cycle regulation.
3.2.4. Canonical Pathway of the Role of BRCA1 in the DNA Damage Response was Activated in Intestinal-Type GC Compared to Diffuse-Type GC
The prediction of molecule activity predictor in IPA demonstrated that the canonical pathway on the role of BRCA1 in the DNA damage response was activated in intestinal-type GC compared to diffuse-type GC (Figure 5). The role of BRCA1 in the DNA damage response pathway was identified as the most significant canonical pathway, with a p-value of 6.6 × 10−12. Gene expression of BRCA1, which is associated with G1/S transition, increased in intestinal-type GC. BRCA1 encodes a 190 kD nuclear phosphorylation protein, which maintains genomic stability and functions as a tumor suppressor. It is interesting that p53 and c21CIP1 are activated in intestinal-type GC in the role of BRCA1 in the DNA damage response pathway. BRCA1 may be involved in the activation of p53. An analysis of the direct relationships of RNA–RNA interactions of microRNA targeting the role of BRCA1 in the DNA damage response pathway revealed the relationships between miRNAs and the targeted molecules (Figure 5, Table 5).
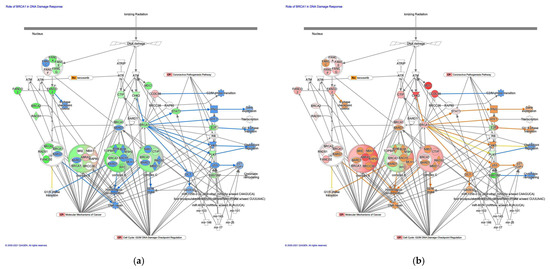
Figure 5.
The role of BRCA1 in the DNA damage response was activated in intestinal-type GC. (a) Gene expression and pathway activity predictions in diffuse-type GC are shown. (b) Gene expression and pathway activity predictions in intestinal-type GC are shown. The genes whose expression was altered in diffuse- and intestinal-type GC are shown in pink (upregulated) or green (downregulated). Predicted activation and inhibition are shown in orange and blue, respectively.

Table 5.
Direct relationships of miRNAs and targeted molecules in the role of BRCA1 in the DNA damage response.
3.2.5. The G2/M DNA Damage Cell Cycle Checkpoint Regulation Pathway in Diffuse- and Intestinal-Type GC
The G2/M DNA damage cell cycle checkpoint regulation pathway was identified as a related canonical pathway in diffuse- and intestinal-type GC (Figure 6). Analysis of the direct relationships of RNA–RNA interactions in the G2/M DNA damage cell cycle checkpoint regulation pathway revealed non-coding RNAs, including nine miRNAs and a biological drug (lipid-encapsulated anti-PLK1 siRNA TKM-080301) (Table 6).
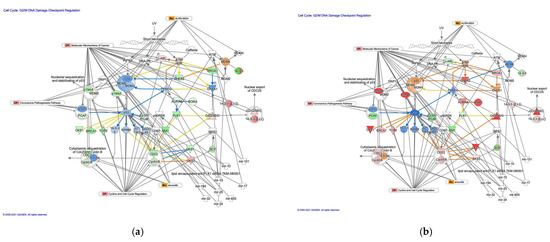
Figure 6.
The G2/M DNA damage cell cycle checkpoint regulation pathway in diffuse- and intestinal-type GC. (a) Gene expression and pathway activity predictions in diffuse-type GC are shown. (b) Gene expression and pathway activity predictions in intestinal-type GC are shown. The genes whose expression was altered in diffuse- and intestinal-type GC are shown in pink (upregulated) or green (downregulated). Predicted activation and inhibition are shown in orange and blue, respectively.

Table 6.
Non-coding RNAs which have direct relationships in the G2/M DNA damage cell cycle checkpoint regulation pathway.
4. Discussion
The network analysis of gene expression data on diffuse- and intestinal-type GC revealed that the activation state of canonical pathways related to cell cycle regulation, including cell cycle control of chromosomal replication, G1/S cell cycle checkpoint regulation, cyclins and cell cycle regulation, and G2/M DNA damage cell cycle checkpoint regulation pathways, altered in diffuse- and intestinal-type GC. A previous study showed that G1/S arrest induces EMT by ribosome biogenesis [20]. This finding of G1/S arrest in EMT may be associated with the finding in the current study in terms of activating the G1/S cell cycle checkpoint regulation pathway in diffuse-type GC. SMAD4, which was activated in diffuse-type GC, is involved in EMT [21]. The silencing of SMAD4 reversed EMT in hepatocytes [22]. The analyses on RNA-RNA relationships highlighted that miRNAs had direct interactions in pathways related to cell cycle regulation. mir-10 (microRNA 99a), mir-17 (microRNA 17), mir-19 (microRNA 19a), mir-194 (microRNA 194-1), mir-224 (microRNA 224), mir-25 (microRNA 25), mir-34 (microRNA 34a), mir-451 (microRNA 451a), mir-605 (microRNA 605), and MYC-targeting siRNA DCR-MYC were identified as molecules having direct relationships (RNA–RNA interactions) in G1/S cell cycle checkpoint regulation pathway. It has been previously revealed that the miR-17/20 cluster is transcriptionally regulated by MYC, E2F, and cyclin D1, and miR-34 is a direct transcriptional target of p53 and regulates the cell cycle [23]. The cell cycle regulation of miRNAs can be involved in cancer. Small nucleolar RNA host gene 7 (SNHG7), an oncogenic long non-coding RNA, promotes cell migration via the miR-34a-Snail-EMT axis in gastric cancer [24]. The involvement of miR-34 in EMT and GC is consistent with the results showing the direct relationship between miR-34 and the G1/S cell cycle checkpoint regulation pathway, which is activated in diffuse-type GC. Another study suggested that miRNA-33a inhibited EMT and the metastasis of GC via the Snail/Slug pathway, which may be related to the miRNA regulation of EMT in intestinal-type GC [25]. MYC-targeting siRNA, a biologic drug targeting MYC, has been identified to have direct relationships in the G1/S cell cycle checkpoint regulation pathway. It was shown previously that the silencing of Aurora kinase B, which is upregulated in GC, decreased the expression of MYC, arrested the cell cycle in the G2/M phase and inhibited the migration of GC [26]. MYC-targeting siRNA might also be involved in regulation of the cell cycle and EMT.
The current study highlights the cell cycle regulation and non-coding RNA regulation in diffuse- and intestinal-type GC. A long non-coding RNA, LINC00460, induced EMT, and cell proliferation and metastasis in head and neck squamous cell carcinoma via translocation of peroxiredoxin-1 into the cell nucleus [27]. Non-coding RNAs, such as microRNAs, have various roles in biological and pathological processes, including the cell cycle, proliferation, EMT, and drug resistance [28]. This study has the potential to identify the target non-coding RNAs for cancer therapy, where the non-coding RNAs have roles as anti-cancer drugs or the targets of anti-cancer drugs. The knowledge gaps include differences in the subtypes of tumor cells in terms of regulation of the cell cycle and EMT, and the influence of the cancer microenvironment on RNA interactions. Researchers have been trying to unravel the RNA networks in cancer subtypes and microenvironments through comprehensive analyses including microarrays, RNA sequencing, genome-wide sequencing, and proteomic analyses of the tumor tissue or cell population, or even a single cell [29,30,31,32,33]. The problem still remains regarding the communication between cells in the orchestrated organs and tissues in human body. In the next five years, rapid advances in artificial intelligence, 3D organoids, and organ modeling may realize a harmonized android of the human body. Investigation into RNA regulation and EMT from the point of view of anti-cancer drug resistance and identification of therapeutic targets would be the future research direction.
The current study has some limitations, as the data of diffuse- and intestinal-type GC have been compared only in terms of cell cycle regulation. To reveal the detailed mechanisms of cell cycle regulation of different subtypes of GC and the potential applicability of targeted therapy, an analysis of the microsatellite instability-high and Epstein-Barr virus-positive subtypes would be needed.
5. Conclusions
Activation stated of canonical pathways related to cell cycle regulation altered in diffuse- and intestinal-type GC. The canonical pathways on G1/S cell cycle checkpoint regulation were activated in diffuse-type GC, while canonical pathways on cell cycle control of chromosomal replication and cyclins and cell cycle regulation were activated in intestinal-type GC. The canonical pathway on role of BRCA1 in DNA damage response was activated in intestinal-type GC compared with diffuse-type GC. Some molecules, such as SMAD4 in the G1/S cell cycle checkpoint regulation pathway, are involved in the regulation of EMT. Cell cycle regulation may also be altered under EMT conditions in diffuse-type GC. The findings of the study showing how the activation states of the canonical pathways related to cell cycle regulation altered in diffuse- and intestinal-type GC highlighted the involvement of cell cycle regulation in cancer malignancy. The precise mechanisms of how the compartment molecules in the cell cycle pathways regulate EMT and CSCs would be beneficial for future investigation.
Author Contributions
Conceptualization, S.T. and H.S.; methodology, S.T.; software, S.T.; formal analysis, S.T.; investigation, S.T.; data curation, S.T., K.A. and H.S.; writing—original draft preparation, S.T.; writing—review and editing, S.T., S.Q. and H.C.; visualization, S.T.; supervision, S.T. and A.H.; project administration, S.T., K.A., H.Y. and H.S.; funding acquisition, S.T., S.Q., R.O. and A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Japan Agency for Medical Research and Development (AMED) (Grant Numbers JP21mk0101216 (S.T.), JP20ak0101093 (S.T., R.O. and A.H.) and JP20mk0101163 (R.O.)) and the Strategic International Collaborative Research Program (Grant Number JP20jm0210059 (S.T. and S.Q.)), the Ministry of Health, Labour and Welfare (MHLW) (Grant Number H30-KAGAKU-IPPAN-002 (S.T. and R.O.)) and JSPS KAKENHI (Grant Number 21K12133 (S.T. and R.O.)).
Data Availability Statement
In this research, the RNA sequencing data of diffuse- and intestinal-type GC, which are publicly available from The Cancer Genome Atlas (TCGA) of the cBioPortal for Cancer Genomics database [10,14,15] and from the National Cancer Institute (NCI) Genomic Data Commons (GDC) data portal, were analyzed [16]. The publicly available data of stomach adenocarcinoma from TCGA (NCI, USA: https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga (accessed on 18 November 2021)) [15], and intestinal- and diffuse-type GC data, which are noted as having chromosomal instability (CIN) (n = 223) and being genomically stable (GS) (n = 50), respectively, from TCGA Research Network publications, were compared [10].
Acknowledgments
The authors would like to thank all colleagues for their support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, S.; Quader, S.; Ono, R.; Cabral, H.; Aoyagi, K.; Hirose, A.; Yokozaki, H.; Sasaki, H. Molecular Network Profiling in Intestinal- and Diffuse-Type Gastric Cancer. Cancers 2020, 12, 3833. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Quader, S.; Cabral, H.; Ono, R. Interplay of EMT and CSC in Cancer and the Potential Therapeutic Strategies. Front. Pharmacol. 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, R.; Shen, Q.; Yang, D.; Peng, H.; Tong, J.; Fu, Q. Overexpression of Circular RNA circ_0013587 Reverses Erlotinib Resistance in Pancreatic Cancer Cells Through Regulating the miR-1227/E-Cadherin Pathway. Front. Oncol. 2021, 11, 754146. [Google Scholar] [CrossRef]
- Chen, S.; Wu, H.; Zhu, L.; Jiang, M.; Wei, S.; Luo, J.; Liu, A. MiR-199b-5p Promotes Gastric Cancer Progression by Regulating HHIP Expression. Front. Oncol. 2021, 11, 728393. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Wang, W.; Zhang, L.; Huang, P. Candidate oncogene circularNOP10 mediates gastric cancer progression by regulating miR-204/SIRT1 pathway. J. Gastrointest. Oncol. 2021, 12, 1428–1443. [Google Scholar] [CrossRef]
- Tanabe, S.; Aoyagi, K.; Yokozaki, H.; Sasaki, H. Gene expression signatures for identifying diffuse-type gastric cancer associated with epithelial-mesenchymal transition. Int. J. Oncol. 2014, 44, 1955–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazăr, D.; Tăban, S.; Ardeleanu, C.; Dema, A.; Sporea, I.; Cornianu, M.; Lazăr, E.; Vernic, C. The immunohistochemical expression of E-cadherin in gastric cancer; correlations with clinicopathological factors and patients’ survival. Rom. J. Morphol. Embryol. 2008, 49, 459–467. [Google Scholar]
- Wilson, M.M.; Weinberg, R.A.; Lees, J.A.; Guen, V.J. Emerging Mechanisms by which EMT Programs Control Stemness. Trends Cancer 2020, 6, 775–780. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zheng, K.; Huang, Y.; Xiong, H.; Su, J.; Chen, R.; Zou, Y. PARP inhibitors in gastric cancer: Beacon of hope. J. Exp. Clin. Cancer. Res. 2021, 40, 211. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.D.; Rizzo, A.; Brandi, G. DNA damage response alterations in gastric cancer: Knocking down a new wall. Future Oncol. 2021, 17, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Kawabata, T.; Aoyagi, K.; Yokozaki, H.; Sasaki, H. Gene expression and pathway analysis of CTNNB1 in cancer and stem cells. World J. Stem Cells 2016, 8, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Grossman, R.L.; Heath, A.P.; Ferretti, V.; Varmus, H.E.; Lowy, D.R.; Kibbe, W.A.; Staudt, L.M. Toward a Shared Vision for Cancer Genomic Data. N. Engl. J. Med. 2016, 375, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Lee, J.H.; Berger, J.M. Cell Cycle-Dependent Control and Roles of DNA Topoisomerase II. Genes 2019, 10, 859. [Google Scholar] [CrossRef] [Green Version]
- Ning, Y.; Zheng, H.; Zhan, Y.; Liu, S.; Yang, Y.; Zang, H.; Wen, Q.; Zhang, Y.; Fan, S. Overexpression of P4HA1 associates with poor prognosis and promotes cell proliferation and metastasis of lung adenocarcinoma. J. Cancer 2021, 12, 6685–6694. [Google Scholar] [CrossRef]
- Prakash, V.; Carson, B.B.; Feenstra, J.M.; Dass, R.A.; Sekyrova, P.; Hoshino, A.; Petersen, J.; Guo, Y.; Parks, M.M.; Kurylo, C.M.; et al. Ribosome biogenesis during cell cycle arrest fuels EMT in development and disease. Nat. Commun. 2019, 10, 2110. [Google Scholar] [CrossRef]
- Chen, B.; Ma, J.; Li, C.; Wang, Y. Long noncoding RNA KCNQ1OT1 promotes proliferation and epithelial-mesenchymal transition by regulation of SMAD4 expression in lens epithelial cells. Mol. Med. Rep. 2018, 18, 16–24. [Google Scholar] [CrossRef]
- Kaimori, A.; Potter, J.; Kaimori, J.Y.; Wang, C.; Mezey, E.; Koteish, A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J. Biol. Chem. 2007, 282, 22089–22101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Baserga, R.; Chen, L.; Wang, C.; Lisanti, M.P.; Pestell, R.G. microRNA, cell cycle, and human breast cancer. Am. J. Pathol. 2010, 176, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, Y.; Zhang, Y.; Cheng, L.; Zhou, X.; Chen, K. SNHG7 accelerates cell migration and invasion through regulating miR-34a-Snail-EMT axis in gastric cancer. Cell Cycle 2020, 19, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.D.; Cheng, J.T.; Chandoo, A.; Sun, X.W.; Zhang, L.; Lu, M.D.; Sun, W.J.; Huang, Y.P. microRNA-33a prevents epithelial-mesenchymal transition, invasion, and metastasis of gastric cancer cells through the Snail/Slug pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G147–G160. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Z.; Wang, G.H.; Zhou, Y.M.; Deng, J.P.; Feng, Y.; Chen, J.Q.; Tian, L. AURKB Promotes the Metastasis of Gastric Cancer, Possibly by Inducing EMT. Cancer Manag. Res. 2020, 12, 6947–6958. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cao, W.; Wu, K.; Qin, X.; Wang, X.; Li, Y.; Yu, B.; Zhang, Z.; Wang, X.; Yan, M.; et al. LncRNA LINC00460 promotes EMT in head and neck squamous cell carcinoma by facilitating peroxiredoxin-1 into the nucleus. J. Exp. Clin. Cancer Res. 2019, 38, 365. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Y.; Yang, Y.; Ma, Y.; Wang, F.; Xue, A.; Zhu, J.; Yang, H.; Chen, Q.; Chen, M.; Ye, L.; et al. Potential regulatory role of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed. Pharmacother. 2020, 121, 109627. [Google Scholar] [CrossRef]
- Liu, D.; Xu, X.; Wen, J.; Xie, L.; Zhang, J.; Shen, Y.; Jiang, G.; Chen, J.; Fan, M. Integrated Genome-Wide Analysis of Gene Expression and DNA Copy Number Variations Highlights Stem Cell-Related Pathways in Small Cell Esophageal Carcinoma. Stem Cells Int. 2018, 2018, 3481783. [Google Scholar] [CrossRef] [Green Version]
- Duan, W.; Wang, K.; Duan, Y.; Chen, X.; Chu, X.; Hu, P.; Xiong, B. Combined Analysis of RNA Sequence and Microarray Data Reveals a Competing Endogenous RNA Network as Novel Prognostic Markers in Malignant Pleural Mesothelioma. Front. Oncol. 2021, 11, 615234. [Google Scholar] [CrossRef]
- Fei, T.; Chen, Y.; Xiao, T.; Li, W.; Cato, L.; Zhang, P.; Cotter, M.B.; Bowden, M.; Lis, R.T.; Zhao, S.G.; et al. Genome-wide CRISPR screen identifies HNRNPL as a prostate cancer dependency regulating RNA splicing. Proc. Natl. Acad. Sci. USA 2017, 114, E5207–E5215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Roulis, M.; Kaklamanos, A.; Schernthanner, M.; Bielecki, P.; Zhao, J.; Kaffe, E.; Frommelt, L.S.; Qu, R.; Knapp, M.S.; Henriques, A.; et al. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature 2020, 580, 524–529. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).