Effect of Orally Administered Collagen Peptides from Bovine Bone on Skin Aging in Chronologically Aged Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Collagen Peptides (CPs) Preparation
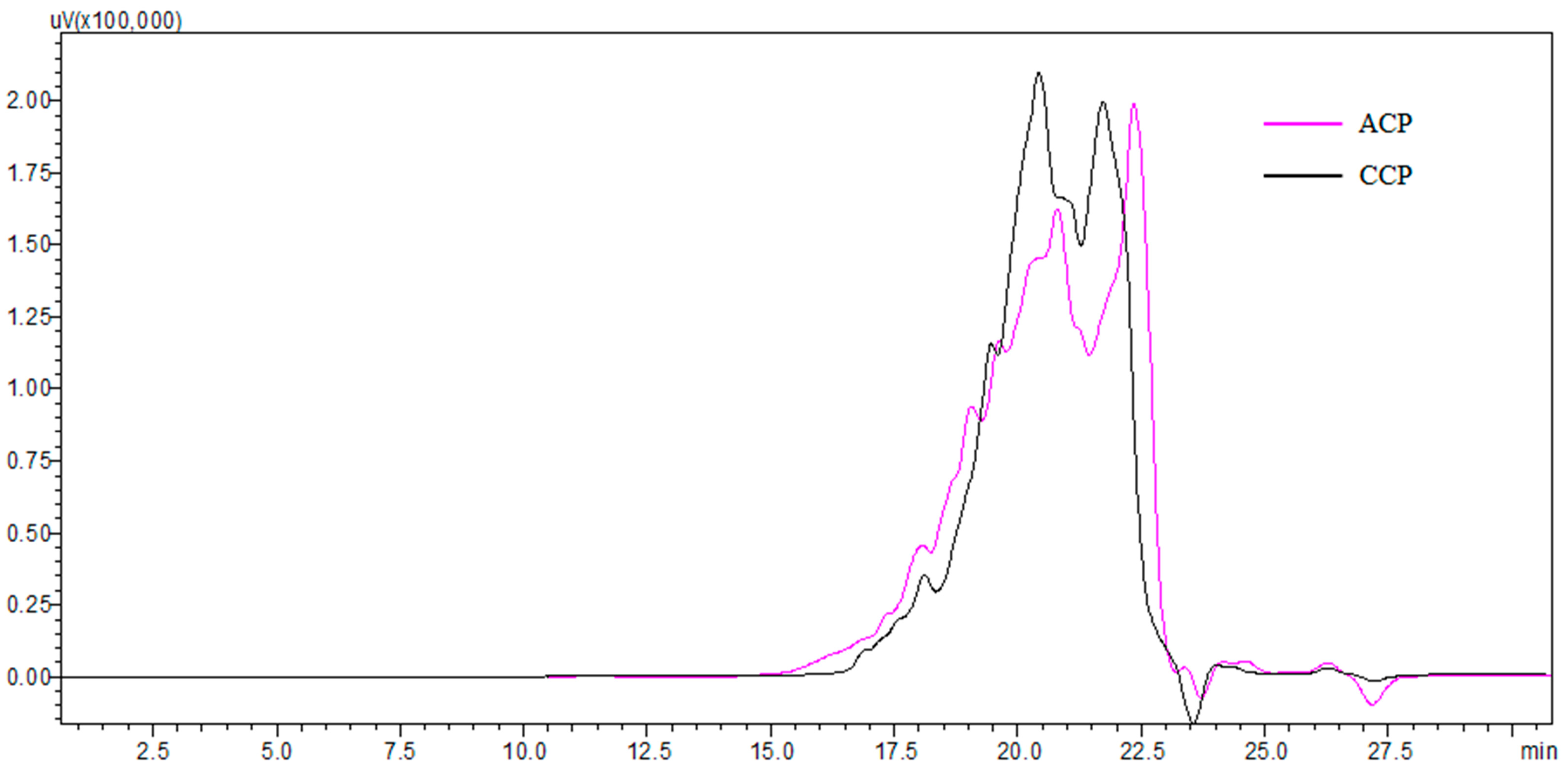
2.3. Molecular Weight Distribution
2.4. Amino Acid Composition
2.5. Animals, Diets, and Treatments
2.6. Measurement of Degree of Skin Laxity (DSL)
2.7. Measurement of Spleen Index (SI) and Thymus Index (TI)
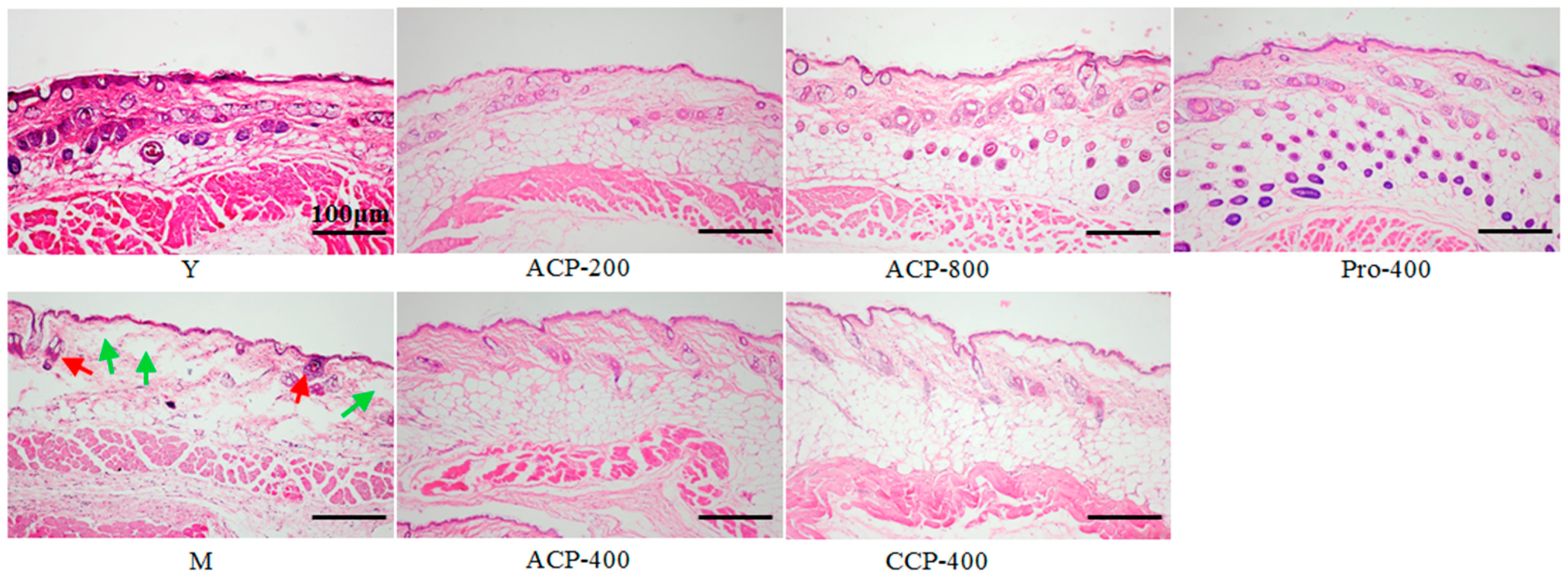
2.8. Histological Analysis
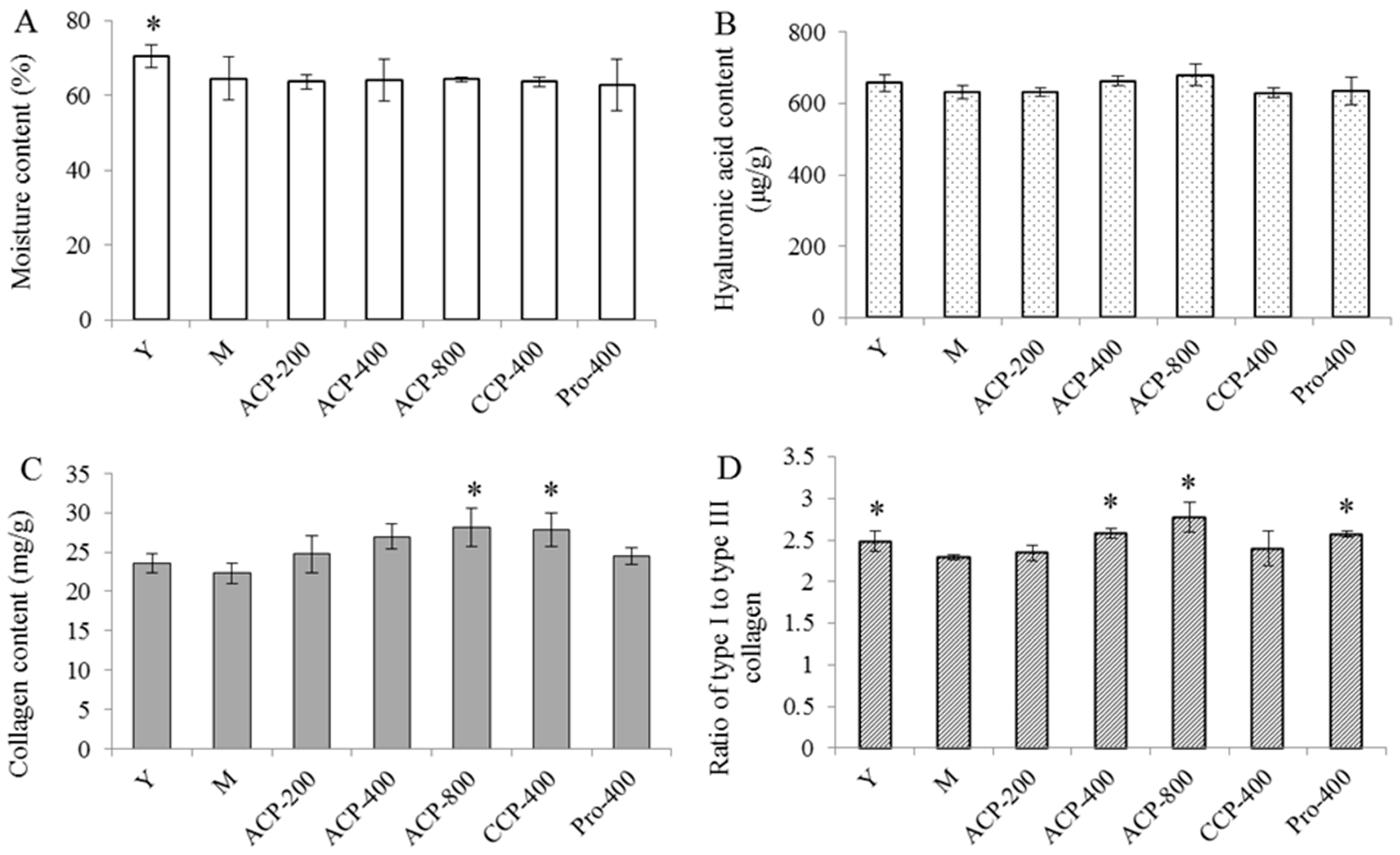
2.9. Measurement of Moisture Content
2.10. Determination of Hyaluronic Acid (HA) Content
2.11. Determination of Collagen Content
2.12. Ratio of Type I to Type III Collagen
2.13. Antioxidant Indicators Analysis
2.14. Statistical Analysis
3. Results
3.1. Characterization of Collagen Peptides
3.2. Degree of Skin Laxity
3.3. Body Weight, Spleen Index (SI) and Thymus Index (TI)
3.4. Skin Histology
3.5. Skin Components
3.6. Antioxidant Indicators
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Durai, P.C.; Thappa, D.M.; Kumari, R.; Malathi, M. Aging in elderly: Chronological versus photoaging. Indian J. Dermatol. 2012, 57, 343–352. [Google Scholar] [PubMed]
- Helfrich, Y.R.; Sachs, D.L.; Voorhees, J.J. Overview of skin aging and photoaging. Dermatol. Nurs. 2008, 20, 177–183. [Google Scholar] [PubMed]
- Dobos, G.; Lichterfeld, A.; Blume-Peytavi, U.; Kottner, J. Evaluation of skin ageing: A systematic review of clinical scales. Brit. J. Dermatol. 2015, 172, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Zhu, Q.; Li, T.; Lu, H.; Wei, N.; Huang, Y.; Shi, R.; Ma, X.; Wang, X.; et al. Anti-skin-aging effect of epigallocatechin gallate by regulating epidermal growth factor receptor pathway on aging mouse model induced by d-Galactose. Mech. Ageing Dev. 2017, 164, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Townley, J.P.; Barnes, T.M.; Greive, K.A. An antiaging skin care system containing alpha hydroxy acids and vitamins improves the biomechanical parameters of facial skin. Clin. Cosmet. Investig. Dermatol. 2015, 8, 9–17. [Google Scholar] [PubMed]
- Latreille, J.; Kesse-Guyot, E.; Malvy, D.; Andreeva, V.; Galan, P.; Tschachler, E.; Hercberg, S.; Guinot, C.; Ezzedine, K. Association between dietary intake of n-3 polyunsaturated fatty acids and severity of skin photoaging in a middle-aged Caucasian population. J. Dermatol. Sci. 2013, 72, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Fanian, F.; Mac-Mary, S.; Jeudy, A.; Lihoreau, T.; Messikh, R.; Ortonne, J.P.; Sainthillier, J.M.; Elkhyat, A.; Guichard, A.; Kenari, K.H.; et al. Efficacy of micronutrient supplementation on skin aging and seasonal variation: A randomized, placebo-controlled, double-blind study. Clin. Interv. Aging 2013, 8, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Satoh, T.; Wakabayashi, H.; Yamauchi, K.; Abe, F.; Nomura, Y. Oral administration of bovine lactoferrin attenuates ultraviolet B-induced skin photodamage in hairless mice. J. Dairy Sci. 2014, 97, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Hou, H.; Zhao, X.; Zhang, Z.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J. Food Sci. 2009, 74, H183–H188. [Google Scholar] [CrossRef] [PubMed]
- Zague, V. A new view concerning the effects of collagen hydrolysate intake on skin properties. Arch. Dermatol. Res. 2008, 300, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Oesser, S.; Adam, M.; Babel, W.; Seifert, J. Oral administration of 14C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J. Nutr. 1999, 129, 1891–1895. [Google Scholar] [PubMed]
- Fan, J.; Zhuang, Y.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients 2013, 5, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Li, B.; Zhang, Z.; Xue, C.; Yu, G.; Wang, J.; Bao, Y.; Bu, L.; Sun, J.; Peng, Z. Moisture absorption and retention properties, and activity in alleviating skin photodamage of collagen polypeptide from marine fish skin. Food Chem. 2012, 135, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Meng, M.; Cheng, X.; Li, B.; Wang, C. The effect of collagen hydrolysates from silver carp (Hypophthalmichthys molitrix) skin on UV-induced photoaging in mice: Molecular weight affects skin repair. Food Funct. 2017, 8, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Segger, D.; Degwert, J.; Schunck, M.; Zague, V.; Oesser, S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: A double-blind, placebo-controlled study. Skin Pharmacol. Physiol. 2014, 27, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.R.; Park, J. Ingestion of BioCell Collagen®, a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin. Interv. Aging 2012, 7, 267–273. [Google Scholar] [PubMed]
- Nur, A.T.; Che, M.Y.; Rn, R.M.H.; Aina, M.A.; Amin, I. Use of principal component analysis for differentiation of gelatine sources based on polypeptide molecular weights. Food Chem. 2014, 151, 286–292. [Google Scholar]
- Ali, M.E.; Sultana, S.; Hamid, S.B.; Hossain, M.A.; Yehya, W.A.; Kader, M.A.; Bhargava, S.K. Gelatin controversies in food, pharmaceuticals and personal care products: Authentication methods, current status and future challenges. Crit. Rev. Food Sci. Nutr. 2016, 29, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Song, S.; Wang, X.; Qin, Y.; Si, S.; Guo, Y. Combined oral administration of bovine collagen peptides with calcium citrate inhibits bone loss in ovariectomized rats. PLoS ONE 2015, 10, e0135019. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sugihara, F.; Suzuki, K.; Inoue, N.; Venkateswarathirukumara, S. A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis. J. Sci. Food Agric. 2015, 95, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Humiski, L.M.; Aluko, R.E. Physicochemical and bitterness properties of enzymatic pea protein hydrolysates. J. Food Sci. 2007, 72, S605–S611. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Ren, X.; Ma, H.; Li, S.; Xu, K.; Oladejo, A.O. Effect of low-frequency ultrasonic-assisted enzymolysis on the physicochemical and antioxidant properties of corn protein hydrolysates. J. Food Qual. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Kanth, S.V.; Venba, R.; Madhan, B.; Chandrababu, N.K.; Sadulla, S. Studies on the influence of bacterial collagenase in leather dyeing. Dyes Pigment. 2008, 76, 338–347. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Hsu, F.W.; Chang, H.S.; Lin, L.C.; Sakata, R. Effect of different acids on the extraction of pepsin-solubilised collagen containing melanin from silky fowl feet. Food Chem. 2009, 113, 563–567. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wu, H.Y.; Wu, D.; Wang, Y.Y.; Chang, J.H.; Zhai, Z.B.; Meng, A.M.; Liu, P.X.; Zhang, L.A.; Fan, F.Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010, 5, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Chung, J.H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, L.; Luo, Y.; Zhang, S.; Li, B. Effects of collagen peptides intake on skin ageing and platelet release in chronologically aged mice revealed by cytokine array analysis. J. Cell. Mol. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ohara, H.; Ito, K.; Iida, H.; Matsumoto, H. Improvement in the moisture content of the stratum corneum following 4 weeks of collagen hydrolysate ingestion. Nippon Shokuhin Kogaku Kaishi 2009, 56, 137–145. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nakayama, J. Upregulated expression of La ribonucleoprotein domain family member 6 and collagen type I gene following water-filtered broad-spectrum near-infrared irradiation in a 3-dimensional human epidermal tissue culture model as revealed by microarray analysis. Australas. J. Dermatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sadick, N.S.; Harth, Y. A 12-week clinical and instrumental study evaluating the efficacy of a multisource radiofrequency home-use device for wrinkle reduction and improvement in skin tone, skin elasticity, and dermal collagen content. J. Cosmet. Laser Ther. 2016, 18, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Desprez, P.Y.; Campisi, J.; Velarde, M.C. Cell autonomous and non-autonomous effects of senescent cells in the skin. J. Investig. Dermatol. 2015, 135, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Boschnakow, A. Chronological and photoaging of the human sebaceous gland. Clin. Exp. Dermatol. 2001, 26, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Lovell, C.R.; Smolenski, K.A.; Duance, V.C.; Light, N.D.; Young, S.; Dyson, M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987, 117, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Haratake, A.; Watase, D.; Fujita, T.; Setoguchi, S.; Matsunaga, K.; Takata, J. Effects of oral administration of collagen peptides on skin collagen content and its underlying mechanism using a newly developed low collagen skin mice model. J. Funct. Foods 2015, 16, 174–182. [Google Scholar] [CrossRef]
- Ohara, H.; Ichikawa, S.; Matsumoto, H.; Akiyama, M.; Fujimoto, N.; Kobayashi, T.; Tajima, S. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J. Dermatol. 2010, 37, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Kamiyama, M.; Shimizu, M.; Kamiyama, S.; Taguchi, Y.; Sone, H.; Morimatsu, F.; Shirakawa, H.; Furukawa, Y.; Komai, M. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J. Agric. Food Chem. 2010, 58, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, T.M.; Wilhelm, K.P. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part I: Cellular and molecular perspectives of skin ageing. Int. J. Cosmet. Sci. 2008, 30, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fisher, G.J. Ultraviolet (UV) light irradiation induced signal transduction in skin photoaging. J. Dermatol. Sci. 2015, 1, S1–S8. [Google Scholar] [CrossRef]
- Liang, J.; Pei, X.; Zhang, Z.; Wang, N.; Wang, J.; Li, Y. The protective effects of long-term oral administration of marine collagen hydrolysate from chum salmon on collagen matrix homeostasis in the chronological aged skin of sprague-dawley male rats. J. Food Sci. 2010, 75, H230–H238. [Google Scholar] [CrossRef] [PubMed]
- Zague, V.; de Freitas, V.; Rosa, M.D.C.; de Castro, G.Á.; Jaeger, R.G.; Machado-Santelli, G.M. Collagen hydrolysate intake increases skin collagen expression and suppresses matrix metalloproteinase 2 activity. J. Med. Food 2011, 14, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Maltese, G.; Psefteli, P.; Rizzo, B.; Srivastava, S.; Gnudi, L.; Mann, G.E.; Siow, R.C. The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J. Cell. Mol. Med. 2017, 21, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Park, S.Y.; Hwang, E.; Zhang, M.; Seo, S.A.; Lin, P.; Yi, T.H. Thymus vulgaris alleviates UVB irradiation induced skin damage via inhibition of MAPK/AP-1 and activation of Nrf2-ARE antioxidant system. J. Cell. Mol. Med. 2017, 21, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, Y.; Iwai, K.; Morimatsu, F.; Iwamoto, T.; Mori, T.; Oda, C.; Taira, T.; Park, E.Y.; Nakamura, Y.; Sato, K. Effect of Prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J. Agric. Food Chem. 2009, 57, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Bouglé, D.; Bouhallab, S. Dietary bioactive peptides: Human studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhou, Y.; Lu, J.; Chen, A.; Li, Y.; Zheng, G. Enzymatic hydrolysis of Alaska pollack (Theragra chalcogramma) skin and antioxidant activity of the resulting hydrolysate. J. Sci. Food Agric. 2010, 90, 635–640. [Google Scholar] [CrossRef] [PubMed]
| Amino Acid | Relative Content (g/100 g) a,b | |
|---|---|---|
| ACP | CCP | |
| Asp | 5.68 | 5.17 |
| Glu | 10.51 | 11.53 |
| Ser | 3.38 | 3.17 |
| Gly | 19.84 | 21.28 |
| His | 3.27 | 2.97 |
| Thr | 7.90 | 8.51 |
| Ala | 4.09 | 4.68 |
| Pro | 12.47 | 12.18 |
| Arg | 8.61 | 8.47 |
| Tyr | 2.28 | 1.79 |
| Val | 3.06 | 2.90 |
| Met | 1.79 | 1.27 |
| Cys | 2.39 | 2.08 |
| Ile | 4.11 | 3.93 |
| Leu | 0.70 | 0.08 |
| Phe | 9.31 | 9.36 |
| Lys | 0.61 | 0.62 |
| Total | 100.00 | 100.00 |
| Group a | Degree of Skin Laxity (DSL, mm) | ||||
|---|---|---|---|---|---|
| Week 0 | Week 2 | Week 4 | Week 6 | Week 8 | |
| Y | 14.90 ± 2.32 * | 19.15 ± 1.57 * | 19.75 ± 1.03 * | 19.60 ± 0.91 * | 20.40 ± 1.48 * |
| M | 22.25 ± 2.40 | 22.40 ± 1.67 | 23.80 ± 2.25 | 22.50 ± 1.30 | 23.00 ± 1.26 |
| ACP-200 | 23.05 ± 0.56 | 23.50 ± 1.64 | 23.11 ± 1.26 | 22.00 ± 1.31 | 21.22 ± 1.47 * |
| ACP-400 | 22.45 ± 1.88 | 21.55 ± 1.78 | 21.65 ± 1.57 | 20.15 ± 1.34 * | 20.25 ± 1.47 * |
| ACP-800 | 22.30 ± 1.81 | 22.72 ± 2.06 | 22.05 ± 2.26 | 21.60 ± 1.78 | 19.80 ± 0.90 * |
| CCP-400 | 22.40 ± 1.57 | 22.35 ± 1.41 | 21.25 ± 2.03 | 22.10 ± 1.92 | 19.95 ± 1.65 * |
| Pro-400 | 21.65 ± 1.23 | 21.30 ± 2.28 | 22.80 ± 0.81 | 21.70 ± 1.00 | 19.75 ± 1.44 * |
| Group a | Body Weight (g) | Spleen Index b (mg/g) | Thymus Index b (mg/g) | ||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 2 | Week 4 | Week 6 | Week 8 | |||
| Y | 28.28 ± 0.73 | 30.27 ± 1.62 | 31.71 ± 1.30 | 32.63 ± 1.81 | 32.70 ± 1.52 | 3.48 ± 0.69 | 1.82 ± 0.39 |
| M | 47.14 ± 5.39 | 45.55 ± 4.67 | 45.55 ± 4.69 | 46.52 ± 3.40 | 46.33 ± 3.81 | 3.83 ± 1.14 | 1.55 ± 0.53 |
| ACP-200 | 47.41 ± 5.47 | 46.28 ± 6.07 | 46.44 ± 4.41 | 47.41 ± 4.13 | 46.83 ± 3.34 | 3.72 ± 0.06 | 1.71 ± 0.65 |
| ACP-400 | 47.50 ± 5.57 | 46.32 ± 4.91 | 46.90 ± 4.48 | 46.64 ± 4.90 | 46.35 ± 4.90 | 3.24 ± 1.24 | 2.06 ± 0.80 |
| ACP-800 | 47.75 ± 5.67 | 46.88 ± 4.68 | 48.36 ± 7.42 | 49.40 ± 6.63 | 47.39 ± 6.57 | 3.86 ± 1.39 | 1.55 ± 0.62 |
| CCP-400 | 47.92 ± 5.73 | 46.82 ± 4.61 | 48.13 ± 6.55 | 48.84 ± 4.88 | 47.38 ± 4.94 | 3.87 ± 0.94 | 1.62 ± 0.66 |
| Pro-400 | 47.24 ± 5.39 | 46.96 ± 6.14 | 46.32 ± 5.86 | 47.65 ± 5.90 | 47.44 ± 5.69 | 3.64 ± 1.04 | 2.02 ± 0.86 |
| Group a | SOD (U/mg Protein) | CAT (U/mg Protein) | MDA Equivalents (nmol/mg Protein) |
|---|---|---|---|
| Y | 36.594 ± 1.142 * | 10.412 ± 1.143 * | 2.209 ± 0.278 * |
| M | 26.877 ± 3.880 | 4.650 ± 1.582 | 3.135 ± 0.302 |
| ACP-200 | 38.746 ± 0.753 * | 8.324 ± 0.890 * | 2.347 ± 0.209 * |
| ACP-400 | 39.823 ± 3.410 * | 11.327 ± 1.096 * | 2.261 ± 0.107 * |
| ACP-800 | 40.036 ± 4.820 * | 12.012 ± 0.752 * | 2.154 ± 0.325 * |
| CCP-400 | 39.796 ± 1.211 * | 9.354 ± 1.856 * | 2.204 ± 0.201 * |
| Pro-400 | 32.646 ± 1.691 | 3.318 ± 0.665 | 2.456 ± 0.316 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Zhang, S.; Zhang, L.; Li, B. Effect of Orally Administered Collagen Peptides from Bovine Bone on Skin Aging in Chronologically Aged Mice. Nutrients 2017, 9, 1209. https://doi.org/10.3390/nu9111209
Song H, Zhang S, Zhang L, Li B. Effect of Orally Administered Collagen Peptides from Bovine Bone on Skin Aging in Chronologically Aged Mice. Nutrients. 2017; 9(11):1209. https://doi.org/10.3390/nu9111209
Chicago/Turabian StyleSong, Hongdong, Siqi Zhang, Ling Zhang, and Bo Li. 2017. "Effect of Orally Administered Collagen Peptides from Bovine Bone on Skin Aging in Chronologically Aged Mice" Nutrients 9, no. 11: 1209. https://doi.org/10.3390/nu9111209
APA StyleSong, H., Zhang, S., Zhang, L., & Li, B. (2017). Effect of Orally Administered Collagen Peptides from Bovine Bone on Skin Aging in Chronologically Aged Mice. Nutrients, 9(11), 1209. https://doi.org/10.3390/nu9111209






