Impact of Brewers’ Spent Grain-Containing Biscuit on Postprandial Glycaemic Response in Individuals with Metabolic Syndrome: A Crossover Randomised Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. BSG Fermentation and Biscuits Baking
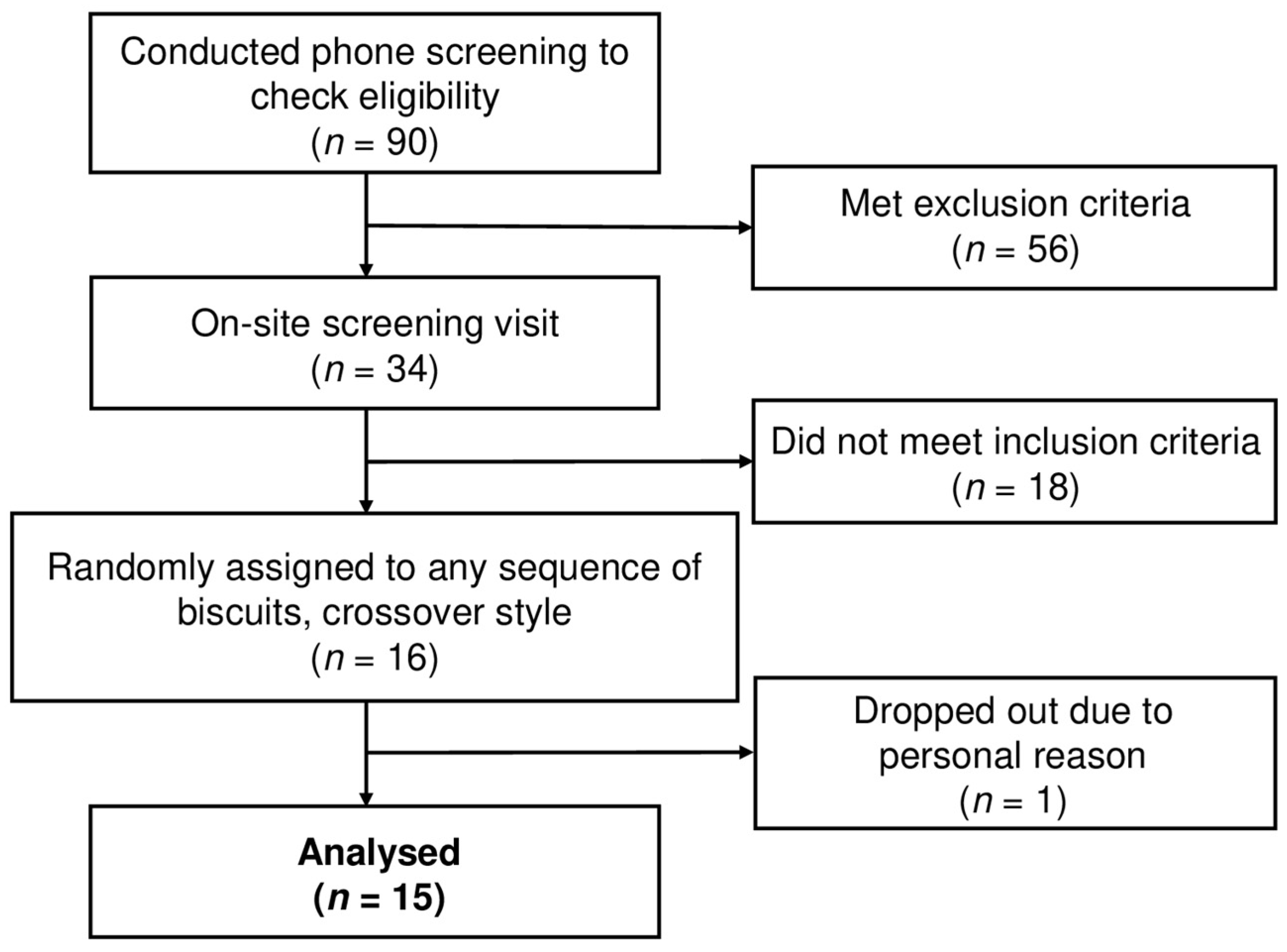
2.2. Study Design and Subject Recruitment
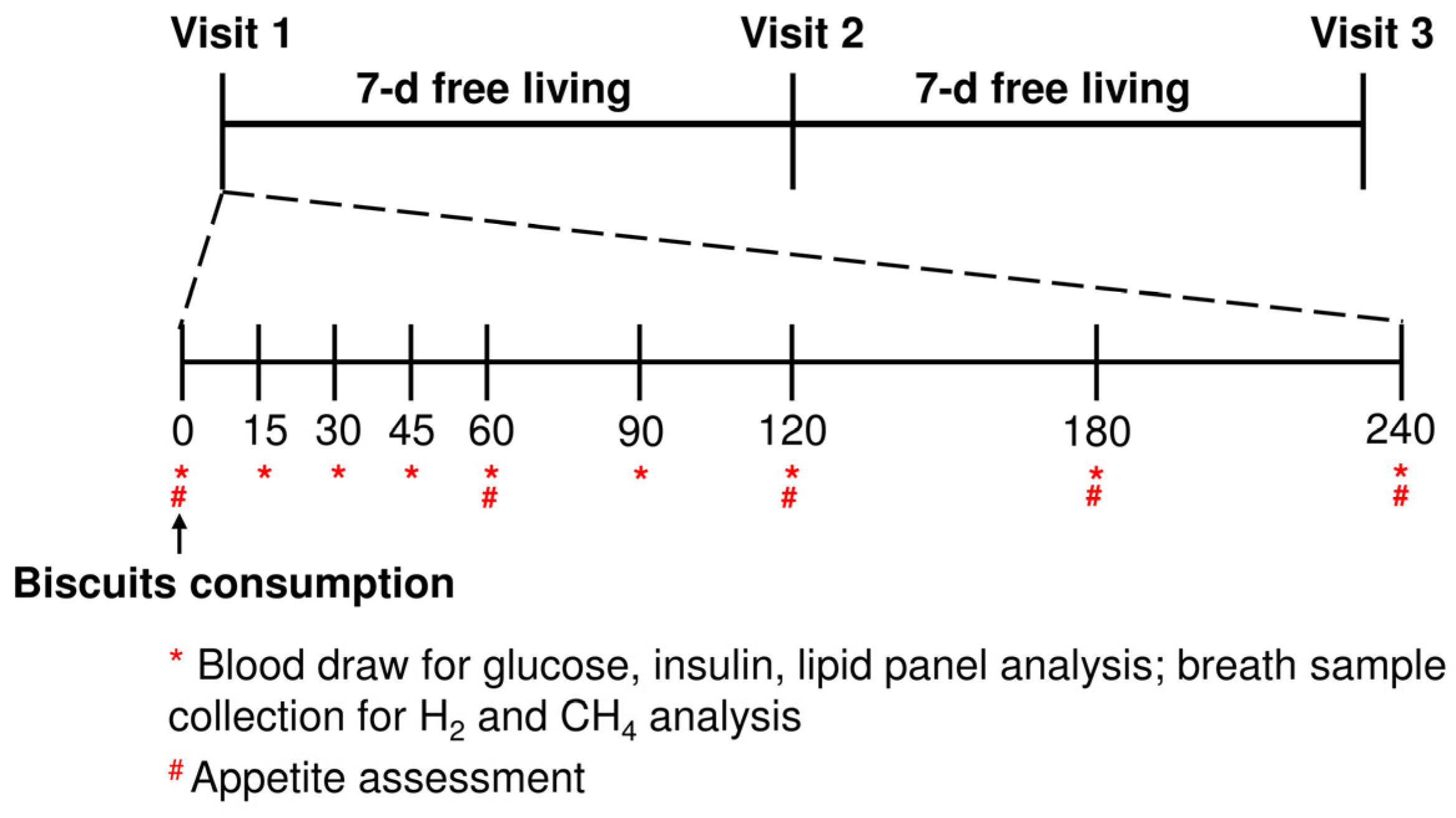
2.3. Study Design
2.4. Anthropometric and Blood Pressure Measurement
2.5. Blood Sample Processing and Biochemical Analysis
2.6. Breath Analysis
2.7. Appetite Sensation
2.8. Power Calculation and Statistical Analysis
3. Results
3.1. Subjects’ Baseline Characteristics
3.2. Postprandial Glucose and Insulin Response
3.3. Postprandial Lipid Panel Response
3.4. Breath Analysis
3.5. Subjective Appetite Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gin, H.; Rigalleau, V. Post-prandial hyperglycemia. Post-prandial hyperglycemia and diabetes. Diabetes Metab. 2000, 26, 265–272. [Google Scholar]
- Balkau, B.; Shipley, M.; Jarrett, R.J.; Pyörälä, K.; Pyörälä, M.; Forhan, A.; Eschwège, E. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men: 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care 1998, 21, 360–367. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Niedowicz, D.M.; Daleke, D.L. The role of oxidative stress in diabetic complications. Cell Biochem. Biophys. 2005, 43, 289–330. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Toh, D.W.K.; Koh, E.S.; Kim, J.E. Lowering breakfast glycemic index and glycemic load attenuates postprandial glycemic response: A systematically searched meta-analysis of randomized controlled trials. Nutrition 2020, 71, 110634. [Google Scholar] [CrossRef] [PubMed]
- Pladevall, M.; Singal, B.; Williams, L.K.; Brotons, C.; Guyer, H.; Sadurni, J.; Falces, C.; Serrano-Rios, M.; Gabriel, R.; Shaw, J.E. A single factor underlies the metabolic syndrome: A confirmatory factor analysis. Diabetes Care 2006, 29, 113–122. [Google Scholar] [CrossRef][Green Version]
- Olawoye, B.; Adeniyi, D.M.; Oyekunle, A.O.; Kadiri, O.; Fawale, S.O. Economic evaluation of cookie made from blend of brewers’ spent grain (BSG), groundnut cake and sorghum flour. Open Agric. 2017, 2, 401–410. [Google Scholar] [CrossRef]
- Petrović, J.S.; Pajin, B.S.; Kocić-Tanackov, S.D.; Pejin, J.D.; Fišteš, A.Z.; Bojanić, N.Đ.; Lončarević, I.S. Quality properties of cookies supplemented with fresh brewer’s spent grain. Food Feed Res. 2017, 44, 57–63. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Jenkins, D.J.; Wolever, T.M.; Rogovik, A.L.; Jovanovski, E.; Božikov, V.; Rahelić, D.; Vuksan, V. Comparable postprandial glucose reductions with viscous fiber blend enriched biscuits in healthy subjects and patients with diabetes mellitus: Acute randomized controlled clinical trial. Croat. Med. J. 2008, 49, 772. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.J.; Eisenmann, J.C.; Norman, G.J.; Ortiz, K.A.; Young, P.C. Dietary fiber and nutrient density are inversely associated with the metabolic syndrome in US adolescents. J. Am. Diet. Assoc. 2011, 111, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Galisteo, M.; Duarte, J.; Zarzuelo, A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J. Nutr. Biochem. 2008, 19, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Dikeman, C.L.; Murphy, M.R.; Fahey, G.C. Dietary Fibers Affect Viscosity of Solutions and Simulated Human Gastric and Small Intestinal Digesta. J. Nutr. 2006, 136, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.L.; Otto, B.; Reich, S.C.; Weickert, M.O.; Steiniger, J.; Machowetz, A.; Rudovich, N.N.; Möhlig, M.; Katz, N.; Speth, M.; et al. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur. J. Clin. Nutr. 2007, 61, 334–341. [Google Scholar] [CrossRef]
- Fărcaş, A.; Tofană, M.; Socaci, S.; Mudura, E.; Scrob, S.N.A.; Salanţă, L.; Mureşan, V. Brewers’ spent grain–A new potential ingredient for functional foods. J. Agroaliment. Process Technol. 2014, 20, 137–141. [Google Scholar]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Lamas, D.L.; Gende, L.B. Valorisation of brewers’ spent grain for the development of novel beverage and food products. Appl. Food Res. 2023, 3, 100314. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Teo, S.Q.; Heng, C.W.; Lee, D.P.S.; Gan, A.X.; Kim, J.E. Impact of solid-state fermented Brewer’s spent grains incorporation in biscuits on nutritional, physical and sensorial properties. LWT 2023, 182, 114840. [Google Scholar] [CrossRef]
- Nuttall, F.Q.; Mooradian, A.D.; Gannon, M.C.; Billington, C.; Krezowski, P. Effect of Protein Ingestion on the Glucose and Insulin Response to a Standardized Oral Glucose Load. Diabetes Care 1984, 7, 465–470. [Google Scholar] [CrossRef]
- Gutierrez-Barrutia, M.B.; Cozzano, S.; Arcia, P.; del Castillo, M.D. In Vitro Digestibility and Bioaccessibility of Nutrients and Non-Nutrients Composing Extruded Brewers’ Spent Grain. Nutrients 2022, 14, 3480. [Google Scholar] [CrossRef]
- Farago, C.V.; de Melo, G.B.; Escher, G.B.; Marcon, M.V.; Granato, D.; Danesi, E.D.G. Cereal bars made from brewers’ spent grain, apple and Spirulina platensis: Antioxidant activity and antihyperglycaemic effects. Res. Soc. Dev. 2021, 10, 5. [Google Scholar]
- Schettino, R.; Verni, M.; Acin-Albiac, M.; Vincentini, O.; Krona, A.; Knaapila, A.; Cagno, R.D.; Gobbetti, M.; Rizzello, C.G.; Coda, R. Bioprocessed Brewers’ Spent Grain Improves Nutritional and Antioxidant Properties of Pasta. Antioxidants 2021, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Bondy, S.C. Dietary fibers and their fermented short-chain fatty acids in prevention of human diseases. Bioact. Carbohydr. Diet. Fibre. 2019, 17, 100170. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kratz, M.; Damman, C.J.; Hullarg, M. Mechanisms Linking the Gut Microbiome and Glucose Metabolism. J. Clin. Endocrinol. Metab. 2016, 101, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hoek, A.M.; Heijboer, A.C.; Corssmit, E.P.; Voshol, P.J.; Romijn, J.A.; Havekes, L.M.; Pijl, H. PYY3–36 reinforces insulin action on glucose disposal in mice fed a high-fat diet. Diabetes 2004, 53, 1949–1952. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- World Health Organization. WHO STEPS Surveillance Manual: The WHO STEPwise Approach to Chronic Disease Risk Factor Surveillance; Report No.: 9241593830; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Bergmeyer, H. Determination with hexokinase and glucose-6-phosphate dehydrogenase. Methods Enzym. Anal. 1997, 3, 1196–1201. [Google Scholar]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Flint, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Raza, G.S.; Maukonen, J.; Makinen, M.; Niemi, P.; Niiranen, L.; Hibberd, A.A.; Poutanen, K.; Buchert, J.; Herzig, K.-H. Hypocholesterolemic Effect of the Lignin-Rich Insoluble Residue of Brewer’s Spent Grain in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2019, 67, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.P.S.; Gan, A.X.; Kim, J.E. Incorporation of biovalorised okara in biscuits: Improvements of nutritional, antioxidant, physical, and sensory properties. LWT 2020, 134, 109902. [Google Scholar] [CrossRef]
- de Carvalho, C.M.; de Paula, T.P.; Viana, L.V.; Machado, V.M.; de Almeida, J.C.; Azevedo, M.J. Plasma glucose and insulin responses after consumption of breakfasts with different sources of soluble fiber in type 2 diabetes patients: A randomized crossover clinical trial. Am. J. Clin. Nutr. 2017, 106, 1238–1245. [Google Scholar] [CrossRef]
- Luhovyy, B.L.; Mollard, R.C.; Yurchenko, S.; Nunez, M.F.; Berengut, S.; Liu, T.T.; Smith, C.E.; Pelkman, C.L.; Anderson, G.H. The Effects of Whole Grain High-Amylose Maize Flour as a Source of Resistant Starch on Blood Glucose, Satiety, and Food Intake in Young Men. J. Food Sci. 2014, 79, H2550–H2556. [Google Scholar] [CrossRef]
- Ullah, H.; Esposito, C.; Piccinocchi, R.; De Lellis, L.F.; Santarcangelo, C.; Minno, A.D.; Baldi, A.; Buccato, D.G.; Khan, A.; Piccinocchi, G.; et al. Postprandial Glycemic and Insulinemic Response by a Brewer’ Spent Grain Extract-Based Food Supplement in Subjects with Slightly Impaired Glucose Tolerance: A Monocentric, Randomized, Cross-Over, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 3916. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Zheng, B.; Li, T.; Liu, R.H. Assessment of the Phenolic Profiles, Hypoglycemic Activity, and Molecular Mechanism of Different Highland Barley (Hordeum vulgare L.) Varieties. Int. J. Mol. Sci. 2020, 21, 1175. [Google Scholar] [CrossRef]
- Steemburgo, T.; Dall’Alba, V.; Almeida, J.; Zelmanovitz, T.; Gross, J.; De Azevedo, M. Intake of soluble fibers has a protective role for the presence of metabolic syndrome in patients with type 2 diabetes. Eur. J. Clin. Nutr. 2009, 63, 127–133. [Google Scholar] [CrossRef]
- Ames, N.; Blewett, H.; Storsley, J.; Thandapilly, S.J.; Zahradka, P.; Taylor, C. A double-blind randomised controlled trial testing the effect of a barley product containing varying amounts and types of fibre on the postprandial glucose response of healthy volunteers. Br. J. Nutr. 2015, 113, 1373–1383. [Google Scholar] [CrossRef]
- Lee, D.P.S.; Gan, A.X.; Sutanto, C.N.; Toh, K.Q.X.; Khoo, C.M.; Kim, J.E. Postprandial glycemic and circulating SCFA concentrations following okara- and biovalorized okara-containing biscuit consumption in middle-aged and older adults: A crossover randomized controlled trial. Food Funct. 2022, 13, 9687–9699. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.D.; Schulz, M.; Fang, F.; Wolever, T.M.; D’Agostino, R.B., Jr.; Sparks, K.C.; Mayer-Davis, E.J. Dietary glycemic index and glycemic load, carbohydrate and fiber intake, and measures of insulin sensitivity, secretion, and adiposity in the Insulin Resistance Atherosclerosis Study. Diabetes Care 2005, 28, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.C.; Martín-Gari, M.; Cassanye, A.; Granado-Serrano, A.B.; Portero-Otín, M. Characterization of the post-prandial insulinemic response and low glycaemic index of a soy beverage. PLoS ONE 2017, 12, e0182762. [Google Scholar] [CrossRef] [PubMed]
- Rietman, A.; Schwarz, J.; Tome, D.; Koke, F.J.; Mensink, M. High dietary protein intake, reducing or eliciting insulin resistance? Eur. J. Clin. Nutr. 2014, 68, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.P.S.; Low, J.H.M.; Chen, J.R.; Zimmermann, D.; Actis-Goretta, L.; Kim, J.E. The influence of different foods and food ingredients on acute postprandial triglyceride response: A systematic literature review and meta-analysis of randomized controlled trials. Adv. Nutr. 2020, 11, 1529–1543. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, I.; Yokoyama, W.; Davis, P.; Hudson, C.; Backus, R.; Richter, D.; Knuckles, B.; Schneeman, B.O. Postprandial lipid, glucose, insulin, and cholecystokinin responses in men fed barley pasta enriched with β-glucan. Am. J. Clin. Nutr. 1999, 69, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Khossousi, A.; Binns, C.; Dhaliwal, S.; Pal, S. The acute effects of psyllium on postprandial lipaemia and thermogenesis in overweight and obese men. Br. J. Nutr. 2008, 99, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Le Marchand, L.; Wilkens, L.R.; Harwood, P.; Cooney, R.V. Use of breath hydrogen and methane as markers of colonic fermentation in epidemiologic studies: Circadian patterns of excretion. Environ. Health Perspect. 1992, 98, 199–202. [Google Scholar] [CrossRef]
- Glitsø, L.V.; Gruppen, H.; Schols, H.A.; Højsgaard, S.; Sandström, B.; Bach Knudsen, K.E. Degradation of rye arabinoxylans in the large intestine of pigs. J. Sci. Food Agric. 1999, 79, 961–969. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Regina, A.; Klingner, B.; Zajac, I.; Chapron, S.; Berbezy, P.; Bird, A.R. High-Amylose Wheat Lowers the Postprandial Glycemic Response to Bread in Healthy Adults: A Randomized Controlled Crossover Trial. J. Nutr. 2019, 149, 1335–1345. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Campbell, W.W. A novel fiber composite ingredient incorporated into a beverage and bar blunts postprandial serum glucose and insulin responses: A randomized controlled trial. Nutr. Res. 2016, 36, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Marciani, L.; Pritchard, S.E.; Hellier-Woods, C.; Costigan, C.; Hoad, C.; Gowland, P.A.; Spiller, R.C. Delayed gastric emptying and reduced postprandial small bowel water content of equicaloric whole meal bread versus rice meals in healthy subjects: Novel MRI insights. Eur. J. Clin. Nutr. 2013, 67, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Giezenaar, C.; Chapman, I.; Luscombe-Marsh, N.; Feinle-Bisset, C.; Horowitz, M.; Soenen, S. Ageing Is Associated with Decreases in Appetite and Energy Intake—A Meta-Analysis in Healthy Adults. Nutrients 2016, 8, 28. [Google Scholar] [CrossRef]
- Akimoto, S.; Miyasaka, K. Age-associated changes of appetite-regulating peptides. Geriatr. Gerontol. Int. 2010, 10, S107–S119. [Google Scholar] [CrossRef] [PubMed]

| Nutrient (per 90 g Biscuits) | Control | ABSG | FBSG |
|---|---|---|---|
| Energy (kcal) | 455 ± 5 | 440 ± 5 | 428 ± 5 |
| Fat (g) | 18.9 ± 0.0 | 20.0 ± 0.2 | 19.6 ± 0.2 |
| Protein (g) | 9.0 ± 0.1 | 10.4 ± 0.0 | 10.7 ± 0.0 |
| ACHO (g) | 61.2 ± 1.2 | 49.2 ± 0.9 | 47.1 ± 1.4 |
| TDF (g) | 1.9 ± 0.4 | 10.7 ± 0.4 | 10.4 ± 0.1 |
| IDF (g) | 1.2 ± 0.1 | 9.9 ± 0.3 | 9.3 ± 0.1 |
| SDF (g) | 0.7 ± 0.2 | 0.8 ± 0.2 | 1.1 ± 0.1 |
| Phytic acid (g) | 0.7 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 |
| Ash (g) | 0.4 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.2 |
| Characteristics | Total | Male | Female |
|---|---|---|---|
| Number of subjects (n) | 15 | 10 | 5 |
| Age (years) | 63 ± 10 | 61 ± 10 | 66 ± 12 |
| WC (cm) | 94.5 ± 12.0 | 96.6 ± 13.7 | 90.4 ± 6.5 |
| HDL (mmol/L) | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.8 ± 0.2 |
| Fasting glucose (mmo/L) | 6.2 ± 0.4 | ||
| TG (mmol/L) | 1.3 ± 0.2 | ||
| SBP (mmHg) | 130.5 ± 18.5 | ||
| DBP (mmHg) | 71.2 ± 11.2 | ||
| Insulin (mU/L) | 10.0 ± 1.4 | ||
| HOMA-IR | 2.61 ± 0.4 | ||
| T2DM medication (n) | 5 | 3 | 2 |
| Cholesterol-lowering medication (n) | 9 | 6 | 3 |
| Antihypertension medication (n) | 6 | 3 | 3 |
| The number of MetS criteria met | |||
| 3 (n) | 8 | ||
| 4 (n) | 6 | ||
| 5 (n) | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Leong, Z.N.; Zhang, W.; Jin, X.; Kong, J.W.; Chan, G.C.T.; Kim, J.E. Impact of Brewers’ Spent Grain-Containing Biscuit on Postprandial Glycaemic Response in Individuals with Metabolic Syndrome: A Crossover Randomised Controlled Trial. Nutrients 2024, 16, 909. https://doi.org/10.3390/nu16060909
Xu Y, Leong ZN, Zhang W, Jin X, Kong JW, Chan GCT, Kim JE. Impact of Brewers’ Spent Grain-Containing Biscuit on Postprandial Glycaemic Response in Individuals with Metabolic Syndrome: A Crossover Randomised Controlled Trial. Nutrients. 2024; 16(6):909. https://doi.org/10.3390/nu16060909
Chicago/Turabian StyleXu, Yujing, Zi Ning Leong, Weijia Zhang, Xinrui Jin, Jia Wen Kong, Gregory Chung Tsing Chan, and Jung Eun Kim. 2024. "Impact of Brewers’ Spent Grain-Containing Biscuit on Postprandial Glycaemic Response in Individuals with Metabolic Syndrome: A Crossover Randomised Controlled Trial" Nutrients 16, no. 6: 909. https://doi.org/10.3390/nu16060909
APA StyleXu, Y., Leong, Z. N., Zhang, W., Jin, X., Kong, J. W., Chan, G. C. T., & Kim, J. E. (2024). Impact of Brewers’ Spent Grain-Containing Biscuit on Postprandial Glycaemic Response in Individuals with Metabolic Syndrome: A Crossover Randomised Controlled Trial. Nutrients, 16(6), 909. https://doi.org/10.3390/nu16060909






