Recovery Effects of Oral Administration of Glucosylceramide and Beet Extract on Skin Barrier Destruction by UVB in Hairless Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Test Sample and Schedule
2.4. Measurement of Water Contents of Stratum Corneum, and TEWL
2.5. Collection of Mouse Stratum Corneum
2.6. Extraction Method and Lipid Analysis by HPTLC
2.7. Measurement of Stratum Corneum Mass
2.8. Hematoxylin and Eosin Staining
2.9. Measurement of Epidermis Thickness
2.10. Data and Statistical Analysis
3. Results
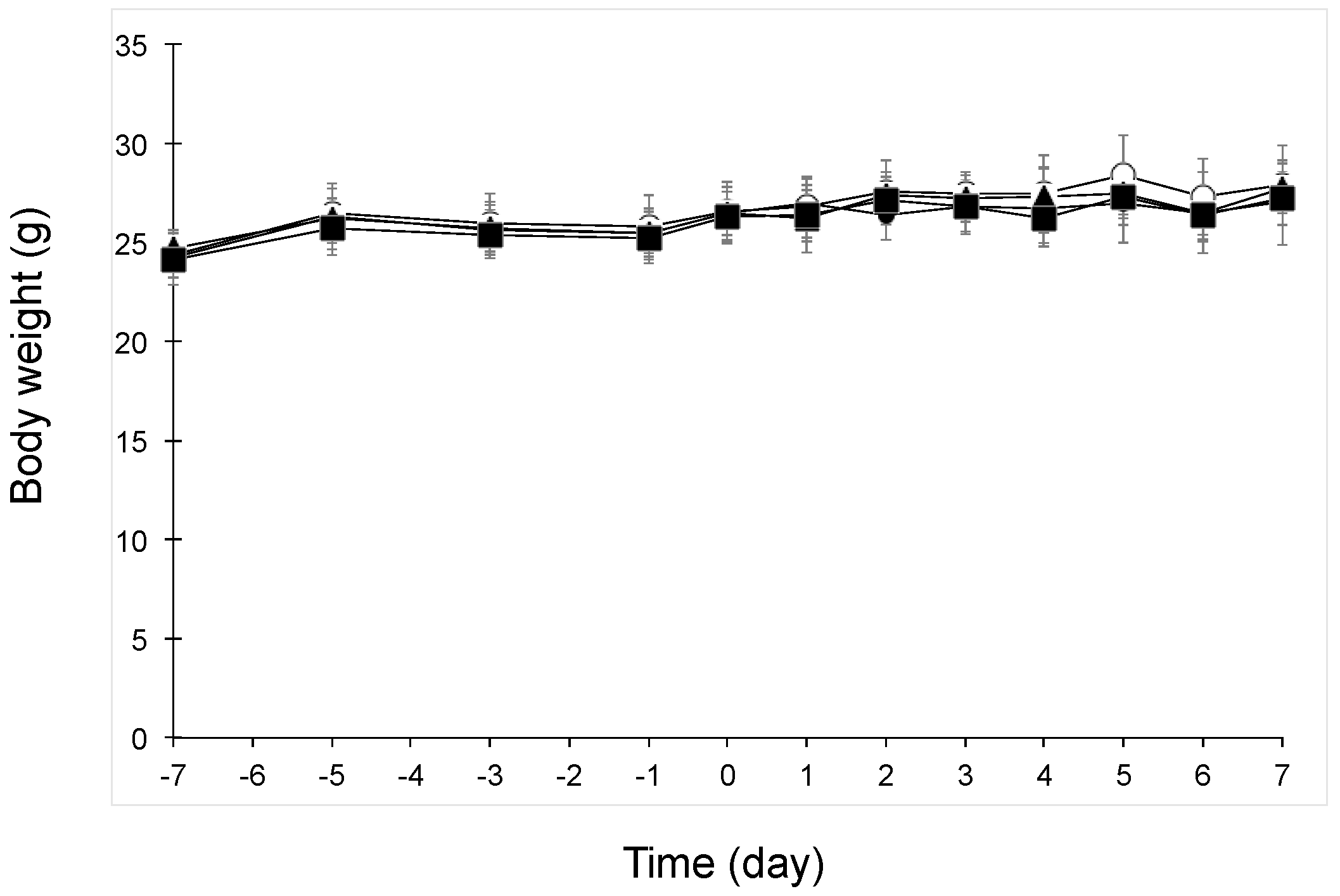
3.1. Body Weight Change after Oral Administration of Beet GlcCer or Beet Extract in UVB-Irradiated Mice
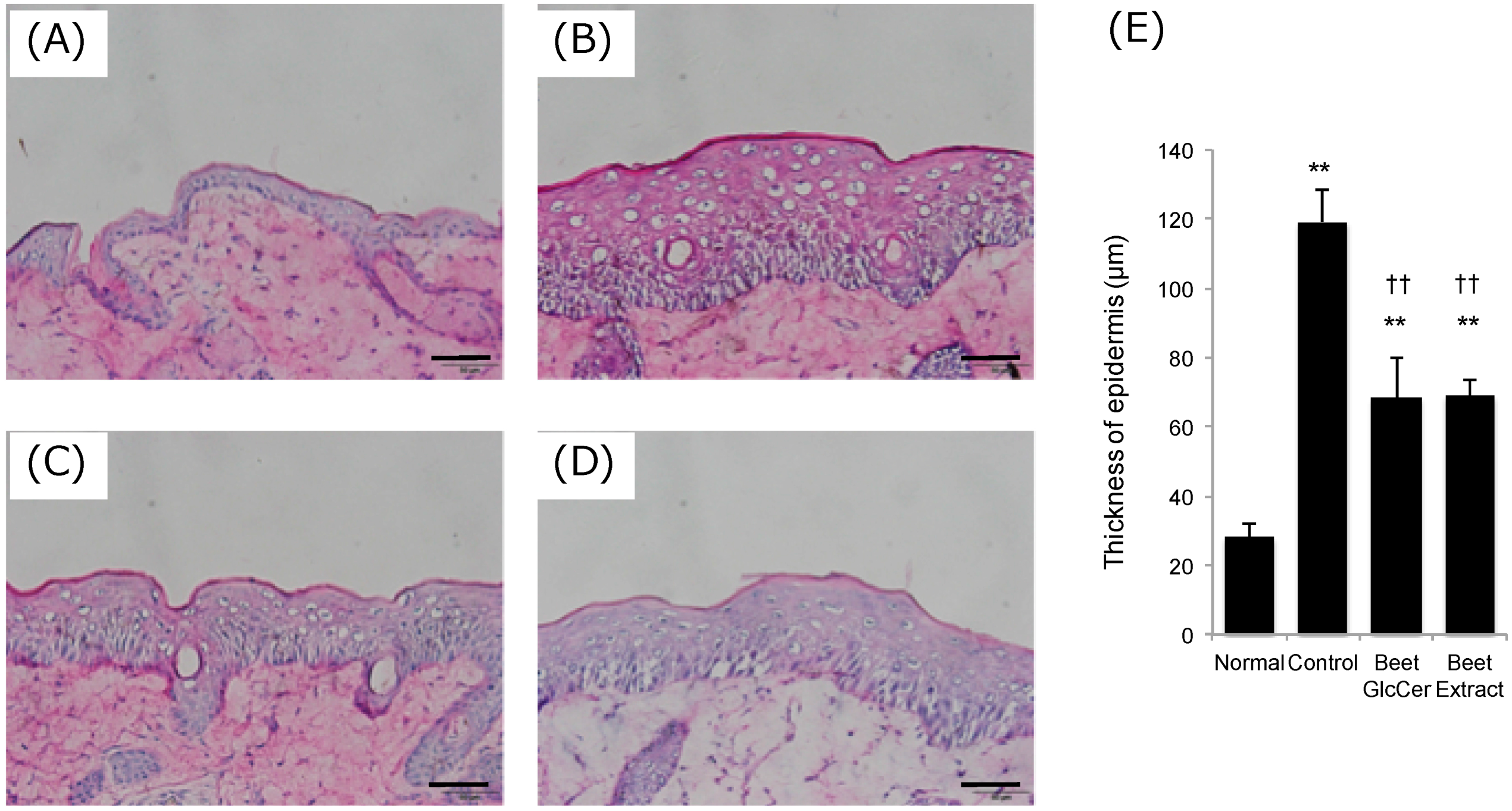
3.2. Epidermal Hyperplasia Following UVB Irradiation
3.3. Comparison of Ceramide Contents in Stratum Corneum among Mouse Groups
3.4. TEWL and Water Contents in Stratum Corneum
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Gray, G.M.; Yardley, H.J. Different populations of pig epidermal cells: Isolation and lipid composition. J. Lipid Res. 1975, 16, 441–447. [Google Scholar] [PubMed]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Serizawa, S.; Ito, M.; Sato, Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch. Dermatol. Res. 1991, 283, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Mitsutake, S.; Ishikawa, J.; Takagi, Y.; Akiyama, M.; Shimizu, H.; Tomiyama, T.; Igarashi, Y. Dietary glucosylceramide improves skin barrier function in hairless mice. J. Dermatol. Sci. 2006, 44, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Nakano, Y.; Ueda, O.; Mori, H.; Nakashima, M.; Noda, A.; Ishizaki, C.; Mizoguchi, M. Oral Intake of Glucosylceramide Improves Relatively Higher Level of Transepidermal Water Loss in Mice and Healthy Human Subjects. J. Health Sci. 2008, 54, 559–566. [Google Scholar] [CrossRef]
- Kawano, K.-I.; Umemura, K. Oral intake of beet extract provides protection against skin barrier impairment in hairless mice. Phytother. Res. 2013, 27, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Sugawara, T.; Hirose, M.; Aida, K.; Sakai, S.; Fujii, A.; Hirata, T. Dietary sphingolipids improve skin barrier functions via the upregulation of ceramide synthases in the epidermis. Exp. Dermatol. 2012, 21, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Shimada, H.; Uchiyama, T.; Ueda, O.; Nakashima, M.; Matsuoka, Y. Dietary glucosylceramide enhances cornified envelope formation via transglutaminase expression and involucrin production. Lipids 2011, 46, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Kishimoto, S.; Tezuka, Y.; Nishigori, H.; Nomoto, K.; Hamada, U.; Yonei, Y. Double-blind study on effects of glucosyl ceramide in beet extract on skin elasticity and fibronectin production in human dermal fibroblasts. Anti-Aging Mag. 2010, 7, 129–142. [Google Scholar] [CrossRef]
- Shirakura, Y.; Kikuchi, K.; Matsumura, K.; Mukai, K.; Mitsutake, S.; Igarashi, Y. 4,8-Sphingadienine and 4-hydroxy-8-sphingenine activate ceramide production in the skin. Lipids Health Dis. 2012, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Kawada, C.; Hasegawa, T.; Watanabe, M.; Nomura, Y. Dietary glucosylceramide enhances tight junction function in skin epidermis via induction of claudin-1. Biosci. Biotechnol. Biochem. 2013, 77, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Aida, K.; Takakuwa, N.; Kinoshita, M.; Sugawara, H.; Imai, H.; Ono, J.; Ohnishi, M. Properties and physiological effects of plant cerebroside species as functional lipids. Adv. Res. Plant Lipids 2003, 233–236. [Google Scholar] [CrossRef]
- Takakuwa, N.; Saito, K.; Oda, Y. Content of Functional Lipid Ceramide in Crop Tissues and By-Products from Their Processing. Atarashii Kenkyu Seika 2005, 173–177. [Google Scholar]
- Ra, J.; Lee, D.E.; Kim, S.H.; Jeong, J.W.; Ku, H.K.; Kim, T.Y.; Choi, I.D.; Jeung, W.; Sim, J.H.; Ahn, Y.T. Effect of oral administration of Lactobacillus plantarum HY7714 on epidermal hydration in ultraviolet B-irradiated hairless mice. J. Microbiol. Biotechnol. 2014, 24, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Haratake, A.; Uchida, Y.; Schmuth, M.; Tanno, O.; Yasuda, R.; Epstein, J.H.; Elias, P.M.; Holleran, W.M. UVB-induced alterations in permeability barrier function: Roles for epidermal hyperproliferation and thymocyte-mediated response. J. Investig. Dermatol. 1997, 108, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Haruta-Ono, Y.; Ueno, H.; Ueda, N.; Kato, K.; Yoshioka, T. Investigation into the dosage of dietary sphingomyelin concentrate in relation to the improvement of epidermal function in hairless mice. Anim. Sci. J. 2012, 83, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lee, S.M.; Lee, D.Y.; Son, Y.; Chung, D.K.; Baek, N.I.; Kim, J. A glycosidic spinasterol from Koreana stewartia promotes procollagen production and inhibits matrix metalloproteinase-1 expression in UVB-irradiated human dermal fibroblasts. Biol. Pharm. Bull. 2011, 34, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.Y.; Sun, Z.W.; Shin, H.S.; Lee, D.G.; Yi, T.H. The protective effects of fucosterol against skin damage in UVB-irradiated human dermal fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Kupper, T.S.; Chua, A.O.; Flood, P.; McGuire, J.; Gubler, U. Interleukin 1 gene expression in cultured human keratinocytes is augmented by ultraviolet irradiation. J. Clin. Investig. 1987, 80, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Kono, T.; Sauder, D.N.; McKenzie, R.C. IL-8 gene expression and production in human keratinocytes and their modulation by UVB. J. Investig. Dermatol. 1993, 101, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.M.; Sharma, M.R.; Werth, V.P. UVB and proinflammatory cytokines synergistically activate TNF-alpha production in keratinocytes through enhanced gene transcription. J. Investig. Dermatol. 2009, 129, 994–1001. [Google Scholar] [CrossRef] [PubMed]


| Group | UVB Irradiation (mJ/cm2) | Composition of Test Sample (mg/Mouse/Day) | ||
|---|---|---|---|---|
| GlcCer | Beet Ethanol Extract | Processed Starch | ||
| Normal | None | 0 | 0 | 22 |
| Control | 200 | 0 | 0 | 22 |
| Beet GlcCer | 200 | 0.3 | 0 | 22 |
| Beet Extract | 200 | (0.3) 1 | 8.0 | 22 |
| Events/Day | −7 d | −6 d | −5 d | −4 d | −3 d | −2 d | −1 d | 0 d | 1 d | 2 d | 3 d | 4 d | 5 d | 6 d | 7 d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample administration | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | |
| UV irradiation | ◯ | ||||||||||||||
| Body weight | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | |||
| Water content, TEWL | ◯ | ◯ | ◯ | ||||||||||||
| Ceramide content | ◯ | ||||||||||||||
| HE staining | ◯ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokudome, Y.; Masutani, N.; Uchino, S.; Fukai, H. Recovery Effects of Oral Administration of Glucosylceramide and Beet Extract on Skin Barrier Destruction by UVB in Hairless Mice. Nutrients 2017, 9, 1178. https://doi.org/10.3390/nu9111178
Tokudome Y, Masutani N, Uchino S, Fukai H. Recovery Effects of Oral Administration of Glucosylceramide and Beet Extract on Skin Barrier Destruction by UVB in Hairless Mice. Nutrients. 2017; 9(11):1178. https://doi.org/10.3390/nu9111178
Chicago/Turabian StyleTokudome, Yoshihiro, Noriomi Masutani, Shohei Uchino, and Hisano Fukai. 2017. "Recovery Effects of Oral Administration of Glucosylceramide and Beet Extract on Skin Barrier Destruction by UVB in Hairless Mice" Nutrients 9, no. 11: 1178. https://doi.org/10.3390/nu9111178
APA StyleTokudome, Y., Masutani, N., Uchino, S., & Fukai, H. (2017). Recovery Effects of Oral Administration of Glucosylceramide and Beet Extract on Skin Barrier Destruction by UVB in Hairless Mice. Nutrients, 9(11), 1178. https://doi.org/10.3390/nu9111178




