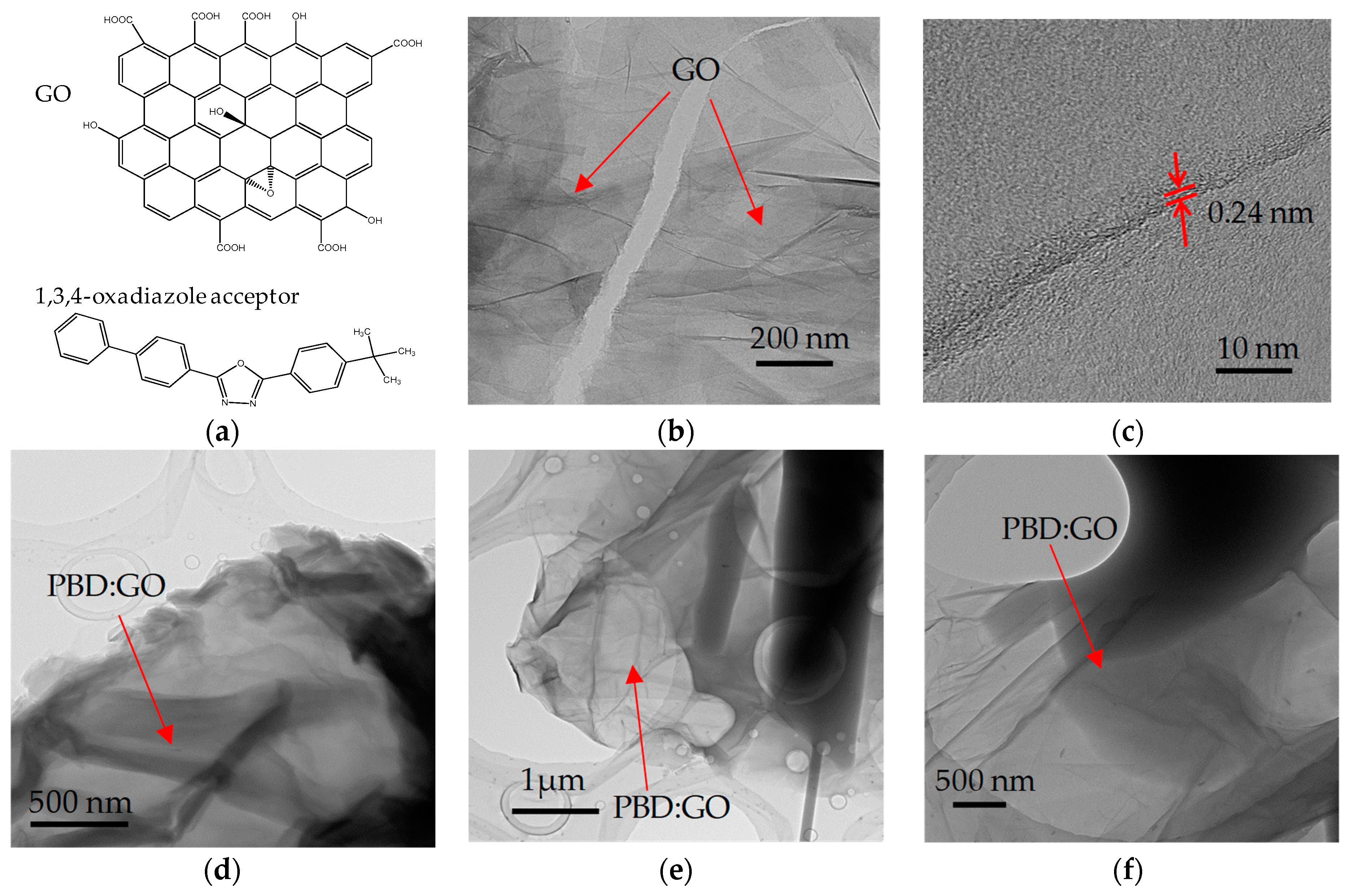
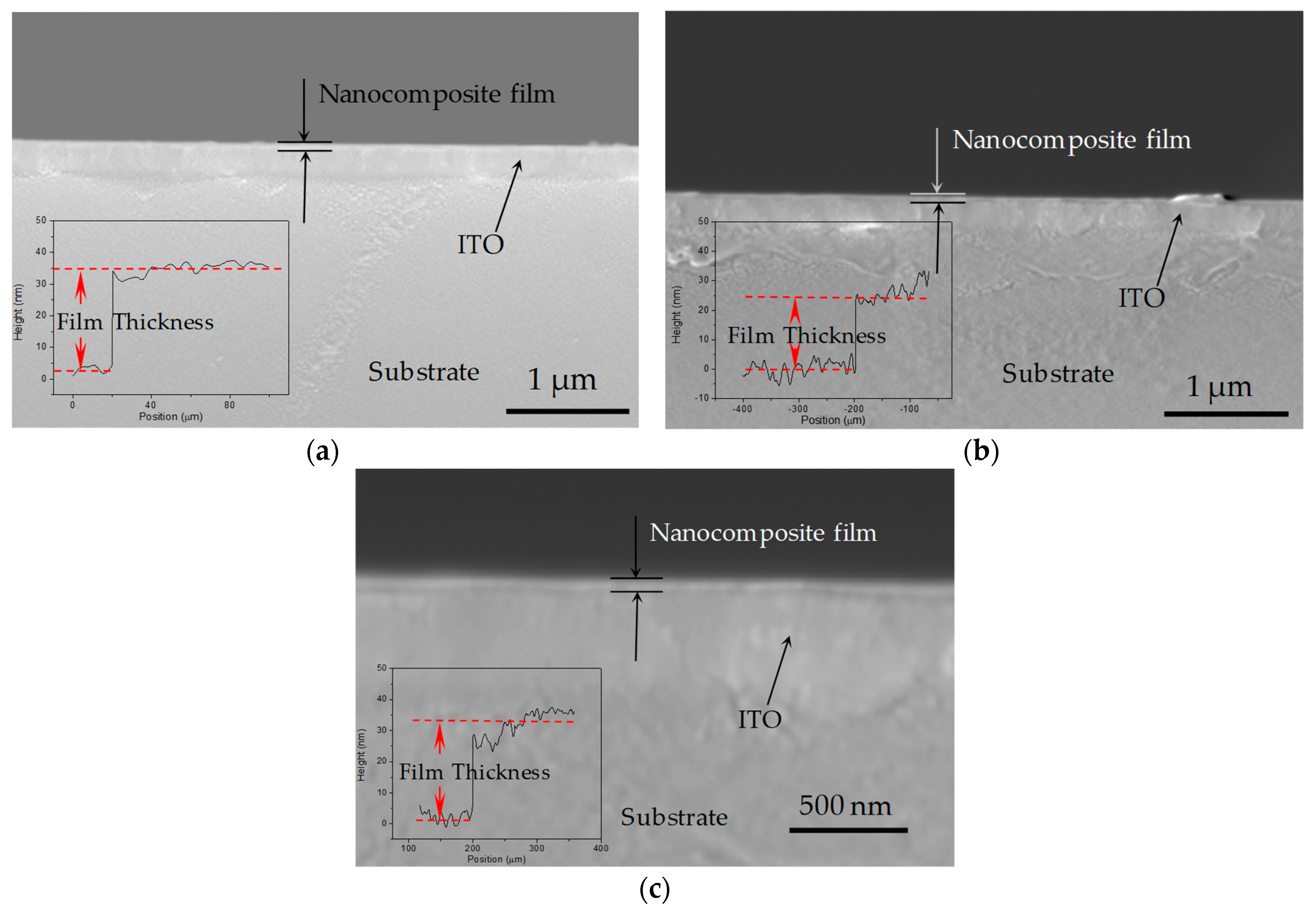
3.2. Memory Behavior Measurments
I-
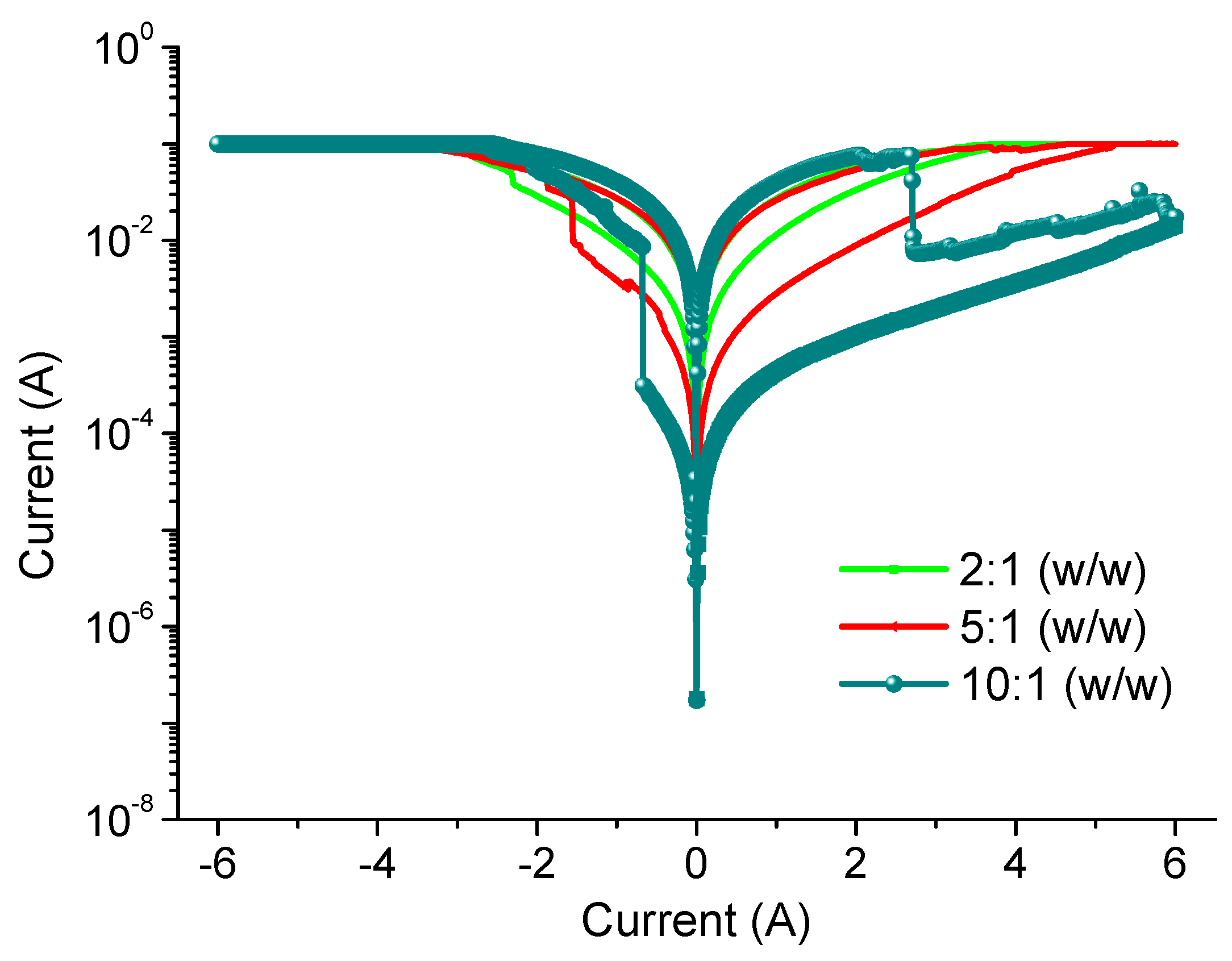
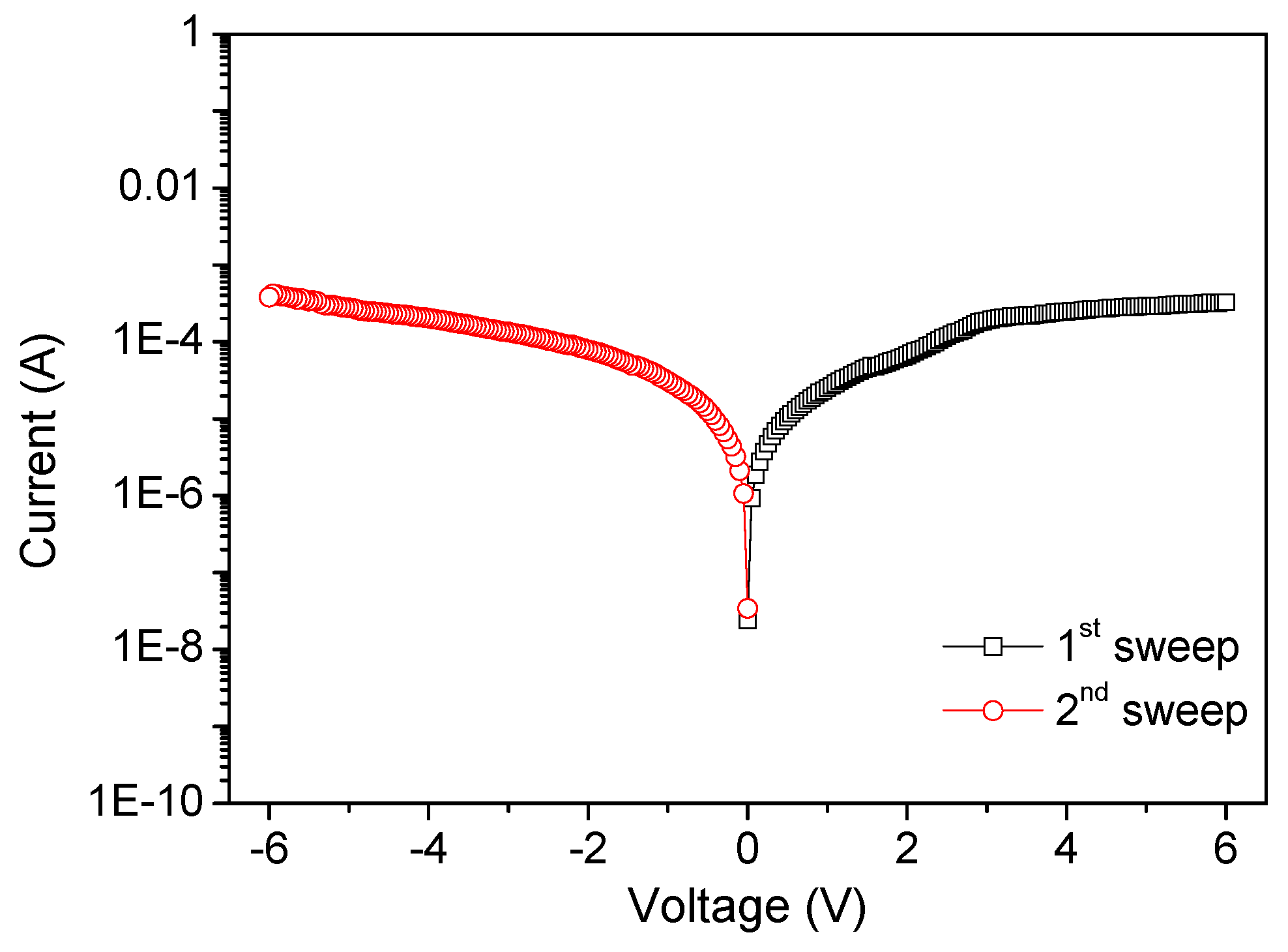
V measurements were performed by two-terminal methods to investigate the charge transport in GO films. The devices with diverse chemical proportions of PBD:GO exhibit similar electrical behaviors when measured under ambient conditions. The devices have the capability for excellent switching performance, allowing the possibility for use in nonvolatile memory. Electrical experiments were conducted with the chemical component PBD:GO, with a weight ratio of 2:1, 5:1, and 10:1 (w/w), as an active layer sandwiched between ITO and Ni electrodes. The device with PBD:GO of 2:1 (w/w) exhibits electrically bistable switching behavior, as illustrated by the
I-
V characteristics in
Figure 3. In the first voltage scan from 0 V to −6 V, the current grows abruptly at a threshold voltage of about −2.3 V, switching from an OFF-state to an ON-state. The device keeps in ON-state during the subsequent sweep from 0 to −6 V (sweep 2). The distinct electrical states in the voltage range of about 0 V to −6 V, where the ON/OFF current ratio is more than 3.5, allow a voltage of −0.1 V to read the ‘‘0’’ signal (before setting) and ‘‘1’’ signal (after setting) of the memory device. For the subsequent positive voltage scan (sweep 3), an abrupt decrease in the current can be observed at about +3.2 V. The device can be reset to the OFF-state (sweep 4) for a nonvolatile rewritable memory device. Further decreases in GO content result in a significant change in the conductivity of the composite film, and the devices with PBD:GO of 5:1 (w/w) also exhibit rewritable resistive switching behavior. The threshold voltage, when the devices switch from an OFF-state to an ON-state, can reach −1.55 V. Interestingly, the ratio between low- and high-conductive states can be stably obtained up to 11, resulting in a nonvolatile memory device. Furthermore, the rewritable memory device with 10:1 (w/w) of PBD:GO is initially in an OFF-state; when the bias voltage increases, a transition from an OFF-state to an ON-state occurs at a lower threshold voltage of −0.68 V, presumably because of the oxidation of 1,3,4-oxadiazole groups. The ON/OFF current ratio is approximately 130 when read at −0.1 V. A nonvolatile ON state is thus observed. The ON state can be retained after removing the power supply (sweep 2). After reading the ON state in the negative sweep, a positively biased sweep with a sufficient magnitude of −2.71 V (sweep 3) can program the ON state back to the initial OFF state (erase process). The OFF state of the device can be read (sweep 4) and re-programmed to the ON state again in subsequent negative sweeps, thus completing the write-read-erase-read- rewrite cycles for a nonvolatile rewritable memory. The resistive switching observed may be the result of trapping/detrapping of the PBD:GO active layer, which acts as a capacitor. We further investigated the endurance cycles, device uniformity, and retention ability of the devices, which are parameters required for application as a nonvolatile memory device.
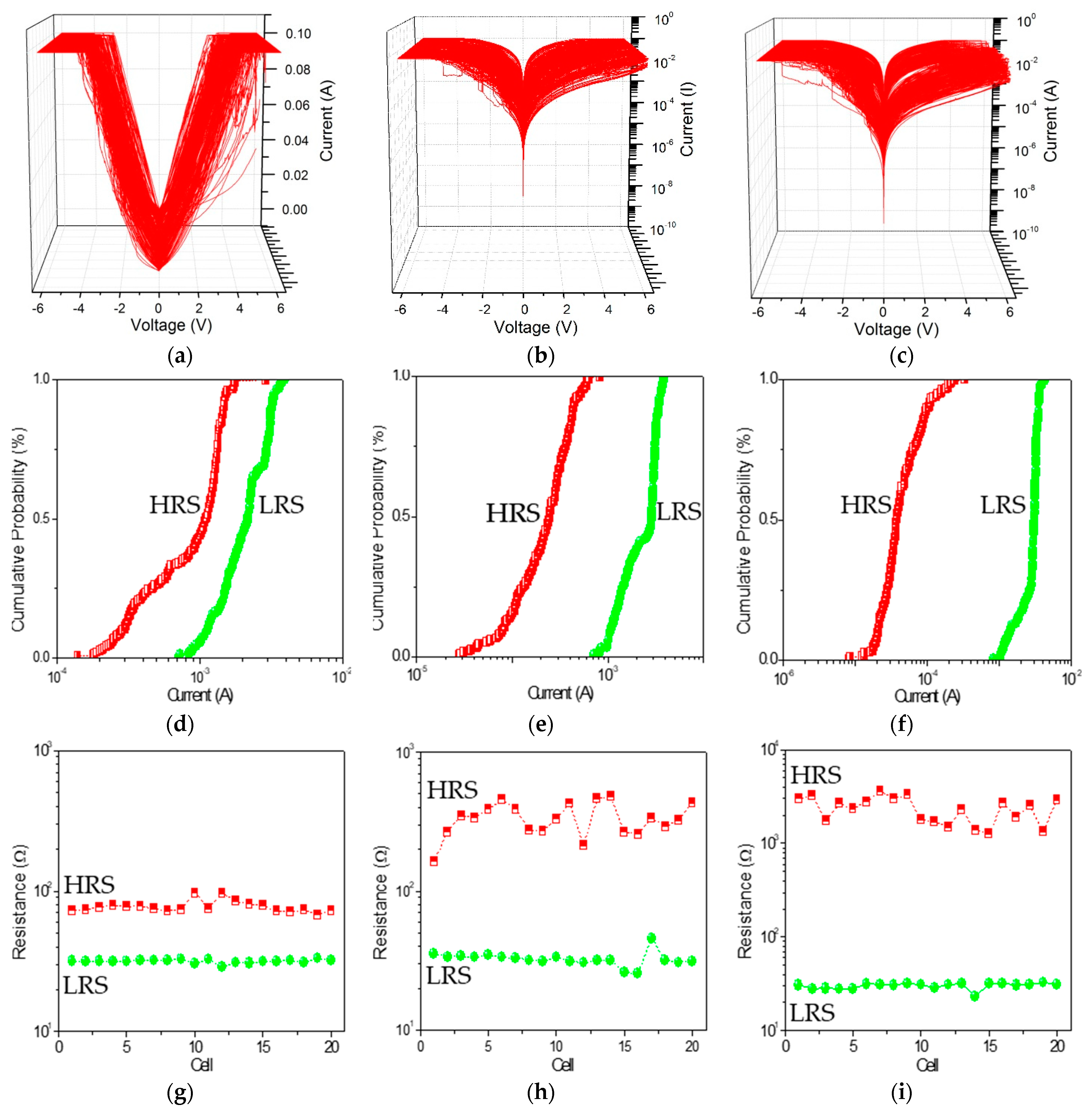
Nanocomposites can exert an exceptional influence on nano elements in a long-term stability, specifically, to enhance the ON/OFF current ratio of the bistable resistive switching. PBD:GO nanocomposite can be treated as an organic storage material for bistably electrical performance. 200 consecutive cycles of
I-
V characteristics for ITO/PBD:GO/Ni were carried out and plotted in
Figure 4a–c. The rewritable cycles can be repeatedly performed without significant degradation after several hundred rewritable cycles. The cumulative distribution of
IHRS and
ILRS at
Vread = −0.1 V is displayed in
Figure 4e,f. The mean (standard deviation) of
IHRS at a read voltage of −1 V is 0.97 mA (0.49 mA), 0.26 mA (0.15 mA), and 0.55 μA (0.0.46 μA), while that of
ILRS is 2.20 mA (0.80 μA), 2.40 mA (0.93 μA), and 2.80 mA (0.75 μA). For the resistive switching separately ITO/PBD:GO(2:1)/Ni, ITO/PBD:GO(5:1)/Ni, and ITO/PBD:GO(10:1)/Ni, the device uniformity of 20 electrically bistable samples were considered and calculated. Device-to-device profiles of
IHRS and
ILRS at
Vread = −0.1 V are illustrated in
Figure 4g–i. The extent of
IHRS and
ILRS are signed by the red and green dashed lines. The observations show that the nanocomposite PBD:GO (10:1 w/w) has better memory characteristics. An ON/OFF state resistance ratio
RON/
ROFF of more than 10
2 at −0.1 V has been achieved in the bistable memory device ITO/PBD:GO(10:1)/Ni. No significant degradation of the device in both the ON and OFF states is observed after 10
6 s of the continuous stress test (
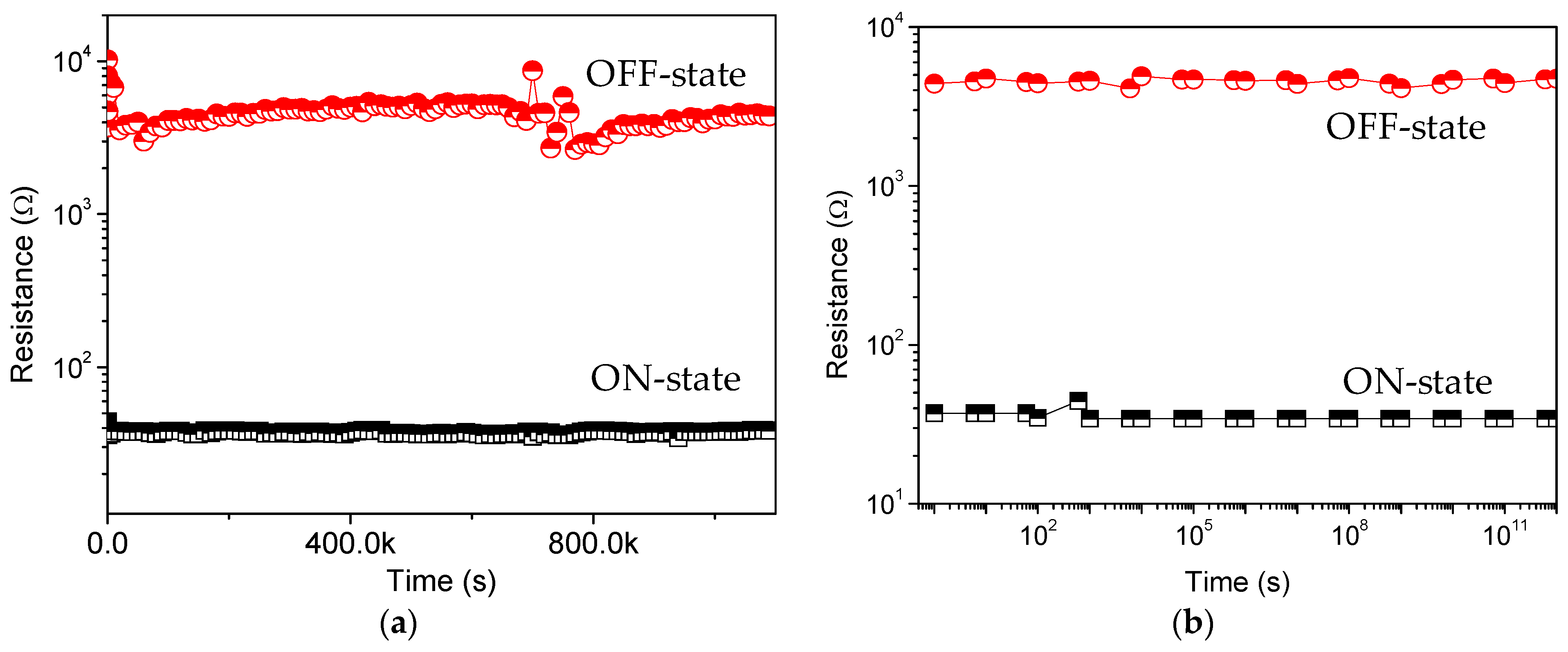
Figure 5a), indicating that both the composite materials and the electrode/nanocomposite interfaces are stable.
RON/
ROFF in the bistable devices is high enough to promise a low misreading rate through precise control of the ON and OFF states. The effect of continuous read pulses with a read voltage of −0.1 V in the ON and OFF states is also studied. As shown in
Figure 5b, more than 10
12 read cycles are conducted on the ITO/PBD:GO/Ni devices, and no resistance degradation is observed for the ON and OFF states. Neither the voltage stress nor the read pulses cause state transition because the applied voltage (−0.1 V) is lower than the switching threshold voltage. Thus, both states are stable under voltage stress and are insensitive to read pulses. These results confirm that the device has excellent rewritability for nonvolatile memory device applications. Thus, a larger ON/OFF ratio with a low driving voltage is desirable in the field of nano-scaled molecular electronics.
3.3. Thermal and Optical Measurements
In order to understand the performance and mechanism of the switching behavior of the PBD:GO device, the thermal and optical measurements were also investigated. To determine the thermal stability of the samples, TGA and differential thermo-gravimetric (DTG) of PBD, GO, and PBD:GO composites were carried out under a nitrogen atmosphere at a heating rate of 10 °C/min. The TGA and DTG curves obtained from PBD:GO nanocomposite samples are shown in
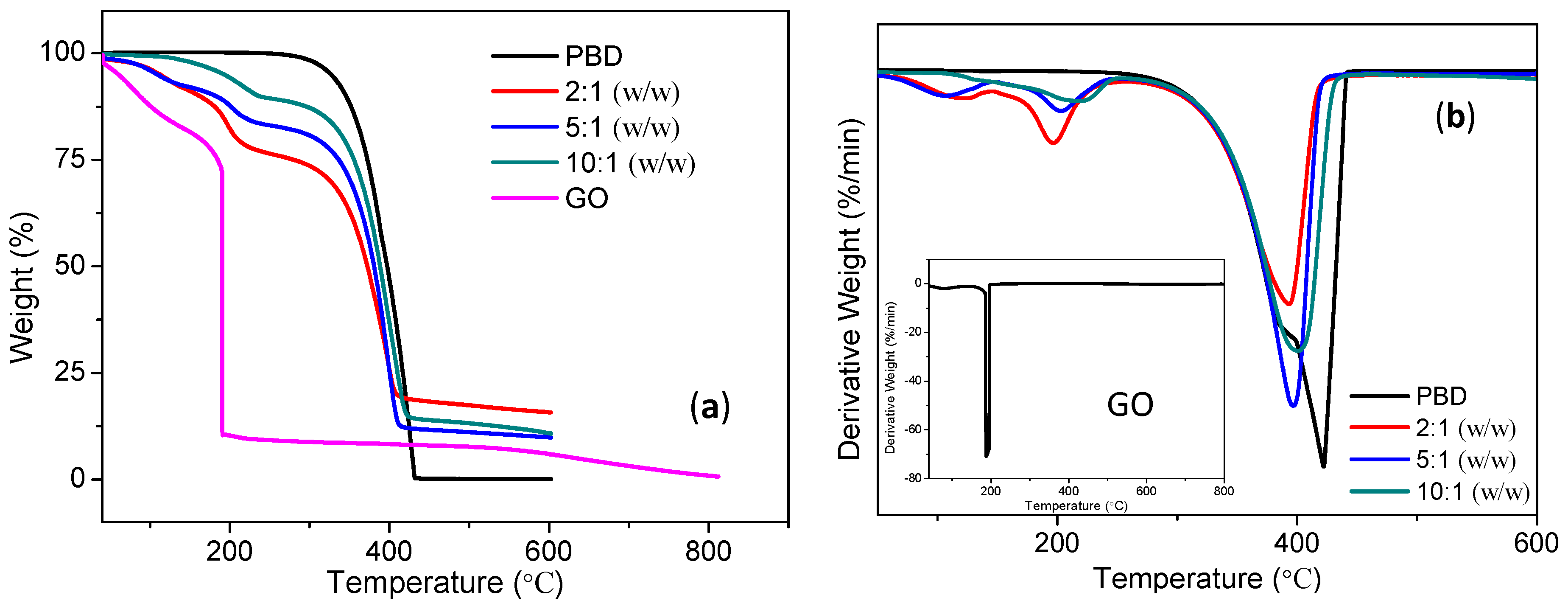
Figure 6. It can be observed that distinct degradation behaviors occur. In
Figure 6a, the decomposition temperature with a weight loss of about 5% for PBD and GO is 327 °C and 60 °C, while that for PBD:GO with 2:1 (w/w), 5:1 (w/w), and 10:1 (w/w) is respectively 112 °C, 109 °C, 193 °C. It can be seen that PBD completely degrades at ~400 °C, while GO has 0.67% weight when the temperature approaches 800 °C. The thermal degradation process for PBD:GO composites has two steps. The DTG curves of the PMMA:GO composites exhibited in
Figure 6b have strong peaks at 395 °C, 397 °C, and 401 °C, suggesting that the incorporation of different amounts of GO significantly shifts the temperature of thermal degradation, and the magnitude of the shift is dependent on the amount of GO. Most likely, small molecules that have inhibiting effects on the thermo-oxidative degradation of PBD are adsorbed on the surface of GO.
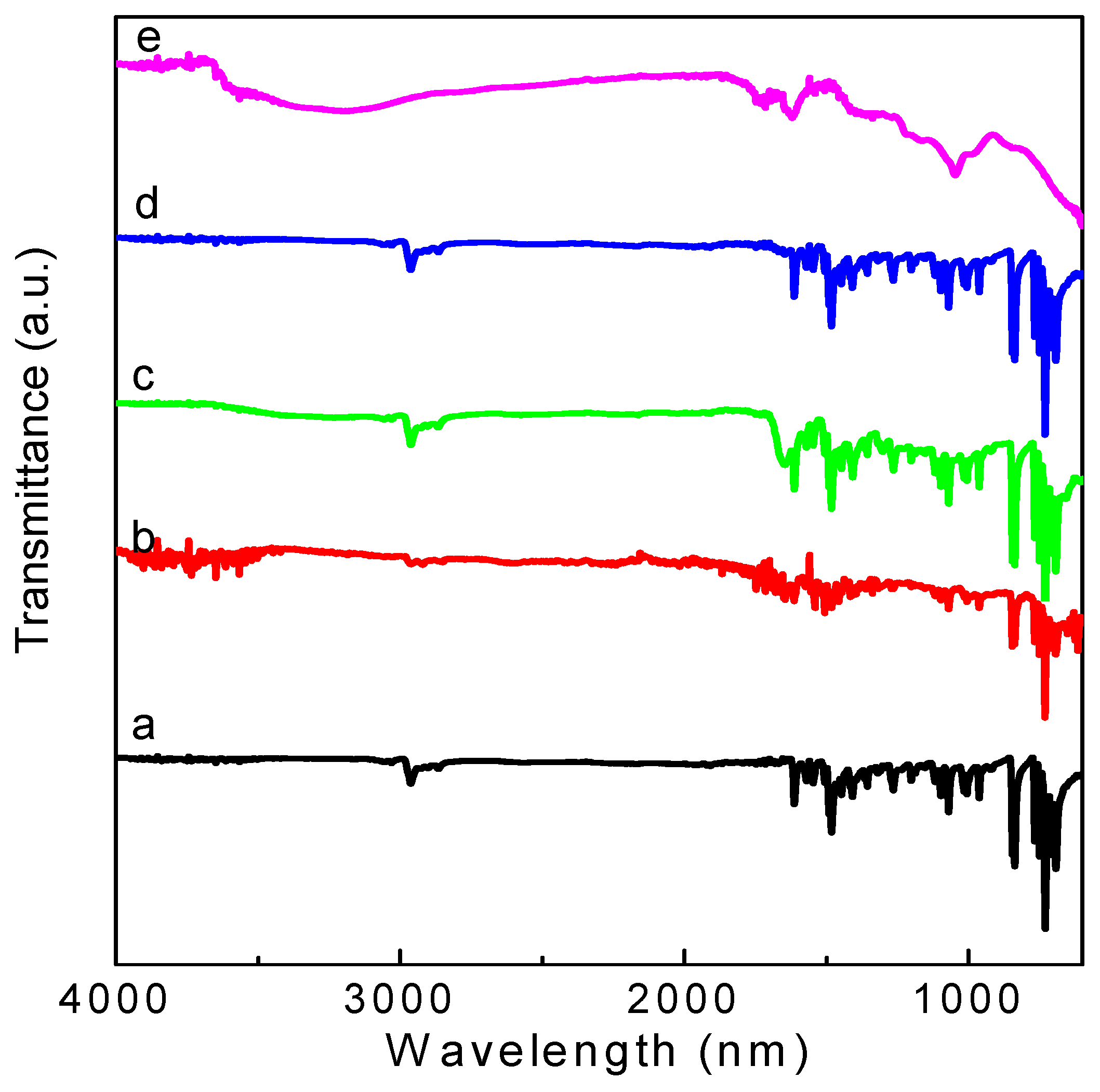
As shown in
Figure 7, the main characteristic absorption bands in the FTIR spectra of GO are located at 1732 cm
−1 (C = O carbonyl stretching of -COOH), 1418 cm
−1 (O-H deformation vibration), 1171 cm
−1 (C-OH stretching), and 1047 cm
−1 (C-O stretching). The broad peaks for GO at 3400 cm
−1 and 1620 cm
−1 are derived from the stretching vibration of hydroxyl (–OH) group and the skeletal vibration of the graphene sheets [
17], respectively. The above mentioned phenomena present various oxygen functional groups on the GO surface. FTIR spectra for the PBD:GO blends are almost the same as those of PBD. The spectra provide evidence that oxadiazole units were successfully incorporated into the blends. The incorporation of the 1,3,4-oxadiazole acceptor is evidenced by the medium intensity at 1570 cm
−1 for –C = N– stretching of the oxadiazole ring, as well as the medium-strong band at 1015 cm
−1 derived from the C–O–C vibration or heteroatom ring deformation of the oxadiazole ring. Moreover, C = C linkages in aromatic rings are responsible for the absorption peaks at 1613 cm
−1 and 1490 cm
−1.
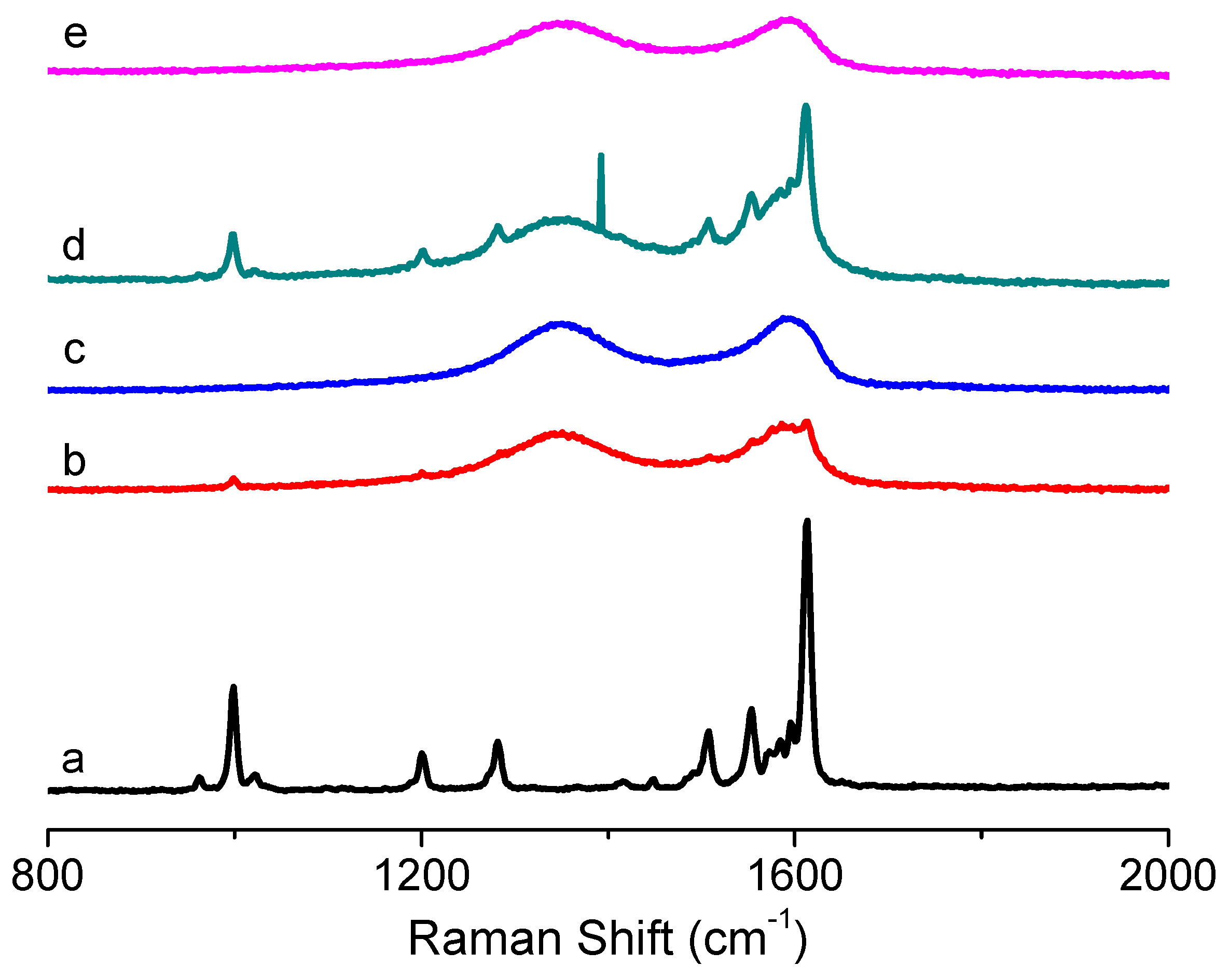
Figure 8 shows the Raman spectra of PBD:GO compared with those of GO and PBD. For PBD, the obvious peak stemming from the C–O–C stretching mode of the 1,3,4-oxadiazole ring is at 999 cm
−1. The stronger Raman activity can be found at 1613 cm
−1 now that it has the C–C stretching pattern in the benzene ring. Under excitation with a 532 nm laser, the Raman spectrum of GO displayed two prominent bands at about 1354 cm
−1 (D-band) and 1596 cm
−1 (G-band), with the
ID/
IG of 0.93. The D-band has usually been used to monitor the process of covalent functionalization, which transforms sp
2 to sp
3 sites, while the G-bands can be utilized to estimate the level of chemical modification. The D-band and G-band of PBD:GO with 2:1, 5:1, and 10:1 (w/w) are located at 1355 cm
−1 and 1595 cm
−1, 1350 cm
−1 and 1587 cm
−1, and 1342 cm
−1 and 1584 cm
−1 respectively, with a blue-shift compared to those of GO. This change implies a decrease in the average size of the sp
2 domains upon reduction of GO. After absorbing the small molecules to the surface of GO, the G-bands were largely enhanced and blue-shifted to 1584 cm
−1. The
ID/
IG ratio decreased from 0.93 to 0.67.
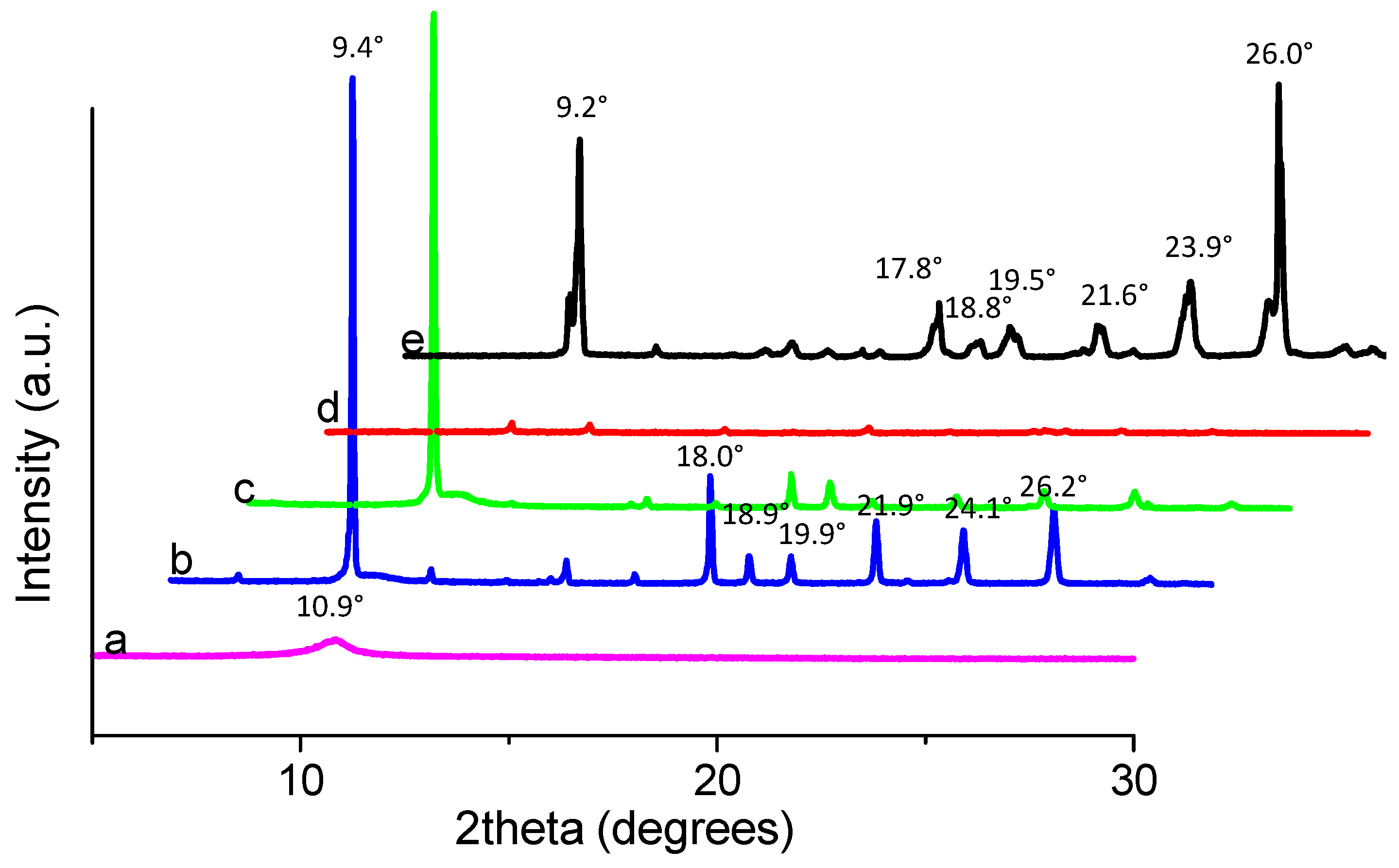
To understand the change in the interlayer of GO films with different chemical ratios, X-ray diffraction (XRD) was used. The XRD patterns of the three compounds are shown in
Figure 9. The diffraction peaks of the compounds, compared with those of PBD, occur at larger degrees. For example, PBD shows diffraction peaks at 2
θ = 9.2° (9.6 Å), while the compounds are at 2
θ = 9.4° (9.4 Å), which demonstrates that compounds have a closer intermolecular staking distance. In reality, the composite with the chemical component ratio PBD:GO of 10:1 (w/w) has the same peaks as the composites with ratios of 2:1 and 5:1 (w/w), but its intensities are so weak that the diffraction peaks for the composite with PBD:GO of 10:1 (w/w) seem inconspicuous. For PBD:GO blends, the intensity for various peaks is obviously associated with the weight ratio of the chemical component, decreasing with incremental PBD content. The small molecules absorbing to the surface of GO may destroy the crystal structure of the PBD to some extent and reduce its crystallinity degree. The increasing content of PBD is responsible for the intensity reduction. This phenomenon may be due to the interaction between PBD and GO that leads to the change of the crystal structure of the samples [
18]. The peak for the (002) facet of graphene at 2
θ = 10.9° (8.1 Å) is also shown. According to the XRD of PBD, the other peaks arise at 2
θ = 17.8° (4.98 Å), 18.8° (4.72 Å), 19.5° (4.55 Å), 21.6° (4.11 Å), 23.9° (3.72 Å), and 26° (3.42 Å), while others for the composites correspondingly exhibit at 2
θ = 18° (4.92 Å), 18.9° (4.69 Å), 19.9° (4.46 Å), 21.9° (4.06 Å), 24.1° (3.69 Å), and 26.2° (3.4 Å). These results indicate that intermolecular π-π stacking, which would facilitate charge transport through the neighboring molecules, became stronger [
19].
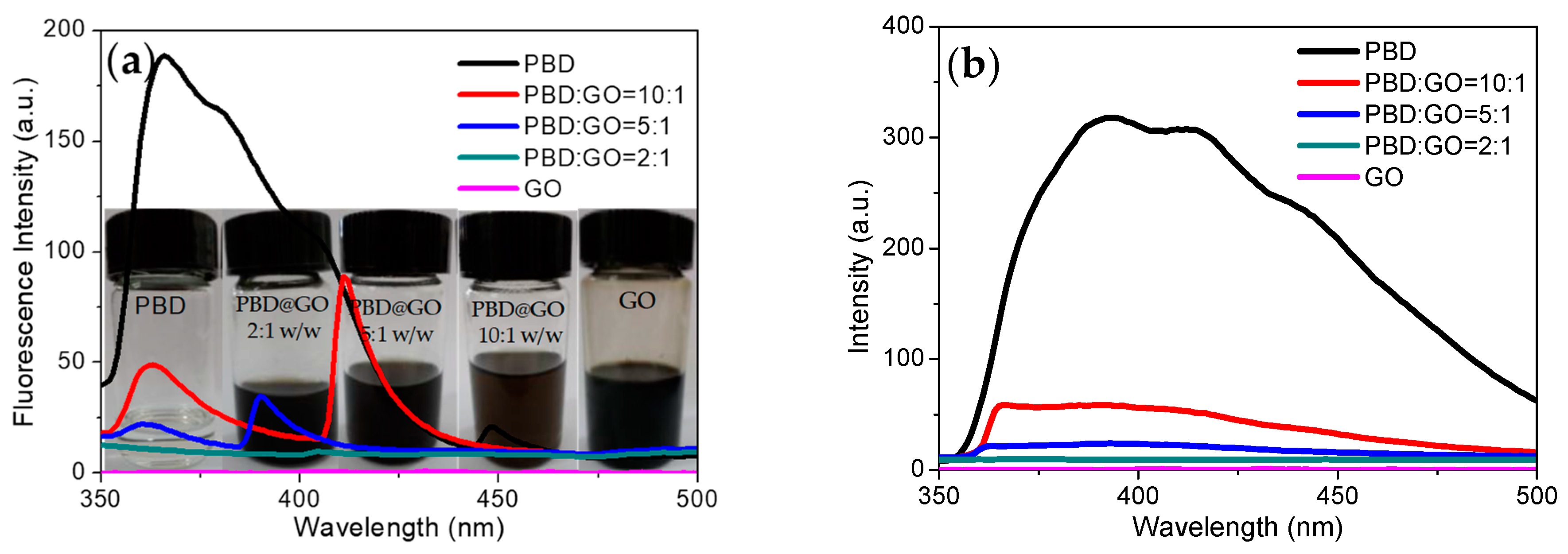
For the fluorescence studies, fluorescence excitation and emission of these compounds in the NMP solution get readily quenched by GO, with increasing amounts of GO, as shown in
Figure 10. Quenching occurs due to the interaction between the molecule and GO. This was explored by studying the spectral behavior as a function of the chemical component ratio. By increasing the chemical ratio of PBD moiety in composites, the absorption spectra are broadened, and absorption increases. The fluorescence excitation spectra of the composites shown in
Figure 10a, in contrast with those of PBD, show splitting of the bands into two sharp red-shifted bands when blending PBD with GO. This was explored by studying the spectral behavior as a function of the chemical component ratio of PBD and GO. From
Figure 10a, it is seen that the fluorescence excitation spectrum intensity decreases with increasing GO content. Up to a chemical component ratio of 5:1 (w/w), the fluorescence excitation spectra show distinct splitting into two sets of bands. As small molecule, there exists the possibility of intermolecular aggregation for PBD.
Figure 10b shows fluorescence emission spectra dependent on the chemical composition between GO and PBD. As the chemical component of PBD:GO changes from 10:1 to 2:1 (w/w), the maximum emission appears in a blue-shift and fluorescence intensity decreases when excited at 360 nm. The fluorescence intensity decreases with the incremental content of GO in composites. The fluorescent quenching effect of GO could be caused by GOs interaction with PBD. The fluorescence of the fluorescent derivatives could be quenched by trace of charged molecules (quenchers) via charge transfer or energy transfer [
1]. The fluorescent small molecule would be absorbed on the surface of the GO sheets due to van der Waals forces between the nucleobases and the basal plane of the GO sheets. The absorption of PBD onto GO facilitates fluorescence quenching, since GO sheets act as a quencher (GO acceptor) that can absorb the energy from the fluorescent small molecule. The GO solution shows no detected fluorescence under various conditions. The added PBD was absorbed by the quencher GO, and quenched chromophore remained within the quenchers active sphere. Fluorescence quenching for systems of this type occurs intramolecularly.
3.4. Resistance-Switchable Mechanism
To determine whether the resistance-switchable effect stems from the PBD:GO nanocomposite, a control experiment was conducted with GO as an active layer sandwiched between ITO and Ni electrodes. As exhibited in
Figure 11, no hysteresis effects were observed in the
I-
V performance with single high resistivity. This result shows that the bistability and memory phenomena of the PBD:GO memory devices are derived from PBD:GO, which interestingly relies on the weight ratio between PBD and GO.
I-
V performance results illustrate that PBD attachment to the GO framework may help to tune the initial high resistivity [
20,
21].
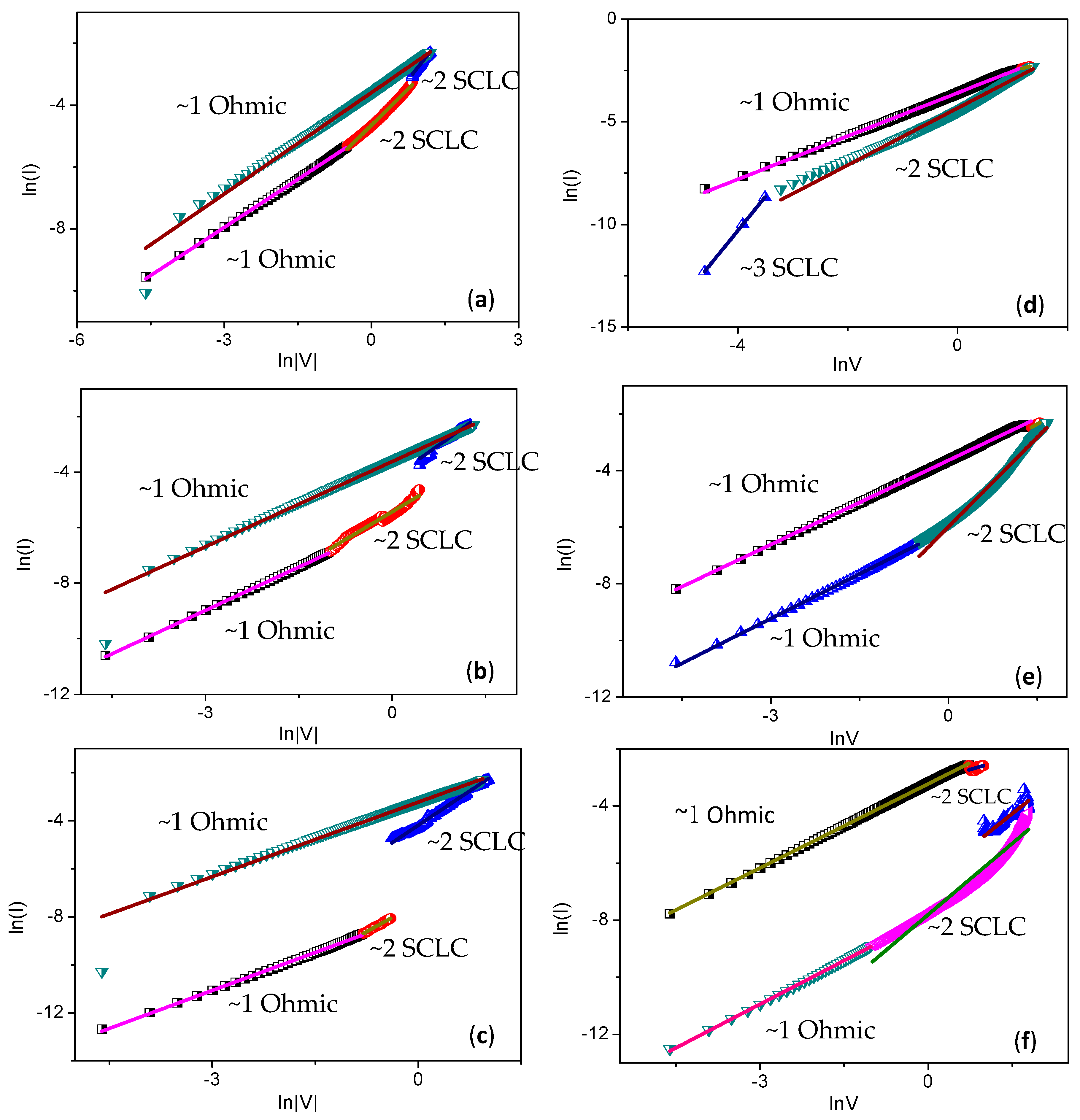
The conduction mechanism of LRS and HRS in the PBD:GO nanocomposite layer can be explained by Ohmic and space-charge-limited conduction (SCLC) (
Figure 12). To interpret the conduction mechanism, log
I-log
V plots were drawn for the write and erase processes. Fitting results suggest that SCLC is the conduction mechanism in the HRS region, whereas Ohmic conduction is dominant in the LRS region. In the write process,
I-
V characteristics in the HRS region consist of two distinct linear regions at low voltage, in which the relationship between current and voltage follows Ohm’s law (
I ∝
V), the quantity of thermally generated free charge carriers exceeds that of injected carriers, and the curve is linear. In this region, traps are partially filled, and Ohmic behavior is observed. The region at high voltage conforms to the trap-filled limit current law (
I ∝
V2). During transition voltage from Ohmic to SCLC, the electric field across the device is sufficient, so all traps are filled with charge carriers. In this quadratic region, the injected carriers are more dominant than thermally generated ones. The current, which is switchable from HRS to LRS, dramatically increases. When scanning again, the
I-
V curve is linear due to an Ohmic conduction mechanism. During the erase process, Ohmic conduction was also observed for LRS. The conduction from SCLC to Ohmic, after the current shifts from LRS to HRS, suddenly decreases. Therefore, the superior ReRAM properties of multilayers of GO blended with a 1,3,4-oxadiazole acceptor nanocomposite, according to the fitting results, are attributed to the electron traps caused by the strong electron acceptors [
22].