Abstract
Neurogenic bladder dysfunction (NB) represents a challenge in pediatric urology. Intravesical botulin toxin-A (BTX-A) bladder injection is part of the armamentarium for the treatment of this condition, usually after failed first-line medical strategies and before the escalation to more invasive options such as neuromodulation or augmented cystoplasty in severe cases. However, there is still a lack of consensus about the appropriate treatment modality for the pediatric population. A review of the last 10 years’ research was performed on the PubMed database by two authors. Articles doubly selected and meeting the inclusion criteria were collected and analyzed for their study type, demographics, neurological disease(s) at diagnosis, BTX-A treatment modality and duration, previous treatment, clinical and urodynamic parameters, adverse events, outcomes, and follow-ups. A total of 285 studies were initially selected, 16 of which matched the inclusion criteria. A cohort of 630 patients was treated with BTX-A at a median age of 9.7 years, 40% of which had a diagnosis of myelomeningocele. The results of the selected publications show the overall efficacy and safety of BTX-A injections in children and confirmed BTX-A as a valuable strategy for NB treatment in pediatric population. Nevertheless, up to now, the literature on this topic offers scarce uniformity among the published series and poor protocol standardization.
Key Contribution:
The clinical outcomes of the studies under investigation were variable, showing the overall efficacy and safety of BTX-A injections with a <1% incidence of postoperative complications. For this reason, BTX-A injections are a valuable strategy for NB treatment in children.
1. Introduction
Neurogenic bladder dysfunction (NB) is a complex disease which often affects patient life. Beyond treatment burden, these patients often experience significant physical limitations to daily activities and are at risk of social exclusion. In children, the most common cause of NB is reported to be myelomeningocele (MMC). In these patients, daily activities are frequently affected by urinary leakage. Moreover, detrusor overactivity (DO) often results in decreased bladder capacity, low compliance, high pressure, and hydronephrosis. The risk of urinary tract infection (UTI) and renal function deterioration can therefore seriously affect these children’s prognoses and quality of life [1]. Children with NB are classically treated with anticholinergic drugs such as oxybutynin or β-3 agonists and undergo daily clean intermittent catheterization (CIC) to reduce intravesical pressure and protect renal function [2]. However, in 10–15% of patients, these therapies fail due to a refractory overactive bladder or the onset of side effects (such as dry mouth, constipation, and blurred vision). Consequently, the bladder pressure remains high, and urinary symptoms persist. In those resistant patients, intravesical injection of BTX-A is considered an alternative as it can improve symptoms, avoiding surgical interventions such as continent urinary diversion with bladder augmentation [3]. The rationale is that BTX-A is capable of blocking the presynaptic release of acetylcholine from the parasympathetic efferent nerves. The efficacy of this action may result not only from an inhibitory effect on the detrusor muscle but also from some effects which are mediated by alterations in the afferent sensory nerve input. Many studies have demonstrated the efficacy of intravesical BTX-A bladder injection in improving symptoms. However, there is a lack of consensus about the appropriate treatment modality in the pediatric population. This study is a review of select studies published on this topic in the last 10 years, with the final aim being to shed some light on this unclarified yet highly clinically relevant medical problem.
2. Material and Methods
A literature review was performed using the PubMed, Cochrane, Ovid-Embase, and Scopus databases and while limiting the research to the last 10 years (January 2013–December 2023). The following keywords were used: “botulinum toxin”, “children”, “adolescent”, “neuropathic bladder”, and “neurogenic bladder”. Articles published in English and involving only patients under 18 years were included in the review. Review articles, case reports, commentaries, editorials, letters, abstracts, and adult series (>18 years) were excluded. Articles describing the use of BTX-A for not-neuropathic bladder dysfunction were also excluded.
All fully published English-language clinical studies on BTX-A were reviewed independently by two authors (V.C. and A.Z.). Only the articles which met the inclusion criteria and were selected by both authors were included (Figure 1). The references of each article were also screened for further research.
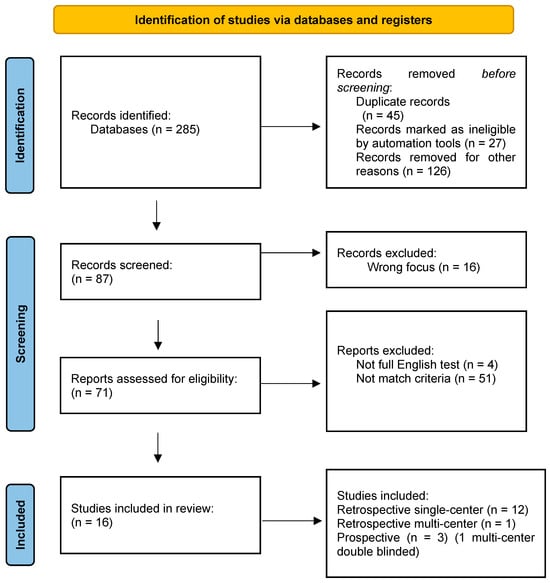
Figure 1.
Prisma flow chart: study selection.
For each article, the study type, anagraphic data, neurological disease(s) at diagnosis, BTX-A treatment modality and duration, previous treatment, clinical and urodynamic parameters, adverse events, outcomes, and follow-up data were collected when available.
3. Results
A total of 285 studies were collected and screened, and 16 of them matched the inclusion criteria; 12 were retrospective single-center studies, 1 was a retrospective multi-center study, and 3 were prospective, 1 of which was a multicentric, randomized double-blinded study. An overall cohort of 630 patients was reviewed. The median age at the first BTX-A injection was 9.7 years (IQR range: 8.5–11.3). The most common diagnosis was myelomeningocele (250/630, 40%). The other diagnoses were transverse myelitis, lipomeningocele, NB following tumor resection, trauma, sacrococcygeal teratoma, and caudal regression syndrome. Indications of BTX-A treatment varied among the studies and included both clinical and urodynamics parameters. All patients received BTX-A treatment after failed or insufficient conservative treatment with CIC, anticholinergic agents, or a combination of both. The dosage of BTX-A was 10 IU/kg in 8 series [4,5,6,7,8,9,10,11]. Austin et al. performed a comparison among three groups of patients which received 50 IU, 100 IU, and 200 IU, regardless of the patients’ weights [12]. All of the authors diluted the botulinum toxin in normal saline. The number of punctures during the single procedure ranged from 20 to 40. The trigone and bladder neck were excluded as well as the ureteral orifices.
Eight authors performed repeated injections based on symptom recurrence and patients’ satisfaction [5,6,9,10,11,13,14,15]. The time interval between injections was not always reported; when described, it was 13.1 months [5], 11.9 months [6], and 14 months [15]. The reason for re-intervention was a lack of response or detriment of symptoms in all cases. No peri-operative complications were described. The most frequent adverse events were hematuria (5/552, 0.9%) and temporary urinary retention (2/552, 0.4%). Complications were not reported in five papers.
UTIs post BTX-A treatment were reported in 72/552 (13%) patients, even if it was not clear if those were part of the chronic symptoms of the patients or if the UTI rate increased after treatment.
The clinical, demographics, urodynamics parameters, and outcomes are shown in Table 1.

Table 1.
Clinical, demographics, and urodynamics parameters and outcomes.
4. Discussion
NBs in children, such as those caused by spina bifida and other congenital or acquired conditions, pose significant clinical challenges. These conditions can lead to neurogenic detrusor overactivity (NDO) which, if not managed properly, can result in renal damage and reduced quality of life. BTX-A has emerged as a promising treatment option for managing these dysfunctions. Even with different outcomes, several studies have shown the overall clinical efficacy of BTX-A injections, which are usually adopted as a second-line treatment after anticholinergic drugs and CIC or in combination with either.
As mentioned, the outcomes were reported rather heterogeneously, and it might be difficult to objectively compare the results. First of all, not all of the papers described the post-treatment urodynamic parameters. However, when reported, an increase in bladder capacity and compliance and a reduction in the maximum detrusor pressure were described. Furthermore, two papers reported improvement in preexisting vesicoureteral reflux (VUR).
Early intervention seems to be crucial in managing neurogenic bladder dysfunctions to preserve kidney function. Dik et al. emphasized the importance of initiating BTX-A treatment early in spina bifida patients to prevent renal deterioration. Through this approach, it seems possible to maintain better control of overactive bladders and protect renal health [1].
The British Association of Paediatric Urologists provided comprehensive guidelines for the management of NB in children, including BTX-A as a treatment option [2]. The authors detailed these strategies, highlighting the role of BTX-A in managing bladder dysfunctions when standard therapies fail. The consensus supports BTX-A for its efficacy and relatively minimal invasiveness compared with surgical interventions such as bladder augmentation with urinary diversion.
Figueroa et al., in their single-center experience with BTX-A injections in 17 children, found that dose adjustments and repeated injections significantly improve bladder function and reduce the need for more invasive procedures [3].
Moreover, Sharifiaghdas et al., in their series of 35 children, demonstrated notable improvements in both the post-treatment clinical and radiological parameters, highlighting the therapeutic potential of BTX-A in managing NB [4].
Botulin injections are generally adopted for patients refractory to medical treatment, thus underscoring the need to properly select NB patients who may benefit from the procedure. Danacioglu et al. identified factors predicting the success of BTX-A treatment in children with neurogenic bladders due to myelomeningocele, and they found that some preoperative urodynamic parameters, such as the presence of a low-compliance bladder without DOA potentially predicting therapeutic outcomes, enabling better patient selection and treatment planning [5]. Similarly, Madec et al. discussed the long-term efficacy of repeated BTX-A injections, suggesting that continuous and repeated administration, with a medium interval of 11.9 months, is a sustainable option for managing NB over the long term [6].
The same urodynamic parameters have recently been shown to predict the outcome of relieving bladder outlet obstruction in kidney transplant patients in the adult population, a finding which underlines the relevant clinical value of low-compliance bladders in determining the success of endoscopic procedures in incontinent patients as a whole [16].
Several single-center studies, such as those proposed by Peeraully et al. and Peyronnet et al. [13,15], provide valuable insights into the practical application of BTX-A in pediatric patients. Peeraully et al. reported a decade-long experience with BTX-A injections, noting improvements in bladder function and patients’ quality of life in 71.4% of the patients treated with repeated injections [15]. Peyronnet et al. conducted a multicenter study on children with spina bifida, reinforcing the efficacy of BTX-A treatment in improving bladder compliance and reducing incontinence [13]. In their series, 62.3% of patients were treated with repeated injections, with an overall clinical success rate of 66%. Additionally, Sekerci et al. [14] reported the outcomes of up to five repeated BTX-A injections in children with refractory NDO, demonstrating sustained efficacy and manageable safety profiles over multiple treatment cycles.
As shown, different treatment modalities exist worldwide, and the need for a summary was already detected by Wu et al. in 2021 [20]. In his review on botulin injections in children, 16 articles were selected, and all but one reported improvement in clinical parameters such as incontinence, VUR, UTIs, and hydronephrosis. Moreover, although the urodynamics parameters considered in the included studies were various, a decrease in detrusor pressure and improvement in bladder capacity and compliance were described.
After the publication of this review, four more articles were published, all of which are retrospective single-center studies [4,5,6,21]. Therefore, the current literature still lacks prospective trials on BTX-A treatment, since most of the studies are retrospective.
Three prospective studies were included in this review [7,8,12]. Hui et al. conducted a prospective multicentric trial, investigating the safety and efficacy of trigonal BTX-A injections for children with NDO secondary to spinal cord injuries. BTX-A injections are usually performed along the bladder’s mucosal surface, avoiding the trigonal and bladder neck area. Despite this, the authors treated 33 patients with trigonal injections, noting a reduction in urinary incontinence episodes, increased voiding volumes, and improvement in the Incontinence Quality of Life questionnaire in all patients. Their results confirmed that this approach is both safe and effective, providing substantial symptom relief without significant adverse effects [7].
In a prospective multicentric, randomized double-blind trial by Austin et al., the population was divided into three groups according to the BTX-A dose injected. The first group (group 50 U) received 50–72 IU; the second group (group 100 U) received 96–144 IU; and the third group (group 200 U) received 168–200 IU. The authors observed a dose-dependent increase in functional bladder capacity which was statistically significant for the 200 U versus 50 U doses (p = 0.0055). A significant improvement from the baseline in storage pressures was also seen in the 200 U arm when compared with the 50 U group (p = 0.0157). There was an increase in the maximum cystometric capacity in all dosage groups, even if there were no statistically significant differences. The duration of the BTX-A effect, based on the median time for patients to require retreatment, did not differ significantly between groups. Reductions in UI episodes were similar across doses. The authors are also conducting a long-term extension study to evaluate the continued safety and efficacy following repeated treatments [12].
Mohajerzadeh et al., in their prospective trial, described a significant reduction in post-void residual volume and an increased cystometric bladder capacity after the injections. However, the study failed to demonstrate any significant improvement in the flow average and peak flow time [21].
Similar conclusions were found by Marte et al. [19]. The authors, in this retrospective single-center study, observed a positive effect on dryness and quality of life, with 38/47 patients achieving dryness with CIC while 9/47 patients improved their incontinence but still needed pads.
Tarcan et al. also reported important improvements, with 30/31 patients being dry with CIC, a 53% reduction in the maximum detrusor pressure, and a 51.5% increase in maximum cystometric capacity 6 weeks after the injections. Moreover, a 324% increase in mean bladder compliance and a 57% increase in mean intermittent catheterization volumes was found [18].
This review suffers from different biases. The main limitations are certainly the small number of studies which matched the inclusion criteria, the retrospective nature of many studies included, and the difficulties in perfectly matching the results of each cohort.
A larger number of high level of evidence data is needed in the future in order to assess the results induced by BTX-A treatment in children. In particular, a longer follow-up period is advised to evaluate the long-term effects of BTX-A injections on the bladder wall.
5. Conclusions
BTX-A represents a significant advancement in the management of neurological bladder dysfunctions in children. It offers a mini-invasive treatment which can improve bladder function, protect renal health, and enhance quality of life. The last 10 years of research have confirmed its efficacy and safety, providing prospective studies to reinforce the level of evidence on this topic. Patients must be counseled for possible repeated injections to maintain the clinical results. For this reason, the treatment itself is strictly tailored to patients’ responses, and successful outcomes depend on appropriate patient selection, careful dose management, and consideration of long-term treatment strategies. Some questions remain unanswered, such as the overall length of multiple cycles of injections and the differences between the outcomes of injection alone versus BTX-A combined with other medications. Studies have investigated different outcomes which do not appear to be uniform and with unstandardized BTX-A protocols, with inevitable bias in comparing the results. Further studies with larger sample sizes and adequate control groups should be conducted to confirm these observations.
Continued research and clinical experience will further refine BTX-A’s use and optimize the outcomes for pediatric patients with neurogenic bladder disorders.
Author Contributions
Conceptualization, A.Z., V.C., A.M. and L.G.; methodology, A.Z. and V.C.; investigation, A.Z. and V.C.; resources, A.Z., V.C. and M.A.C.; data curation, A.Z. and V.C.; writing—original draft preparation, A.Z. and A.M.; writing—review and editing, A.M., M.A.C. and L.G.; supervision, L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was performed with the Health Innovation Factory (HIF) Department Research Center at the University of Verona.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dik, P.; Klijn, A.J.; van Gool, J.D.; de Jong-de Vos van Steenwijk, C.C.E.; de Jong, T.P.V.M. Early Start to Therapy Preserves Kidney Function in Spina Bifida Patients. Eur. Urol. 2006, 49, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Featherstone, N.; Nagappan, P.; McCarthy, L.; O’Toole, S. British Association of Paediatric Urologists Consensus Statement on the Management of the Neuropathic Bladder. J. Pediatr. Urol. 2016, 12, 76–87. [Google Scholar] [CrossRef]
- Figueroa, V.; Romao, R.; Pippi Salle, J.L.; Koyle, M.A.; Braga, L.H.P.; Bägli, D.J.; Lorenzo, A.J. Single-Center Experience with Botulinum Toxin Endoscopic Detrusor Injection for the Treatment of Congenital Neuropathic Bladder in Children: Effect of Dose Adjustment, Multiple Injections, and Avoidance of Reconstructive Procedures. J. Pediatr. Urol. 2014, 10, 368–373. [Google Scholar] [CrossRef]
- Sharifiaghdas, F.; Narouie, B.; Rostaminejad, N.; Hamidi Madani, M.; Manteghi, M.; Rouientan, H.; Ahmadzade, M.; Dadpour, M. Intravesical Botulinum Toxin-A Injection in Pediatric Overactive Neurogenic Bladder with Detrusor Overactivity: Radiologic and Clinical Outcomes. Urologia 2023, 90, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Danacioglu, Y.O.; Keser, F.; Ersoz, C.; Polat, S.; Avci, A.E.; Kalkan, S.; Silay, M.S. Factors Predicting the Success of Intradetrusor Onabotulinum Toxin-A Treatment in Children with Neurogenic Bladders Due to Myelomeningocele: The Outcomes of a Large Cohort. J. Pediatr. Urol. 2021, 17, 520.e1–520.e7. [Google Scholar] [CrossRef]
- Madec, F.X.; Suply, E.; Forin, V.; Chamond, O.; lalanne, A.; Irtan, S.; Audry, G.; Lallemant, P. Repeated Detrusor Injection of Botulinum Toxin A for Neurogenic Bladder in Children: A Long Term Option? Prog. Urol. 2022, 32, 319–325. [Google Scholar] [CrossRef]
- Hui, C. Safety and Efficacy of Trigonal BTX-A Injections for Children with Neurological Detrusor Overactivity Secondary to Spinal Cord Injury. J. Pediatr. Surg. 2020, 55, 2736–2739. [Google Scholar] [CrossRef] [PubMed]
- Ladi-Seyedian, S.S.; Sharifi-Rad, L.; Kajbafzadeh, A.M. Botulinum Toxin Type A Therapy: Intravesical Injection or Electromotive Drug Administration. Urology 2020, 142, 190–194. [Google Scholar] [CrossRef]
- Greer, T.; Abbott, J.; Breytenbach, W.; McGuane, D.; Barker, A.; Khosa, J.; Samnakay, N. Ten Years of Experience with Intravesical and Intrasphincteric OnabotulinumtoxinA in Children. J. Pediatr. Urol. 2016, 12, 94.e1–94.e6. [Google Scholar] [CrossRef]
- Khan, M.K.; VanderBrink, B.A.; DeFoor, W.R.; Minevich, E.; Jackson, E.; Noh, P.; Reddy, P.P. Botulinum Toxin Injection in the Pediatric Population with Medically Refractory Neuropathic Bladder. J. Pediatr. Urol. 2016, 12, 104.e1–104.e6. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, J.H.; Lee, Y.S.; Han, S.W.; Im, Y.J. Preoperative Urodynamic Factors Predicting Outcome of Botulinum Toxin-A Intradetrusor Injection in Children with Neurogenic Detrusor Overactivity. Urology 2014, 84, 1480–1484. [Google Scholar] [CrossRef]
- Austin, P.F.; Franco, I.; Dobremez, E.; Kroll, P.; Titanji, W.; Geib, T.; Jenkins, B.; Hoebeke, P.B. OnabotulinumtoxinA for the Treatment of Neurogenic Detrusor Overactivity in Children. Neurourol. Urodyn. 2021, 40, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Peyronnet, B.; Even, A.; Capon, G.; De Seze, M.; Hascoet, J.; Biardeau, X.; Baron, M.; Perrouin-Verbe, M.A.; Boutin, J.M.; Saussine, C.; et al. Intradetrusor Injections of Botulinum Toxin A in Adults with Spinal Dysraphism. J. Urol. 2018, 200, 875–880. [Google Scholar] [CrossRef]
- Sekerci, C.A.; Tanidir, Y.; Garayev, A.; Akbal, C.; Tarcan, T.; Simsek, F. Clinical and Urodynamic Results of Repeated Intradetrusor Onabotulinum Toxin A Injections in Refractory Neurogenic Detrusor Overactivity: Up to 5 Injections in a Cohort of Children with Myelodysplasia. Urology 2018, 111, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Peeraully, R.; Lam, C.; Mediratta, N.; Patel, R.; Williams, A.; Shenoy, M.; Fraser, N. Intradetrusor Injection of Botulinum Toxin A in Children: A 10-Year Single Centre Experience. Int. Urol. Nephrol. 2019, 51, 1321–1327. [Google Scholar] [CrossRef]
- Righetto, M.; Mancini, M.; Modonutti, D.; Calpista, A.; Beltrami, P.; Dal Moro, F. Patients with renal transplant and moderate-to-severe LUTS benefit from urodynamic evaluation and early transurethral resection of the prostate. World J. Urol. 2021, 39, 4397–4404. [Google Scholar] [CrossRef] [PubMed]
- Mohajerzadeh, L.; Tabari, A.K.; Atqiaee, K.; Vosoughi, A.; Lotfollahzadeh, S. The Effects of Botulinum Toxin Injection on Urodynamic Changes in Pediatric Population with Neurospastic Bladder: First Trial in Iran. J. Pediatr. Surg. 2020, 55, 2517–2520. [Google Scholar] [CrossRef]
- Tarcan, T.; Akbal, C.; Şekerci, C.A.; Top, T.; Şimşek, F. Intradetrusor Injections of Onabotulinum Toxin-A in Children with Urinary Incontinence due to Neurogenic Detrusor Overactivity Refractory to Antimuscarinic Treatment. Korean J. Urol. 2014, 55, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Marte, A. Onabotulinumtoxin A for Treating Overactive/Poor Compliant Bladders in Children and Adolescents with Neurogenic Bladder Secondary to Myelomeningocele. Toxins 2013, 5, 16–24. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Chang, S.-J.; Yang, S.S.-D.; Hsu, C.-K. Botulinum Toxin Injection for Medically Refractory Neurogenic Bladder in Children: A Systematic Review. Toxins 2021, 13, 447. [Google Scholar] [CrossRef]
- Softness, K.A.; Thaker, H.; Theva, D.; Rajender, A.; Cilento, B.G.; Bauer, S.B. Onabotulinumtoxin A (Botox): A Reasonable Alternative for Refractory Neurogenic Bladder Dysfunction in Children and Young Adults. Neurourol. Urodyn. 2021, 40, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).