Factors Impacting the Reduction in Neophobia Prevalence in Phenylketonuria Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Data Analysis
2.4. Hypothesis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zurflüh, M.R.; Zschocke, J.; Lindner, M.; Feillet, F.; Chery, C.; Burlina, A.; Stevens, R.C.; Thöny, B.; Blau, N. Molecular genetics of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Hum. Mutat. 2008, 29, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Al Hafid, N.; Christodoulou, J. Phenylketonuria: A review of current and future treatments. Transl. Pediatr. 2015, 4, 304–317. [Google Scholar] [CrossRef]
- Evans, S.; Daly, A.; Chahal, S.; Ashmore, C.; MacDonald, J.; MacDonald, A. The influence of parental food preference and neophobia on children with phenylketonuria (PKU). Mol. Genet. Metab. 2018, 14, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Pinto, A.; Faria, A.; Teixeira, D.; van Wegberg, A.M.J.; Ahring, K.; Feillet, F.; Calhau, C.; MacDonald, A.; Moreira-Rosário, A.; et al. Is the Phenylalanine-Restricted Diet a Risk Factor for Overweight or Obesity in Patients with Phenylketonuria (PKU)? A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3443. [Google Scholar] [CrossRef] [PubMed]
- Shoraka, H.R.; Haghdoost, A.A.; Baneshi, M.R.; Bagherinezhad, Z.; Zolala, F. Global prevalence of classic phenylketonuria based on Neonatal Screening Program Data: Systematic Review and Meta-Analysis. Clin. Exp. Pediatr. 2020, 63, 34–43. [Google Scholar] [CrossRef]
- Raport Privind Procedurile Utilizate, Indicatorii de Performanță, Standarde, și Modele de Raportare Periodică in Cadrul Institutului Național de Endocrinologie C.I. Parhon, Ministerului Sanătății (Programul Național de Tratament Dietetic pentru Boli Rare) și Institutului Național de Sănătate Publică (Programul Național de Management Al Registrelor Naționale): Modulul 9. Available online: https://acad.ro/proiecteFSE/OPERE_SCRISE-SIPOCA13/3.1/3.1_Modulul_9.pdf (accessed on 17 September 2023).
- Pliner, P.; Hobden, K. Development of a scale to measure the trait of food neophobia in humans. Appetite 1992, 19, 105–120. [Google Scholar] [CrossRef]
- Galloway, A.T.; Lee, Y.; Birch, L.L. Predictors and consequences of food neophobia and pickiness in young girls. J. Am. Diet. Assoc. 2003, 103, 692–698. [Google Scholar] [CrossRef]
- Knaapila, A.; Tuorila, H.; Silventoinen, K.; Keskitalo, K.; Kallela, M.; Wessman, M.; Peltonen, L.; Cherkas, L.F.; Spector, T.D.; Perola, M. Food neophobia shows heritable variation in humans. Physiol. Behav. 2007, 91, 573–578. [Google Scholar] [CrossRef]
- Tonon, T.; Martinez, C.; Poloni, S.; Nalin, T.; MacDonald, A.; Schwartz, I.V.D. Food neophobia in patients with phenylketonuria. J. Endocrinol. Metab. 2019, 9, 108–112. [Google Scholar] [CrossRef]
- MacDonald, A.; Rylance, G.; Asplin, D.; Booth, I.W. Abnormal feeding behaviors in phenylketonuria. J. Hum. Nutr. Diet. 1997, 10, 163–170. [Google Scholar] [CrossRef]
- Menella, J.A.; Kennedy, J.M.; Beauchamp, G.K. Vegetable acceptance by infants: Effects of formula flavours. Early Hum. Dev. 2006, 82, 463–468. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Mennella, J.A. Flavor perception in human infants: Development and functional significance. Digestion 2011, 83 (Suppl. S1), 1–6. [Google Scholar] [CrossRef]
- Mennella, J.A.; Beauchamp, G.K. Flavour experiences during formula feeding are related to preferences during childhood. Early Hum. Dev. 2002, 68, 71–82. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Mennella, J.A. Early flavor learning and its impact on later feeding behavior. J. Pediatr. Gastroenterol. Nutr. 2009, 48 (Suppl. S1), 25–30. [Google Scholar] [CrossRef]
- Ramalho, C.; Sampaio, M.; Rocha, N.; Poínhos, R. Food Neophobia among Primary School Children and Their Caregivers. Acta Port. Nutr. 2016, 7, 10–13. [Google Scholar]
- Białek-Dratwa, A.; Szczepańska, E.; Szymańska, D.; Grajek, M.; Krupa-Kotara, K.; Kowalski, O. Neophobia—A Natural Developmental Stage or Feeding Difficulties for Children? Nutrients 2022, 14, 1521. [Google Scholar] [CrossRef] [PubMed]
- Łoboś, P.; Januszewicz, A. Food neophobia in children. Pediatr. Endocrinol. Diabetes Metab. 2019, 25, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Gedrich, K. Determinants of nutritional behavior: A multitude of levers for successful intervention? Appetite 2003, 41, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Green, A. The First Treatment for PKU: The Pioneers-Birmingham 1951. Int. J. Neonatal Screen. 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Child Growth Standards; World Health Organization: Geneva, Switzerland, 2006; ISBN 924154693X. [Google Scholar]
- Canadian Paediatric Society. A health professional’s guide for using the new WHO growth charts. Paediatr. Child Health 2010, 15, 84–98. [Google Scholar] [CrossRef]
- Barrena, R.; Sánchez, M. Neophobia, personal consumer values and novel food acceptance. Food Qual. Prefer. 2013, 27, 72–84. [Google Scholar] [CrossRef]
- Taherdoost, H. What Is the Best Response Scale for Survey and Questionnaire Design; Review of Different Lengths of Rating Scale/Attitude Scale/Likert Scale. Int. J. Acad. Res. Manag. 2019, 8, ffhal-02557308f. [Google Scholar]
- D’Antuono, L.F.; Bignami, C. Perception of typical Ukrainian foods among an Italian population. Food Qual. Prefer. 2012, 25, 1–8. [Google Scholar] [CrossRef]
- Choe, J.Y.; Cho, M.S. Food neophobia and willingness to try non-traditional foods for Koreans. Food Qual. Prefer. 2011, 22, 671–677. [Google Scholar] [CrossRef]
- Olabi, A.; Najm, N.E.O.; Baghdadi, O.K.; Morton, J.M. Food neophobia levels of Lebanese and American college students. Food Qual. Prefer. 2009, 20, 353–362. [Google Scholar] [CrossRef]
- Ribeiro de Andrade Previato, H.D.; Behrens, J.H. Translation and validation of the Food Neophobia Scale (FNS) to the Brazilian Portuguese. Nutr. Hosp. 2015, 32, 925–930. [Google Scholar] [CrossRef]
- JASP Team. JASP, version 0.18.1; JASP Team: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Pliner, P.; Salvy, S.-J. Food neophobia in humans. In The Psychology of Food Choice; Shepherd, R., Raats, M., Eds.; CABI Publishing: Oxfordshire, UK, 2006; pp. 75–92. [Google Scholar]
- Kauer, J.; Rozin, P.; Pelchat, M.L. Adulty picky eating: Is there more to it. Soc. Study Ingestion Behav. 2002. [Google Scholar]
- Smith, A.M.; Roux, S.; Naidoo, N.R.; Venter, D.J. Food choices of tactile defensive children. Nutrition 2005, 21, 14–19. [Google Scholar] [CrossRef]
- MacDonald, A.; Rylance, G.W.; Asplin, D.A.; Hall, K.; Harris, G.; Booth, I.W. Feeding problems in young PKU children. Acta Paediatr. 1994, 407, 73–74. [Google Scholar] [CrossRef]
- Evans, S.; Daly, A.; Chahal, S.; MacDonald, J.; MacDonald, A. Food acceptance and neophobia in children with phenylketonuria: A prospective controlled study. J. Hum. Nutr. Diet. 2016, 29, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.C.; An, R.; Lee, S.Y.; Donovan, S.M. Correlates of picky eating and food neophobia in young children: A systematic review and meta-analysis. Nutr. Rev. 2017, 75, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Rossbach, S.; Foterek, K.; Schmidt, I.; Hilbig, A.; Alexy, U. Food neophobia in German adolescents: Determinants and association with dietary habits. Appetite 2016, 101, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Addessi, E.; Galloway, T.A.; Visalberghi, E.; Birch, L.L. Specific social influences on the acceptance of novel foods in 2–5-year-old children. Appetite 2005, 45, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Levy, H.L.; Waisbren, S.E. PKU in adolescents: Rationale and psychosocial factors in diet continuation. Acta Paediatr. Suppl. 1994, 407, 92–97. [Google Scholar] [CrossRef]
- Kenneson, A.; Singh, R.H. Natural history of children and adults with phenylketonuria in the NBS-PKU Connect registry. Mol Genet. Metab. 2021, 134, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.M.; Grosse, S.D.; Garcia, S.P.; Bach, J.; Kleyn, M.; Simon, N.J.E.; Prosser, L.A. The financial and time burden associated with phenylketonuria treatment in the United States. Mol. Genet. Metab. 2019, 21, 100523. [Google Scholar] [CrossRef] [PubMed]
- Matic, J.; Zeltner, N.A.; Häberle, J. Normal Growth in PKU Patients Under Low-Protein Diet in a Single-Center Cross-Sectional Study. JIMD Rep. 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Yong, C.; Kuang, X.; Liu, Y.; Xiang, C.; Xi, Y.; Huo, J.; Liang, J.; Zou, H.; Lin, Q. Parental food neophobia, feeding practices, and preschooler’s food neophobia: A cross-sectional study in China. Appetite 2023, 185, 106547. [Google Scholar] [CrossRef]
- Pliner, P. Development of measures of food neophobia in children. Appetite 1994, 23, 147–163. [Google Scholar] [CrossRef]
- Tuorila, H.; Meiselman, H.L.; Bell, R.; Cardello, A.V.; Johnson, W. Role of sensory and cognitive information in the enhancement of certainty and liking for novel and familiar foods. Appetite 1994, 23, 231–246. [Google Scholar] [CrossRef]
- Pliner, P.; Lähteenmäki, L.; Tuorila, H. Correlates of human food neophobia. Appetite 1998, 30, 93. [Google Scholar] [CrossRef]
- Tuorila, H.; Lähteenmäki, L.; Pohjalainen, L.; Lotti, L. Food neofobia among the Finns and related responses to familiar and unfamiliar foods. Food Qual. Prefer. 2001, 12, 29–37. [Google Scholar] [CrossRef]
- Kim, S.-J.; Park, H.-J.; Lee, K.-H. Comparison of Food Neophobia Scale and Food Involvement Scale Between Koreans and East-South Asians. J. Korean Soc. Food Cult. 2011, 26, 429–436. [Google Scholar] [CrossRef]
- Yuan, W.L.; Rigal, N.; Monnery-Patris, S.; Chabanet, C.; Forhan, A.; Charles, M.A.; de Lauzon-Guillain, B. Early determinants of food liking among 5y-old children: A longitudinal study from the EDEN mother–child cohort. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Kozioł-Kozakowska, A.; Piórecka, B.; Schlegel-Zawadzka, M. Prevalence of food neophobia in pre-school children from southern Poland and its association with eating habits, dietary intake and anthropometric parameters: A cross-sectional study. Public Health Nutr. 2018, 21, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Maratos, F.A.; Staples, P. Attentional biases towards familiar and unfamiliar foods in children. The role of food neophobia. Appetite 2015, 91, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Faith, M.S.; Heo, M.; Keller, K.L.; Pietrobelli, A. Child food neophobia is heritable, associated with less compliant eating, and moderates familial resemblance for BMI. Obesity 2013, 21, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.L.; Whittaker, M.N.; Said, H.; Dwivedi, G.; Qu, P.; Musunuru, K.; Ahrens-Nicklas, R.C.; Alameh, M.G.; Wang, X. A base editing strategy using mRNA-LNPs for in vivo correction of the most frequent phenylketonuria variant. HGG Adv. 2024, 5, 100253. [Google Scholar] [CrossRef]
- Falciglia, G.A.; Couch, S.C.; Gribble, L.S.; Pabst, S.M.; Frank, R. Food neophobia in childhood affects dietary variety. J. Am. Diet. Assoc. 2000, 100, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
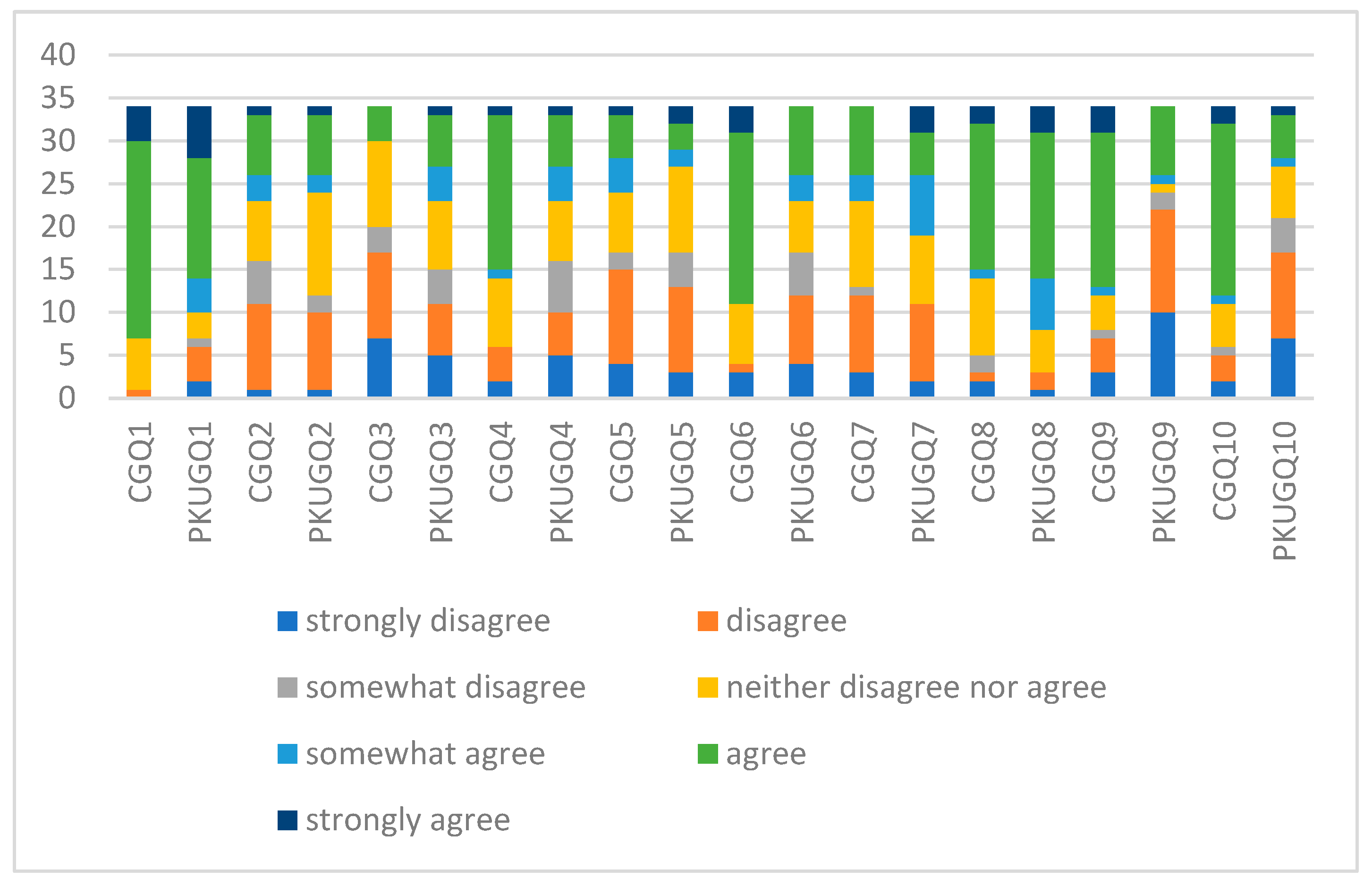
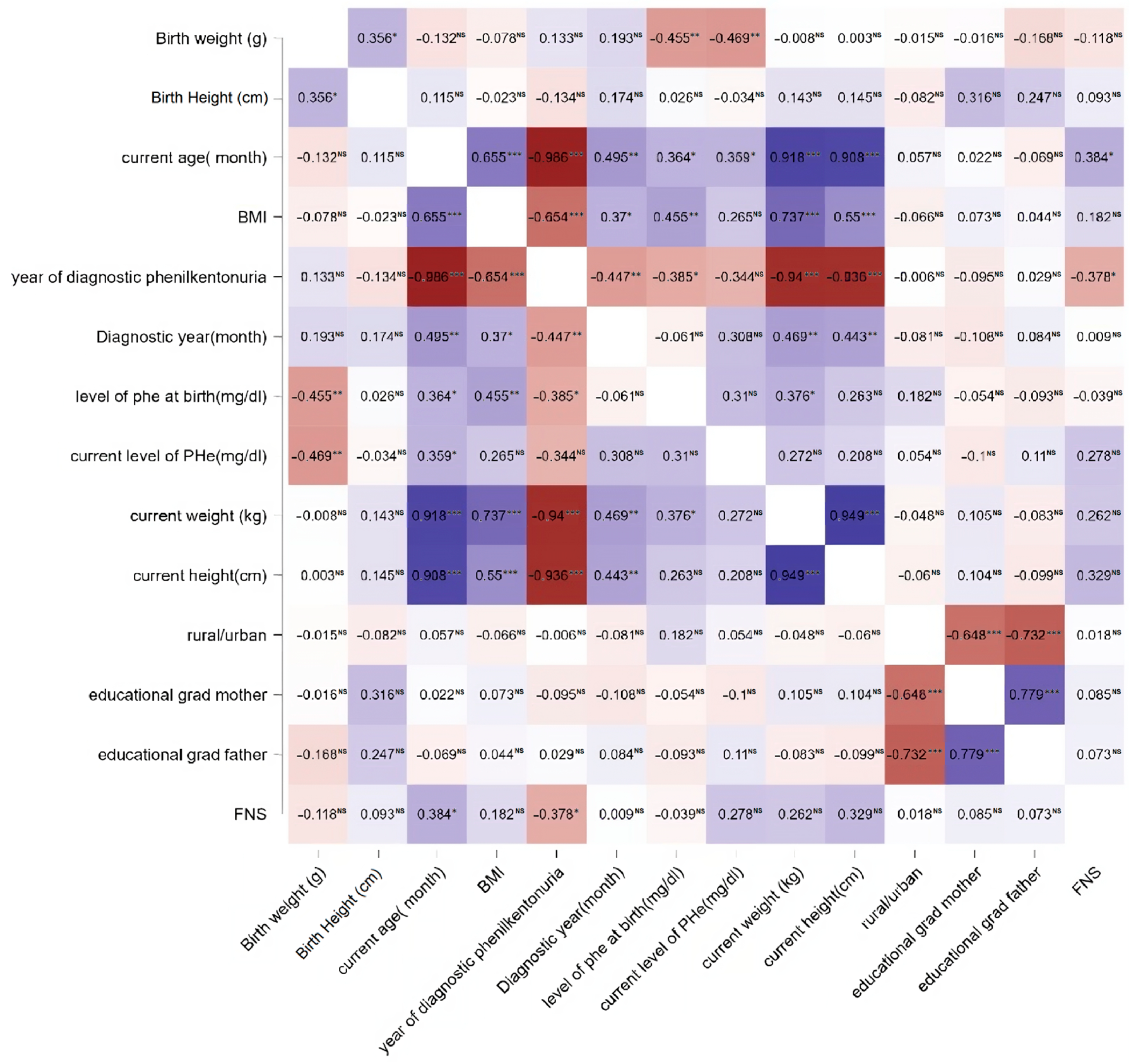
| Parameters | PKUG (n = 34) | CG (n = 34) | p-Value |
|---|---|---|---|
| Sex % (n) | |||
| Males | 67.6 (23) | 67.6 (23) | 1 |
| Females | 32.4 (11) | 32.4 (11) | |
| Age at diagnosis in days (median, IQR) | 21(15, 41) | ||
| Age in years (median, IQR) | 7.05 (4.9, 15.2) | 5.75 (3.8, 14.87) | 0.615 |
| N0. | PAH Variant | Zygosity Status | ACMG [30] Classification | PKU Phenotype |
|---|---|---|---|---|
| 001 | NM_000277.3:c.1066-11G>A | Heterozygous | Pathogenic | Classic or moderate |
| 001 | NM_000277.3:c.1222C>T NP_000268.1:p.(Arg408Trp) | Heterozygous | Pathogenic | Classic |
| 002 | NM_000277.3:c.472C>T NP_000268.1:p.Arg158Trp | Heterozygous | Pathogenic | Classic |
| 002 | NM_000277.3:c.1222C>T NP_000268.1: p.Arg408Trp | Heterozygous | Pathogenic | Classic |
| 003 | NM_000277.3:c.1222C>T NP_000268.1: p.Arg408Trp | Homozygous | Pathogenic | Classic |
| 004 | NM_000277.3:c.1222C>T NP_000268.1:p.Arg408Trp | Homozygous | Pathogenic | Classic |
| 005 | NM_000277.3:c.754C>T NP_000268.1: p.Arg252Trp | Heterozygous | Pathogenic | Classic |
| 005 | NM_000277.3:c.782G>A NP_000268.1:p.Arg261 GLN | Heterozygous | Pathogenic | Classic or moderate |
| 006 | NM_000277.3:c.1222C>T NP_000268.1:p.Arg408Trp | Heterozygous | Pathogenic | Classic |
| 006 | NM_000277.3:c.782G>A NP_000268.1:p.Arg261Gln | Heterozygous | Pathogenic | Classic or moderate |
| 007 | NM_000277.3:c.1222C>T NP_000268.1:p.(Arg408Trp) | Homozygous | Pathogenic | Classic |
| 008 | NM_000277.3:c.1222C>T NP_000268.1:p.Arg408Trp | Homozygous | Pathogenic | Classic |
| 009 | NM_000277.3:c.1315+1G>A | Heterozygous | Pathogenic | Classic |
| 009 | NM_000277.3:c.533A>G NP_000268.1:p.Glu178Gly | Heterozygous | Pathogenic | Mild |
| 010 | NM_000277.3:c.1222C>T NP_000268.1:p.(Arg408Trp) | Homozygous | Pathogenic | Classic |
| 011 | NM_000277.3:c.1222C>T NP_000268.1:p.(Arg408Trp) | Homozygous | Pathogenic | Classic |
| 012 | NM_000277.3:c.1222C>T NP_000268.1:p.(Arg408Trp) | Homozygous | Pathogenic | Classic |
| 013 | NM_000277.3:c.1222C>T NP_000268.1:p.(Arg408Trp) | Homozygous | Pathogenic | Classic |
| 014 | NM_000277.3:c.1222C>T NP_000268.1:p.(Arg408Trp) | Homozygous | Pathogenic | Classic |
| 015 | NM_000277.3:c.1222C>T NP_000268.1:p.(Arg408Trp) | Homozygous | Pathogenic | Classic |
| 016 | NM_000277.3:c.1222C>T NP_000268.1:p.(Arg408Trp) | Homozygous | Pathogenic | Classic |
| 017 | NM_000277.3:c.1222C>T NP_000268.1:p.(Arg408Trp) | Homozygous | Pathogenic | Classic |
| Age Group (Years) | Group and Sex | FNS < 30 (%) | 31–35 FNS (%) | >35 FNS (%) |
|---|---|---|---|---|
| Under 2 | PKUG—male | 33.33 | 33.33 | 33.33 |
| PKUG—female | 0.00 | 0.00 | 0.00 | |
| Total PKUG | 33.33 | 33.33 | 33.33 | |
| CG male | 33.33 | 0.00 | 66.67 | |
| CG female | 0.00 | 0.00 | 0.00 | |
| Total CG | 33.33 | 0.00 | 66.67 | |
| 2–7.9 | PKUG—male | 18.18 | 9.09 | 72.73 |
| PKUG—female | 25.00 | 25.00 | 50.00 | |
| Total PKUG | 14.29 | 14.29 | 71.43 | |
| CG male | 0.00 | 8.33 | 91.67 | |
| CG female | 0.00 | 0.00 | 100.00 | |
| Total CG | 0.00 | 6.67 | 93.33 | |
| 8–13.9 | PKUG—male | 0.00 | 0.00 | 100.00 |
| PKUG—female | 0.00 | 50.00 | 50.00 | |
| Total PKUG | 0.00 | 16.67 | 83.33 | |
| CG male | 0.00 | 0.00 | 100.00 | |
| CG female | 0.00 | 0.00 | 100.00 | |
| Total CG | 0.00 | 0.00 | 100.00 | |
| 14–17.9 | PKUG—male | 0.00 | 25.00 | 75.00 |
| PKUG—female | 50.00 | 0.00 | 50.00 | |
| Total PKUG | 16.67 | 16.67 | 66.67 | |
| CG male | 25.00 | 0.00 | 75.00 | |
| CG female | 0.00 | 0.00 | 100.00 | |
| Total CG | 16.67 | 0.00 | 83.33 | |
| Over 18 | PKUG—male | 0.00 | 0.00 | 100.00 |
| PKUG -female | 0.00 | 0.00 | 100.00 | |
| Total PKUG | 0.00 | 0.00 | 100.00 | |
| CG male | 0.00 | 0.00 | 100.00 | |
| CG female | 0.00 | 0.00 | 100.00 | |
| Total CG | 0.00 | 0.00 | 100.00 |
| Parameters | PKUG (n = 34) | CG (n = 34) | p-Value |
|---|---|---|---|
| Development median (IQR) | 80 (43.7, 99.9) | 75.5 (22.5, 97) | 0.473 |
| Primary caretaker % (n) | 0.003 | ||
| Mother | 91.2 (31) | 41.2 (14) | |
| Other | 8.8 (3) | 58.8 (20) | |
| Mother education % (n) | 0.634 | ||
| Primary school | 23.5 (8) | 23.5 (8) | |
| Vocational school | 14.7 (5) | 14.7 (5) | |
| High school | 14.7 (5) | 26.5 (9) | |
| University | 47.1 (16) | 35.2 (12) | |
| Father education % (n) | 0.279 | ||
| Primary school | 17.6 (6) | 20.6 (7) | |
| Vocational school | 17.6 (6) | 38.2 (13) | |
| High school | 23.5 (8) | 14.7 (5) | |
| University | 41.2 (14) | 26.5 (9) | |
| FNS median (IQR) | 38.5 (30, 46) | 45 (40, 49) | 0.013 |
| Level of Phe at birth median (IQR) | 1089.6 (484.3, 1785.8) | ||
| Level of Phe at present median (IQR) | 260.3 (183.4, 381.3) |
| Independent Samples t-Test Rural/Urban | W | p-Value | Hodges–Lehmann Estimate | Rank-Biserial Correlation |
|---|---|---|---|---|
| FNS | 141.000 | 0.931 | −3.769 × 10–5 | −0.021 |
| Birth weight (g) | 146.500 | 0.945 | 2.580 × 10–5 | 0.017 |
| Birth height (cm) | 157.500 | 0.651 | 1.000 | 0.094 |
| Current age (months) | 134.500 | 0.756 | −4.331 | −0.066 |
| BMI | 155.000 | 0.717 | 0.330 | 0.076 |
| Time to diagnosis (years) | 145.000 | 0.986 | 4.815 × 10–6 | 0.007 |
| Age at diagnosis (months) | 157.500 | 0.653 | 2.685 | 0.094 |
| Level of Phe at birth (µmol/L) | 107.500 | 0.313 | −5.000 | −0.210 |
| Current level of Phe (µmol/L) | 126.500 | 0.772 | −0.500 | −0.063 |
| Current weight (kg) | 152.000 | 0.796 | 1.577 | 0.056 |
| Current height (cm) | 154.000 | 0.743 | 2.451 | 0.069 |
| No | Hypothesis | Result |
|---|---|---|
| H1 | There is a variation of distribution for FN score among PKU group and control group. | Rejected |
| H2 | There is a relationship between the patients’ current BMI and the development of neophobia. | Rejected |
| H3 | There is a relationship between diet compliance and neophobia. | Rejected |
| H4 | There is a relationship between a patient’s Phe level at the diagnostic moment and the development of neophobia. | Rejected |
| H5 | There is a correlation between parental educational level and the patient’s development of neophobia. | Accepted |
| H6 | There is a correlation between the patient’s age and the onset of neophobia. | Accepted |
| H7 | There is a relationship between length of period from birth to diagnostic and neophobia. | Accepted |
| H8 | There is a variation of distribution for FN score among urban and rural patients. | Rejected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugi, M.-A.; Jugănaru, I.; Isac, R.; Simina, I.-E.; Munteanu, A.-I.; Mang, N.; Brad, G.-F.; Nicoară, D.-M.; Cîrnatu, D.; Mărginean, O. Factors Impacting the Reduction in Neophobia Prevalence in Phenylketonuria Patients. Nutrients 2024, 16, 768. https://doi.org/10.3390/nu16060768
Bugi M-A, Jugănaru I, Isac R, Simina I-E, Munteanu A-I, Mang N, Brad G-F, Nicoară D-M, Cîrnatu D, Mărginean O. Factors Impacting the Reduction in Neophobia Prevalence in Phenylketonuria Patients. Nutrients. 2024; 16(6):768. https://doi.org/10.3390/nu16060768
Chicago/Turabian StyleBugi, Meda-Ada, Iulius Jugănaru, Raluca Isac, Iulia-Elena Simina, Andrei-Ioan Munteanu, Niculina Mang, Georgiana-Flavia Brad, Delia-Maria Nicoară, Daniela Cîrnatu, and Otilia Mărginean. 2024. "Factors Impacting the Reduction in Neophobia Prevalence in Phenylketonuria Patients" Nutrients 16, no. 6: 768. https://doi.org/10.3390/nu16060768
APA StyleBugi, M.-A., Jugănaru, I., Isac, R., Simina, I.-E., Munteanu, A.-I., Mang, N., Brad, G.-F., Nicoară, D.-M., Cîrnatu, D., & Mărginean, O. (2024). Factors Impacting the Reduction in Neophobia Prevalence in Phenylketonuria Patients. Nutrients, 16(6), 768. https://doi.org/10.3390/nu16060768






