Characteristics of Snakebite-Related Infection in French Guiana
Abstract
:1. Introduction
2. Results
2.1. Clinical and Biological Manifestations at Admission
2.2. Initial Management
2.3. Wound Infection
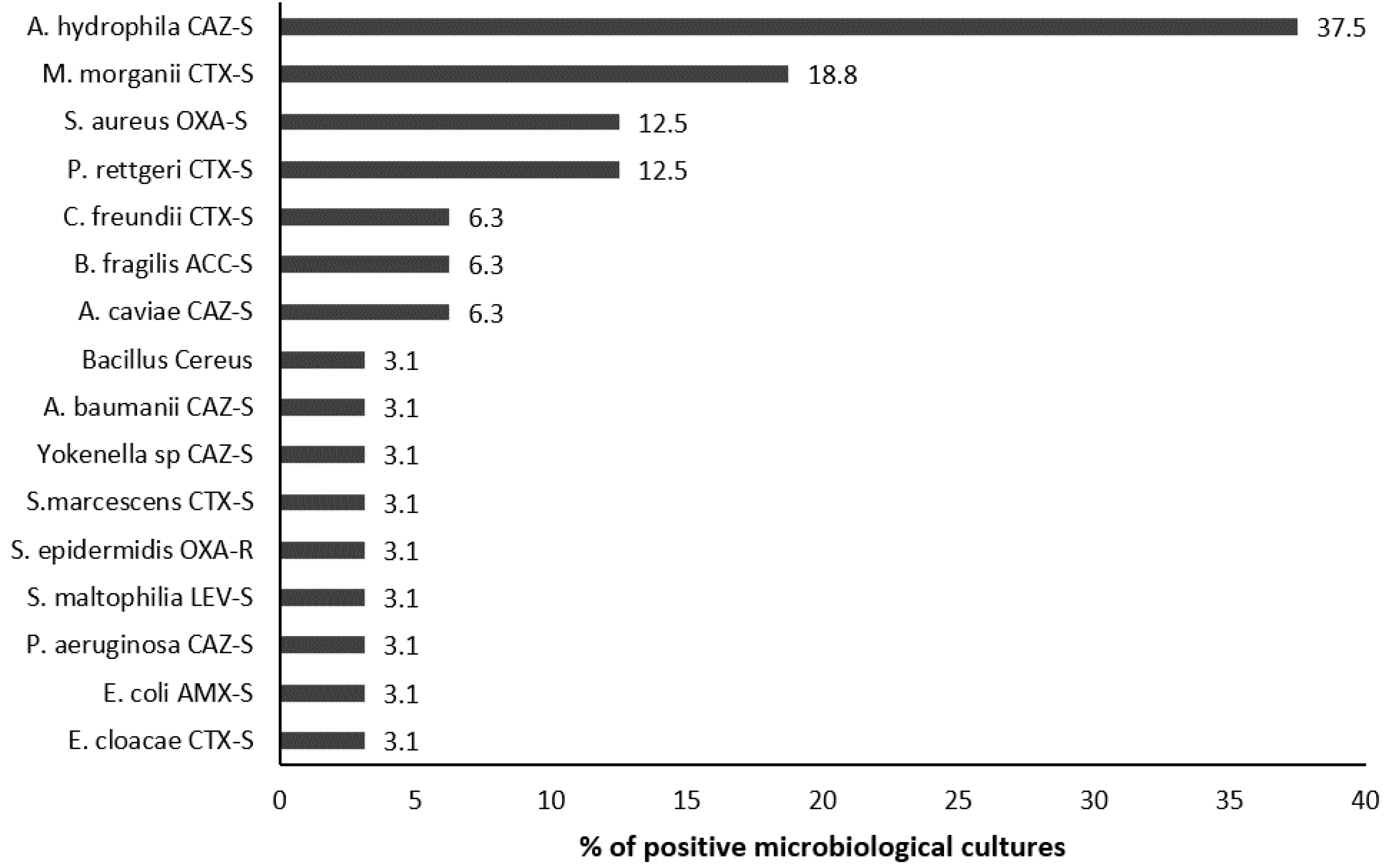
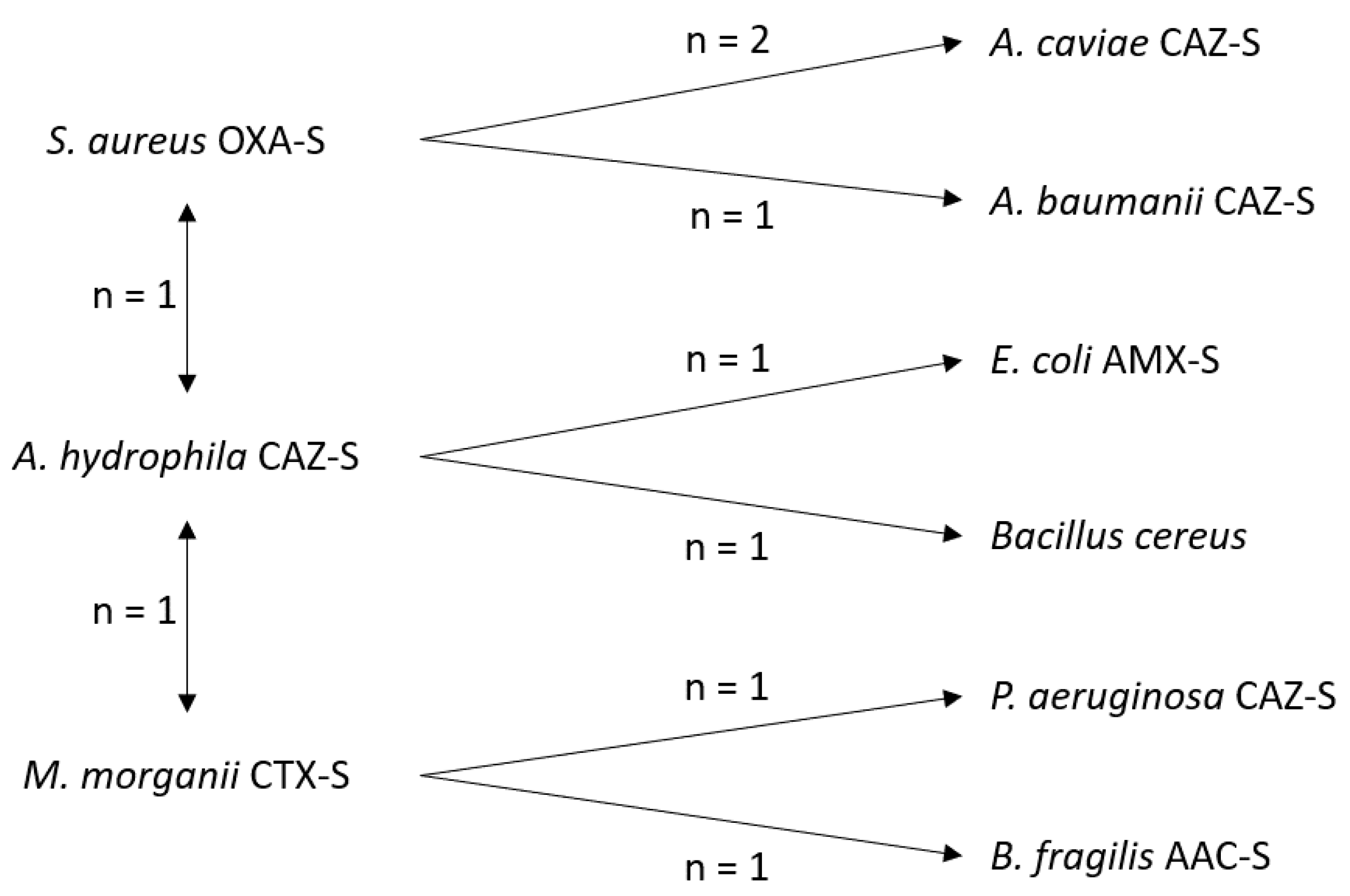
2.4. Microbiological Analyses
2.5. Management of Wound Infection
2.6. Independent Factors Associated with Wound Infection
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Management of Snakebite Envenoming
5.2. Diagnosis and Management of Snakebite-Related Wound Infection
5.3. Ethical Statement
5.4. Definitions
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mutricy, R.; Heckmann, X.; Douine, M.; Marty, C.; Jolivet, A.; Lambert, V.; Perotti, F.; Boels, D.; Larréché, S.; Chippaux, J.-P.; et al. High Mortality Due to Snakebites in French Guiana: Time Has Come to Re-Evaluate Medical Management Protocols. PLoS Negl. Trop. Dis. 2018, 12, e0006482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallel, H.; Mayence, C.; Houcke, S.; Mathien, C.; Mehdaoui, H.; Gutiérrez, J.M.; Megarbane, B.; Hommel, D.; Resiere, D. Severe Snakebite Envenomation in French Guiana: When Antivenom Is Not Available. Toxicon 2018, 146, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Resiere, D.; Monteiro, W.; Houcke, S.; Pujo, J.M.; Mathien, C.; Mayence, C.; Neviere, R.; Hommel, D.; de Almeida Gonçalves Sachett, J.; Mehdaoui, H.; et al. Bothrops Snakebite Envenomings in the Amazon Region. Curr. Trop. Med. Rep. 2020, 7, 48–60. [Google Scholar] [CrossRef]
- Garg, A.; Sujatha, S.; Garg, J.; Acharya, N.S.; Chandra Parija, S. Wound Infections Secondary to Snakebite. J. Infect. Dev. Ctries. 2009, 3, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Wagener, M.; Naidoo, M.; Aldous, C. Wound Infection Secondary to Snakebite. South Afr. Med. J. 2017, 107, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Resiere, D.; Mehdaoui, H.; Névière, R.; Olive, C.; Severyns, M.; Beaudoin, A.; Florentin, J.; Brouste, Y.; Banydeen, R.; Cabié, A.; et al. Infectious Complications Following Snakebite by Bothrops Lanceolatus in Martinique: A Case Series. Am. J. Trop. Med. Hyg. 2020, 102, 232–240. [Google Scholar] [CrossRef]
- Resiere, D.; Gutiérrez, J.M.; Névière, R.; Cabié, A.; Hossein, M.; Kallel, H. Antibiotic Therapy for Snakebite Envenoming. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190098. [Google Scholar] [CrossRef]
- Chen, C.-M.; Wu, K.-G.; Chen, C.-J.; Wang, C.-M. Bacterial Infection in Association with Snakebite: A 10-Year Experience in a Northern Taiwan Medical Center. J. Microbiol. Immunol. Infect. 2011, 44, 456–460. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.-C.; Liu, P.-Y.; Hung, D.-Z.; Lai, W.-C.; Huang, S.-T.; Hung, Y.-M.; Yang, C.-C. Bacteriology of Naja Atra Snakebite Wound and Its Implications for Antibiotic Therapy. Am. J. Trop. Med. Hyg. 2016, 94, 1129–1135. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Hsueh, J.-H.; Liu, W.-C.; Yang, K.-C.; Hsu, K.-C.; Lin, C.-T.; Ho, Y.-Y.; Chen, L.-W. Contributing Factors for Complications and Outcomes in Patients With Snakebite: Experience in a Medical Center in Southern Taiwan. Ann. Plast. Surg. 2017, 78, S31–S36. [Google Scholar] [CrossRef]
- Sachett, J.A.G.; da Silva, I.M.; Alves, E.C.; Oliveira, S.S.; Sampaio, V.S.; do Vale, F.F.; Romero, G.A.S.; dos Santos, M.C.; Marques, H.O.; Colombini, M.; et al. Poor Efficacy of Preemptive Amoxicillin Clavulanate for Preventing Secondary Infection from Bothrops Snakebites in the Brazilian Amazon: A Randomized Controlled Clinical Trial. PLoS Negl. Trop. Dis. 2017, 11, e0005745. [Google Scholar] [CrossRef]
- Résière, D.; Olive, C.; Kallel, H.; Cabié, A.; Névière, R.; Mégarbane, B.; Gutiérrez, J.M.; Mehdaoui, H. Oral Microbiota of the Snake Bothrops Lanceolatus in Martinique. Int. J. Environ. Res. Public Health 2018, 15, 2122. [Google Scholar] [CrossRef] [Green Version]
- Glaser, H.S.R. Bactericidal Activity of Crotalus Venom in Vitro. Copeia 1948, 1948, 245–247. [Google Scholar] [CrossRef]
- Aloof-Hirsch, S.; de Vries, A.; Berger, A. The Direct Lytic Factor of Cobra Venom: Purification and Chemical Characterization. Biochim. Biophys. Acta 1968, 154, 53–60. [Google Scholar] [CrossRef]
- Bustillo, S.; Leiva, L.C.; Acosta, O.; Bal de Kier Joffé, E.; Gorodner, J.O. Antimicrobial Activity of Bothrops Alternatus Venom from the Northeast of Argentine. Rev. Latinoam. De Microbiol. 2008, 50, 79–82. [Google Scholar]
- Blaylock, R.S. Antibacterial Properties of KwaZulu Natal Snake Venoms. Toxicon 2000, 38, 1529–1534. [Google Scholar] [CrossRef]
- Kallel, H.; Hommel, D.; Mehdaoui, H.; Megarbane, B.; Resiere, D. Snakebites in French Guiana: Conclusions of an International Symposium. Toxicon 2018, 146, 91–94. [Google Scholar] [CrossRef]
- Jorge, M.T.; Ribeiro, L.A.; Da Silva, M.L.R.; Kusano, E.J.U.; de Mendonça, J.S. Microbiological Studies of Abscesses Complicating Bothrops Snakebite in Humans: A Prospective Study. Toxicon 1994, 32, 743–748. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, 147–159. [Google Scholar] [CrossRef]
- Lam, K.K.; Crow, P.; Ng, K.H.L.; Shek, K.C.; Fung, H.T.; Ades, G.; Grioni, A.; Tan, K.S.; Yip, K.T.; Lung, D.C.; et al. A Cross-Sectional Survey of Snake Oral Bacterial Flora from Hong Kong, SAR, China. Emerg. Med. J. 2011, 28, 107–114. [Google Scholar] [CrossRef]
- Jorge, M.T.; de Mendonça, J.S.; Ribeiro, L.A.; da Silva, M.L.R.; Kusano, E.J.U.; Cordeiro, C.L. Bacterial Flora of the Oral Cavity, Fangs and Venom of Bothrops Jararaca: Possible Source of Infection at the Local Bite. Rev. Do Inst. De Med. Trop. De São Paulo 1990, 32, 6–10. [Google Scholar] [CrossRef]
- Shaikh, I.K.; Dixit, P.P.; Pawade, B.S.; Potnis-Lele, M.; Kurhe, B.P. Assessment of Cultivable Oral Bacterial Flora from Important Venomous Snakes of India and Their Antibiotic Susceptibilities. Curr. Microbiol. 2017, 74, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Saravia-Otten, P.; Gutierrez, J.M.; Arvidson, S.; Thelestam, M.; Flock, J.-I. Increased Infectivity of Staphylococcus Aureus in an Experimental Model of Snake Venom-Induced Tissue Damage. J. Infect. Dis. 2007, 196, 748–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallel, H.; Resiere, D.; Houcke, S.; Hommel, D.; Pujo, J.M.; Martino, F.; Carles, M.; Mehdaoui, H. Critical Care Medicine in the French Territories in the Americas: Current Situation and Prospects. Rev. Panam. De Salud Pública 2021, 45, e46. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-W.; Wang, J.-D.; Huang, J.-A.; Hu, S.-Y.; Wang, L.-M.; Tsan, Y.-T. Wound Infections Secondary to Snakebite in Central Taiwan. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 272–276. [Google Scholar] [CrossRef] [Green Version]
- Ki, V.; Rotstein, C. Bacterial Skin and Soft Tissue Infections in Adults: A Review of Their Epidemiology, Pathogenesis, Diagnosis, Treatment and Site of Care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Cefalu, J.E.; Barrier, K.M.; Davis, A.H. Wound Infections in Critical Care. Crit. Care Nurs. Clin. 2017, 29, 81–96. [Google Scholar] [CrossRef]
- da Siva, A.M.; Monteiro, W.M.; Bernarde, P.S. Popular Names for Bushmaster (Lachesis Muta) and Lancehead (Bothrops Atrox) Snakes in the Alto Juruá Region: Repercussions for Clinical-Epidemiological Diagnosis and Surveillance. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180140. [Google Scholar] [CrossRef]
- da Silva, J.L.; da Siva, A.M.; do Amaral, G.L.G.; Ortega, G.; Monteiro, W.M.; Bernarde, P.S. The Deadliest Snake According to Ethnobiological Perception of the Population of the Alto Juruá Region, Western Brazilian Amazonia. Rev. Soc. Bras. Med. Trop. 2019, 53, e20190305. [Google Scholar] [CrossRef] [Green Version]
- Soussy, C.J.; Carret, G.; Cavallo, J.D.; Chardon, H.; Chidiac, C.; Choutet, P.; Courvalin, P.; Dabernat, H.; Drugeon, H.; Dubreuil, L.; et al. Antibiogram Committee of the French Microbiology Society. Report 2000–2001. Pathol. Biol. 2000, 48, 832–871. [Google Scholar]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Muñoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F.; Bock, S.A.; Branum, A.; Brown, S.G.A.; Camargo, C.A.; Cydulka, R.; Galli, S.J.; et al. Second Symposium on the Definition and Management of Anaphylaxis: Summary Report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. J. Allergy Clin. Immunol. 2006, 117, 391–397. [Google Scholar] [CrossRef] [PubMed]

| Total Population | Wound Infection | Without Wound Infection | p | ||||
|---|---|---|---|---|---|---|---|
| Nb | Result | Nb | Result | Nb | Result | ||
| Age (years) | 172 | 41 (28–52) | 55 | 46 (31.7–54.6) | 117 | 40 (25.4–50.8) | 0.340 |
| Gender, male | 172 | 119 (69.2%) | 55 | 39 (70.9%) | 117 | 80 (68.4%) | 0.737 |
| BMI | 119 | 23.8 (21–26.9) | 37 | 23.5 (20.3–26.9) | 82 | 23.8 (21.3–26.9) | 0.462 |
| Medical history | 172 | 42 (24.4%) | 55 | 12 (21.8%) | 117 | 30 (25.6%) | |
| Arterial hypertension | 172 | 13 (8%) | 55 | 2 (3.6%) | 117 | 11 (9.4%) | 0.182 |
| Alcohol abuse | 172 | 6 (3%) | 55 | 1 (1.8%) | 117 | 5 (4.3%) | 0.413 |
| Snake bite (SB) | |||||||
| Time from SB to hospital (hh:mm) | 171 | 9:00 (2:02–21:00) | 55 | 12:00 (3:30–22:58) | 116 | 7:07 (1:43–19:03) | 0.064 |
| Identification of the snake | 172 | 78 (45.3%) | 55 | 21 (38.2%) | 117 | 57 (48.7%) | 0.195 |
| Anatomic site of the bite | |||||||
| Upper limb | 172 | 34 (19.8%) | 55 | 10 (18.2%) | 117 | 24 (20.5%) | 0.328 |
| Lower limb | 172 | 137 (79.7%) | 55 | 44 (80%) | 117 | 93 (79.5%) | |
| Head | 172 | 1 (0.6%) | 55 | - | 117 | 1 (0.8%) | - |
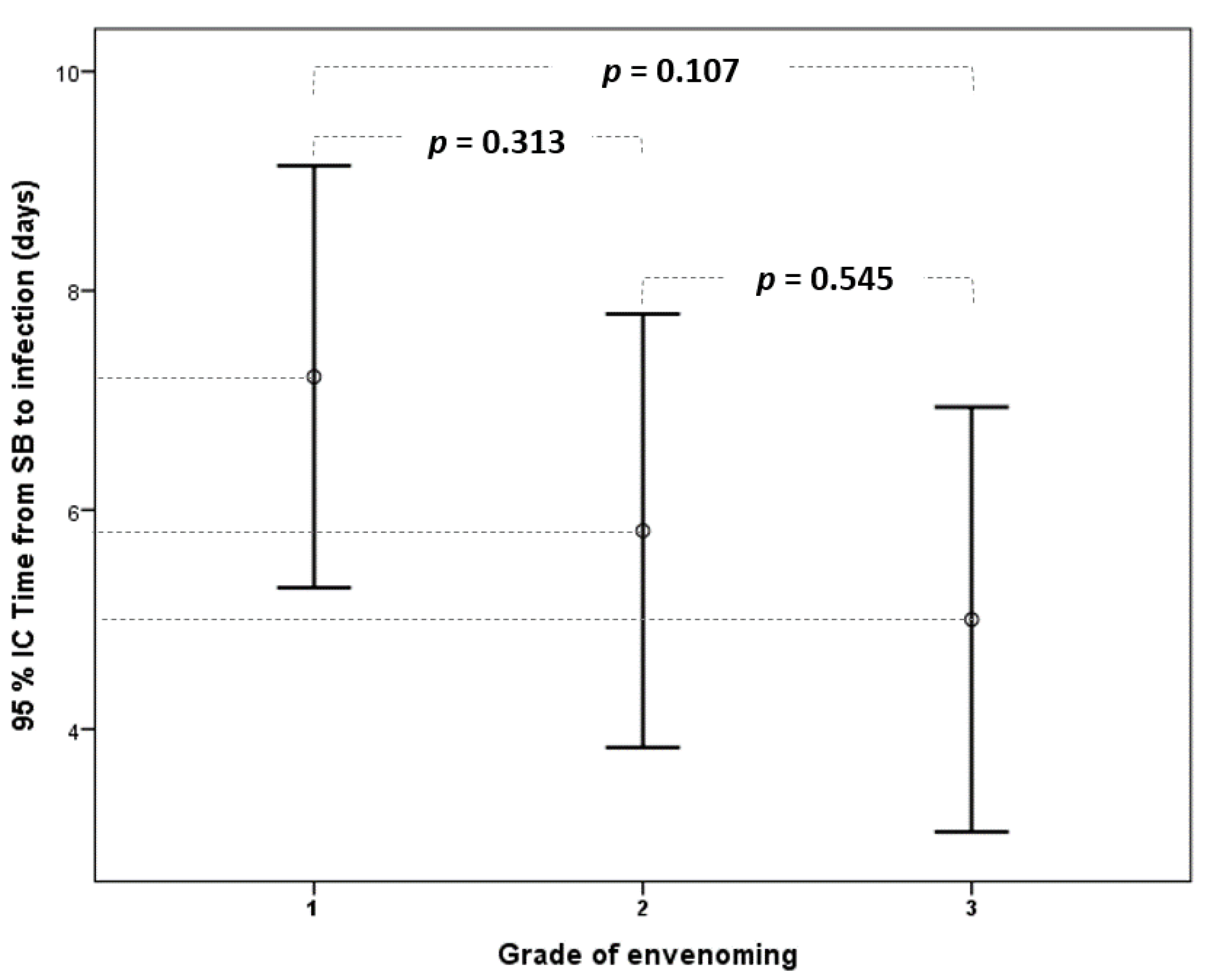
| Grade of envenoming | |||||||
| Grade 1 | 172 | 74 (43%) | 55 | 14 (25.5%) | 117 | 60 (51.3%) | Reference |
| Grade 2 | 172 | 60 (34.9%) | 55 | 21 (38.2%) | 117 | 39 (33.3%) | 0.035 |
| Grade 3 | 172 | 38 (22.1%) | 55 | 20 (36.4%) | 117 | 18 (15.4%) | <0.001 |
| Clinical presentation at admission | |||||||
| Edema | 172 | 165 (95.9%) | 55 | 55 (100%) | 117 | 110 (94%) | 0.064 |
| Local hemorrhage | 172 | 22 (12.8%) | 55 | 8 (14.5%) | 117 | 14 (12%) | 0.637 |
| Necrosis | 172 | 21 (12.2%) | 55 | 17 (30.9%) | 117 | 4 (3.4%) | <0.001 |
| Blisters | 172 | 22 (12.8%) | 55 | 14 (25.5%) | 117 | 8 (6.8%) | 0.001 |
| Pain | 171 | 165 (96.5%) | 54 | 53 (98.1%) | 117 | 112 (95.7%) | 0.424 |
| Temperature (°C) | 165 | 36.9 (36.5–37.3) | 49 | 37 (36.6–37.4) | 116 | 36.9 (36.5–37.3) | 0.045 |
| Shock | 172 | 2 (1.2%) | 55 | 1 (1.8%) | 117 | 1 (0.9%) | 0.583 |
| Acute renal injury | 172 | 28 (16.3%) | 55 | 12 (21.8%) | 117 | 16 (13.7%) | 0.177 |
| Time to normal renal function (days) | 28 | 5 (28–10) | 12 | 4 (2–23) | 16 | 6 (2–10) | 0.958 |
| Systemic hemorrhage (SH) | 172 | 24 (14%) | 55 | 7 (12.7%) | 117 | 17 (14.5%) | 0.750 |
| Time from SB to SH (hours) | 24 | 5 (0–24) | 7 | 4 (1–8) | 17 | 7 (0–24) | 0.125 |
| Biological parameters at admission | |||||||
| Fibrinolysis | 172 | 151 (87.8%) | 55 | 45 (81.8%) | 117 | 106 (90.6%) | 0.101 |
| Thrombocytopenia | 172 | 49 (28.5%) | 55 | 25 (45.5%) | 117 | 24 (20.5%) | 0.001 |
| Hemolysis | 172 | 38 (22.1%) | 55 | 15 (27.3%) | 117 | 23 (19.7%) | 0.262 |
| Rhabdomyolysis | 172 | 54 (31.4%) | 55 | 26 (47.3%) | 117 | 28 (23.9%) | 0.002 |
| White blood count (/mm3) | 167 | 10.4 (8.3–13.6) | 53 | 12 (9.4–14.6) | 114 | 10.2 (8–13.2) | 0.016 |
| C-reactive protein (mg/L) | 153 | 8.7 (2.8–32.2) | 46 | 27.6 (5.6–83.2) | 107 | 6.4 (2.7–19.6) | 0.002 |
| Procalcitonin (µmol/L) | 10 | 0.1 (0–0.5) | 0 | - | 10 | 0.1 (0–0.5) | - |
| Nb | Result | |
|---|---|---|
| Abscess | 55 | 38 (69.1%) |
| Necrotizing fasciitis | 55 | 9 (16.4%) |
| Cellulitis | 55 | 12 (21.8%) |
| Isolated microorganism | 55 | 32 (58.2%) |
| Time from SB to WI (days) | 55 | 6 (3–8) |
| Antibiotic duration for WI (days) | 36 | 7 (5–8) |
| Total Population | Wound Infection | Without Wound Infection | p | ||||
|---|---|---|---|---|---|---|---|
| Nb | Result | Nb | Result | Nb | Result | ||
| Renal replacement therapy | 172 | 9 (5.2%) | 55 | 4 (7.3%) | 117 | 5 (4.3%) | 0.410 |
| Noradrenaline | 172 | 2 (1.2%) | 55 | 1 (1.8%) | 117 | 1 (0.9%) | 0.583 |
| Mechanical ventilation | 172 | 3 (1.7%) | 55 | 2 (3.6%) | 117 | 1 (0.9%) | 0.194 |
| Antibiotics at admission | 172 | 63 (36.6%) | 55 | 22 (40%) | 117 | 41 (35%) | 0.391 |
| Antivenom therapy (AV) | 172 | 115 (66.9%) | 55 | 32 (58.2%) | 117 | 83 (70.9%) | 0.097 |
| Time from SB to AV | 83 | 9:00 (5:22–20:40) | 22 | 10:00 (6:00–19:45) | 61 | 9:00 (5:15–21:00) | 1 |
| Adverse reaction to AV | 116 | 20 (17.2%) | 33 | 7 (21.2%) | 83 | 13 (15.7%) | 0.284 |
| Transfusion | 172 | 17 (9.9%) | 55 | 9 (16.4%) | 117 | 8 (6.8%) | 0.51 |
| Surgery | 172 | 48 (27.9%) | 55 | 43 (78.2%) | 117 | 5 (4.3%) | <0.001 |
| Necrosectomy | 48 | 23 (47.9%) | 43 | 21 (48.8%) | 5 | 2 (40%) | 0.476 |
| Time from SB to surgery (days) | 48 | 7 (5–9) | 43 | 7 (5–9) | 5 | 5 (3–8) | 0.865 |
| Length of stay in ICU (days) | 138 | 3 (3–5) | 43 | 4 (3–8) | 95 | 3 (3–4) | 0.370 |
| Length of hospital stay (days) | 172 | 9 (6–13) | 55 | 14 (11–23) | 117 | 7 (5–11) | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Wound Infection (n = 55) | Without Wound Infection (n = 117) | p | OR | 95% CI | p |
| Blisters | 14 (25.5%) | 8 (6.8%) | 0.001 | 2.812 | 0.909–8.697 | 0.073 |
| Thrombocytopenia | 25 (45.5%) | 24 (20.5%) | 0.001 | 3.374 | 1.594–7.163 | 0.002 |
| Necrosis | 17 (30.9%) | 4 (3.4%) | <0.001 | 13.15 | 4.042–42.841 | <0.001 |
| Rhabdomyolysis | 26 (47.3%) | 28 (23.9%) | 0.002 | 2.294 | 1.015–5.187 | 0.046 |
| Grade 2 | 21 (38.2%) | 39 (33.3%) | 0.035 | 1.947 | 0.822–4.615 | 0.130 |
| Grade 3 | 20 (36.4%) | 18 (15.4%) | <0.001 | 0.875 | 0.263–2.916 | 0.828 |
| Grade | ||||
|---|---|---|---|---|
| 1 (Mild) | 2 (Moderate) | 3 (Severe) | ||
| Coagulation disorder | present | present | present | |
| Local symptoms | Pain | present | present | present |
| Swelling | Not exceeding elbow or knee | Exceeding elbow or knee | Beyond the root of the limb | |
| Blister | absent | present | present | |
| Necrosis | absent | absent | present | |
| Local or systemic bleeding | absent | present | present | |
| Systemic manifestations (Hypotension, renal injury, coma, respiratory failure, …) | absent | absent | present | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houcke, S.; Resiere, D.; Lontsingoula, G.R.; Cook, F.; Lafouasse, P.; Pujo, J.M.; Demar, M.; Matheus, S.; Hommel, D.; Kallel, H. Characteristics of Snakebite-Related Infection in French Guiana. Toxins 2022, 14, 89. https://doi.org/10.3390/toxins14020089
Houcke S, Resiere D, Lontsingoula GR, Cook F, Lafouasse P, Pujo JM, Demar M, Matheus S, Hommel D, Kallel H. Characteristics of Snakebite-Related Infection in French Guiana. Toxins. 2022; 14(2):89. https://doi.org/10.3390/toxins14020089
Chicago/Turabian StyleHoucke, Stéphanie, Dabor Resiere, Guy Roger Lontsingoula, Fabrice Cook, Pierre Lafouasse, Jean Marc Pujo, Magalie Demar, Severine Matheus, Didier Hommel, and Hatem Kallel. 2022. "Characteristics of Snakebite-Related Infection in French Guiana" Toxins 14, no. 2: 89. https://doi.org/10.3390/toxins14020089
APA StyleHoucke, S., Resiere, D., Lontsingoula, G. R., Cook, F., Lafouasse, P., Pujo, J. M., Demar, M., Matheus, S., Hommel, D., & Kallel, H. (2022). Characteristics of Snakebite-Related Infection in French Guiana. Toxins, 14(2), 89. https://doi.org/10.3390/toxins14020089





