Abstract
Venom is a key evolutionary innovation of venomous organisms in the long-term process of survival adaptation. As one of the oldest arthropods, scorpions produce venom rich in bioactive peptides that also constitute a valuable pharmacological resource. Omics-driven discovery and structural biology have expanded the peptide catalog and clarified structure–function principles across disulfide-bridged (DBPs) and non-disulfide-bridged peptides (NDBPs). Within this arsenal, ion-channel targeting neurotoxins predominantly modulate Nav, Kv, Calcium, Chloride, and TRP channels to achieve predation, defense, and competition. Owing to their unique mechanisms of action and significant therapeutic potential, scorpion venom peptides have attracted sustained interest as leads and scaffolds for drug development. This review synthesizes current knowledge of scorpion venom composition, with an emphasis on the pivotal role of neurotoxins, covering their molecular diversity, structural features, and modes of ion-channel modulation, as well as emerging applications in disease treatment.
Key Contribution:
This review delivers a synthesis of the molecular diversity, structural scaffolds, and structure–function determinants of scorpion venom neurotoxins, with a mechanistic emphasis on how these peptides modulate major ion-channel targets. By linking toxin sequence and scaffold features to channel selectivity, pharmacological profiles, and translational considerations, it clarifies their utility as molecular probes and as prioritized lead scaffolds for drug discovery.
1. Introduction
“Survival of the fittest,” a central tenet of Darwin’s theory of evolution, holds that under constraints of space and resources, individuals with advantageous heritable traits are more likely to survive and reproduce [1]. Venomous animals span both vertebrate and invertebrate lineages and occupy diverse ecological niches [2,3], deploying toxins to subdue prey, deter predators, and mediate intra- and interspecific competition [4,5,6]. Under sustained natural selection, most venom systems have repeatedly evolved into powerful biochemical arsenals, representing a striking case of convergent evolution [7,8,9,10].
Scorpions, ancient arthropods with an evolutionary history of over 400 million years, are among the earliest fully terrestrialized animal lineages [11,12]. The oldest known scorpion taxa include Dolichophonus loudonensis and Eramoscorpius brucensis, whose fossil records date back to the Silurian period (approximately 430 million years ago), providing critical evidence for understanding early scorpion morphology and the evolutionary transition toward a fully terrestrial lifestyle [13,14,15]. Unlike insects and spiders, which underwent extensive morphological diversification, scorpions have largely retained key ancestral features such as venom glands, book lungs, and pectines, and they have adapted to extreme and fluctuating environments, earning them the moniker “living fossils” [11,16,17,18]. Fossil evidence provides some of the earliest records of scorpions, and anatomical interpretations suggest that a venom apparatus emerged early in their evolutionary history [12,15,17]. Scorpion venom is a complex mixture of bioactive molecules, including neurotoxins, proteases, protease inhibitors, mucopolysaccharides, free amino acids, and inorganic ions [19,20]. This biochemical repertoire underpins both predation and defense, facilitating survival across diverse and often hostile habitats.
To date, approximately 2900 scorpion species have been described, with distributions across all continents except Antarctica [21]. Scorpion envenomation is a widespread public health concern, particularly in tropical and subtropical regions such as the Middle East, North Africa, southern India, Mexico, and Brazil [22,23]. Globally, an estimated 1.2 million stings occur each year [11,24,25]. Most cases cause only local pain and swelling [26]; however, approximately 30% reportedly progress to severe systemic symptoms, including fever, vomiting, convulsions, abnormalities in blood pressure and heart rate, and respiratory distress, and, in rare cases, coma or death [24,25]. Nearly all fatal stings are attributed to species in the family Buthidae [27,28], which are generally more toxic than members of Scorpionidae or Hemiscorpiidae [28,29].
The principal bioactive components responsible for scorpion venom toxicity are small peptides, typically 20–70 amino acids in length [30], that target multiple physiological receptors [27,30]. Among these, neurotoxins that modulate ion channels account for most envenomation symptoms. The first characterized scorpion neurotoxin, AaH I, was purified from the venom of Androctonus australis in 1964 and acts on mammalian voltage-gated sodium channels as an α-NaTx [31]. Since then, scorpion venoms have yielded a wide variety of peptides targeting voltage-gated sodium (Nav), potassium (Kv), calcium (Cav), chloride (ClC) channels; acid-sensing ion channels (ASICs), and transient receptor potential (TRP) channels [26,32,33,34]. These toxins modulate ion-channel function by shifting the voltage dependence, altering activation and inactivation kinetics, and changing conductance and open probability [35,36]. Ion channels are essential for neural signaling, muscle contraction, cardiac rhythm, endocrine secretion, and immune responses [37,38,39,40], and their dysfunction is associated with neurological and cardiovascular disorders [41,42], autoimmune and inflammatory conditions [43,44,45], and several cancers [46,47]. Owing to their potency and high selectivity for defined ion-channel isoforms and other receptors, scorpion neurotoxins serve as powerful molecular probes and promising leads or scaffolds for therapeutic design [48,49,50].
Advances in genomic and transcriptomic sequencing, high-resolution mass spectrometry, and structural biology have driven venom research into an era of molecular precision, enabling systematic discovery, annotation, and structure–function analysis of neurotoxin peptides [32,51,52,53,54,55,56,57,58]. To date, numerous scorpion neurotoxins have been identified and functionally characterized [50,59,60], which not only support the development of effective clinical treatments for envenomation, but also provide potent probes and regulators for ion-channel pharmacology [50,61]. This review synthesizes current knowledge of scorpion venom composition and diversity, highlights the molecular mechanisms and pharmacological functions of key neurotoxins, and evaluates their potential as therapeutic scaffolds. These insights lay a foundation for the efficient utilization and transformation of scorpion venom peptides in drug discovery.
2. Diversity of Scorpion Venom Components
Scorpion venom is a complex biochemical mixture composed of diverse constituents. In addition to non-protein components such as lipids, free amino acids, nucleotides, and inorganic ions, the biologically active fraction is dominated by small peptides and enzymes [55]. Disulfide-bridged peptides (DBPs) and non-disulfide-bridged peptides (NDBPs) constitute major components of scorpion venom peptides (SVPs) [50,62,63]. NDBPs are typically small peptides consisting of 13–56 amino acids, lacking disulfide bonds, and often showing no precise conserved sequence–function relationship [64,65]. They frequently exhibit multiple bioactivities without a single defined molecular target and have attracted increasing research interest for their broad pharmacological potential, including antimicrobial, antiviral, anticancer, and immunomodulatory effects [66,67]. DBPs usually comprise 30–70 amino acids and are stabilized by disulfide bridges that maintain compact tertiary structures [30]. Many DBPs act as highly specific and potent ion-channel modulators. The well-defined structure–function relationships make DBPs valuable templates for drug discovery and molecular engineering [68].
3. Non-Disulfide-Bridged Peptides
Non-disulfide-bridged peptides (NDBPs) constitute more than one-third of known scorpion venom peptides [64,69,70]. They are typically short peptides of 13–56 amino acids that usually lack cysteines and display marked sequence and functional diversity [64] (Table 1). Most NDBPs bear C-terminal amidation (often on Lys, Arg, or His), which confers overall cationicity and promotes interaction with negatively charged biological membranes [71,72]. Structurally, NDBPs are predominantly α-helical peptides and could be grouped into three classes based on the distribution of α-helices along the backbone [64]. Class I comprises a single α-helix flanked by disordered coil regions at both termini; representatives include HsAP, BmKb1, Pandinin 2, BmKn2, IsCT, Meucin-24, Im-1, AamAP1, AamAP2, and Mauriporin [73,74,75,76,77,78,79,80] (Figure 1a). Class II contains two α-helices connected by a flexible coil, such as BmKbpp, Opistoporin 1, Hadrurin, Pandinin 1, and Parabutoporin [80,81,82,83,84] (Figure 1b). Class III consists of peptides that are essentially fully α-helical structures, with only a few identified members, such as StCT2 and Imcroporin [85,86] (Figure 1c). Although NDBPs lack conserved motifs or defined molecular targets, their multifunctional pharmacological activities, particularly antimicrobial, antiviral, and bradykinin-potentiating effects, make them an attractive reservoir of lead molecules [87].

Table 1.
Representative scorpion non-disulfide-bridged peptides (NDBPs).
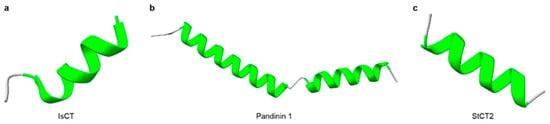
Figure 1.
Three-dimensional structures of representative scorpion non-disulfide-bridged peptides (NDBPs). (a) The structure of Class I NDBP (IsCT). (b) The structure of Class II NDBP (Pandinin 1). (c) The structure of Class III NDBP (StCT2). α-Helical regions are highlighted in green. The structure of IsCT was obtained from RCSB PDB (PDB: 1T51), the structures of Pandinin 1 and StCT2 were predicted with AlphaFold3.
The global rise of multidrug-resistant pathogens has intensified interest in antimicrobial peptides (AMPs) [107], which are small, cationic peptides composed of approximately 15–150 amino acids [108]. Owing to their broad-spectrum activity, short length, structural simplicity, and relatively low propensity to induce resistance, AMPs are promising alternatives to conventional antibiotics. Most scorpion-derived NDBPs are functionally categorized as AMPs. Their positively charged surfaces interact with negatively charged bacterial membranes, disrupt membrane integrity, and form pores that cause leakage of cellular contents and bacterial death [109]. Scorpion AMPs such as Imcroporin, StCT1, Ctriporin, Pantinin 1, Pantinin 2, and Pantinin 3, retain potent activity even against methicillin-resistant Staphylococcus aureus (MRSA) and other resistant strains [85,86,110,111]. However, many antimicrobial NDBPs display variable hemolytic and cytotoxic effects. For instance, VmCT2 from Vaejovis mexicanus shows 84% hemolysis at 50 μM, whereas VsCT1 from Vaejovis subcristatus exhibits only 12% under the same conditions [112]. Therefore, structural optimization and rational peptide design are essential to improve selectivity and safety for clinical applications.
Beyond antimicrobial activity, NDBPs exhibit broad bioactivity profiles. Several scorpion-derived NDBPs show anticancer effects, including Mauriporin, Pantinin, RK1, TsAP-1, TsAP-2, Gonearrestide, AaeAP1a, and AaeAP2a [80,113,114,115,116]. BmKn2 from Olivierus martensii (O. martensii) induces apoptosis in human oral squamous carcinoma (HSC4) and epidermoid carcinoma (KB) cells through a p53-dependent intrinsic pathway while sparing normal gingival (HGC) and dental pulp (DPC) cells [117]. Some NDBPs, such as Peptide T, Peptide K12, TsHpt, and BmKbpp, show bradykinin-potentiating activity by inhibiting angiotensin-converting enzyme (ACE)-mediated degradation of bradykinin, thereby promoting vasodilation and lower blood-pressure [91,99,101,118,119]. Recent studies also report in vitro antiviral activity against hepatitis C virus (HCV), herpes simplex virus (HSV), and human immunodeficiency virus (HIV) [50]. Examples include Hp1090 and Ctry2459, which inhibit HCV replication [102,103], and Hp1036, Hp1239, Eval418, and Kn2-7, which suppress HSV and HIV [90,104,105]. Moreover, several NDBPs act as immunomodulators, capable of modulating innate and adaptive immune responses through interactions with host cell membranes or immune receptors [120]. Collectively, scorpion-derived NDBPs offer versatile molecular templates for antimicrobial, anticancer, antiviral, and immunotherapeutic development.
4. Disulfide-Bridged Peptides
Disulfide-bridged peptides (DBPs) are typically 30–70 amino acids in length and contain three to four intramolecular disulfide bonds, which confer high structural stability and resistance to proteolysis [121]. Most DBPs adopt compact, cystine-stabilized folds that enable precise molecular recognition [122]. Unlike NDBPs, DBPs are usually specific ion channel-acting peptides, including voltage-gated sodium (Nav), potassium (Kv), calcium (Cav), chloride (ClC) channels, and transient receptor potential (TRP) channels [123]. These channel proteins are central to normal cellular physiology [123]. Scorpion DBPs bind their targets with high affinity and selectivity, altering ion permeability or gating characteristics, which may produce neurotoxic, cardiotoxic, or cytotoxic effects [123]. Because of their potency, selectivity, and structural diversity, scorpion DBPs have attracted considerable pharmacological interest and serve as powerful molecular probes for dissecting ion-channel function and as promising leads for the treatment of channelopathies [124].
4.1. Toxins Targeting Voltage-Gated Sodium Channels (NaTx)
Voltage-gated sodium channels (Nav) are expressed in most excitable cells and play key roles in the initiation and propagation of action potentials [125] (Figure 2a). Nav channels are primary pharmacological targets for local anesthetics, antiarrhythmics, and antiepileptics [26,126]. Among scorpion venom components, sodium-channel toxins (NaTx) are the principal neurotoxins that act directly on Nav channels, disrupting their normal function and inducing various characteristic envenomation symptoms [26]. NaTxs typically consist of 60–76 amino acids; most contain eight cysteines forming four disulfide bridges that lock an α-helix and two antiparallel β-strands into a compact and stable cysteine-stabilized α/β (CSαβ) fold conformation [125,127,128], which represents the most typical and conserved structural scaffold among scorpion peptide toxins [129,130]. In a minority of NaTx, only three disulfide bonds are present, yet the overall CSαβ topology is retained (Table 2).
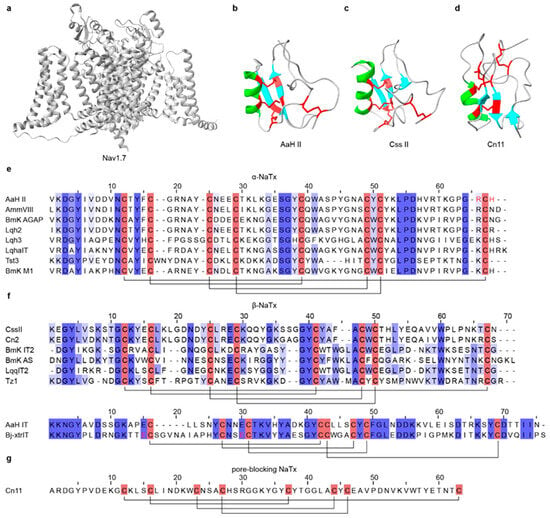
Figure 2.
Three-dimensional structures and sequence alignments of representative scorpion NaTx and their target channel. (a) The structure of the Nav1.7 channel (PDB: 6J8G). (b) The structures of α-NaTx (AaH II, PDB: 1AHO; Ts1, PDB: 1B7D). (c) The structures of β-NaTx (Css II, PDB: 2LI7; Cn2, PDB: 1CN2). (d) Structure of a pore-blocking NaTx, Cn11 (PDB: 1PE4). (e) Multiple sequence alignment of representative α-NaTx. (f) Multiple sequence alignment of representative β-NaTx. (g) Multiple sequence alignment of representative pore-blocking NaTx. In the three-dimensional structures, α-helices are highlighted in green, β-strands are highlighted in cyan, and disulfide bonds are shown in red. In the sequence alignments, cysteine residues are highlighted in red, disulfide bonds are indicated by black connecting lines, and functionally important amino acid residues are marked in red. All structures were obtained from RCSB PDB.

Table 2.
Representative scorpion toxins targeting sodium channels (NaTx).
For well-characterized NaTxs, classification is typically based on their biophysical effects and experimentally validated binding sites on sodium channels, distinguishing gating modifiers from pore blockers. However, functional classification schemes may overlook some phylogenetically divergent potential NaTxs with specialized functions [32,168]. Gating modifiers NaTx constitute the vast majority and are further divided into α-NaTx and β-NaTx, whereas pore-blocking NaTx are rarely reported [169]. α-NaTx binds to site 3 in domain IV of Nav channels and interfere with the normal movement of the S4 voltage sensor during fast inactivation, thereby suppressing or delaying channel inactivation [170,171,172,173]. The resulting persistent sodium influx drives sustained neuronal firing and can produce symptoms such as pain, muscle spasms, convulsions, and respiratory paralysis [26,174] (Figure 2e). AaH II, an α-NaTx from Androctonus australis, is a mammal-specific toxin of 64 residues [175]; it inhibits fast inactivation of Nav1.2 and Nav1.6, prolonging action potentials and evoking intense pain and muscle convulsion [131] (Figure 2b). Lqh-2 shows mammalian-like toxicity by impairing the Nav channel inactivation [155,176,177]. By target preference, α-NaTx are commonly further divided into three subgroups: classical α-toxins (mammalian-preferring), insect α-toxins (insect-preferring), and α-like toxins (active on both mammalian and insect NaV channels) [27,169]. β-NaTx binds to site 4 in domain II of Nav channels [171,178]. Rather than inhibiting inactivation, they interact with the voltage-sensing domain to modify activation kinetics and shift the activation threshold toward more negative potentials, rendering the channels hyperexcitable [179,180] (Figure 2f). CssII, a β-NaTx from Centruroides suffusus, specifically targets Nav1.6 by binding to the extracellular end of the domain II voltage sensor, stabilizing an activated conformation and facilitating channel opening [137]. Cn2 from Centruroides noxius acts through the same mechanism and shares high sequence similarity with CssII [139] (Figure 2c). In contrast, pore-blocking NaTx are exceedingly rare; Cn11 from Centruroides noxius is a well-characterized example proposed to inhibit Nav channels by physically occluding the ion-conducting pore rather than by modifying channel gating [140] (Figure 2d,g).
Given the direct involvement of Nav channels in pain perception and transmission, scorpion-derived Nav modulators are being explored as analgesic leads [181]. Numerous NaTx have been reported to exert analgesic effects by selectively modulating Nav channel subtypes, particularly Nav1.1, Nav1.6, Nav1.7, Nav1.8, and Nav1.9 [182,183]. Several peptides from O. martensii exhibit analgesic activity, including BmKBTx [141], BmNaL-3SS2 [141], and DKK-SP2 [142], which specifically target Nav1.7. BmK IT2 [184] and BmK IT-AP [185], display dual bioactivity, being insecticidal and analgesic in mice. Additional Nav channel-targeting peptides with analgesic activity include LqqIT2 from Leiurus quinquestriatus [185], AmmVIII from Androctonus mauretanicus [185], and BotAF from Buthus occitanus [158]. Moreover, studies have shown that voltage-gated sodium channels are often aberrantly upregulated in advanced epithelial cancers, where they contribute to invasion and metastasis [186,187,188]. Thus, Nav inhibitors are being considered for oncology applications. Notably, BmK AGAP from O. martensii exhibits both analgesic and anticancer activities; it suppresses the migration and invasion of human breast cancer cells (MDA-MB-231 and MCF-7), and inhibits epithelial–mesenchymal transition (EMT) and the acquisition of stem-like phenotypes [145,189].
4.2. Toxins Targeting Voltage-Gated Potassium Channels
Voltage-gated potassium channels (Kv) form a large and evolutionarily conserved ion-channel family expressed in virtually all cell types. They shape action potentials in excitable cells, such as neurons, cardiomyocytes, and muscle fibers, in coordination with Nav channels. Kv channels are also involved in the regulation of cell volume, proliferation, and migration in diverse cell types [190,191]. Notably, Kv1.3 is highly expressed in macrophages, microglia, platelets, B cells, T lymphocytes, and the central nervous system (CNS), and is implicated in the pathogenesis and treatment of autoimmune diseases and cancers [192,193] (Figure 3a).
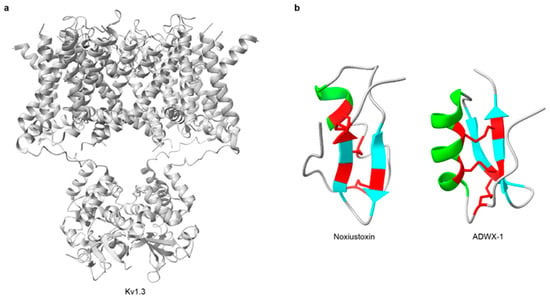
Figure 3.
Three-dimensional structures of representative scorpion KTx and their target channel. (a) The structure of the Kv1.3 channel (PDB: 7SSX). (b) The structures of KTx (Noxiustoxin, PDB: 1SXM; ADWX-1, PDB: 2K4U). In the three-dimensional structures, α-helices are highlighted in green, β-strands are highlighted in cyan, and disulfide bonds are shown in red. All structures were obtained from RCSB PDB.
Potassium-channel toxins (KTx) are among the most common and extensively studied scorpion neurotoxins [194]. These short peptides typically comprise 23–64 amino acids and contain 2-4 disulfide bridges that form the characteristic cystine-stabilized α/β (CSαβ) fold [124,192,195] (Table 3). Noxiustoxin was the first isolated short-chain scorpion toxin; it specifically reduced K+ permeability in the squid giant axon without altering voltage-dependent gating [196,197] (Figure 3b). Since the discovery, more than 199 distinct KTx peptides have been identified in scorpion venoms [50]. For instance, charybdotoxin, purified from Leiurus quinquestriatus, is a high-affinity, reversible blocker of Ca2+-activated K+ channels that occludes the external pore without affecting voltage-dependent gating [198,199]. Based on sequence features, structural motifs, and disulfide-bond patterns, KTx are grouped into: α-KTx, β-KTx, γ-KTx, κ-KTx, δ-KTx, λ-KTx, and ε-KTx [124,200,201] (Figure 4). Among these, α-KTx is the largest and best characterized, with 31 subfamilies currently identified. α-KTx peptides generally contain 23–42 residues, which typically block K+ flux either by physically occluding the pore [195] or by interacting with negatively charged residues near the outer vestibule of the channel [202]. It also should be noted, however, that this classification is predominantly based on function and does not necessarily represent actual evolutionary relationships, as phylogenetic analyses have demonstrated that some functionally defined KTx groups do not share a recent common ancestor [203,204].

Table 3.
Representative scorpion toxins targeting potassium channels (KTx).

Figure 4.
Sequence alignments of representative scorpion KTx. (a) Multiple sequence alignment of representative α-KTx. (b) Multiple sequence alignment of representative β-KTx. (c) Multiple sequence alignment of representative γ-KTx. (d) Multiple sequence alignment of representative κ-KTx. (e) Multiple sequence alignment of representative δ-KTx. (f) Multiple sequence alignment of representative ε-KTx. (g) Multiple sequence alignment of representative λ-KTx. In the sequence alignments, cysteine residues are highlighted in red, disulfide bonds are indicated by black connecting lines, and functionally important amino acid residues are marked in red.
Several scorpion KTx selectively target the Kv1.3 channel and have shown therapeutic potential in autoimmune disease models [193], including ImKTx88 [231,248], Vm24 [232,233], HsTX1 [249,250], Ts6 [234], Ts15 [234], etc. ADWX-1, a 37-residue derivative of BmKTX, is a highly selective Kv1.3 blocker with remarkable affinity and efficacy [251]. It preferentially inhibits Kv1.3 on CCR7− effector memory T (TEM) cells, suppressing Ca2+-dependent activation and proinflammatory cytokine release, thereby effectively alleviating experimental autoimmune encephalomyelitis (EAE) [224]. Ts6 and Ts15 from Tityus serrulatus potently inhibit Kv1.3, and Ts15 also blocks Kv2.1. Both peptides reduce T cell-mediated delayed-type hypersensitivity (DTH) responses in vivo [234], highlighting their potential for autoimmune therapy.
Beyond immunomodulation, KTx also exhibit analgesic, antiviral, and anticancer activities. Heterolaxin from Heterometrus laoticus and leptucin from Hemiscorpius lepturus are representatives with pain-relieving effects [239,246]. Two defensins from O. martensii, BmKDfsin3 [252] and BmKDfsin4 [253], exhibit both antimicrobial and Kv1.3-blocking effects. BmKDfsin3 inhibits hepatitis C virus (HCV) infection in a dose-dependent manner by suppressing p38 MAPK activation at noncytotoxic concentrations [226,254]. BmKDfsin4 dose-dependently reduces hepatitis B virus (HBV) DNA and viral protein production [255]. Additional KTx with antiviral activity include Smp76 from Scorpio maurus, which acts via an interferon-β (IFN-β) like mechanism to inhibit established infections, with significant inhibitory effects on dengue virus (DENV) and Zika virus (ZIKV) [236,237]. Ev37 from Euscorpiops validus, which alkalizes intracellular acidic organelles to prevent low-pH-dependent fusion between viral and endosomal membranes, thereby blocking release of the viral genome into the cytoplasm [256]. Ev37 has been reported to inhibit DENV-2, HCV, ZIKV, and herpes simplex virus type 1 (HSV-1) infections [238]. In oncology, margatoxin (MgTx) and iberiotoxin (IbTx) are promising leads. MgTx, a 39-residue α-KTx with three disulfide bridges, blocks mammalian Kv1.3 and suppresses proliferation of human lung adenocarcinoma A549 cells [257,258]. The large-conductance Ca2+-activated K+ channel (BK) is widely expressed in glioma and may regulate tumor growth [259,260,261]. IbTx, a BK-specific blocker [262], inhibits proliferation of human 1321N1 astrocytoma cells and induces S-phase arrest [259,263]. Collectively, KTx are rich sources of pharmacological tools and therapeutic leads. Their potency, isoform selectivity, and compact scaffolds support applications in immune modulation, antiviral therapy, pain management, and cancer treatment.
4.3. Toxins Targeting Calcium Channels (CaTx)
Calcium ions (Ca2+) play a key role in numerous physiological processes, including neurotransmission, muscle contraction, hormone secretion, and intracellular signaling [264,265,266]. Ca2+ influx across the plasma membrane and release from intracellular stores are mediated by voltage-gated calcium channels (VGCCs) and ryanodine receptors (RyRs), respectively [267,268] (Figure 5a). Among scorpion venom components, a subset of peptides collectively termed CaTx, acts directly or indirectly on these channels, thereby modulating Ca2+ permeability and intracellular Ca2+ levels [269,270]. Toxins that target calcium channels are valuable probes for basic physiological research and hold significant pharmacological potential. CaTx typically contains 33–36 amino acids and 2-4 disulfide bridges [54,271] (Table 4). Based on structural characteristics, they are grouped into two motifs: the inhibitor cystine knot (ICK) fold and the disulfide-directed hairpin (DDH) fold [272]. Most scorpion CaTx act on intracellular Ca2+-release channels (RyRs), whereas only a few target VGCCs [273,274].

Figure 5.
Three-dimensional structures and sequence alignment of representative scorpion CaTx and their intracellular target. (a) The structure of the RyR1 channel (PDB: 8SEN). (b) The structures of CaTx (φ-LITX-Lw1a, PDB: 2KYJ; Imperatoxin A, PDB: 1IE6). (c) Multiple sequence alignment of representative CaTx. In the three-dimensional structures, α-helices are highlighted in green, β-strands are highlighted in cyan, and disulfide bonds are shown in red. In the sequence alignment, cysteine residues are highlighted in red, disulfide bonds are indicated by black connecting lines, and functionally important amino acid residues are marked in red. All structures were obtained from RCSB PDB.

Table 4.
Representative scorpion toxins targeting calcium channels (CaTx).
Notable VGCC-active examples include kurtoxin and kurtoxin-like I. Kurtoxin, identified from Pandinus transvaalicus, shares sequence similarity with NaTx and selectively interacts with VGCCs [274]. It binds the α1G subunit of T-type calcium channels and suppresses activity by altering voltage-dependent gating kinetics [270,275]. Thus, kurtoxin could serve as a molecular tool for studying the functions of T-type calcium channels. Kurtoxin-like I from Parabuthus granulatus differs from kurtoxin by six residues yet exhibits similar function on T-type channels [276]. Calcins are small scorpion peptide toxins of approximately 33–35 residues with three disulfide bridges that adopt a stable ICK fold [278] (Figure 5c). They possess positively charged surfaces and can penetrate cell membranes. Calcins specifically target RyRs in the endoplasmic and sarcoplasmic reticulum, where they modulate channel opening to regulate Ca2+ release from intracellular stores [282,283]. To date, numerous calcin-like sequences have been described, primarily through transcriptomic and proteomic studies [54,284,285]; however, detailed functional characterization has been reported for only a limited subset, including urocalcin [278], vejocalcin [278], hemicalcin [279], hadrucalcin [273], maurocalcin [286], opicalcin 1 [278], opicalcin 2 [278], imperatoxin A [278,287], and intrepicalcin [277]. Liotoxins are a subclass of CaTx that also act on RyRs but adopt a DDH rather than an ICK fold [50,275]. The peptide ϕ-LITX-Lw1a, the first DDH motif CaTx isolated from scorpion venom, efficiently targets and activates mammalian RyRs [272] (Figure 5b). A key functional difference between calcins and liotoxins is cell permeability: calcins are cell-penetrating due to amphipathic α-helical regions, whereas liotoxins lack such structural features and do not rapidly cross the plasma membrane to reach intracellular RyRs [272].
RyRs are intracellular Ca2+ release channels located on endoplasmic and sarcoplasmic reticulum membranes and are essential for Ca2+ homeostasis [288]. Among the three isoforms, RyR2 is the principal channel in cardiomyocytes and is critical for excitation–contraction coupling [266,289,290]. Dysfunction of RyR2 has been linked to severe cardiac disorders, including ventricular arrhythmia, heart failure, and sudden cardiac death [291,292]. Consequently, pharmacological modulation of RyR2 is a promising therapeutic strategy. Maurocalcin, a 33-residue peptide toxin, increases RyR2 sensitivity to cytosolic Ca2+, thereby enhancing channel opening and regulating Ca2+ release in cardiomyocytes [293], which suggests utility in certain types of arrhythmias. Imperatoxin A (IpTxa), from Pandinus imperator, is an intracellular RyR1 activator that promotes Ca2+ release from the sarcoplasmic reticulum [287] (Figure 5b). Remarkably, IpTxa is cell-penetrant and can deliver otherwise impermeant cargos into cells [294], providing valuable strategies for targeted intracellular delivery.
4.4. Toxins Targeting Chloride Channels (ClTx)
Voltage-gated chloride channels (ClCs) are homodimeric proteins that conduct Cl−, NO3−, SCN−, and other anions and participate in numerous physiological processes, including cell growth, pH regulation, modulation of cellular excitability, ionic homeostasis, etc [123]. In mammals, ClC-1, ClC-2, and ClC-K are expressed at the plasma membrane, where they stabilize membrane potential and maintain excitability, with essential roles in skeletal muscle relaxation and renal water–salt balance [295,296]. By contrast, ClC-3 through ClC-7 function predominantly as Cl−/H+ exchangers on intracellular organelle membranes, maintaining organelle ionic homeostasis and acidic environments [297,298].
Currently, only a limited number of scorpion toxin peptides that target chloride channels (ClTx) have been identified, including Ammp2 [299], chlorotoxin [300,301,302] (Figure 6a), I5A [303] (Figure 6b), Lqh7-1 [304], Lqh2-2 [304], Lqh8-6 [304], BmKCT [305], Bs14, and PBITX1 [306,307] (Table 5 and Figure 6c). Among these, chlorotoxin (CTx) is the first to be identified and the best characterized ClTx from Leiurus quinquestriatus. CTx is a 36-residue peptide with four disulfide bonds that inhibits Cl− influx across the plasma membrane [308]. CTx shows selectivity for glioblastoma cells, suppressing migration and invasion while exerting minimal effects on normal cells, highlighting its potential as an anticancer molecule [309]. CTx binds matrix metalloproteinase 2 (MMP-2), which is overexpressed in glioblastoma, reducing gelatinase activity in membrane-associated complexes and downregulating MMP-2 expression [302,310]. CTx also blocks ClC-3 channels, inhibiting cell volume changes and reducing migratory speed, and is ultimately endocytosed after binding [311]. Because of its high tumor specificity, biotinylated or fluorescently labeled CTx has been used as a glioma-specific tracer [300]. A homologous peptide, BmKCT from O. martensii, consists of 35 residues with four disulfide bonds and similarly binds glioma cells, inhibiting proliferation and migration with minimal effects on normal cells [305]. BmKCT is therefore another promising diagnostic and therapeutic candidate for glioblastoma. Additionally, three short-chain peptides, Lqh2-2, Lqh7-1, and Lqh8-6, were isolated from Leiurus quinquestratus [304,312]. Electrophysiology and intracellular Ca2+ imaging indicate that these peptides inhibit calcium-activated chloride channel currents in vascular smooth muscle cells, which may be consistent with targeting the TMEM16A/Ano1 channel [304]. These peptides provide useful pharmacological tools for studying calcium-activated chloride channels (CaCCs).

Figure 6.
Three-dimensional structures and sequence alignment of representative scorpion ClTx. (a) The structure of Chlorotoxin (PDB: 1CHL) and (b) I5A (PDB: 1SIS). (c) Multiple sequence alignment of representative ClTx. In the three-dimensional structures, α-helices are highlighted in green, β-strands are highlighted in cyan, and disulfide bonds are shown in red. In the sequence alignment, cysteine residues are highlighted in red, disulfide bonds are indicated by black connecting lines, and functionally important amino acid residues are marked in red. All structures were obtained from RCSB PDB.

Table 5.
Representative scorpion toxins targeting chloride channels (ClTx).
4.5. Toxins Targeting TRP Channels
Transient receptor potential (TRP) channels are nonselective cation channels mainly expressed on the plasma membrane of peripheral and central neurons [314,315,316], and are central to cellular signal transduction, sensory perception, and homeostasis [317,318] (Figure 7a). These receptors can be activated by chemical ligands, and also by physical stimuli, including osmotic stress, mechanical force, temperature, and light [319,320,321]. Scorpion neurotoxins also modulate TRP channels. To date, a limited set of TRP-targeting toxins from scorpions has been described, including BmP01, WaTx, Tx203, and OdK1 [322,323] (Table 6 and Figure 7c). WaTx, isolated from Urodacus manicatus, is a cell-penetrant peptide that targets TRPA1 (Figure 7b). WaTx prolongs the channel’s open state while reducing Ca2+ permeability, thereby eliciting acute pain and pain hypersensitivity without triggering neurogenic inflammation [324,325]. This pharmacological profile makes WaTx a valuable probe for elucidating TRPA1 function in nociception and neuronal signal transduction [324]. BmP01, isolated from O. martensii, activates TRPV1 in an acid-dependent manner via a “one-two” punch mechanism [325] (Figure 7b). Likewise, OdK1 and Tx203, members of the α-KTx8 subfamily, significantly potentiate TRPV1 under acidic conditions [323,326]. Collectively, these TRP-targeting toxins provide distinctive pharmacological tools for probing nociception and thermosensation, and promote the development of therapies for pain and other channel-related disorders.

Figure 7.
Three-dimensional structures and sequence alignment of representative scorpion toxins targeting TRP channels and their target. (a) The structure of the TRPV1 channel (PDB: 9P6B). (b) The structures of BmP01 (PDB: 1WM7) and WaTx (PDB: 6OFA). (c) Multiple sequence alignment of representative TRPTx. In the three-dimensional structures, α-helices are highlighted in green, β-strands are highlighted in cyan, and disulfide bonds are shown in red. In the sequence alignment, cysteine residues are highlighted in red, disulfide bonds are indicated by black connecting lines, and functionally important amino acid residues are marked in red. All structures were obtained from RCSB PDB.

Table 6.
Representative scorpion toxins targeting TRP channels.
5. Enzymes
Unlike many snake venoms, enzymes in scorpion venom are generally not the primary toxic agents; instead, they play auxiliary roles that facilitate venom diffusion and promote tissue penetration [329]. With advances in omics, diverse enzymes have been identified in scorpion venoms, including phospholipases, hyaluronidases, metalloproteinases, and serine proteases [51,330,331]. Elucidating their structure–function relationships, molecular evolution, and interactions with peptide toxins are important directions for future scorpion venom research.
Metalloproteinases and serine proteases are two major proteolytic enzyme classes in scorpion venom. Metalloproteinases degrade fibrinogen and neuropeptides, thereby contributing to neurotoxicity and inflammation [332,333,334], and promote host tissue damage and degradation of immune defense proteins [335]. Serine proteases help regulate inflammatory responses and coordinate multiple physiological processes [336,337]. Both classes may post-translationally modify venom peptides and facilitate venom spread within prey, thereby amplifying overall toxicity [200,338,339]. Phospholipases hydrolyze ester bonds in phospholipids and, in snake venoms, contribute to predation and defense by disrupting cell membranes and modulating signaling pathways [340,341]. In scorpion venoms, such as those of Scorpio maurus and Anuroctonus phaiodactylus, phospholipase A2 (PLA2) is the most common type and exhibits multiple toxic effects, including hemolytic, inflammatory, myotoxic, cardiotoxic, and anticoagulant activities [340,342,343]. Hyaluronidases degrade hyaluronic acid in the extracellular matrix and are generally regarded as “spreading factors” that increase tissue permeability [344,345,346,347]. Studies on Tityus serrulatus hyaluronidase indicate that it is crucial for venom diffusion from the sting site to the bloodstream and for biodistribution to target organs [346].
6. Non-Peptidic Small Molecules
In addition to bioactive peptides and enzymes, scorpion venom contains a range of non-peptidic small molecules, although research on these constituents remains limited [348]. Studies on snake and spider venoms have demonstrated that small molecules contribute substantially to toxicity and physiological regulation, suggesting similar roles in scorpions [349,350]. Systematic identification and functional characterization of these compounds would clarify the overall mechanisms of venom action and inform improved prevention and clinical management of scorpion envenomation. To date, only a few have been characterized [348]. Examples include an alkaloid from Megacormus gertschi [351]; two antibacterial 1,4-benzoquinone derivatives from Diplocentrus melici [352]; adenosine with anticoagulant activity in Heterometrus laoticus [353,354]; and citrate in Centruroides sculpturatus [355]. Analysis of Heterometrus waigiensis venom further revealed several small molecules, including adenosine, AMP, citrate, glutamic acid, and aspartic acid [348]. In addition, scorpion venoms contain other small-molecule components, such as nucleotides, lipids, amines, heterocyclic compounds, and inorganic salts [20,75].
7. Discussion
Scorpion venom is an evolutionarily refined biochemical arsenal rich in bioactive molecules with distinct pharmacological profiles. Among these, neurotoxins stand out for their selectivity, potency, and structural stability. They act with high selectivity on ion channels or other membrane receptors, thereby modulating a range of physiological processes, including neural transmission, immune response, tumor progression, and pain perception [50,356]. Accordingly, they may serve as precise molecular probes for studying ion-channel function and as templates for rational therapeutic design [61]. Although only a limited number of venom-derived peptide drugs have reached development or clinical use [62], notable examples include Captopril, the first blood pressure medication developed through structural modification of a snake venom peptide [357,358]; Ziconotide, a cone snail peptide approved for the treatment of chronic pain [359]; Eptifibatide, a snake venom-derived antiplatelet drug used in cardiovascular interventions [360,361]. Clinical translation of scorpion peptides is still in its early stages. Chlorotoxin, initially isolated from Leiurus quinquestriatus, is the only scorpion peptide that has advanced to clinical trials to date [308].
Despite ongoing venom research identifying numerous peptides with promising in vitro pharmacological properties, clinical translation is constrained by conventional validation processes that typically verify target engagement without accounting for in vivo limitations such as rapid proteolysis, short half-life, inadequate tissue penetration, and off-target liabilities [362,363,364]. Bridging this gap may require developability prioritization and peptides engineering. Artificial intelligence and bioinformatics can mine omics-scale venom resources, improve toxin annotation, and rank candidates by integrating predicted potency with sequence features linked to stability and toxicity, while interpretable models can highlight residues and motifs that guide rational redesign [365,366,367]. These optimized scaffolds can then be strengthened through chemical strategies such as sequence minimization, incorporation of noncanonical residues, cyclization or stapling, and disulfide surrogates, as well as polymer or glycan conjugation when appropriate, to improve conformation, protease resistance, and pharmacokinetics while preserving activity [368,369,370,371]. In parallel, precision nanocarriers and peptide-mediated delivery systems can protect peptides in circulation, tune biodistribution, and enable intracellular delivery, thereby converting strong in vitro bioactivity into therapeutic exposure in vivo [372,373]. Collectively, combining computational design, structural stabilization, and delivery engineering can shift venom peptide research toward better candidates explicitly optimized for translational performance.
Integrative investigations encompassing genomics, transcriptomics, proteomics, and structure biology have uncovered considerable molecular diversity and functional intricacy driven by evolutionary adaptation, thereby enhancing our comprehension of venom biology [11,51,54,55]. High-quality scorpion genomes, together with deep RNA sequencing of venom glands, now enable the comprehensive recovery of expressed toxin repertoires and place them in an explicit evolutionary framework [11,374,375]. Transcriptomics not only increases the number of discovered peptides, but also reveals extensive sequence diversity within toxin families, including low-abundance paralogs and lineage variants [55,285,330,376]. It allows for studying toxin function by analyzing sequence diversity and evolutionary relationships, which is essential for understanding peptide scaffolding [32,69]. Additionally, comparative analyses clarify peptide homology across taxa, highlight scaffold conservation versus hypervariable functional regions, and help distinguish convergent functional recruitment from true shared ancestry, thereby providing a rational basis for selecting peptide scaffolds with stability and target selectivity [329,377]. Proteomics adds an indispensable orthogonal layer by confirming which transcripts are translated into secreted venom components, resolving mature peptide processing and post-translational modifications, and prioritizing candidates with both biological activity and technical operation [51,54,285,330]. When coupled with structural determination and functional assays, this multi omics framework links toxin evolution to mechanism and ultimately to drug scaffold optimization.
Future progress will benefit from discovery-to-development pipelines. High-throughput omics and high-resolution structure determination can accelerate target validation and mechanism elucidation [378,379]. AI-assisted design and peptidomimetic strategies can improve stability, selectivity, and pharmacokinetics [380,381]. Chemical modification and sequence minimization, backbone cyclization, and disulfide surrogates can reduce proteolysis and immunogenicity while preserving activity [382,383]. Formulation with nanocarriers or conjugation to tumor-homing ligands can enhance delivery and tissue selectivity [384,385]. With continued advances, research on scorpion peptides is entering a phase of molecular precision and bioengineering. Scorpion-derived peptides are expected to yield candidates with high specificity and low toxicity for applications in ion-channel modulation, oncology, antiviral therapy, and immune regulation.
Author Contributions
Conceptualization, B.L. and Y.H.; writing—original draft preparation, Y.H. and P.M.K.; writing—review and editing, B.L., Y.H. and P.M.K.; visualization, Y.H., J.W. and M.G.; supervision, B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Science and Technology Projects of Xizang Autonomous Region, China (XZ202402ZD0005), National Natural Science Foundation of China (32471557 and 31900375), and the Science and Technology Department of Gansu Province (23JRRA1085).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Skinner, M.K. Environmental Epigenetics and a Unified Theory of the Molecular Aspects of Evolution: A Neo-Lamarckian Concept that Facilitates Neo-Darwinian Evolution. Genome Biol. Evol. 2015, 7, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Dufton, M.J. Venomous mammals. Pharmacol. Ther. 1992, 53, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, K.; Harris, R.J. Radiating pain: Venom has contributed to the diversification of the largest radiations of vertebrate and invertebrate animals. BMC Ecol. Evol. 2021, 21, 150. [Google Scholar] [CrossRef]
- Michálek, O.; King, G.F.; Pekár, S. Prey specificity of predatory venoms. Biol. Rev. Camb. Philos. Soc. 2024, 99, 2253–2273. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Jin, A.H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef]
- Brodie, E.D., 3rd. Toxins and venoms. Curr. Biol. 2009, 19, R931–R935. [Google Scholar] [CrossRef]
- Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A.B. The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef]
- Barua, A.; Koludarov, I.; Mikheyev, A.S. Co-option of the same ancestral gene family gave rise to mammalian and reptilian toxins. BMC Biol. 2021, 19, 268. [Google Scholar] [CrossRef]
- Surm, J.M.; Moran, Y. Insights into how development and life-history dynamics shape the evolution of venom. EvoDevo 2021, 12, 1. [Google Scholar] [CrossRef]
- Zancolli, G.; Reijnders, M.; Waterhouse, R.M.; Robinson-Rechavi, M. Convergent evolution of venom gland transcriptomes across Metazoa. Proc. Natl. Acad. Sci. USA 2022, 119, e2111392119. [Google Scholar] [CrossRef]
- Cao, Z.; Yu, Y.; Wu, Y.; Hao, P.; Di, Z.; He, Y.; Chen, Z.; Yang, W.; Shen, Z.; He, X.; et al. The genome of Mesobuthus martensii reveals a unique adaptation model of arthropods. Nat. Commun. 2013, 4, 2602. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, J.A.; Erik Tetlie, O.; Prendini, L. Reinterpretation of the Silurian Scorpion Proscorpius Osborni (Whitfield): Integrating Data from Palaeozoic and Recent Scorpions. Palaeontology 2008, 51, 303–320. [Google Scholar] [CrossRef]
- Waddington, J.; Rudkin, D.M.; Dunlop, J.A. A new mid-Silurian aquatic scorpion-one step closer to land? Biol. Lett. 2015, 11, 20140815. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, J.A.; Selden, P.A.J.A. Scorpion fragments from the Silurian of Powys, Wales. Arachnology 2013, 16, 27–32. [Google Scholar] [CrossRef]
- Dunlop, J.A.; Garwood, R.J. A review of fossil scorpion higher systematics. PeerJ 2024, 12, e18557. [Google Scholar] [CrossRef]
- Aria, C. The origin and early evolution of arthropods. Biol. Rev. Camb. Philos. Soc. 2022, 97, 1786–1809. [Google Scholar] [CrossRef]
- Anderson, E.P.; Schiffbauer, J.D.; Jacquet, S.M.; Lamsdell, J.C.; Kluessendorf, J.; Mikulic, D.G.J.P. Stranger than a scorpion: A reassessment of Parioscorpio venator, a problematic arthropod from the Llandoverian Waukesha Lagerstätte. Palaeontology 2021, 64, 429–474. [Google Scholar] [CrossRef]
- Howard, R.J.; Edgecombe, G.D.; Legg, D.A.; Pisani, D.; Lozano-Fernandez, J. Exploring the evolution and terrestrialization of scorpions (Arachnida: Scorpiones) with rocks and clocks. Org. Divers. Evol. 2019, 19, 71–86. [Google Scholar] [CrossRef]
- Oukkache, N.; Rosso, J.P.; Alami, M.; Ghalim, N.; Saïle, R.; Hassar, M.; Bougis, P.E.; Martin-Eauclaire, M.F. New analysis of the toxic compounds from the Androctonus mauretanicus mauretanicus scorpion venom. Toxicon 2008, 51, 835–852. [Google Scholar] [CrossRef]
- Ortiz, E.; Gurrola, G.B.; Schwartz, E.F.; Possani, L.D. Scorpion venom components as potential candidates for drug development. Toxicon 2015, 93, 125–135. [Google Scholar] [CrossRef]
- Rein, J.O. The Scorpion Files. Trondheim: Norwegian University of Science and Technology. 2017. Available online: https://www.ntnu.no/ub/scorpion-files/ (accessed on 23 January 2017).
- Vasconez-Gonzalez, J.; Alexander-León, H.; Noboa-Lasso, M.L.; Izquierdo-Condoy, J.S.; Puente-Villamarín, E.; Ortiz-Prado, E. Scorpionism: A neglected tropical disease with global public health implications. Front. Public Health 2025, 13, 1603857. [Google Scholar] [CrossRef] [PubMed]
- Bouchaala, K.; Bahloul, M.; Hadded, A.; Haddar, O.; Hamida, C.B. Intravenous Levosimendan for Acute Heart Failure with Renal Impairment Following Severe Scorpion Envenomation: Case Report and Literature Review. Am. J. Trop. Med. Hyg. 2025, 113, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P.; Goyffon, M. Epidemiology of scorpionism: A global appraisal. Acta Trop. 2008, 107, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Abroug, F.; Ouanes-Besbes, L.; Tilouche, N.; Elatrous, S. Scorpion envenomation: State of the art. Intensive Care Med. 2020, 46, 401–410. [Google Scholar] [CrossRef]
- He, D.; Lei, Y.; Qin, H.; Cao, Z.; Kwok, H.F. Deciphering Scorpion Toxin-Induced Pain: Molecular Mechanisms and Ion Channel Dynamics. Int. J. Biol. Sci. 2025, 21, 2921–2934. [Google Scholar] [CrossRef]
- Quintero-Hernández, V.; Jiménez-Vargas, J.M.; Gurrola, G.B.; Valdivia, H.H.; Possani, L.D. Scorpion venom components that affect ion-channels function. Toxicon 2013, 76, 328–342. [Google Scholar] [CrossRef]
- Dehghani, R.; Ghorbani, A.; Varzandeh, M.; Karami-Robati, F. Toxicity Mechanism of Dangerous Scorpion Stings in Iran. J. Arthropod-Borne Dis. 2023, 17, 105–119. [Google Scholar] [CrossRef]
- Dehghani, R.; Kamiabi, F.; Mohammadi, M. Scorpionism by Hemiscorpius spp. in Iran: A review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 8. [Google Scholar] [CrossRef]
- Xin, K.; Sun, R.; Xiao, W.; Lu, W.; Sun, C.; Lou, J.; Xu, Y.; Chen, T.; Wu, D.; Gao, Y. Short Peptides from Asian Scorpions: Bioactive Molecules with Promising Therapeutic Potential. Toxins 2025, 17, 114. [Google Scholar] [CrossRef]
- M’Barek, S.; Fajloun, Z.; Cestèle, S.; Devaux, C.; Mansuelle, P.; Mosbah, A.; Jouirou, B.; Mantegazza, M.; Van Rietschoten, J.; El Ayeb, M.; et al. First chemical synthesis of a scorpion alpha-toxin affecting sodium channels: The Aah I toxin of Androctonus australis hector. J. Pept. Sci. 2004, 10, 666–677. [Google Scholar] [CrossRef]
- Santibáñez-López, C.E.; Aharon, S.; Ballesteros, J.A.; Gainett, G.; Baker, C.M.; González-Santillán, E.; Harvey, M.S.; Hassan, M.K.; Abu Almaaty, A.H.; Aldeyarbi, S.M.; et al. Phylogenomics of Scorpions Reveal Contemporaneous Diversification of Scorpion Mammalian Predators and Mammal-Active Sodium Channel Toxins. Syst. Biol. 2022, 71, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Diego-García, E.; Abdel-Mottaleb, Y.; Schwartz, E.F.; de la Vega, R.C.; Tytgat, J.; Possani, L.D. Cytolytic and K+ channel blocking activities of beta-KTx and scorpine-like peptides purified from scorpion venoms. Cell. Mol. Life Sci. 2008, 65, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Petroff, E.Y.; Price, M.P.; Snitsarev, V.; Gong, H.; Korovkina, V.; Abboud, F.M.; Welsh, M.J. Acid-sensing ion channels interact with and inhibit BK K+ channels. Proc. Natl. Acad. Sci. USA 2008, 105, 3140–3144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Tonggu, L.; Gamal El-Din, T.M.; Banh, R.; Pomès, R.; Zheng, N.; Catterall, W.A. Structural basis for voltage-sensor trapping of the cardiac sodium channel by a deathstalker scorpion toxin. Nat. Commun. 2021, 12, 128. [Google Scholar] [CrossRef]
- Campos, F.V.; Coronas, F.I.; Beirão, P.S. Voltage-dependent displacement of the scorpion toxin Ts3 from sodium channels and its implication on the control of inactivation. Br. J. Pharmacol. 2004, 142, 1115–1122. [Google Scholar] [CrossRef]
- Camerino, D.C.; Tricarico, D.; Desaphy, J.F. Ion channel pharmacology. Neurother. J. Am. Soc. Exp. Neurother. 2007, 4, 184–198. [Google Scholar] [CrossRef]
- Musio, C. Ion Channels and Neurological Disease. Life 2024, 14, 758. [Google Scholar] [CrossRef]
- Dai, G. Signaling by Ion Channels: Pathways, Dynamics and Channelopathies. Mo. Med. 2023, 120, 367–373. [Google Scholar]
- Ohya, S. Recent Developments in Ion Channel and Ion-Related Signaling. Int. J. Mol. Sci. 2023, 24, 4419. [Google Scholar] [CrossRef]
- Coste, B.; Delmas, P. PIEZO Ion Channels in Cardiovascular Functions and Diseases. Circ. Res. 2024, 134, 572–591. [Google Scholar] [CrossRef]
- Baker, E.H. Ion channels and the control of blood pressure. Br. J. Clin. Pharmacol. 2000, 49, 185–198. [Google Scholar] [CrossRef]
- Vaeth, M.; Feske, S. Ion channelopathies of the immune system. Curr. Opin. Immunol. 2018, 52, 39–50. [Google Scholar] [CrossRef]
- Estadella, I.; Navarro-Pérez, M.; Colomer-Molera, M.; Dustin, M.L.; Sorkin, A.; Capera, J.; Felipe, A. Molecular determinants for the endocytosis of the voltage-gated K(+) channel Kv1.3. Sci. Signal. 2025, 18, eado8924. [Google Scholar] [CrossRef] [PubMed]
- Winge, M.C.G.; Nasrallah, M.; Jackrazi, L.V.; Guo, K.Q.; Fuhriman, J.M.; Szafran, R.; Ramanathan, M.; Gurevich, I.; Nguyen, N.T.; Siprashvili, Z.; et al. Repurposing an epithelial sodium channel inhibitor as a therapy for murine and human skin inflammation. Sci. Transl. Med. 2024, 16, eade5915. [Google Scholar] [CrossRef] [PubMed]
- Rifat, A.; Bickel, T.; Kreis, P.; Trimbuch, T.; Onken, J.; Ivanov, A.; Albertini, G.; Beule, D.; Mazzanti, M.; Singh, H.; et al. The chloride intracellular channel 1 (CLIC1) is essential for microglial morphodynamics and neuroinflammation. Sci. Adv. 2025, 11, eads9181. [Google Scholar] [CrossRef] [PubMed]
- Son, G.Y.; Tu, N.H.; Santi, M.D.; Loya Lopez, S.; Souza Bomfim, G.H.; Vinu, M.; Zhou, F.; Chaloemtoem, A.; Alhariri, R.; Idaghdour, Y.; et al. The Ca(2+) channel ORAI1 is a regulator of oral cancer growth and nociceptive pain. Sci. Signal. 2023, 16, eadf9535. [Google Scholar] [CrossRef]
- Beraldo-Neto, E.; Ferreira, V.F.; Vigerelli, H.; Fernandes, K.R.; Juliano, M.A.; Nencioni, A.L.A.; Pimenta, D.C. Unraveling neuroprotection with Kv1.3 potassium channel blockade by a scorpion venom peptide. Sci. Rep. 2024, 14, 27888. [Google Scholar] [CrossRef]
- Nosouhian, M.; Rastegari, A.A.; Shahanipour, K.; Ahadi, A.M.; Sajjadieh, M.S. Anticancer potentiality of Hottentotta saulcyi scorpion curd venom against breast cancer: An in vitro and in vivo study. Sci. Rep. 2024, 14, 24607. [Google Scholar] [CrossRef]
- Xia, Z.; He, D.; Wu, Y.; Kwok, H.F.; Cao, Z. Scorpion venom peptides: Molecular diversity, structural characteristics, and therapeutic use from channelopathies to viral infections and cancers. Pharmacol. Res. 2023, 197, 106978. [Google Scholar] [CrossRef]
- Xu, X.; Duan, Z.; Di, Z.; He, Y.; Li, J.; Li, Z.; Xie, C.; Zeng, X.; Cao, Z.; Wu, Y.; et al. Proteomic analysis of the venom from the scorpion Mesobuthus martensii. J. Proteom. 2014, 106, 162–180. [Google Scholar] [CrossRef]
- Pineda, S.S.; Chin, Y.K.; Undheim, E.A.B.; Senff, S.; Mobli, M.; Dauly, C.; Escoubas, P.; Nicholson, G.M.; Kaas, Q.; Guo, S.; et al. Structural venomics reveals evolution of a complex venom by duplication and diversification of an ancient peptide-encoding gene. Proc. Natl. Acad. Sci. USA 2020, 117, 11399–11408. [Google Scholar] [CrossRef]
- Modahl, C.M.; Brahma, R.K.; Koh, C.Y.; Shioi, N.; Kini, R.M. Omics Technologies for Profiling Toxin Diversity and Evolution in Snake Venom: Impacts on the Discovery of Therapeutic and Diagnostic Agents. Annu. Rev. Anim. Biosci. 2020, 8, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Cid-Uribe, J.I.; Veytia-Bucheli, J.I.; Romero-Gutierrez, T.; Ortiz, E.; Possani, L.D. Scorpion venomics: A 2019 overview. Expert Rev. Proteom. 2020, 17, 67–83. [Google Scholar] [CrossRef]
- Solano-Godoy, J.A.; Betancourt-Osorio, M.; Orjuela-Rodriguez, M.; Guerrero-Vargas, J.A.; Sepulveda-Arias, J.C. Scorpion venom gland transcriptomics: A systematic review. Toxicon 2025, 267, 108563. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Duda, T.F., Jr. Extensive and continuous duplication facilitates rapid evolution and diversification of gene families. Mol. Biol. Evol. 2012, 29, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. Venomics: Integrative venom proteomics and beyond. Biochem. J. 2017, 474, 611–634. [Google Scholar] [CrossRef]
- Huang, J.; Pan, X.; Yan, N. Structural biology and molecular pharmacology of voltage-gated ion channels. Nat. Rev. Mol. Cell Biol. 2024, 25, 904–925. [Google Scholar] [CrossRef]
- Kalapothakis, Y.; Miranda, K.; Molina, D.A.M.; Conceição, I.; Larangote, D.; Op den Camp, H.J.M.; Kalapothakis, E.; Chávez-Olórtegui, C.; Borges, A. An overview of Tityus cisandinus scorpion venom: Transcriptome and mass fingerprinting reveal conserved toxin homologs across the Amazon region and novel lipolytic components. Int. J. Biol. Macromol. 2023, 225, 1246–1266. [Google Scholar] [CrossRef]
- Romero-Gutierrez, T.; Peguero-Sanchez, E.; Cevallos, M.A.; Batista, C.V.F.; Ortiz, E.; Possani, L.D. A Deeper Examination of Thorellius atrox Scorpion Venom Components with Omic Techonologies. Toxins 2017, 9, 399. [Google Scholar] [CrossRef]
- Peter Muiruri, K.; Zhong, J.; Yao, B.; Lai, R.; Luo, L. Bioactive peptides from scorpion venoms: Therapeutic scaffolds and pharmacological tools. Chin. J. Nat. Med. 2023, 21, 19–35. [Google Scholar] [CrossRef]
- Uzair, B.; Bint, E.I.S.; Khan, B.A.; Azad, B.; Mahmood, T.; Rehman, M.U.; Braga, V.A. Scorpion Venom Peptides as a Potential Source for Human Drug Candidates. Protein Pept. Lett. 2018, 25, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Panayi, T.; Diavoli, S.; Nicolaidou, V.; Papaneophytou, C.; Petrou, C.; Sarigiannis, Y. Short-Chained Linear Scorpion Peptides: A Pool for Novel Antimicrobials. Antibiotic 2024, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Almaaytah, A.; Albalas, Q. Scorpion venom peptides with no disulfide bridges: A review. Peptides 2014, 51, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.C.; Corzo, G.; Hahin, R. Scorpion venom peptides without disulfide bridges. IUBMB Life 2005, 57, 13–21. [Google Scholar] [CrossRef]
- Zeng, X.C.; Luo, F.; Li, W.X. Molecular dissection of venom from Chinese scorpion Mesobuthus martensii: Identification and characterization of four novel disulfide-bridged venom peptides. Peptides 2006, 27, 1745–1754. [Google Scholar] [CrossRef]
- Zhijian, C.; Feng, L.; Yingliang, W.; Xin, M.; Wenxin, L. Genetic mechanisms of scorpion venom peptide diversification. Toxicon 2006, 47, 348–355. [Google Scholar] [CrossRef]
- Tyler, T.J.; Durek, T.; Craik, D.J. Native and Engineered Cyclic Disulfide-Rich Peptides as Drug Leads. Molecules 2023, 28, 3189. [Google Scholar] [CrossRef]
- Rodríguez de la Vega, R.C.; Schwartz, E.F.; Possani, L.D. Mining on scorpion venom biodiversity. Toxicon 2010, 56, 1155–1161. [Google Scholar] [CrossRef]
- Nascimento, D.G.; Rates, B.; Santos, D.M.; Verano-Braga, T.; Barbosa-Silva, A.; Dutra, A.A.; Biondi, I.; Martin-Eauclaire, M.F.; De Lima, M.E.; Pimenta, A.M. Moving pieces in a taxonomic puzzle: Venom 2D-LC/MS and data clustering analyses to infer phylogenetic relationships in some scorpions from the Buthidae family (Scorpiones). Toxicon 2006, 47, 628–639. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J.; Chen, Y. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef]
- Jiménez-Vargas, J.M.; Ramírez-Carreto, S.; Corzo, G.; Possani, L.D.; Becerril, B.; Ortiz, E. Structural and functional characterization of NDBP-4 family antimicrobial peptides from the scorpion Mesomexovis variegatus. Peptides 2021, 141, 170553. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Villegas, E.; Montoya-Rosales, A.; Rivas-Santiago, B.; Corzo, G. Characterization of antibacterial and hemolytic activity of synthetic pandinin 2 variants and their inhibition against Mycobacterium tuberculosis. PLoS ONE 2014, 9, e101742. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.C.; Wang, S.X.; Zhu, Y.; Zhu, S.Y.; Li, W.X. Identification and functional characterization of novel scorpion venom peptides with no disulfide bridge from Buthus martensii Karsch. Peptides 2004, 25, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Corzo, G.; Naoki, H.; Andriantsiferana, M.; Nakajima, T. Purification, structure-function analysis, and molecular characterization of novel linear peptides from scorpion Opisthacanthus madagascariensis. Biochem. Biophys. Res. Commun. 2002, 293, 1514–1522. [Google Scholar] [CrossRef]
- Gao, B.; Xu, J.; Rodriguez Mdel, C.; Lanz-Mendoza, H.; Hernández-Rivas, R.; Du, W.; Zhu, S. Characterization of two linear cationic antimalarial peptides in the scorpion Mesobuthus eupeus. Biochimie 2010, 92, 350–359. [Google Scholar] [CrossRef]
- Almaaytah, A.; Zhou, M.; Wang, L.; Chen, T.; Walker, B.; Shaw, C. Antimicrobial/cytolytic peptides from the venom of the North African scorpion, Androctonus amoreuxi: Biochemical and functional characterization of natural peptides and a single site-substituted analog. Peptides 2012, 35, 291–299. [Google Scholar] [CrossRef]
- Miyashita, M.; Sakai, A.; Matsushita, N.; Hanai, Y.; Nakagawa, Y.; Miyagawa, H. A novel amphipathic linear peptide with both insect toxicity and antimicrobial activity from the venom of the scorpion Isometrus maculatus. Biosci. Biotechnol. Biochem. 2010, 74, 364–369. [Google Scholar] [CrossRef]
- Nie, Y.; Zeng, X.C.; Yang, Y.; Luo, F.; Luo, X.; Wu, S.; Zhang, L.; Zhou, J. A novel class of antimicrobial peptides from the scorpion Heterometrus spinifer. Peptides 2012, 38, 389–394. [Google Scholar] [CrossRef]
- Mabunda, I.G.; Offor, B.C.; Muller, B.; Motadi, L.R.; Piater, L.A. Scorpion venoms from the Buthidae family: A dual study of proteomic composition and anticancer potentials. Toxicon 2025, 266, 108542. [Google Scholar] [CrossRef]
- Moerman, L.; Bosteels, S.; Noppe, W.; Willems, J.; Clynen, E.; Schoofs, L.; Thevissen, K.; Tytgat, J.; Van Eldere, J.; Van Der Walt, J.; et al. Antibacterial and antifungal properties of alpha-helical, cationic peptides in the venom of scorpions from southern Africa. Eur. J. Biochem. 2002, 269, 4799–4810. [Google Scholar] [CrossRef]
- Torres-Larios, A.; Gurrola, G.B.; Zamudio, F.Z.; Possani, L.D. Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus. Eur. J. Biochem. 2000, 267, 5023–5031. [Google Scholar] [CrossRef] [PubMed]
- Corzo, G.; Escoubas, P.; Villegas, E.; Barnham, K.J.; He, W.; Norton, R.S.; Nakajima, T. Characterization of unique amphipathic antimicrobial peptides from venom of the scorpion Pandinus imperator. Biochem. J. 2001, 359, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Remijsen, Q.; Verdonck, F.; Willems, J. Parabutoporin, a cationic amphipathic peptide from scorpion venom: Much more than an antibiotic. Toxicon 2010, 55, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, Z.; Zhang, R.; Wu, Y.; Li, W.; Cao, Z. StCT2, a new antibacterial peptide characterized from the venom of the scorpion Scorpiops tibetanus. Peptides 2012, 36, 213–220. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, Y.; Dai, C.; Zhao, R.; Li, S.; Wu, Y.; Cao, Z.; Li, W. Imcroporin, a new cationic antimicrobial peptide from the venom of the scorpion Isometrus maculates. Antimicrob. Agents Chemother. 2009, 53, 3472–3477. [Google Scholar] [CrossRef]
- Pucca, M.B.; Cerni, F.A.; Pinheiro-Junior, E.L.; Zoccal, K.F.; Bordon, K.C.F.; Amorim, F.G.; Peigneur, S.; Vriens, K.; Thevissen, K.; Cammue, B.P.A.; et al. Non-disulfide-bridged peptides from Tityus serrulatus venom: Evidence for proline-free ACE-inhibitors. Peptides 2016, 82, 44–51. [Google Scholar] [CrossRef]
- Gao, B.; Tian, C.; Zhu, S. Inducible antibacterial response of scorpion venom gland. Peptides 2007, 28, 2299–2305. [Google Scholar] [CrossRef]
- Xia, Z.; Wang, H.; Chen, W.; Wang, A.; Cao, Z. Scorpion Venom Antimicrobial Peptide Derivative BmKn2-T5 Inhibits Enterovirus 71 in the Early Stages of the Viral Life Cycle In Vitro. Biomolecules 2024, 14, 545. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, L.; Zhong, M.; Zhang, Y.; Han, C.; Li, Q.; Yang, J.; Zhou, D.; Shi, W.; He, B.; et al. Anti-HIV-1 activity of a new scorpion venom peptide derivative Kn2-7. PLoS ONE 2012, 7, e34947. [Google Scholar] [CrossRef]
- Zeng, X.C.; Wang, S.; Nie, Y.; Zhang, L.; Luo, X. Characterization of BmKbpp, a multifunctional peptide from the Chinese scorpion Mesobuthus martensii Karsch: Gaining insight into a new mechanism for the functional diversification of scorpion venom peptides. Peptides 2012, 33, 44–51. [Google Scholar] [CrossRef]
- de la Salud Bea, R.; Petraglia, A.F.; Ascuitto, M.R.; Buck, Q.M. Antibacterial Activity and Toxicity of Analogs of Scorpion Venom IsCT Peptides. Antibiotics 2017, 6, 13. [Google Scholar] [CrossRef]
- Almaaytah, A.; Tarazi, S.; Alsheyab, F.; Al-Balas, Q.; Mukattash, T. Antimicrobial and Antibiofilm Activity of Mauriporin, a Multifunctional Scorpion Venom Peptide. Int. J. Pept. Res. Ther. 2014, 20, 397–408. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Soon, T.N. 44P Investigation of scorpion venom-derived anticancer peptides inhibition of metastatic cancer cells growth and induction of apoptosis. Ann. Oncol. 2021, 32, S18. [Google Scholar] [CrossRef]
- Yuan, W.; Cao, L.; Ma, Y.; Mao, P.; Wang, W.; Zhao, R.; Wu, Y.; Cao, Z.; Li, W. Cloning and functional characterization of a new antimicrobial peptide gene StCT1 from the venom of the scorpion Scorpiops tibetanus. Peptides 2010, 31, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, J.; Chai, J.; Gao, Y.; Abdel-Rahman, M.A.; Xu, X. Scorpion Peptide Smp24 Exhibits a Potent Antitumor Effect on Human Lung Cancer Cells by Damaging the Membrane and Cytoskeleton In Vivo and In Vitro. Toxins 2022, 14, 438. [Google Scholar] [CrossRef]
- Nguyen, T.; Guo, R.; Chai, J.; Wu, J.; Liu, J.; Chen, X.; Abdel-Rahman, M.A.; Xia, H.; Xu, X. Smp24, a Scorpion-Venom Peptide, Exhibits Potent Antitumor Effects against Hepatoma HepG2 Cells via Multi-Mechanisms In Vivo and In Vitro. Toxins 2022, 14, 717. [Google Scholar] [CrossRef]
- Rawson, K.M.; Lacey, M.M.; Strong, P.N.; Miller, K. Improving the Therapeutic Index of Smp24, a Venom-Derived Antimicrobial Peptide: Increased Activity against Gram-Negative Bacteria. Int. J. Mol. Sci. 2022, 23, 7979. [Google Scholar] [CrossRef]
- Ferreira, L.A.; Alves, E.W.; Henriques, O.B. Peptide T, a novel bradykinin potentiator isolated from Tityus serrulatus scorpion venom. Toxicon 1993, 31, 941–947. [Google Scholar] [CrossRef]
- Duzzi, B.; Silva, C.C.F.; Kodama, R.T.; Cajado-Carvalho, D.; Squaiella-Baptistão, C.C.; Portaro, F.C.V. New Insights into the Hypotensins from Tityus serrulatus Venom: Pro-Inflammatory and Vasopeptidases Modulation Activities. Toxins 2021, 13, 846. [Google Scholar] [CrossRef]
- Meki, A.R.; Nassar, A.Y.; Rochat, H. A bradykinin-potentiating peptide (peptide K12) isolated from the venom of Egyptian scorpion Buthus occitanus. Peptides 1995, 16, 1359–1365. [Google Scholar] [CrossRef]
- Hong, W.; Zhang, R.; Di, Z.; He, Y.; Zhao, Z.; Hu, J.; Wu, Y.; Li, W.; Cao, Z. Design of histidine-rich peptides with enhanced bioavailability and inhibitory activity against hepatitis C virus. Biomaterials 2013, 34, 3511–3522. [Google Scholar] [CrossRef]
- Yan, R.; Zhao, Z.; He, Y.; Wu, L.; Cai, D.; Hong, W.; Wu, Y.; Cao, Z.; Zheng, C.; Li, W. A new natural α-helical peptide from the venom of the scorpion Heterometrus petersii kills HCV. Peptides 2011, 32, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Li, T.; Song, Y.; Zhang, R.; Zeng, Z.; Han, S.; Zhang, X.; Wu, Y.; Li, W.; Cao, Z. Inhibitory activity and mechanism of two scorpion venom peptides against herpes simplex virus type 1. Antivir. Res. 2014, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhang, R.; Hong, W.; Cheng, Y.; Wang, H.; Lang, Y.; Ji, Z.; Wu, Y.; Li, W.; Xie, Y.; et al. Histidine-rich Modification of a Scorpion-derived Peptide Improves Bioavailability and Inhibitory Activity against HSV-1. Theranostics 2018, 8, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Kovařík, F.J.E. Taxonomic reassessment of the genera Lychas, Mesobuthus, and Olivierus, with descriptions of four new genera (Scorpiones: Buthidae). Euscorpius 2019, 2019, 1–27. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42, fux053. [Google Scholar] [CrossRef]
- Rincón-Cortés, C.A.; Bayona-Rojas, M.A.; Reyes-Montaño, E.A.; Vega-Castro, N.A. Antimicrobial Activity Developed by Scorpion Venoms and Its Peptide Component. Toxins 2022, 14, 740. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Z.; Wang, W.; Zhao, Y.; Yin, X.; Meng, Y.; Zhao, P.; Wang, M.; Liu, X.; Wang, X.; et al. Development of Antibacterial Peptides with Membrane Disruption and Folate Pathway Inhibitory Activities against Methicillin-Resistant Staphylococcus aureus. J. Med. Chem. 2024, 67, 1044–1060. [Google Scholar] [CrossRef]
- Fan, Z.; Cao, L.; He, Y.; Hu, J.; Di, Z.; Wu, Y.; Li, W.; Cao, Z. Ctriporin, a new anti-methicillin-resistant Staphylococcus aureus peptide from the venom of the scorpion Chaerilus tricostatus. Antimicrob. Agents Chemother. 2011, 55, 5220–5229. [Google Scholar] [CrossRef]
- Zeng, X.C.; Zhou, L.; Shi, W.; Luo, X.; Zhang, L.; Nie, Y.; Wang, J.; Wu, S.; Cao, B.; Cao, H. Three new antimicrobial peptides from the scorpion Pandinus imperator. Peptides 2013, 45, 28–34. [Google Scholar] [CrossRef]
- Ramírez-Carreto, S.; Quintero-Hernández, V.; Jiménez-Vargas, J.M.; Corzo, G.; Possani, L.D.; Becerril, B.; Ortiz, E. Gene cloning and functional characterization of four novel antimicrobial-like peptides from scorpions of the family Vaejovidae. Peptides 2012, 34, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Khamessi, O.; Ben Mabrouk, H.; ElFessi-Magouri, R.; Kharrat, R. RK1, the first very short peptide from Buthus occitanus tunetanus inhibits tumor cell migration, proliferation and angiogenesis. Biochem. Biophys. Res. Commun. 2018, 499, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lyu, P.; Xi, X.; Ge, L.; Mahadevappa, R.; Shaw, C.; Kwok, H.F. Triggering of cancer cell cycle arrest by a novel scorpion venom-derived peptide-Gonearrestide. J. Cell. Mol. Med. 2018, 22, 4460–4473. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, C.; Du, Q.; Wei, R.; Wang, L.; Zhou, M.; Chen, T.; Shaw, C. Two peptides, TsAP-1 and TsAP-2, from the venom of the Brazilian yellow scorpion, Tityus serrulatus: Evaluation of their antimicrobial and anticancer activities. Biochimie 2013, 95, 1784–1794. [Google Scholar] [CrossRef]
- Du, Q.; Hou, X.; Wang, L.; Zhang, Y.; Xi, X.; Wang, H.; Zhou, M.; Duan, J.; Wei, M.; Chen, T.; et al. AaeAP1 and AaeAP2: Novel antimicrobial peptides from the venom of the scorpion, Androctonus aeneas: Structural characterisation, molecular cloning of biosynthetic precursor-encoding cDNAs and engineering of analogues with enhanced antimicrobial and anticancer activities. Toxins 2015, 7, 219–237. [Google Scholar] [CrossRef]
- Satitmanwiwat, S.; Changsangfa, C.; Khanuengthong, A.; Promthep, K.; Roytrakul, S.; Arpornsuwan, T.; Saikhun, K.; Sritanaudomchai, H. The scorpion venom peptide BmKn2 induces apoptosis in cancerous but not in normal human oral cells. Biomed. Pharmacother. 2016, 84, 1042–1050. [Google Scholar] [CrossRef]
- Verano-Braga, T.; Figueiredo-Rezende, F.; Melo, M.N.; Lautner, R.Q.; Gomes, E.R.; Mata-Machado, L.T.; Murari, A.; Rocha-Resende, C.; Elena de Lima, M.; Guatimosim, S.; et al. Structure-function studies of Tityus serrulatus Hypotensin-I (TsHpt-I): A new agonist of B(2) kinin receptor. Toxicon 2010, 56, 1162–1171. [Google Scholar] [CrossRef]
- Riordan, J.F. Angiotensin-I-converting enzyme and its relatives. Genome Biol. 2003, 4, 225. [Google Scholar] [CrossRef]
- Hancock, R.E.; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000, 8, 402–410. [Google Scholar] [CrossRef]
- Possani, L.D.; Merino, E.; Corona, M.; Bolivar, F.; Becerril, B. Peptides and genes coding for scorpion toxins that affect ion-channels. Biochimie 2000, 82, 861–868. [Google Scholar] [CrossRef]
- Saucedo, A.L.; Flores-Solis, D.; Rodríguez de la Vega, R.C.; Ramírez-Cordero, B.; Hernández-López, R.; Cano-Sánchez, P.; Noriega Navarro, R.; García-Valdés, J.; Coronas-Valderrama, F.; de Roodt, A.; et al. New tricks of an old pattern: Structural versatility of scorpion toxins with common cysteine spacing. J. Biol. Chem. 2012, 287, 12321–12330. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.C.; Viana, G.M.M.; Nencioni, A.L.A.; Pimenta, D.C.; Beraldo-Neto, E. Scorpion Peptides and Ion Channels: An Insightful Review of Mechanisms and Drug Development. Toxins 2023, 15, 238. [Google Scholar] [CrossRef] [PubMed]
- Pashmforoosh, N.; Baradaran, M. Peptides with Diverse Functions from Scorpion Venom: A Great Opportunity for the Treatment of a Wide Variety of Diseases. Iran. Biomed. J. 2023, 27, 84–99. [Google Scholar] [CrossRef]
- Bosmans, F.; Tytgat, J. Voltage-gated sodium channel modulation by scorpion alpha-toxins. Toxicon 2007, 49, 142–158. [Google Scholar] [CrossRef]
- Cerni, F.; Oliveira, I.; Cordeiro, F.; Bordon, K.; Ferreira, I.; Monteiro, W.; Arantes, E.; Cunha, T.; Pucca, M. The nociceptive response induced by different classes of Tityus serrulatus neurotoxins: The important role of Ts5 in venom-induced nociception. PLoS Neglected Trop. Dis. 2023, 17, e0011057. [Google Scholar] [CrossRef] [PubMed]
- Alami, M.; Ouafik, L.; Céard, B.; Legros, C.; Bougis, P.E.; Martin-Eauclaire, M.F. Characterisation of the gene encoding the alpha-toxin Amm V from the scorpion Androctonus mauretanicus mauretanicus. Toxicon 2001, 39, 1579–1585. [Google Scholar] [CrossRef]
- Arnon, T.; Potikha, T.; Sher, D.; Elazar, M.; Mao, W.; Tal, T.; Bosmans, F.; Tytgat, J.; Ben-Arie, N.; Zlotkin, E. BjalphaIT: A novel scorpion alpha-toxin selective for insects-unique pharmacological tool. Insect Biochem. Mol. Biol. 2005, 35, 187–195. [Google Scholar] [CrossRef]
- Otvos, L., Jr. Antibacterial peptides and proteins with multiple cellular targets. J. Pept. Sci. 2005, 11, 697–706. [Google Scholar] [CrossRef]
- Rodríguez de la Vega, R.C.; Possani, L.D. Overview of scorpion toxins specific for Na+ channels and related peptides: Biodiversity, structure-function relationships and evolution. Toxicon 2005, 46, 831–844. [Google Scholar] [CrossRef]
- Abbas, F.; Blömer, L.A.; Millet, H.; Montnach, J.; De Waard, M.; Canepari, M. Analysis of the effect of the scorpion toxin AaH-II on action potential generation in the axon initial segment. Sci. Rep. 2024, 14, 4967. [Google Scholar] [CrossRef]
- Loret, E.P.; Mansuelle, P.; Rochat, H.; Granier, C. Neurotoxins active on insects: Amino acid sequences, chemical modifications, and secondary structure estimation by circular dichroism of toxins from the scorpion Androctonus australis Hector. Biochemistry 1990, 29, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, F.; Ergene, E.; Sogut, I.; Hatipoglu, I.; Basalp, A.; Sivas, H.; Kanbak, G. Biological assays on the effects of Acra3 peptide from Turkish scorpion Androctonus crassicauda venom on a mouse brain tumor cell line (BC3H1) and production of specific monoclonal antibodies. Toxicon 2013, 76, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, F.; García, B.I.; Coronas, F.I.; Restano-Cassulini, R.; Korkmaz, F.; Sahin, Y.; Corzo, G.; Possani, L.D. Purification and cDNA cloning of a novel neurotoxic peptide (Acra3) from the scorpion Androctonus crassicauda. Peptides 2012, 37, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Gaudioso-Tyzra, C.; Bonnet, C.; Gabriac, M.; Amsalem, M.; Lonigro, A.; Padilla, F.; Crest, M.; Martin-Eauclaire, M.F.; Delmas, P. The scorpion toxin Amm VIII induces pain hypersensitivity through gain-of-function of TTX-sensitive Na+ channels. Pain 2013, 154, 1204–1215. [Google Scholar] [CrossRef]
- Valdez-Cruz, N.A.; Batista, C.V.; Zamudio, F.Z.; Bosmans, F.; Tytgat, J.; Possani, L.D. Phaiodotoxin, a novel structural class of insect-toxin isolated from the venom of the Mexican scorpion Anuroctonus phaiodactylus. Eur. J. Biochem. 2004, 271, 4753–4761. [Google Scholar] [CrossRef]
- Estrada, G.; Restano-Cassulini, R.; Ortiz, E.; Possani, L.D.; Corzo, G. Addition of positive charges at the C-terminal peptide region of CssII, a mammalian scorpion peptide toxin, improves its affinity for sodium channels Nav1.6. Peptides 2011, 32, 75–79. [Google Scholar] [CrossRef]
- Saucedo, A.L.; del Rio-Portilla, F.; Picco, C.; Estrada, G.; Prestipino, G.; Possani, L.D.; Delepierre, M.; Corzo, G. Solution structure of native and recombinant expressed toxin CssII from the venom of the scorpion Centruroides suffusus suffusus, and their effects on Nav1.5 sodium channels. Biochim. Biophys. Acta 2012, 1824, 478–487. [Google Scholar] [CrossRef]
- Schiavon, E.; Pedraza-Escalona, M.; Gurrola, G.B.; Olamendi-Portugal, T.; Corzo, G.; Wanke, E.; Possani, L.D. Negative-shift activation, current reduction and resurgent currents induced by β-toxins from Centruroides scorpions in sodium channels. Toxicon 2012, 59, 283–293. [Google Scholar] [CrossRef]
- Ramirez-Dominguez, M.E.; Olamendi-Portugal, T.; Garcia, U.; Garcia, C.; Arechiga, H.; Possani, L.D. Cn11, the first example of a scorpion toxin that is a true blocker of Na(+) currents in crayfish neurons. J. Exp. Biol. 2002, 205, 869–876. [Google Scholar] [CrossRef]
- Lin, S.; Wang, X.; Hu, X.; Zhao, Y.; Zhao, M.; Zhang, J.; Cui, Y. Recombinant Expression, Functional Characterization of Two Scorpion Venom Toxins with Three Disulfide Bridges from the Chinese Scorpion Buthus martensii Karsch. Protein Pept. Lett. 2017, 24, 235–240. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zhu, Y.; Zhang, L.; Ji, J.; Gui, M.; Li, C.; Song, Y. Study of Anti-Inflammatory and Analgesic Activity of Scorpion Toxins DKK-SP1/2 from Scorpion Buthus martensii Karsch (BmK). Toxins 2021, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.F.; Bai, Z.T.; Liu, T.; Pang, X.Y.; Ji, Y.H. The binding of BmK IT2 on mammal and insect sodium channels by surface plasmon resonance assay. Pharmacol. Res. 2006, 54, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, S.; Zhang, Y.; Qin, C.; Xu, L.; Li, W.; Cao, Z.; Li, W.; Wu, Y. Expression of recombinant α-toxin BmKM9 from scorpion Buthus martensii Karsch and its functional characterization on sodium channels. Peptides 2018, 99, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Cui, Y.; Zhou, Y.; Bao, Y.M.; Yang, W.Y.; Liu, Y.F.; Wu, C.F.; Zhang, J.H. Location of the analgesic domain in Scorpion toxin BmK AGAP by mutagenesis of disulfide bridges. Biochem. Biophys. Res. Commun. 2010, 394, 330–334. [Google Scholar] [CrossRef]
- Goudet, C.; Huys, I.; Clynen, E.; Schoofs, L.; Wang, D.C.; Waelkens, E.; Tytgat, J. Electrophysiological characterization of BmK M1, an alpha-like toxin from Buthus martensi Karsch venom. FEBS Lett. 2001, 495, 61–65. [Google Scholar] [CrossRef]
- Wang, C.G.; Gilles, N.; Hamon, A.; Le Gall, F.; Stankiewicz, M.; Pelhate, M.; Xiong, Y.M.; Wang, D.C.; Chi, C.W. Exploration of the functional site of a scorpion alpha-like toxin by site-directed mutagenesis. Biochemistry 2003, 42, 4699–4708. [Google Scholar] [CrossRef]
- He, H.; Liu, Z.; Dong, B.; Zhou, J.; Zhu, H.; Ji, Y. Molecular determination of selectivity of the site 3 modulator (BmK I) to sodium channels in the CNS: A clue to the importance of Nav1.6 in BmK I-induced neuronal hyperexcitability. Biochem. J. 2010, 431, 289–298. [Google Scholar] [CrossRef]
- Zhu, M.M.; Tan, M.; Cheng, H.W.; Ji, Y.H. The alpha-like scorpion toxin BmK I enhances membrane excitability via persistent sodium current by preventing slow inactivation and deactivation of rNav1.2a expressed in Xenopus Oocytes. Toxicol. In Vitro 2009, 23, 561–568. [Google Scholar] [CrossRef]
- Tan, M.; Zhu, M.M.; Liu, Y.; Cheng, H.W.; Ji, Y.H. Effects of BmK AS on Nav1.2 expressed in Xenopus laevis oocytes. Cell Biol. Toxicol. 2008, 24, 143–149. [Google Scholar] [CrossRef]
- Liu, Z.R.; Zhang, H.; Wu, J.Q.; Zhou, J.J.; Ji, Y.H. PKA phosphorylation reshapes the pharmacological kinetics of BmK AS, a unique site-4 sodium channel-specific modulator. Sci. Rep. 2014, 4, 3721. [Google Scholar] [CrossRef]
- Chen, J.; Feng, X.H.; Shi, J.; Tan, Z.Y.; Bai, Z.T.; Liu, T.; Ji, Y.H. The anti-nociceptive effect of BmK AS, a scorpion active polypeptide, and the possible mechanism on specifically modulating voltage-gated Na+ currents in primary afferent neurons. Peptides 2006, 27, 2182–2192. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Song, Y.; Cao, Y.; Ban, M.; Zhang, Z.; Sun, Y.; Feng, Y.; Li, C. Scorpion Neurotoxin Syb-prII-1 Exerts Analgesic Effect through Nav1.8 Channel and MAPKs Pathway. Int. J. Mol. Sci. 2022, 23, 7065. [Google Scholar] [CrossRef] [PubMed]
- Martin-Eauclaire, M.F.; Abbas, N.; Sauze, N.; Mercier, L.; Berge-Lefranc, J.L.; Condo, J.; Bougis, P.E.; Guieu, R. Involvement of endogenous opioid system in scorpion toxin-induced antinociception in mice. Neurosci. Lett. 2010, 482, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Sautière, P.; Cestèle, S.; Kopeyan, C.; Martinage, A.; Drobecq, H.; Doljansky, Y.; Gordon, D. New toxins acting on sodium channels from the scorpion Leiurus quinquestriatus hebraeus suggest a clue to mammalian vs. insect selectivity. Toxicon 1998, 36, 1141–1154. [Google Scholar] [CrossRef]
- Karbat, I.; Kahn, R.; Cohen, L.; Ilan, N.; Gilles, N.; Corzo, G.; Froy, O.; Gur, M.; Albrecht, G.; Heinemann, S.H.; et al. The unique pharmacology of the scorpion alpha-like toxin Lqh3 is associated with its flexible C-tail. FEBS J. 2007, 274, 1918–1931. [Google Scholar] [CrossRef]
- Jinn, T.R.; Tu, W.C.; Lu, C.I.; Tzen, J.T. Enhancing insecticidal efficacy of baculovirus by early expressing an insect neurotoxin, LqhIT2, in infected Trichoplusia ni larvae. Appl. Microbiol. Biotechnol. 2006, 72, 1247–1253. [Google Scholar] [CrossRef]
- Maatoug, R.; Jebali, J.; Guieu, R.; De Waard, M.; Kharrat, R. BotAF, a new Buthus occitanus tunetanus scorpion toxin, produces potent analgesia in rodents. Toxicon 2018, 149, 72–85. [Google Scholar] [CrossRef]
- Chow, C.Y.; Chin, Y.K.; Walker, A.A.; Guo, S.; Blomster, L.V.; Ward, M.J.; Herzig, V.; Rokyta, D.R.; King, G.F. Venom Peptides with Dual Modulatory Activity on the Voltage-Gated Sodium Channel Na(V)1.1 Provide Novel Leads for Development of Antiepileptic Drugs. ACS Pharmacol. Transl. Sci. 2020, 3, 119–134. [Google Scholar] [CrossRef]
- Karbat, I.; Cohen, L.; Gilles, N.; Gordon, D.; Gurevitz, M. Conversion of a scorpion toxin agonist into an antagonist highlights an acidic residue involved in voltage sensor trapping during activation of neuronal Na+ channels. FASEB J. 2004, 18, 683–689. [Google Scholar] [CrossRef]
- Oren, D.A.; Froy, O.; Amit, E.; Kleinberger-Doron, N.; Gurevitz, M.; Shaanan, B. An excitatory scorpion toxin with a distinctive feature: An additional alpha helix at the C terminus and its implications for interaction with insect sodium channels. Structure 1998, 6, 1095–1103. [Google Scholar] [CrossRef]
- Cohen, L.; Lipstein, N.; Karbat, I.; Ilan, N.; Gilles, N.; Kahn, R.; Gordon, D.; Gurevitz, M. Miniaturization of scorpion beta-toxins uncovers a putative ancestral surface of interaction with voltage-gated sodium channels. J. Biol. Chem. 2008, 283, 15169–15176. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F.K.; Bochi, G.V.; Pereira, A.L.; Adamante, G.; Ferro, P.R.; Dal-Toé De Prá, S.; Milioli, A.M.; Damiani, A.P.; da Silveira Prestes, G.; Dalenogare, D.P.; et al. TsNTxP, a non-toxic protein from Tityus serrulatus scorpion venom, induces antinociceptive effects by suppressing glutamate release in mice. Eur. J. Pharmacol. 2019, 855, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Dang, B.; Kubota, T.; Correa, A.M.; Bezanilla, F.; Kent, S.B. Total chemical synthesis of biologically active fluorescent dye-labeled Ts1 toxin. Angew. Chem. Int. Ed. 2014, 53, 8970–8974. [Google Scholar] [CrossRef] [PubMed]
- Tibery, D.V.; Nunes, J.A.A.; da Mata, D.O.; Menezes, L.F.S.; de Souza, A.C.B.; Fernandes-Pedrosa, M.F.; Treptow, W.; Schwartz, E.F. Unveiling Tst3, a Multi-Target Gating Modifier Scorpion α Toxin from Tityus stigmurus Venom of Northeast Brazil: Evaluation and Comparison with Well-Studied Ts3 Toxin of Tityus serrulatus. Toxins 2024, 16, 257. [Google Scholar] [CrossRef]
- Borges, A.; Alfonzo, M.J.; García, C.C.; Winand, N.J.; Leipold, E.; Heinemann, S.H. Isolation, molecular cloning and functional characterization of a novel beta-toxin from the Venezuelan scorpion, Tityus zulianus. Toxicon 2004, 43, 671–684. [Google Scholar] [CrossRef]
- Leipold, E.; Hansel, A.; Borges, A.; Heinemann, S.H. Subtype specificity of scorpion beta-toxin Tz1 interaction with voltage-gated sodium channels is determined by the pore loop of domain 3. Mol. Pharmacol. 2006, 70, 340–347. [Google Scholar] [CrossRef]
- Sunagar, K.; Undheim, E.A.; Chan, A.H.; Koludarov, I.; Muñoz-Gómez, S.A.; Antunes, A.; Fry, B.G. Evolution stings: The origin and diversification of scorpion toxin peptide scaffolds. Toxins 2013, 5, 2456–2487. [Google Scholar] [CrossRef]
- Couraud, F.; Jover, E.; Dubois, J.M.; Rochat, H. Two types of scorpion receptor sites, one related to the activation, the other to the inactivation of the action potential sodium channel. Toxicon 1982, 20, 9–16. [Google Scholar] [CrossRef]
- Rogers, J.C.; Qu, Y.; Tanada, T.N.; Scheuer, T.; Catterall, W.A. Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3–S4 extracellular loop in domain IV of the Na+ channel alpha subunit. J. Biol. Chem. 1996, 271, 15950–15962. [Google Scholar] [CrossRef]
- Catterall, W.A.; Cestèle, S.; Yarov-Yarovoy, V.; Yu, F.H.; Konoki, K.; Scheuer, T. Voltage-gated ion channels and gating modifier toxins. Toxicon 2007, 49, 124–141. [Google Scholar] [CrossRef]
- Stevens, M.; Peigneur, S.; Tytgat, J. Neurotoxins and their binding areas on voltage-gated sodium channels. Front. Pharmacol. 2011, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Silva, J.R.; Lu, X.; Luo, L.; Wang, Y.; Xu, L.; Aierken, A.; Shynykul, Z.; Kamau, P.M.; Luo, A.; et al. Molecular game theory for a toxin-dominant food chain model. Natl. Sci. Rev. 2019, 6, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Jiao, Y.; Li, Z.; Hua, L.; Fu, J.; Jiang, F.; Liu, T.; Ji, Y. Scorpion toxin BmK I directly activates Nav1.8 in primary sensory neurons to induce neuronal hyperexcitability in rats. Protein Cell 2015, 6, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Martin-Eauclaire, M.F.; Cestèle, S.; Kopeyan, C.; Carlier, E.; Khalifa, R.B.; Pelhate, M.; Rochat, H. Scorpion toxins affecting sodium current inactivation bind to distinct homologous receptor sites on rat brain and insect sodium channels. J. Biol. Chem. 1996, 271, 8034–8045. [Google Scholar] [CrossRef]
- Leipold, E.; Lu, S.; Gordon, D.; Hansel, A.; Heinemann, S.H. Combinatorial interaction of scorpion toxins Lqh-2, Lqh-3, and LqhalphaIT with sodium channel receptor sites-3. Mol. Pharmacol. 2004, 65, 685–691. [Google Scholar] [CrossRef]
- Leipold, E.; Hansel, A.; Olivera, B.M.; Terlau, H.; Heinemann, S.H. Molecular interaction of delta-conotoxins with voltage-gated sodium channels. FEBS Lett. 2005, 579, 3881–3884. [Google Scholar] [CrossRef]
- Chen, R.; Chung, S.H. Conserved functional surface of antimammalian scorpion β-toxins. J. Phys. Chem. B 2012, 116, 4796–4800. [Google Scholar] [CrossRef]
- Cestèle, S.; Catterall, W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie 2000, 82, 883–892. [Google Scholar] [CrossRef]
- Cestèle, S.; Yarov-Yarovoy, V.; Qu, Y.; Sampieri, F.; Scheuer, T.; Catterall, W.A. Structure and function of the voltage sensor of sodium channels probed by a beta-scorpion toxin. J. Biol. Chem. 2006, 281, 21332–21344. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, J.; Liu, H.; Sun, J.; Yu, Y.; Su, Y.; Cui, Y.; Zhao, M.; Zhang, J. Scorpion Toxins Targeting Voltage-gated Sodium Channels Associated with Pain. Curr. Pharm. Biotechnol. 2018, 19, 848–855. [Google Scholar] [CrossRef]
- Lu, W.; Cheng, X.; Chen, J.; Wang, M.; Chen, Y.; Liu, J.; Sang, M.; Zhao, N.; Yan, H.; Cheng, X.; et al. A Buthus martensii Karsch scorpion sting targets Nav1.7 in mice and mimics a phenotype of human chronic pain. Pain 2022, 163, e202–e214. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Hua, L.; Jiao, Y.; Li, Z.; Qin, S.; Fu, J.; Jiang, F.; Liu, T.; Ji, Y. Functional up-regulation of Nav1.8 sodium channel on dorsal root ganglia neurons contributes to the induction of scorpion sting pain. Acta Biochim. Biophys. Sin. 2016, 48, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Wang, C.G.; Wang, M.; Wang, D.C. A depressant insect toxin with a novel analgesic effect from scorpion Buthus martensii Karsch. Biochim. Biophys. Acta 2001, 1549, 9–18. [Google Scholar] [CrossRef]
- Xiong, Y.M.; Lan, Z.D.; Wang, M.; Liu, B.; Liu, X.Q.; Fei, H.; Xu, L.G.; Xia, Q.C.; Wang, C.G.; Wang, D.C.; et al. Molecular characterization of a new excitatory insect neurotoxin with an analgesic effect on mice from the scorpion Buthus martensi Karsch. Toxicon 1999, 37, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Gradek, F.; Lopez-Charcas, O.; Chadet, S.; Poisson, L.; Ouldamer, L.; Goupille, C.; Jourdan, M.L.; Chevalier, S.; Moussata, D.; Besson, P.; et al. Sodium Channel Na(v)1.5 Controls Epithelial-to-Mesenchymal Transition and Invasiveness in Breast Cancer Cells Through its Regulation by the Salt-Inducible Kinase-1. Sci. Rep. 2019, 9, 18652. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A.; Fraser, S.P.; Brackenbury, W.J. In Vivo Evidence for Voltage-Gated Sodium Channel Expression in Carcinomas and Potentiation of Metastasis. Cancers 2019, 11, 1675. [Google Scholar] [CrossRef]
- Fraser, S.P.; Diss, J.K.; Chioni, A.M.; Mycielska, M.E.; Pan, H.; Yamaci, R.F.; Pani, F.; Siwy, Z.; Krasowska, M.; Grzywna, Z.; et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin. Cancer Res. 2005, 11, 5381–5389. [Google Scholar] [CrossRef]
- Liu, Y.F.; Hu, J.; Zhang, J.H.; Wang, S.L.; Wu, C.F. Isolation, purification, and N-terminal partial sequence of an antitumor peptide from the venom of the Chinese scorpion Buthus martensii Karsch. Prep. Biochem. Biotechnol. 2002, 32, 317–327. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Hoffmann, E.K.; Novak, I. Cell volume regulation in epithelial physiology and cancer. Front. Physiol. 2013, 4, 233. [Google Scholar] [CrossRef]
- Girault, A.; Brochiero, E. Evidence of K+ channel function in epithelial cell migration, proliferation, and repair. Am. J. Physiol. Cell Physiol. 2014, 306, C307–C319. [Google Scholar] [CrossRef]
- Oliveira, I.S.; Ferreira, I.G.; Alexandre-Silva, G.M.; Cerni, F.A.; Cremonez, C.M.; Arantes, E.C.; Zottich, U.; Pucca, M.B. Scorpion toxins targeting Kv1.3 channels: Insights into immunosuppression. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e148118. [Google Scholar] [CrossRef] [PubMed]
- Chandy, K.G.; Wulff, H.; Beeton, C.; Pennington, M.; Gutman, G.A.; Cahalan, M.D. K+ channels as targets for specific immunomodulation. Trends Pharmacol. Sci. 2004, 25, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez de la Vega, R.C.; Possani, L.D. Current views on scorpion toxins specific for K+-channels. Toxicon 2004, 43, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, Z.L.; Bingham, J.P. Scorpion toxins specific for potassium (K+) channels: A historical overview of peptide bioengineering. Toxins 2012, 4, 1082–1119. [Google Scholar] [CrossRef]
- Sitges, M.; Possani, L.D.; Bayón, A. Noxiustoxin, a short-chain toxin from the Mexican scorpion Centruroides noxius, induces transmitter release by blocking K+ permeability. J. Neurosci. 1986, 6, 1570–1574. [Google Scholar] [CrossRef]
- Nieto, A.R.; Gurrola, G.B.; Vaca, L.; Possani, L.D. Noxiustoxin 2, a novel K+ channel blocking peptide from the venom of the scorpion Centruroides noxius Hoffmann. Toxicon 1996, 34, 913–922. [Google Scholar] [CrossRef]
- Miller, C. The charybdotoxin family of K+ channel-blocking peptides. Neuron 1995, 15, 5–10. [Google Scholar] [CrossRef]
- Miller, C.; Moczydlowski, E.; Latorre, R.; Phillips, M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature 1985, 313, 316–318. [Google Scholar] [CrossRef]
- Ahmadi, S.; Knerr, J.M.; Argemi, L.; Bordon, K.C.F.; Pucca, M.B.; Cerni, F.A.; Arantes, E.C.; Çalışkan, F.; Laustsen, A.H. Scorpion Venom: Detriments and Benefits. Biomedicines 2020, 8, 118. [Google Scholar] [CrossRef]
- Cremonez, C.M.; Maiti, M.; Peigneur, S.; Cassoli, J.S.; Dutra, A.A.; Waelkens, E.; Lescrinier, E.; Herdewijn, P.; de Lima, M.E.; Pimenta, A.M.; et al. Structural and Functional Elucidation of Peptide Ts11 Shows Evidence of a Novel Subfamily of Scorpion Venom Toxins. Toxins 2016, 8, 288. [Google Scholar] [CrossRef]
- Andreotti, N.; di Luccio, E.; Sampieri, F.; De Waard, M.; Sabatier, J.M. Molecular modeling and docking simulations of scorpion toxins and related analogs on human SKCa2 and SKCa3 channels. Peptides 2005, 26, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Peigneur, S.; Gao, B.; Luo, L.; Jin, D.; Zhao, Y.; Tytgat, J. Molecular diversity and functional evolution of scorpion potassium channel toxins. Mol. Cell. Proteom. 2011, 10, M110.002832. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenkov, A.I.; Vassilevski, A.A.; Kudryashova, K.S.; Nekrasova, O.V.; Peigneur, S.; Tytgat, J.; Feofanov, A.V.; Kirpichnikov, M.P.; Grishin, E.V. Variability of Potassium Channel Blockers in Mesobuthus eupeus Scorpion Venom with Focus on Kv1.1: An integrated transcriptomic and proteomic study. J. Biol. Chem. 2015, 290, 12195–12209. [Google Scholar] [CrossRef] [PubMed]
- Landoulsi, Z.; Miceli, F.; Palmese, A.; Amoresano, A.; Marino, G.; El Ayeb, M.; Taglialatela, M.; Benkhalifa, R. Subtype-selective activation of K(v)7 channels by AaTXKβ2–64, a novel toxin variant from the Androctonus australis scorpion venom. Mol. Pharmacol. 2013, 84, 763–773. [Google Scholar] [CrossRef]
- Pardo-López, L.; García-Valdés, J.; Gurrola, G.B.; Robertson, G.A.; Possani, L.D. Mapping the receptor site for ergtoxin, a specific blocker of ERG channels. FEBS Lett. 2002, 510, 45–49. [Google Scholar] [CrossRef]
- Pardo-Lopez, L.; Zhang, M.; Liu, J.; Jiang, M.; Possani, L.D.; Tseng, G.N. Mapping the binding site of a human ether-a-go-go-related gene-specific peptide toxin (ErgTx) to the channel’s outer vestibule. J. Biol. Chem. 2002, 277, 16403–16411. [Google Scholar] [CrossRef]
- Restano-Cassulini, R.; Korolkova, Y.V.; Diochot, S.; Gurrola, G.; Guasti, L.; Possani, L.D.; Lazdunski, M.; Grishin, E.V.; Arcangeli, A.; Wanke, E. Species diversity and peptide toxins blocking selectivity of ether-a-go-go-related gene subfamily K+ channels in the central nervous system. Mol. Pharmacol. 2006, 69, 1673–1683. [Google Scholar] [CrossRef]
- Jimenez-Vargas, J.M.; Restano-Cassulini, R.; Quintero-Hernández, V.; Gurrola, G.B.; Possani, L.D. Recombinant expression of the toxic peptide ErgTx1 and role of Met35 on its stability and function. Peptides 2011, 32, 560–567. [Google Scholar] [CrossRef]
- Schwartz, A.B.; Kapur, A.; Wang, W.; Huang, Z.; Fardone, E.; Palui, G.; Mattoussi, H.; Fadool, D.A. Margatoxin-bound quantum dots as a novel inhibitor of the voltage-gated ion channel Kv1.3. J. Neurochem. 2017, 140, 404–420. [Google Scholar] [CrossRef]
- Wu, B.M.; Liu, J.D.; Li, Y.H.; Li, J. Margatoxin mitigates CCl4-induced hepatic fibrosis in mice via macrophage polarization, cytokine secretion and STAT signaling. Int. J. Mol. Med. 2020, 45, 103–114. [Google Scholar] [CrossRef]
- Nastainczyk, W.; Meves, H.; Watt, D.D. A short-chain peptide toxin isolated from Centruroides sculpturatus scorpion venom inhibits ether-à-go-go-related gene K(+) channels. Toxicon 2002, 40, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Olamendi-Portugal, T.; Bartok, A.; Zamudio-Zuñiga, F.; Balajthy, A.; Becerril, B.; Panyi, G.; Possani, L.D. Isolation, chemical and functional characterization of several new K(+)-channel blocking peptides from the venom of the scorpion Centruroides tecomanus. Toxicon 2016, 115, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.N.; Sivaraja, V.; Huys, I.; Sasaki, T.; Cheng, B.; Kumar, T.K.; Sato, K.; Tytgat, J.; Yu, C.; San, B.C.; et al. kappa-Hefutoxin1, a novel toxin from the scorpion Heterometrus fulvipes with unique structure and function. Importance of the functional diad in potassium channel selectivity. J. Biol. Chem. 2002, 277, 30040–30047. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Yamaguchi, Y.; Goto, H.; Srinivasan, K.N.; Gopalakrishnakone, P.; Tytgat, J.; Sato, K. Synthesis and characterization of amino acid deletion analogs of κ-hefutoxin 1, a scorpion toxin on potassium channels. Toxicon 2013, 71, 25–30. [Google Scholar] [CrossRef]
- Moreels, L.; Peigneur, S.; Yamaguchi, Y.; Vriens, K.; Waelkens, E.; Zhu, S.; Thevissen, K.; Cammue, B.P.A.; Sato, K.; Tytgat, J. Expanding the pharmacological profile of κ-hefutoxin 1 and analogues: A focus on the inhibitory effect on the oncogenic channel K(v)10.1. Peptides 2017, 98, 43–50. [Google Scholar] [CrossRef]
- Khabiri, M.; Nikouee, A.; Cwiklik, L.; Grissmer, S.; Ettrich, R. Charybdotoxin unbinding from the mKv1.3 potassium channel: A combined computational and experimental study. J. Phys. Chem. B 2011, 115, 11490–11500. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, F.; Feng, J.; Yang, W.; Zeng, D.; Zhao, R.; Cao, Z.; Liu, M.; Li, W.; Jiang, L.; et al. Genomic and structural characterization of Kunitz-type peptide LmKTT-1a highlights diversity and evolution of scorpion potassium channel toxins. PLoS ONE 2013, 8, e60201. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Hu, Y.T.; Yang, W.S.; He, Y.W.; Feng, J.; Wang, B.; Zhao, R.M.; Ding, J.P.; Cao, Z.J.; Li, W.X.; et al. Hg1, novel peptide inhibitor specific for Kv1.3 channels from first scorpion Kunitz-type potassium channel toxin family. J. Biol. Chem. 2012, 287, 13813–13821. [Google Scholar] [CrossRef]
- Camargos, T.S.; Restano-Cassulini, R.; Possani, L.D.; Peigneur, S.; Tytgat, J.; Schwartz, C.A.; Alves, E.M.; de Freitas, S.M.; Schwartz, E.F. The new kappa-KTx 2.5 from the scorpion Opisthacanthus cayaporum. Peptides 2011, 32, 1509–1517. [Google Scholar] [CrossRef]
- Gao, B.; Harvey, P.J.; Craik, D.J.; Ronjat, M.; De Waard, M.; Zhu, S. Functional evolution of scorpion venom peptides with an inhibitor cystine knot fold. Biosci. Rep. 2013, 33, e00047. [Google Scholar] [CrossRef]
- Korolkova, Y.V.; Kozlov, S.A.; Lipkin, A.V.; Pluzhnikov, K.A.; Hadley, J.K.; Filippov, A.K.; Brown, D.A.; Angelo, K.; Strøbaek, D.; Jespersen, T.; et al. An ERG channel inhibitor from the scorpion Buthus eupeus. J. Biol. Chem. 2001, 276, 9868–9876. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Fang, M.; Gao, B.; Chui, R.W.; Vargas, H.M. BeKm-1, a peptide inhibitor of human ether-a-go-go-related gene potassium currents, prolongs QTc intervals in isolated rabbit heart. J. Pharmacol. Exp. Ther. 2011, 337, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, W.H.; Han, S.; Peng, B.W.; Yin, J.; Wu, Y.L.; He, X.H.; Li, W.X. Selective inhibition of CCR7(-) effector memory T cell activation by a novel peptide targeting Kv1.3 channel in a rat experimental autoimmune encephalomyelitis model. J. Biol. Chem. 2012, 287, 29479–29494. [Google Scholar] [CrossRef]
- Han, S.; Yi, H.; Yin, S.J.; Chen, Z.Y.; Liu, H.; Cao, Z.J.; Wu, Y.L.; Li, W.X. Structural basis of a potent peptide inhibitor designed for Kv1.3 channel, a therapeutic target of autoimmune disease. J. Biol. Chem. 2008, 283, 19058–19065. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, F.; Li, S.; Gao, M.; Wang, L.; Sarhan, M.; Abdel-Rahman, M.A.; Li, W.; Kwok, H.F.; Wu, Y.; et al. Inhibitory Activity of a Scorpion Defensin BmKDfsin3 against Hepatitis C Virus. Antibiotic 2020, 9, 33. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, Q.; Hong, W.; Xie, Y.; Liu, Y.; Li, W.; Wu, Y.; Cao, Z. A Scorpion Defensin BmKDfsin4 Inhibits Hepatitis B Virus Replication in Vitro. Toxins 2016, 8, 124. [Google Scholar] [CrossRef]
- Yuan, S.; Gao, B.; Zhu, S. Molecular Dynamics Simulation Reveals Specific Interaction Sites between Scorpion Toxins and K(v)1.2 Channel: Implications for Design of Highly Selective Drugs. Toxins 2017, 9, 354. [Google Scholar] [CrossRef]
- Liu, X.; Tao, J.; Zhang, S.; Lan, W.; Wang, C.; Ji, Y.; Cao, C. Selective Blockade of Neuronal BK (α + β4) Channels Preventing Epileptic Seizure. J. Med. Chem. 2020, 63, 216–230. [Google Scholar] [CrossRef]
- Cao, Z.; Xiao, F.; Peng, F.; Jiang, D.; Mao, X.; Liu, H.; Li, W.; Hu, D.; Wang, T. Expression, purification and functional characterization of a recombinant scorpion venom peptide BmTXKbeta. Peptides 2003, 24, 187–192. [Google Scholar] [CrossRef]
- Huang, J.; Han, S.; Sun, Q.; Zhao, Y.; Liu, J.; Yuan, X.; Mao, W.; Peng, B.; Liu, W.; Yin, J.; et al. Kv1.3 channel blocker (ImKTx88) maintains blood-brain barrier in experimental autoimmune encephalomyelitis. Cell Biosci. 2017, 7, 31. [Google Scholar] [CrossRef]
- Csoti, A.; Del Carmen Nájera Meza, R.; Bogár, F.; Tajti, G.; Szanto, T.G.; Varga, Z.; Gurrola, G.B.; Tóth, G.K.; Possani, L.D.; Panyi, G. sVmKTx, a transcriptome analysis-based synthetic peptide analogue of Vm24, inhibits Kv1.3 channels of human T cells with improved selectivity. Biochem. Pharmacol. 2022, 199, 115023. [Google Scholar] [CrossRef]
- Veytia-Bucheli, J.I.; Jiménez-Vargas, J.M.; Melchy-Pérez, E.I.; Sandoval-Hernández, M.A.; Possani, L.D.; Rosenstein, Y. K(v)1.3 channel blockade with the Vm24 scorpion toxin attenuates the CD4(+) effector memory T cell response to TCR stimulation. Cell Commun. Signal. 2018, 16, 45. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Bertolini, T.B.; Cerni, F.A.; Bordon, K.C.; Peigneur, S.; Tytgat, J.; Bonato, V.L.; Arantes, E.C. Immunosuppressive evidence of Tityus serrulatus toxins Ts6 and Ts15: Insights of a novel K(+) channel pattern in T cells. Immunology 2016, 147, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Cerni, F.A.; Cordeiro, F.A.; Peigneur, S.; Cunha, T.M.; Tytgat, J.; Arantes, E.C. Ts8 scorpion toxin inhibits the Kv4.2 channel and produces nociception in vivo. Toxicon 2016, 119, 244–252. [Google Scholar] [CrossRef] [PubMed]
- El-Bitar, A.M.H.; Sarhan, M.; Abdel-Rahman, M.A.; Quintero-Hernandez, V.; Aoki-Utsubo, C.; Moustafa, M.A.; Possani, L.D.; Hotta, H. Smp76, a Scorpine-Like Peptide Isolated from the Venom of the Scorpion Scorpio maurus palmatus, with a Potent Antiviral Activity Against Hepatitis C Virus and Dengue Virus. Int. J. Pept. Res. Ther. 2020, 26, 811–821. [Google Scholar] [CrossRef]
- Ji, Z.; Li, F.; Xia, Z.; Guo, X.; Gao, M.; Sun, F.; Cheng, Y.; Wu, Y.; Li, W.; Ali, S.A.; et al. The Scorpion Venom Peptide Smp76 Inhibits Viral Infection by Regulating Type-I Interferon Response. Virol. Sin. 2018, 33, 545–556. [Google Scholar] [CrossRef]
- Li, F.; Lang, Y.; Ji, Z.; Xia, Z.; Han, Y.; Cheng, Y.; Liu, G.; Sun, F.; Zhao, Y.; Gao, M.; et al. A scorpion venom peptide Ev37 restricts viral late entry by alkalizing acidic organelles. J. Biol. Chem. 2019, 294, 182–194. [Google Scholar] [CrossRef]
- Bagheri-Ziari, S.; Shahbazzadeh, D.; Sardari, S.; Sabatier, J.M.; Pooshang Bagheri, K. Discovery of a New Analgesic Peptide, Leptucin, from the Iranian Scorpion, Hemiscorpius lepturus. Molecules 2021, 26, 2580. [Google Scholar] [CrossRef]
- Meera, P.; Wallner, M.; Toro, L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl. Acad. Sci. USA 2000, 97, 5562–5567. [Google Scholar] [CrossRef]
- Galvez, A.; Gimenez-Gallego, G.; Reuben, J.P.; Roy-Contancin, L.; Feigenbaum, P.; Kaczorowski, G.J.; Garcia, M.L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J. Biol. Chem. 1990, 265, 11083–11090. [Google Scholar] [CrossRef]
- Tanner, M.R.; Tajhya, R.B.; Huq, R.; Gehrmann, E.J.; Rodarte, K.E.; Atik, M.A.; Norton, R.S.; Pennington, M.W.; Beeton, C. Prolonged immunomodulation in inflammatory arthritis using the selective Kv1.3 channel blocker HsTX1[R14A] and its PEGylated analog. Clin. Immunol. 2017, 180, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, J.A.; Pan, Y.; Di Stefano, I.; Choy, K.H.C.; Reddiar, S.B.; Low, Y.L.; Wai, D.C.C.; Norton, R.S.; Jin, L. Blockade of Microglial Kv1.3 Potassium Channels by the Peptide HsTX1[R14A] Attenuates Lipopolysaccharide-mediated Neuroinflammation. J. Pharm. Sci. 2022, 111, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Vandendriessche, T.; Kopljar, I.; Jenkins, D.P.; Diego-Garcia, E.; Abdel-Mottaleb, Y.; Vermassen, E.; Clynen, E.; Schoofs, L.; Wulff, H.; Snyders, D.; et al. Purification, molecular cloning and functional characterization of HelaTx1 (Heterometrus laoticus): The first member of a new κ-KTX subfamily. Biochem. Pharmacol. 2012, 83, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Esaki, N.; Yamaguchi, Y.; Tytgat, J.; Sato, K. Effects of deletion and insertion of amino acids on the activity of HelaTx1, a scorpion toxin on potassium channels. Toxicon 2016, 111, 1–5. [Google Scholar] [CrossRef]
- Hoang, A.N.; Vo, H.D.; Vo, N.P.; Kudryashova, K.S.; Nekrasova, O.V.; Feofanov, A.V.; Kirpichnikov, M.P.; Andreeva, T.V.; Serebryakova, M.V.; Tsetlin, V.I.; et al. Vietnamese Heterometrus laoticus scorpion venom: Evidence for analgesic and anti-inflammatory activity and isolation of new polypeptide toxin acting on Kv1.3 potassium channel. Toxicon 2014, 77, 40–48. [Google Scholar] [CrossRef]
- Aissaoui, D.; Mlayah-Bellalouna, S.; Jebali, J.; Abdelkafi-Koubaa, Z.; Souid, S.; Moslah, W.; Othman, H.; Luis, J.; ElAyeb, M.; Marrakchi, N.; et al. Functional role of Kv1.1 and Kv1.3 channels in the neoplastic progression steps of three cancer cell lines, elucidated by scorpion peptides. Int. J. Biol. Macromol. 2018, 111, 1146–1155. [Google Scholar] [CrossRef]
- Yuan, X.L.; Zhao, Y.P.; Huang, J.; Liu, J.C.; Mao, W.Q.; Yin, J.; Peng, B.W.; Liu, W.H.; Han, S.; He, X.H. A Kv1.3 channel-specific blocker alleviates neurological impairment through inhibiting T-cell activation in experimental autoimmune encephalomyelitis. CNS Neurosci. Ther. 2018, 24, 967–977. [Google Scholar] [CrossRef]
- Pan, Y.; Kagawa, Y.; Sun, J.; Lucas, D.S.D.; Takechi, R.; Mamo, J.C.L.; Wai, D.C.C.; Norton, R.S.; Jin, L.; Nicolazzo, J.A. Peripheral Administration of the Kv1.3-Blocking Peptide HsTX1[R14A] Improves Cognitive Performance in Senescence Accelerated SAMP8 Mice. Neurotherapeutics 2023, 20, 1198–1214. [Google Scholar] [CrossRef]
- Reddiar, S.B.; de Veer, M.; Paterson, B.M.; Sepehrizadeh, T.; Wai, D.C.C.; Csoti, A.; Panyi, G.; Nicolazzo, J.A.; Norton, R.S. A Biodistribution Study of the Radiolabeled Kv1.3-Blocking Peptide DOTA-HsTX1[R14A] Demonstrates Brain Uptake in a Mouse Model of Neuroinflammation. Mol. Pharm. 2023, 20, 255–266. [Google Scholar] [CrossRef]
- Yin, S.J.; Jiang, L.; Yi, H.; Han, S.; Yang, D.W.; Liu, M.L.; Liu, H.; Cao, Z.J.; Wu, Y.L.; Li, W.X. Different residues in channel turret determining the selectivity of ADWX-1 inhibitor peptide between Kv1.1 and Kv1.3 channels. J. Proteome Res. 2008, 7, 4890–4897. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, Y.; Qu, D.; Xie, Z.; Guo, X.; Zhu, Z.; Chen, Z.; Zhang, L.; Li, W.; Cao, Z.; et al. Ion channel modulation by scorpion hemolymph and its defensin ingredients highlights origin of neurotoxins in telson formed in Paleozoic scorpions. Int. J. Biol. Macromol. 2020, 148, 351–363. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Zuo, Z.; Qin, C.; Liu, Y.; Cao, Z.; Wu, Y. Novel structural determinants and bacterial death-related regulatory effects of the scorpion defensin BmKDfsin4 against gram-positive bacteria. Int. J. Biol. Macromol. 2024, 282, 137151. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, J.M. Antibacterial Peptides. Antibiotic 2020, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, M.P.; Fraser, G.L. Isopropanol ingestion: Case report with pharmacokinetic analysis. Am. J. Emerg. Med. 1989, 7, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yu, C.; Wang, M.; Li, Z.; Wu, Y.; Cao, Z.; Li, W.; He, X.; Han, S. Expression and characterization of a novel scorpine-like peptide Ev37, from the scorpion Euscorpiops validus. Protein Expr. Purif. 2013, 88, 127–133. [Google Scholar] [CrossRef]
- Dueñas-Cuellar, R.A.; Santana, C.J.C.; Magalhães, A.C.M.; Pires, O.R., Jr.; Fontes, W.; Castro, M.S. Scorpion Toxins and Ion Channels: Potential Applications in Cancer Therapy. Toxins 2020, 12, 326. [Google Scholar] [CrossRef]
- Jang, S.H.; Choi, S.Y.; Ryu, P.D.; Lee, S.Y. Anti-proliferative effect of Kv1.3 blockers in A549 human lung adenocarcinoma in vitro and in vivo. Eur. J. Pharmacol. 2011, 651, 26–32. [Google Scholar] [CrossRef]
- Weaver, A.K.; Liu, X.; Sontheimer, H. Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J. Neurosci. Res. 2004, 78, 224–234. [Google Scholar] [CrossRef]
- Wawrzkiewicz-Jałowiecka, A.; Trybek, P.; Machura, Ł.; Dworakowska, B.; Grzywna, Z.J. Mechanosensitivity of the BK Channels in Human Glioblastoma Cells: Kinetics and Dynamical Complexity. J. Membr. Biol. 2018, 251, 667–679. [Google Scholar] [CrossRef]
- Elias, A.F.; Lin, B.C.; Piggott, B.J. Ion Channels in Gliomas-From Molecular Basis to Treatment. Int. J. Mol. Sci. 2023, 24, 2530. [Google Scholar] [CrossRef]
- Roger, S.; Potier, M.; Vandier, C.; Le Guennec, J.Y.; Besson, P. Description and role in proliferation of iberiotoxin-sensitive currents in different human mammary epithelial normal and cancerous cells. Biochim. Biophys. Acta 2004, 1667, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Basrai, D.; Kraft, R.; Bollensdorff, C.; Liebmann, L.; Benndorf, K.; Patt, S. BK channel blockers inhibit potassium-induced proliferation of human astrocytoma cells. Neuroreport 2002, 13, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Bagur, R.; Hajnóczky, G. Intracellular Ca(2+) Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M. Calcium and cell function. J. Physiol. 2020, 598, 1647–1648. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Zalk, R.; Lehnart, S.E.; Marks, A.R. Modulation of the ryanodine receptor and intracellular calcium. Annu. Rev. Biochem. 2007, 76, 367–385. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Quintero-Hernández, V.; Ramírez-Carreto, S.; Romero-Gutiérrez, M.T.; Valdez-Velázquez, L.L.; Becerril, B.; Possani, L.D.; Ortiz, E. Transcriptome analysis of scorpion species belonging to the Vaejovis genus. PLoS ONE 2015, 10, e0117188. [Google Scholar] [CrossRef]
- Sidach, S.S.; Mintz, I.M. Kurtoxin, a gating modifier of neuronal high- and low-threshold ca channels. J. Neurosci. 2002, 22, 2023–2034. [Google Scholar] [CrossRef]
- McDonough, S.I. Gating modifier toxins of voltage-gated calcium channels. Toxicon 2007, 49, 202–212. [Google Scholar] [CrossRef]
- Smith, J.J.; Vetter, I.; Lewis, R.J.; Peigneur, S.; Tytgat, J.; Lam, A.; Gallant, E.M.; Beard, N.A.; Alewood, P.F.; Dulhunty, A.F. Multiple actions of phi-LITX-Lw1a on ryanodine receptors reveal a functional link between scorpion DDH and ICK toxins. Proc. Natl. Acad. Sci. USA 2013, 110, 8906–8911. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.F.; Capes, E.M.; Diego-García, E.; Zamudio, F.Z.; Fuentes, O.; Possani, L.D.; Valdivia, H.H. Characterization of hadrucalcin, a peptide from Hadrurus gertschi scorpion venom with pharmacological activity on ryanodine receptors. Br. J. Pharmacol. 2009, 157, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Bae, C.; Lee, J.; Ryu, J.H.; Kim, H.H.; Kohno, T.; Swartz, K.J.; Kim, J.I. Solution structure of kurtoxin: A gating modifier selective for Cav3 voltage-gated Ca(2+) channels. Biochemistry 2012, 51, 1862–1873. [Google Scholar] [CrossRef] [PubMed]
- Chuang, R.S.; Jaffe, H.; Cribbs, L.; Perez-Reyes, E.; Swartz, K.J. Inhibition of T-type voltage-gated calcium channels by a new scorpion toxin. Nat. Neurosci. 1998, 1, 668–674. [Google Scholar] [CrossRef]
- Olamendi-Portugal, T.; García, B.I.; López-González, I.; Van Der Walt, J.; Dyason, K.; Ulens, C.; Tytgat, J.; Felix, R.; Darszon, A.; Possani, L.D. Two new scorpion toxins that target voltage-gated Ca2+ and Na+ channels. Biochem. Biophys. Res. Commun. 2002, 299, 562–568. [Google Scholar] [CrossRef]
- Vargas-Jaimes, L.; Xiao, L.; Zhang, J.; Possani, L.D.; Valdivia, H.H.; Quintero-Hernández, V. Recombinant expression of Intrepicalcin from the scorpion Vaejovis intrepidus and its effect on skeletal ryanodine receptors. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 936–946. [Google Scholar] [CrossRef]
- Xiao, L.; Gurrola, G.B.; Zhang, J.; Valdivia, C.R.; SanMartin, M.; Zamudio, F.Z.; Zhang, L.; Possani, L.D.; Valdivia, H.H. Structure-function relationships of peptides forming the calcin family of ryanodine receptor ligands. J. Gen. Physiol. 2016, 147, 375–394. [Google Scholar] [CrossRef]
- Shahbazzadeh, D.; Srairi-Abid, N.; Feng, W.; Ram, N.; Borchani, L.; Ronjat, M.; Akbari, A.; Pessah, I.N.; De Waard, M.; El Ayeb, M. Hemicalcin, a new toxin from the Iranian scorpion Hemiscorpius lepturus which is active on ryanodine-sensitive Ca2+ channels. Biochem. J. 2007, 404, 89–96. [Google Scholar] [CrossRef]
- Fajloun, Z.; Kharrat, R.; Chen, L.; Lecomte, C.; Di Luccio, E.; Bichet, D.; El Ayeb, M.; Rochat, H.; Allen, P.D.; Pessah, I.N.; et al. Chemical synthesis and characterization of maurocalcine, a scorpion toxin that activates Ca(2+) release channel/ryanodine receptors. FEBS Lett. 2000, 469, 179–185. [Google Scholar] [CrossRef]
- Estève, E.; Smida-Rezgui, S.; Sarkozi, S.; Szegedi, C.; Regaya, I.; Chen, L.; Altafaj, X.; Rochat, H.; Allen, P.; Pessah, I.N.; et al. Critical amino acid residues determine the binding affinity and the Ca2+ release efficacy of maurocalcine in skeletal muscle cells. J. Biol. Chem. 2003, 278, 37822–37831. [Google Scholar] [CrossRef]
- Dhureja, M.; Arthur, R.; Soni, D.; Upadhayay, S.; Temgire, P.; Kumar, P. Calcium channelopathies in neurodegenerative disorder: An untold story of RyR and SERCA. Expert Opin. Ther. Targets 2023, 27, 1159–1172. [Google Scholar] [CrossRef]
- Haseeb, M.; Thompson, P.D. The effect of statins on RyR and RyR-associated disease. J. Appl. Physiol. 2021, 131, 661–671. [Google Scholar] [CrossRef]
- Santibáñez-López, C.E.; Kriebel, R.; Ballesteros, J.A.; Rush, N.; Witter, Z.; Williams, J.; Janies, D.A.; Sharma, P.P. Integration of phylogenomics and molecular modeling reveals lineage-specific diversification of toxins in scorpions. PeerJ 2018, 6, e5902. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gutiérrez, M.T.; Santibáñez-López, C.E.; Jiménez-Vargas, J.M.; Batista, C.V.F.; Ortiz, E.; Possani, L.D. Transcriptomic and Proteomic Analyses Reveal the Diversity of Venom Components from the Vaejovid Scorpion Serradigitus gertschi. Toxins 2018, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, A.; Kharrat, R.; Fajloun, Z.; Renisio, J.G.; Blanc, E.; Sabatier, J.M.; El Ayeb, M.; Darbon, H. A new fold in the scorpion toxin family, associated with an activity on a ryanodine-sensitive calcium channel. Proteins 2000, 40, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Lee, E.H.; Takeuchi, K.; Takahashi, H.; Shimada, I.; Sato, K.; Shin, S.Y.; Kim, D.H.; Kim, J.I. Molecular basis of the high-affinity activation of type 1 ryanodine receptors by imperatoxin A. Biochem. J. 2004, 377, 385–394. [Google Scholar] [CrossRef]
- Fill, M.; Copello, J.A. Ryanodine receptor calcium release channels. Physiol. Rev. 2002, 82, 893–922. [Google Scholar] [CrossRef]
- Steinberg, C.; Roston, T.M.; van der Werf, C.; Sanatani, S.; Chen, S.R.W.; Wilde, A.A.M.; Krahn, A.D. RYR2-ryanodinopathies: From calcium overload to calcium deficiency. Europace 2023, 25, euad156. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, Y.; Jardin, B.D.; Zhang, X.; Zhou, P.; Guatimosim, S.; Lin, J.; Chen, Z.; Zhang, Y.; Mazumdar, N.; et al. Ryanodine receptor 2 (RYR2) dysfunction activates the unfolded protein response and perturbs cardiomyocyte maturation. Cardiovasc. Res. 2023, 119, 221–235. [Google Scholar] [CrossRef]
- Blayney, L.M.; Lai, F.A. Ryanodine receptor-mediated arrhythmias and sudden cardiac death. Pharmacol. Ther. 2009, 123, 151–177. [Google Scholar] [CrossRef]
- Joshi, P.; Estes, S.; DeMazumder, D.; Knollmann, B.C.; Dey, S. Ryanodine receptor 2 inhibition reduces dispersion of cardiac repolarization, improves contractile function, and prevents sudden arrhythmic death in failing hearts. eLife 2023, 12, e88638. [Google Scholar] [CrossRef] [PubMed]
- De Waard, S.; Montnach, J.; Cortinovis, C.; Chkir, O.; Erfanian, M.; Hulin, P.; Gaborit, N.; Lemarchand, P.; Mesirca, P.; Bidaud, I.; et al. Maurocalcin and its analog MCaE12A facilitate Ca2+ mobilization in cardiomyocytes. Biochem. J. 2020, 477, 3985–3999. [Google Scholar] [CrossRef] [PubMed]
- Gurrola, G.B.; Capes, E.M.; Zamudio, F.Z.; Possani, L.D.; Valdivia, H.H. Imperatoxin A, a Cell-Penetrating Peptide from Scorpion Venom, as a Probe of Ca-Release Channels/Ryanodine Receptors. Pharmaceuticals 2010, 3, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, T.J. CLC chloride channels and transporters: From genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 3–36. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Stein, V.; Weinreich, F.; Zdebik, A.A. Molecular structure and physiological function of chloride channels. Physiol. Rev. 2002, 82, 503–568. [Google Scholar] [CrossRef]
- Graves, A.R.; Curran, P.K.; Smith, C.L.; Mindell, J.A. The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 2008, 453, 788–792. [Google Scholar] [CrossRef]
- Picollo, A.; Pusch, M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 2005, 436, 420–423. [Google Scholar] [CrossRef]
- Rosso, J.P.; Rochat, H. Characterization of ten proteins from the venom of the Moroccan scorpion Androctonus mauretanicus mauretanicus, six of which are toxic to the mouse. Toxicon 1985, 23, 113–125. [Google Scholar] [CrossRef]
- Soroceanu, L.; Gillespie, Y.; Khazaeli, M.B.; Sontheimer, H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998, 58, 4871–4879. [Google Scholar]
- McFerrin, M.B.; Sontheimer, H. A role for ion channels in glioma cell invasion. Neuron Glia Biol. 2006, 2, 39–49. [Google Scholar] [CrossRef]
- Dardevet, L.; Rani, D.; Aziz, T.A.; Bazin, I.; Sabatier, J.M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef]
- Correnti, C.E.; Gewe, M.M.; Mehlin, C.; Bandaranayake, A.D.; Johnsen, W.A.; Rupert, P.B.; Brusniak, M.Y.; Clarke, M.; Burke, S.E.; De Van Der Schueren, W.; et al. Screening, large-scale production and structure-based classification of cystine-dense peptides. Nat. Struct. Mol. Biol. 2018, 25, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Morel, J.L.; Mokrzycki, N.; Lippens, G.; Drobecq, H.; Sautière, P.; Hugues, M. Characterization of a Family of Scorpion Toxins Modulating Ca(2+)-Activated Cl(-) Current in Vascular Myocytes. Toxins 2022, 14, 780. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Sun, Z.; Jiang, D.; Dai, C.; Ma, Y.; Zhao, Z.; Liu, H.; Wu, Y.; Cao, Z.; Li, W. BmKCT toxin inhibits glioma proliferation and tumor metastasis. Cancer Lett. 2010, 291, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Rochat, H.; Bernard, P.; Couraud, F. Scorpion toxins: Chemistry and mode of action. Adv. Cytopharmacol. 1979, 3, 325–334. [Google Scholar]
- Tytgat, J.; Debont, T.; Rostoll, K.; Müller, G.J.; Verdonck, F.; Daenens, P.; van der Walt, J.J.; Possani, L.D. Purification and partial characterization of a ‘short’ insectotoxin-like peptide from the venom of the scorpion Parabuthus schlechteri. FEBS Lett. 1998, 441, 387–391. [Google Scholar] [CrossRef]
- The, M.R.T. Chlorotoxin:Cy5.5. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Ojeda, P.G.; Wang, C.K.; Craik, D.J. Chlorotoxin: Structure, activity, and potential uses in cancer therapy. Biopolymers 2016, 106, 25–36. [Google Scholar] [CrossRef]
- Cohen, G.; Burks, S.R.; Frank, J.A. Chlorotoxin-A Multimodal Imaging Platform for Targeting Glioma Tumors. Toxins 2018, 10, 496. [Google Scholar] [CrossRef]
- Ramsoomair, D.; Ramsoomair, C.K.; Daftari, M.; Himic, V.; Shlobin, N.A.; Wang, S.E.; Ivan, M.E.; Komotar, R.J.; Shah, A.H. Translating Venom to Medicine: A Comprehensive Review on the Role of Chlorotoxin in Glioblastoma Diagnosis and Therapy. Mol. Cancer Ther. 2025, 24, 1867–1877. [Google Scholar] [CrossRef]
- Adjadj, E.; Naudat, V.; Quiniou, E.; Wouters, D.; Sautière, P.; Craescu, C.T. Solution structure of Lqh-8/6, a toxin-like peptide from a scorpion venom--structural heterogeneity induced by proline cis/trans isomerization. Eur. J. Biochem. 1997, 246, 218–227. [Google Scholar] [CrossRef]
- Ali, S.A.; Stoeva, S.; Schütz, J.; Kayed, R.; Abassi, A.; Zaidi, Z.H.; Voelter, W. Purification and primary structure of low molecular mass peptides from scorpion (Buthus sindicus) venom. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 121, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Fallah, H.P.; Ahuja, E.; Lin, H.; Qi, J.; He, Q.; Gao, S.; An, H.; Zhang, J.; Xie, Y.; Liang, D. A Review on the Role of TRP Channels and Their Potential as Drug Targets_An Insight Into the TRP Channel Drug Discovery Methodologies. Front. Pharmacol. 2022, 13, 914499. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.M. TRP Channels as Potential Drug Targets. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Gees, M.; Owsianik, G.; Nilius, B.; Voets, T. TRP channels. Compr. Physiol. 2012, 2, 563–608. [Google Scholar] [CrossRef]
- Minke, B. TRP channels and Ca2+ signaling. Cell Calcium 2006, 40, 261–275. [Google Scholar] [CrossRef]
- Koivisto, A.P.; Szallasi, A. Targeting TRP Channels for Pain, Itch and Neurogenic Inflammation. Int. J. Mol. Sci. 2023, 25, 320. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Caterina, M.J.; Julius, D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef]
- Yang, S.; Yang, F.; Zhang, B.; Lee, B.H.; Li, B.; Luo, L.; Zheng, J.; Lai, R. A bimodal activation mechanism underlies scorpion toxin-induced pain. Sci. Adv. 2017, 3, e1700810. [Google Scholar] [CrossRef]
- Jami, S.; Erickson, A.; Brierley, S.M.; Vetter, I. Pain-Causing Venom Peptides: Insights into Sensory Neuron Pharmacology. Toxins 2017, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Lin King, J.V.; Emrick, J.J.; Kelly, M.J.S.; Herzig, V.; King, G.F.; Medzihradszky, K.F.; Julius, D. A Cell-Penetrating Scorpion Toxin Enables Mode-Specific Modulation of TRPA1 and Pain. Cell 2019, 178, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.A.; Jiang, W.; Luo, L.; Li, B.; Yang, S.; Song, Y.; Lai, R. Scorpion Toxin, BmP01, Induces Pain by Targeting TRPV1 Channel. Toxins 2015, 7, 3671–3687. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, J.; Chandy, K.G.; Garcia, M.L.; Gutman, G.A.; Martin-Eauclaire, M.F.; van der Walt, J.J.; Possani, L.D. A unified nomenclature for short-chain peptides isolated from scorpion venoms: Alpha-KTx molecular subfamilies. Trends Pharmacol. Sci. 1999, 20, 444–447. [Google Scholar] [CrossRef]
- Green, D.; Dong, X. A Pungent and Painful Toxin. Cell 2019, 178, 1279–1281. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, Y.; Clynen, E.; Jalali, A.; Bosmans, F.; Vatanpour, H.; Schoofs, L.; Tytgat, J. The first potassium channel toxin from the venom of the Iranian scorpion Odonthobuthus doriae. FEBS Lett. 2006, 580, 6254–6258. [Google Scholar] [CrossRef]
- Delgado-Prudencio, G.; Cid-Uribe, J.I.; Morales, J.A.; Possani, L.D.; Ortiz, E.; Romero-Gutiérrez, T. The Enzymatic Core of Scorpion Venoms. Toxins 2022, 14, 248. [Google Scholar] [CrossRef]
- Santibáñez-López, C.E.; Cid-Uribe, J.I.; Batista, C.V.; Ortiz, E.; Possani, L.D. Venom Gland Transcriptomic and Proteomic Analyses of the Enigmatic Scorpion Superstitionia donensis (Scorpiones: Superstitioniidae), with Insights on the Evolution of Its Venom Components. Toxins 2016, 8, 367. [Google Scholar] [CrossRef]
- de Oliveira, U.C.; Nishiyama, M.Y., Jr.; Dos Santos, M.B.V.; Santos-da-Silva, A.P.; Chalkidis, H.M.; Souza-Imberg, A.; Candido, D.M.; Yamanouye, N.; Dorce, V.A.C.; Junqueira-de-Azevedo, I.L.M. Proteomic endorsed transcriptomic profiles of venom glands from Tityus obscurus and T. serrulatus scorpions. PLoS ONE 2018, 13, e0193739. [Google Scholar] [CrossRef]
- Bordon, K.C.F.; Santos, G.C.; Martins, J.G.; Wiezel, G.A.; Amorim, F.G.; Crasset, T.; Redureau, D.; Quinton, L.; Procópio, R.E.L.; Arantes, E.C. Pioneering Comparative Proteomic and Enzymatic Profiling of Amazonian Scorpion Venoms Enables the Isolation of Their First α-Ktx, Metalloprotease, and Phospholipase A(2). Toxins 2025, 17, 411. [Google Scholar] [CrossRef]
- Carmo, A.O.; Oliveira-Mendes, B.B.; Horta, C.C.; Magalhães, B.F.; Dantas, A.E.; Chaves, L.M.; Chávez-Olórtegui, C.; Kalapothakis, E. Molecular and functional characterization of metalloserrulases, new metalloproteases from the Tityus serrulatus venom gland. Toxicon 2014, 90, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Cajado-Carvalho, D.; da Silva, C.C.F.; Kodama, R.T.; Mariano, D.O.C.; Pimenta, D.C.; Duzzi, B.; Kuniyoshi, A.K.; Portaro, F.V. Purification and Biochemical Characterization of TsMS 3 and TsMS 4: Neuropeptide-Degrading Metallopeptidases in the Tityus serrulatus Venom. Toxins 2019, 11, 194. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, R.; Di, Z.; Li, Z.; Xu, X.; Hong, W.; Wu, Y.; Zhao, H.; Li, W.; Cao, Z. Molecular diversity of Chaerilidae venom peptides reveals the dynamic evolution of scorpion venom components from Buthidae to non-Buthidae. J. Proteom. 2013, 89, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Doley, R.; Kini, R.M. Protein complexes in snake venom. Cell. Mol. Life Sci. 2009, 66, 2851–2871. [Google Scholar] [CrossRef]
- Gunas, V.; Maievskyi, O.; Raksha, N.; Vovk, T.; Savchuk, O.; Shchypanskyi, S.; Gunas, I. The Activity of Metalloproteases and Serine Proteases in Various Organs after Leiurus macroctenus Envenomation. J. Toxicol. 2023, 2023, 5262729. [Google Scholar] [CrossRef]
- Scieuzo, C.; Salvia, R.; Franco, A.; Pezzi, M.; Cozzolino, F.; Chicca, M.; Scapoli, C.; Vogel, H.; Monti, M.; Ferracini, C.; et al. An integrated transcriptomic and proteomic approach to identify the main Torymus sinensis venom components. Sci. Rep. 2021, 11, 5032. [Google Scholar] [CrossRef]
- Krayem, N.; Gargouri, Y. Scorpion venom phospholipases A(2): A minireview. Toxicon 2020, 184, 48–54. [Google Scholar] [CrossRef]
- Aloulou, A.; Rahier, R.; Arhab, Y.; Noiriel, A.; Abousalham, A. Phospholipases: An Overview. Methods Mol. Biol. 2018, 1835, 69–105. [Google Scholar] [CrossRef]
- Louati, H.; Krayem, N.; Fendri, A.; Aissa, I.; Sellami, M.; Bezzine, S.; Gargouri, Y. A thermoactive secreted phospholipase A2 purified from the venom glands of Scorpio maurus: Relation between the kinetic properties and the hemolytic activity. Toxicon 2013, 72, 133–142. [Google Scholar] [CrossRef]
- Valdez-Cruz, N.A.; Batista, C.V.; Possani, L.D. Phaiodactylipin, a glycosylated heterodimeric phospholipase A from the venom of the scorpion Anuroctonus phaiodactylus. Eur. J. Biochem. 2004, 271, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Tu, A.T.; Hendon, R.R. Characterization of lizard venom hyaluronidase and evidence for its action as a spreading factor. Comp. Biochem. Physiol. B Comp. Biochem. 1983, 76, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Duran-Reynals, F. Studies on a certain spreading factor existing in bacteria and its significance for bacterial invasiveness. J. Exp. Med. 1933, 58, 161–181. [Google Scholar] [CrossRef]
- Oliveira-Mendes, B.B.R.; Miranda, S.E.M.; Sales-Medina, D.F.; Magalhães, B.F.; Kalapothakis, Y.; Souza, R.P.; Cardoso, V.N.; de Barros, A.L.B.; Guerra-Duarte, C.; Kalapothakis, E.; et al. Inhibition of Tityus serrulatus venom hyaluronidase affects venom biodistribution. PLoS Neglected Trop. Dis. 2019, 13, e0007048. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef]
- Evans, E.R.J.; McIntyre, L.; Northfield, T.D.; Daly, N.L.; Wilson, D.T. Small Molecules in the Venom of the Scorpion Hormurus waigiensis. Biomedicines 2020, 8, 259. [Google Scholar] [CrossRef]
- Villar-Briones, A.; Aird, S.D. Organic and Peptidyl Constituents of Snake Venoms: The Picture Is Vastly More Complex Than We Imagined. Toxins 2018, 10, 392. [Google Scholar] [CrossRef]
- Aird, S.D. Ophidian envenomation strategies and the role of purines. Toxicon 2002, 40, 335–393. [Google Scholar] [CrossRef]
- Banerjee, S.; Gnanamani, E.; Lynch, S.R.; Zuñiga, F.Z.; Jiménez-Vargas, J.M.; Possani, L.D.; Zare, R.N. An Alkaloid from Scorpion Venom: Chemical Structure and Synthesis. J. Nat. Prod. 2018, 81, 1899–1904. [Google Scholar] [CrossRef]
- Carcamo-Noriega, E.N.; Sathyamoorthi, S.; Banerjee, S.; Gnanamani, E.; Mendoza-Trujillo, M.; Mata-Espinosa, D.; Hernández-Pando, R.; Veytia-Bucheli, J.I.; Possani, L.D.; Zare, R.N. 1,4-Benzoquinone antimicrobial agents against Staphylococcus aureus and Mycobacterium tuberculosis derived from scorpion venom. Proc. Natl. Acad. Sci. USA 2019, 116, 12642–12647. [Google Scholar] [CrossRef]
- Tran, T.V.; Hoang, A.N.; Nguyen, T.T.T.; Phung, T.V.; Nguyen, K.C.; Osipov, A.V.; Ivanov, I.A.; Tsetlin, V.I.; Utkin, Y.N. Anticoagulant Activity of Low-Molecular Weight Compounds from Heterometrus laoticus Scorpion Venom. Toxins 2017, 9, 343. [Google Scholar] [CrossRef]
- Thien, T.V.; Anh, H.N.; Trang, N.T.T.; Trung, P.V.; Khoa, N.C.; Osipov, A.V.; Dubovskii, P.V.; Ivanov, I.A.; Arseniev, A.S.; Tsetlin, V.I.; et al. Low-molecular-weight compounds with anticoagulant activity from the scorpion Heterometrus laoticus venom. Doklady. Biochem. Biophys. 2017, 476, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.W.; West, P.R.; Odell, G.V.; Hudiburg, S.M.; Ownby, C.L.; Mills, J.N.; Scroggins, B.T.; Shannon, S.B. Arthropod venom citrate inhibits phospholipase A2. Toxicon 1995, 33, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Possani, L.D.; Becerril, B.; Delepierre, M.; Tytgat, J. Scorpion toxins specific for Na+-channels. Eur. J. Biochem. 1999, 264, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Heel, R.C.; Brogden, R.N.; Speight, T.M.; Avery, G.S. Captopril: A preliminary review of its pharmacological properties and therapeutic efficacy. Drugs 1980, 20, 409–452. [Google Scholar] [CrossRef]
- Atkinson, A.B.; Robertson, J.I. Captopril in the treatment of clinical hypertension and cardiac failure. Lancet 1979, 2, 836–839. [Google Scholar] [CrossRef]
- Schmidtko, A.; Lötsch, J.; Freynhagen, R.; Geisslinger, G. Ziconotide for treatment of severe chronic pain. Lancet 2010, 375, 1569–1577. [Google Scholar] [CrossRef]
- PURSUIT Trial Investigators. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N. Engl. J. Med. 1998, 339, 436–443. [Google Scholar] [CrossRef]
- Impact-II Investigators. Randomised placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. Integrilin to Minimise Platelet Aggregation and Coronary Thrombosis-II. Lancet 1997, 349, 1422–1428. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, X.; Guo, M.; Tzeng, C.M. Therapeutic Peptides: Recent Advances in Discovery, Synthesis, and Clinical Translation. Int. J. Mol. Sci. 2025, 26, 5131. [Google Scholar] [CrossRef]
- Stepensky, D. Pharmacokinetics of Toxin-Derived Peptide Drugs. Toxins 2018, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.W.; Czerwinski, A.; Norton, R.S. Peptide therapeutics from venom: Current status and potential. Bioorganic Med. Chem. 2018, 26, 2738–2758. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Torres, M.D.T.; Li, S.; de la Fuente-Nunez, C. Computational exploration of global venoms for antimicrobial discovery with Venomics artificial intelligence. Nat. Commun. 2025, 16, 6446. [Google Scholar] [CrossRef] [PubMed]
- Goles, M.; Daza, A.; Cabas-Mora, G.; Sarmiento-Varón, L.; Sepúlveda-Yañez, J.; Anvari-Kazemabad, H.; Davari, M.D.; Uribe-Paredes, R.; Olivera-Nappa, Á.; Navarrete, M.A.; et al. Peptide-based drug discovery through artificial intelligence: Towards an autonomous design of therapeutic peptides. Brief. Bioinform. 2024, 25, bbae275. [Google Scholar] [CrossRef]
- Gao, H.; Guan, F.; Luo, B.; Zhang, D.; Liu, W.; Shen, Y.; Fan, L.; Xu, G.; Wang, Y.; Tu, T.; et al. DLFea4AMPGen de novo design of antimicrobial peptides by integrating features learned from deep learning models. Nat. Commun. 2025, 16, 9134. [Google Scholar] [CrossRef]
- Bian, H.; Liang, X.; Lu, D.; Lin, J.; Lu, X.; Jin, J.; Zhang, L.; Wu, Y.; Chen, H.; Zhang, W.; et al. In Silico Discovery of Stapled Peptide Inhibitor Targeting the Nur77-PPARγ Interaction and Its Anti-Breast-Cancer Efficacy. Adv. Sci. 2024, 11, e2308435. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Fu, Y.; Xue, J.; Yuan, F.; Qu, T.; Rissanou, A.N.; Wang, Y.; Li, X.; Hu, H. Therapeutic stapled peptides: Efficacy and molecular targets. Pharmacol. Res. 2024, 203, 107137. [Google Scholar] [CrossRef]
- Li, Q.; Chao, W.; Qiu, L. Therapeutic peptides: Chemical strategies fortify peptides for enhanced disease treatment efficacy. Amino Acids 2025, 57, 25. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Liu, C.; Luo, Y.; Chen, D. Peptide-Mediated Nanocarriers for Targeted Drug Delivery: Developments and Strategies. Pharmaceutics 2024, 16, 240. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.K.; Xiu, M.H.; Yang, H.Y.; Shi, C.M. A chromosome-level genome assembly for the desert scorpion Mesobuthus przewalskii from Asian drylands. J. Hered. 2025, 116, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.J.; Coello, A.M.; Glendening, A.M.; Hilliman, S.A., III; Jara, C.F.; Pring, S.S.; Rodríguez Rivera, A.; Santiago Membreño, J.; Nigro, L.; Pauloski, N.; et al. Unveiling the Genetic Blueprint of a Desert Scorpion: A Chromosome-level Genome of Hadrurus arizonensis Provides the First Reference for Parvorder Iurida. Genome Biol. Evol. 2024, 16, evae097. [Google Scholar] [CrossRef] [PubMed]
- Grashof, D.G.B.; Kerkkamp, H.M.I.; Afonso, S.; Archer, J.; Harris, D.J.; Richardson, M.K.; Vonk, F.J.; van der Meijden, A. Transcriptome annotation and characterization of novel toxins in six scorpion species. BMC Genom. 2019, 20, 645. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez-López, C.E.; Francke, O.F.; Ureta, C.; Possani, L.D. Scorpions from Mexico: From Species Diversity to Venom Complexity. Toxins 2015, 8, 2. [Google Scholar] [CrossRef]
- Wu, Q.; Zheng, J.; Sui, X.; Fu, C.; Cui, X.; Liao, B.; Ji, H.; Luo, Y.; He, A.; Lu, X.; et al. High-throughput drug target discovery using a fully automated proteomics sample preparation platform. Chem. Sci. 2024, 15, 2833–2847. [Google Scholar] [CrossRef]
- Paananen, J.; Fortino, V. An omics perspective on drug target discovery platforms. Brief. Bioinform. 2020, 21, 1937–1953. [Google Scholar] [CrossRef]
- Zhang, D.E.; He, T.; Shi, T.; Huang, K.; Peng, A. Trends in the research and development of peptide drug conjugates: Artificial intelligence aided design. Front. Pharmacol. 2025, 16, 1553853. [Google Scholar] [CrossRef]
- Wan, F.; Kontogiorgos-Heintz, D.; de la Fuente-Nunez, C. Deep generative models for peptide design. Digit. Discov. 2022, 1, 195–208. [Google Scholar] [CrossRef]
- Díaz-Perlas, C.; Oller-Salvia, B. Chemically Enhanced Peptide and Protein Therapeutics. Pharmaceutics 2023, 15, 827. [Google Scholar] [CrossRef]
- Gentilucci, L.; De Marco, R.; Cerisoli, L. Chemical modifications designed to improve peptide stability: Incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Hong, L.; Li, W.; Li, Y.; Yin, S. Nanoparticle-based drug delivery systems targeting cancer cell surfaces. RSC Adv. 2023, 13, 21365–21382. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.