The Mediating Role of Psychological Balance on the Effects of Dietary Behavior on Cognitive Impairment in Chinese Elderly
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Samples
2.2. Measures
2.3. Statistical Analysis
3. Results
3.1. General Demographic Characteristics of the Study Participants
3.2. The Seven Food Varieties and Dietary Diversity
3.3. Latent Class Analysis of Dietary Patterns
3.4. Chi-Square Test of Dietary and Cognitive Impairment
3.5. Binary Logistic Regression Analysis Testing the Association between the Dietary and Cognitive Impairment
3.6. Pearson Correlation Analysis and Chain Mediated Effects Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panza, F.; D’Introno, A.; Colacicco, A.M.; Capurso, C.; Del Parigi, A.; Caselli, R.J.; Pilotto, A.; Argentieri, G.; Scapicchio, P.L.; Scafato, E.; et al. Current epidemiology of mild cognitive impairment and other predementia syndromes. Am. J. Geriatr. Psychiatry 2005, 13, 633–644. [Google Scholar] [CrossRef]
- Vos, S.J.B.; Verhey, F.; Frölich, L.; Kornhuber, J.; Wiltfang, J.; Maier, W.; Peters, O.; Rüther, E.; Nobili, F.; Morbelli, S.; et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain 2015, 138, 1327–1338. [Google Scholar] [CrossRef]
- Connors, M.H.; Ames, D.; Boundy, K.; Clarnette, R.; Kurrle, S.; Mander, A.; Ward, J.; Woodward, M.; Brodaty, H. Mortality in Mild Cognitive Impairment: A Longitudinal Study in Memory Clinics. J. Alzheimers Dis. 2016, 54, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Tschanz, J.T.; Welsh-Bohmer, K.A.; Lyketsos, C.G.; Corcoran, C.; Green, R.C.; Hayden, K.; Norton, M.C.; Zandi, P.P.; Toone, L.; West, N.A.; et al. Conversion to dementia from mild cognitive disorder: The Cache County Study. Neurology 2006, 67, 229–234. [Google Scholar] [CrossRef]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef]
- Wu, X.; Hou, G.; Han, P.; Yu, X.; Chen, X.; Song, P.; Zhang, Y.; Zhao, Y.; Xie, F.; Niu, S.; et al. Association between Physical Performance and Cognitive Function in Chinese Community-Dwelling Older Adults: Serial Mediation of Malnutrition and Depression. Clin. Interv. Aging 2021, 16, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- An, R.P.; Liu, G.; Khan, N.; Yan, H.; Wang, Y.F. Dietary Habits and Cognitive Impairment Risk among Oldest-Old Chinese. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2019, 74, 474–483. [Google Scholar] [CrossRef]
- van de Rest, O.; Berendsen, A.A.; Haveman-Nies, A.; de Groot, L.C. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef]
- Dewsbury, L.S.; Lim, C.K.; Steiner, G.Z. The Efficacy of Ketogenic Therapies in the Clinical Management of People with Neurodegenerative Disease: A Systematic Review. Adv. Nutr. 2021, 12, 1571–1593. [Google Scholar] [CrossRef]
- Chen, X.; Maguire, B.; Brodaty, H.; O’Leary, F. Dietary Patterns and Cognitive Health in Older Adults: A Systematic Review. J. Alzheimers Dis. 2019, 69, 595–596. [Google Scholar] [CrossRef]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef]
- Kheirouri, S.; Alizadeh, M. MIND diet and cognitive performance in older adults: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8059–8077. [Google Scholar] [CrossRef]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.F.; Barberger-Gateau, P. Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Panza, F.; Capurso, A. The role of diet in cognitive decline. J. Neural Transm. 2003, 110, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Morris, M.C.; Bennett, D.A.; Berr, C.; Amouyel, P.; Dartigues, J.F.; Tzourio, C.; Chasman, D.I.; Grodstein, F. Fish Intake, Genetic Predisposition to Alzheimer Disease, and Decline in Global Cognition and Memory in 5 Cohorts of Older Persons. Am. J. Epidemiol. 2018, 187, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, H.; Ge, X.; Liu, L.; Wang, T.; Yu, H. Effect of n-3 long-chain polyunsaturated fatty acids on mild cognitive impairment: A meta-analysis of randomized clinical trials. Eur. J. Clin. Nutr. 2020, 74, 548–554. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Lee, S.E.; Kim, S.H.; Chung, H.W.; Kim, W.Y. Psychological Distress Is Associated with Inadequate Dietary Intake in Vietnamese Marriage Immigrant Women in Korea. J. Am. Diet. Assoc. 2010, 110, 779–785. [Google Scholar] [CrossRef]
- Ma, S.R.; Zhu, J.; Xie, S.H.; Chen, R.; Li, X.Q.; Wei, W.Q. Suboptimal dietary quality is associated with mental symptoms among adults aged 40 years and over in China: A population-based cross-sectional study. J. Affect. Disord. 2023, 340, 802–811. [Google Scholar] [CrossRef]
- Castellini, G.; Bryant, E.J.; Stewart-Knox, B.J.; Graffigna, G. Development and validation of the Psychological Food Involvement Scale (PFIS). Food Qual. Prefer. 2023, 105, 104784. [Google Scholar] [CrossRef]
- Wu, F.; Liu, H.X.; Liu, W.B. Association between sensation, perception, negative socio-psychological factors and cognitive impairment. Heliyon 2023, 9, e22101. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Coetzer, R.; Addy, K. Investigating the discrepancy between subjective and objective cognitive impairment following acquired brain injury: The role of psychological affect. Neurorehabilitation 2017, 41, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Wetherell, J.L.; Reynolds, C.A.; Gatz, M.; Pedersen, N.L. Anxiety, cognitive performance, and cognitive decline in normal aging. J. Gerontol. B Psychol. Sci. Soc. Sci. 2002, 57, P246–P255. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.C.C.; Pondé, M.P.; Liu, A.H.; Caron, J. Anxiety and Depression as Longitudinal Predictors of Mild Cognitive Impairment in Older Adults. Can. J. Psychiatry-Rev. Can. Psychiatr. 2017, 62, 343–350. [Google Scholar] [CrossRef]
- Vlachos, G.S.; Scarmeas, N. Dietary interventions in mild cognitive impairment and dementia. Dialogues Clin. Neurosci. 2019, 21, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Center for Healthy, A.; Development, S. The Chinese Longitudinal Healthy Longevity Survey (CLHLS)-Longitudinal Data (1998–2018); Peking University Open Research Data Platform: Beijing, China, 2020. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Feng, Q.; Zeng, Y. The Effects of “Diet–Smoking–Gender” Three-Way Interactions on Cognitive Impairment among Chinese Older Adults. Nutrients 2022, 14, 2144. [Google Scholar] [CrossRef]
- Lv, Y.; Kraus, V.B.; Gao, X.; Yin, Z.; Zhou, J.; Mao, C.; Duan, J.; Zeng, Y.; Brasher, M.S.; Shi, W.; et al. Higher dietary diversity scores and protein-rich food consumption were associated with lower risk of all-cause mortality in the oldest old. Clin. Nutr. 2020, 39, 2246–2254. [Google Scholar] [CrossRef]
- Yin, Z.; Brasher, M.S.; Kraus, V.B.; Lv, Y.; Shi, X.; Zeng, Y. Dietary Diversity Was Positively Associated with Psychological Resilience among Elders: A Population-Based Study. Nutrients 2019, 11, 650. [Google Scholar] [CrossRef]
- Aihemaitijiang, S.; Zhang, L.; Ye, C.; Halimulati, M.; Huang, X.; Wang, R.; Zhang, Z. Long-Term High Dietary Diversity Maintains Good Physical Function in Chinese Elderly: A Cohort Study Based on CLHLS from 2011 to 2018. Nutrients 2022, 14, 1730. [Google Scholar] [CrossRef]
- Jiang, K.; Chen, Y. Can Parent-Child Living Together Improve the Well-being of the Elderly? Evident Based on CLHLS Data. Popul. J. 2016, 38, 77–86. (In Chinese) [Google Scholar] [CrossRef]
- Li, W.; Hu, H.; Li, S.; Xia, L. Social Activities and Health Promotion of the Elderly: A Survey Based on Tracking Data from 2005 to 2014. Popul. Dev. 2018, 24, 90–100. (In Chinese) [Google Scholar]
- Li, A.; Wu, R. Does the Remarriages of the Elderly Improve Their Mental Health? An Empirical Analysis Based on CLHLS. S. China Popul. 2019, 34, 70–80. (In Chinese) [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Yi, Z.; Vaupel, J.W. Functional Capacity and Self–Evaluation of Health and Life of Oldest Old inChina. J. Soc. Issues 2010, 58, 733–748. [Google Scholar] [CrossRef]
- Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [CrossRef]
- Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; Guessous, I. Socio-demographic and lifestyle determinants of dietary patterns in French-speaking Switzerland, 2009–2012. BMC Public Health 2018, 18, 131. [Google Scholar] [CrossRef]
- Tavakoli, S.; Dorosty-Motlagh, A.R.; Hoshiar-Rad, A.; Eshraghian, M.R.; Sotoudeh, G.; Azadbakht, L.; Karimi, M.; Jalali-Farahani, S. Is dietary diversity a proxy measurement of nutrient adequacy in Iranian elderly women? Appetite 2016, 105, 468–476. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006, 67, 1370–1376. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. Fruit, vegetables and prevention of cognitive decline or dementia: A systematic review of cohort studies. J. Nutr. Health Aging 2012, 16, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Mottaghi, T.; Amirabdollahian, F.; Haghighatdoost, F. Fruit and vegetable intake and cognitive impairment: A systematic review and meta-analysis of observational studies. Eur. J. Clin. Nutr. 2018, 72, 1336–1344. [Google Scholar] [CrossRef]
- Dunham, A.; Johnson, E.J. Fruits, Vegetables, and Their Components and Mild Cognitive Impairment and Dementia: A Review. Food Rev. Int. 2013, 29, 409–440. [Google Scholar] [CrossRef]
- Granic, A.; Davies, K.; Adamson, A.; Kirkwood, T.; Hill, T.R.; Siervo, M.; Mathers, J.C.; Jagger, C. Dietary Patterns High in Red Meat, Potato, Gravy, and Butter Are Associated with Poor Cognitive Functioning but Not with Rate of Cognitive Decline in Very Old Adults. J. Nutr. 2016, 146, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Titova, O.E.; Ax, E.; Brooks, S.J.; Sjögren, P.; Cederholm, T.; Kilander, L.; Kullberg, J.; Larsson, E.M.; Johansson, L.; Ahlström, H.; et al. Mediterranean diet habits in older individuals: Associations with cognitive functioning and brain volumes. Exp. Gerontol. 2013, 48, 1443–1448. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Sheng, L.T.; Pan, X.F.; Feng, L.; Yuan, J.M.; Pan, A.; Koh, W.P. Meat consumption in midlife and risk of cognitive impairment in old age: The Singapore Chinese Health Study. Eur. J. Nutr. 2020, 59, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Elias, M.F.; Davey, A.; Alkerwi, A.; Dore, G.A. Higher Cognitive Performance Is Prospectively Associated with Healthy Dietary Choices: The Maine Syracuse Longitudinal Study. J. Prev. Alzheimers Dis. 2015, 2, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Hooda, J.; Shah, A.; Zhang, L. Heme, an essential nutrient from dietary proteins, critically impacts diverse physiological and pathological processes. Nutrients 2014, 6, 1080–1102. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Cheng, H.G. Lower intake of vegetables and legumes associated with cognitive decline among illiterate elderly Chinese: A 3-year cohort study. J. Nutr. Health Aging 2012, 16, 549–552. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, B.M.; Tijhuis, M.; Kalmijn, S.; Kromhout, D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: The Zutphen Elderly Study. Am. J. Clin. Nutr. 2007, 85, 1142–1147. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef]
- Qin, B.; Plassman, B.L.; Edwards, L.J.; Popkin, B.M.; Adair, L.S.; Mendez, M.A. Fish intake is associated with slower cognitive decline in Chinese older adults. J. Nutr. 2014, 144, 1579–1585. [Google Scholar] [CrossRef]
- Muñoz-Garach, A.; Cornejo-Pareja, I.; Martínez-Gonzalez, M.A.; Bulló, M.; Corella, D.; Castañer, O.; Romaguera, D.; Vioque, J.; Alonso-Gómez, A.M.; Wärnberg, J.; et al. Milk and Dairy Products Intake Is Related to Cognitive Impairment at Baseline in Predimed Plus Trial. Mol. Nutr. Food Res. 2021, 65, e2000728. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Ayabe, T.; Kutsukake, T.; Ohya, R.; Takaichi, Y.; Uchida, S.; Yamada, K.; Uchida, K.; Takashima, A.; Nakayama, H. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol. Aging 2018, 72, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Camfield, D.A.; Owen, L.; Scholey, A.B.; Pipingas, A.; Stough, C. Dairy constituents and neurocognitive health in ageing. Br. J. Nutr. 2011, 106, 159–174. [Google Scholar] [CrossRef]
- Lee, J.; Fu, Z.; Chung, M.; Jang, D.J.; Lee, H.J. Role of milk and dairy intake in cognitive function in older adults: A systematic review and meta-analysis. Nutr. J. 2018, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Schulze, M.B.; Fung, T.T.; Meigs, J.B.; Rifai, N.; Manson, J.E.; Hu, F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004, 80, 1029–1035. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, R.; Li, F.; Chen, L.; Wu, K.; Huang, J.; Liu, H.; Huang, Z.; Xu, L.; Yuan, Z.; et al. Association between dietary diversity and cognitive impairment among the oldest-old: Findings from a nationwide cohort study. Clin. Nutr. 2021, 40, 1452–1462. [Google Scholar] [CrossRef]
- Song, Y.; Zeng, L.; Gao, J.; Chen, L.; Sun, C.; Yan, M.; Li, M.; Jiang, H. Adherence to High Dietary Diversity and Incident Cognitive Impairment for the Oldest-Old: A Community-Based, Nationwide Cohort Study. Nutrients 2022, 14, 4530. [Google Scholar] [CrossRef]
- Kumawat, M.; Sharma, T.K.; Singh, I.; Singh, N.; Singh, S.K.; Ghalaut, V.S.; Shankar, V.; Vardey, S.K. Decrease in Antioxidant Status of Plasma and Erythrocytes from Geriatric Population. Dis. Markers 2012, 33, 324696. [Google Scholar] [CrossRef][Green Version]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Shikh, E.V.; Nikolaeva, N.B.; Molchanova, N.B.; Elizarova, E.V. Correction of gut dysbiosis as a promising direction in the prevention of neuroinflammation and cognitive impairment. Vopr. Pitan. 2023, 92, 107–119. [Google Scholar] [CrossRef]
- Su, X.; Zhang, J.; Wang, W.; Ni, C.; Hu, S.; Shao, P.; Li, C.; Hua, Y.; Lang, H.; Wan, Y. Dietary patterns and risk of mild cognitive impairment among Chinese elderly: A cross-sectional study. PLoS ONE 2020, 15, e0235974. [Google Scholar] [CrossRef]
- Salminen, L.E.; Paul, R.H. Oxidative stress and genetic markers of suboptimal antioxidant defense in the aging brain: A theoretical review. Rev. Neurosci. 2014, 25, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C. Nutritional determinants of cognitive aging and dementia. Proc. Nutr. Soc. 2012, 71, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Blusztajn, J.K.; Slack, B.E.; Mellott, T.J. Neuroprotective Actions of Dietary Choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- La Rue, A.; Koehler, K.M.; Wayne, S.J.; Chiulli, S.J.; Haaland, K.Y.; Garry, P.J. Nutritional status and cognitive functioning in a normally aging sample: A 6-y reassessment. Am. J. Clin. Nutr. 1997, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Triana, F.; Verdejo-Bravo, C.; Fernández-Pérez, C.; Martín-Sánchez, F.J. Effect of Milk and Other Dairy Products on the Risk of Frailty, Sarcopenia, and Cognitive Performance Decline in the Elderly: A Systematic Review. Adv. Nutr. 2019, 10, S105–S119. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.J.; Zuniga, K.E. Egg Consumption, Multi-Domain Cognitive Performance, and Short-Term Cognitive Change in a Representative Sample of Older U.S. Adults. J. Am. Coll. Nutr. 2019, 38, 537–546. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Barbagallo, M. The relevance of nutrition for the concept of cognitive frailty. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 61–68. [Google Scholar] [CrossRef]
- Bremner, J.D.; Moazzami, K.; Wittbrodt, M.T.; Nye, J.A.; Lima, B.B.; Gillespie, C.F.; Rapaport, M.H.; Pearce, B.D.; Shah, A.J.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef]

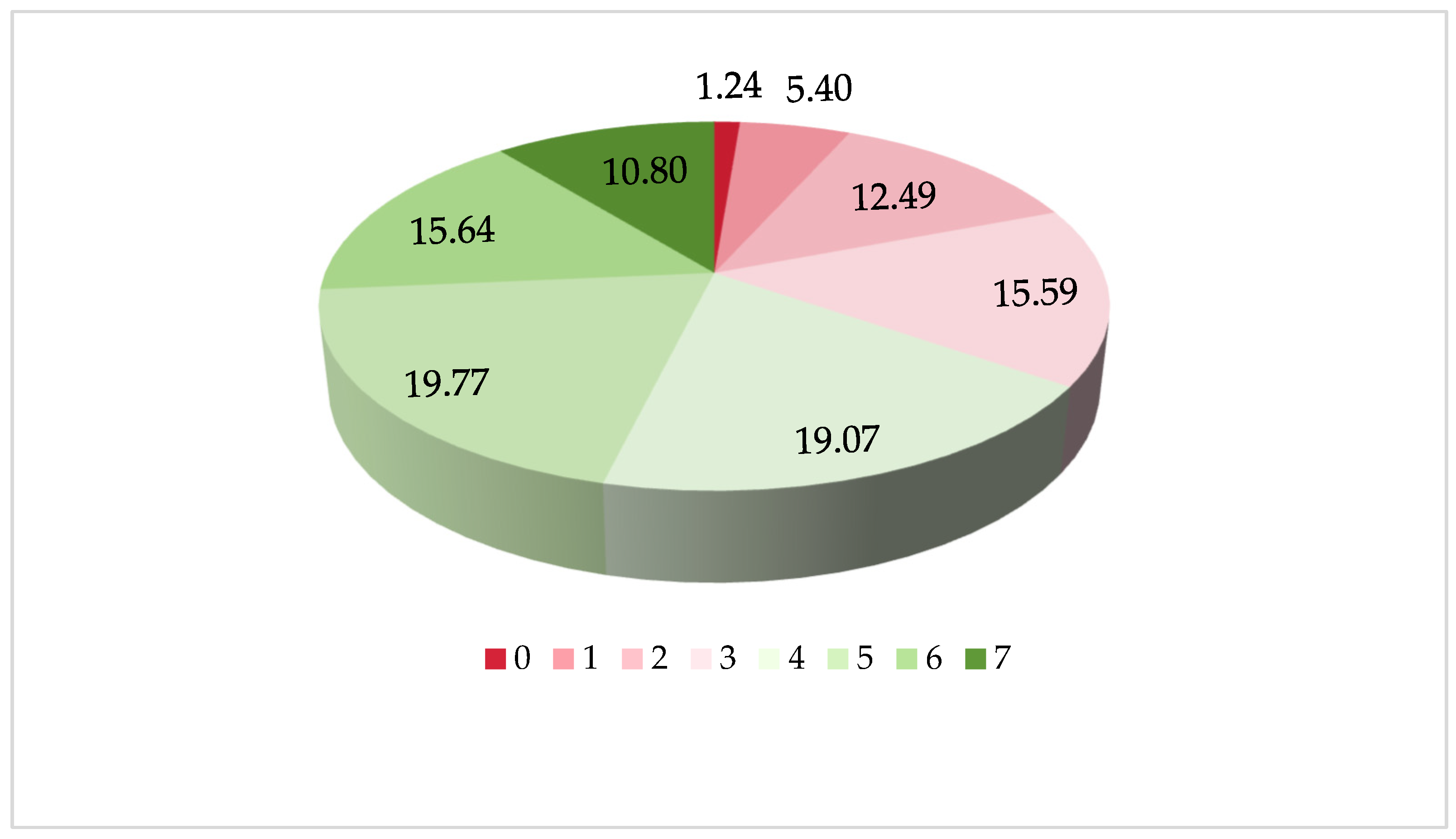


| Variables | Participants | Constitutional Ratio (%) | Cognitive Impairment | |
|---|---|---|---|---|
| Gender | Male | 6435 | 44.94 | 1304 (20.26) |
| Female | 7883 | 55.06 | 2990 (37.93) | |
| Age (years) | 65–74 | 3249 | 22.69 | 168 (5.17) |
| 75–84 | 4166 | 29.10 | 648 (15.55) | |
| 85–94 | 3646 | 25.46 | 1320 (36.20) | |
| ≥95 | 3257 | 22.75 | 2158 (66.26) | |
| Educational level | Below primary school | 6864 | 47.94 | 3172 (46.21) |
| Primary school | 4695 | 32.79 | 829 (17.66) | |
| Junior high school or above | 2759 | 19.27 | 293 (10.62) | |
| Marital status | Married | 6253 | 43.67 | 868 (13.88) |
| Others | 8065 | 56.33 | 3426 (42.48) | |
| Current residence | County | 7955 | 55.56 | 2255 (28.35) |
| Village | 6363 | 44.44 | 2039 (32.04) | |
| Living alone | Yes | 2339 | 16.34 | 664 (28.39) |
| No | 11,979 | 83.66 | 3630 (30.30) | |
| Occupation before age 60 | agriculture | 9124 | 63.72 | 3032 (33.23) |
| non-agricultural | 5194 | 36.28 | 1262 (24.30) | |
| Self-assessed economic situation | Wealthy | 2847 | 19.88 | 646 (22.69) |
| Average | 10,001 | 69.85 | 3091 (30.91) | |
| Poor | 1470 | 10.27 | 557 (37.89) | |
| Self-assessed health | Good | 6737 | 47.05 | 1727 (25.63) |
| Poor | 7581 | 52.95 | 2567 (33.86) | |
| Social participation | Yes | 2533 | 17.69 | 274 (10.82) |
| No | 11,785 | 82.31 | 4020 (34.11) |
| Model | AIC | BIC | ssaBIC | LRT p Value | BLRT p Value | Entropy |
|---|---|---|---|---|---|---|
| 1-class | 120,673.950 | 120,726.935 | 120,704.689 | - | - | - |
| 2-class | 113,663.213 | 113,776.752 | 113,729.083 | <0.001 | <0.001 | 0.600 |
| 3-class | 113,080.619 | 113,254.712 | 113,181.620 | <0.001 | <0.001 | 0.745 |
| 4-class | 112,421.239 | 112,655.886 | 112,557.371 | <0.001 | <0.001 | 0.695 |
| 5-class | 112,113.300 | 112,408.502 | 112,284.563 | <0.001 | <0.001 | 0.654 |
| 6-class | 111,906.943 | 112,262.699 | 112,113.337 | <0.001 | <0.001 | 0.746 |
| Variables | Cognitive Impairment | χ2 | p | |
|---|---|---|---|---|
| Vegetables | Yes | 3516 (27.46) | 367.374 | <0.001 |
| No | 778 (51.32) | |||
| Fruits | Yes | 1632 (24.99) | 142.824 | <0.001 |
| No | 2662 (34.18) | |||
| Red Meat | Yes | 3045 (27.46) | 122.027 | <0.001 |
| No | 1249 (37.70) | |||
| Fish | Yes | 1671 (24.70) | 171.180 | <0.001 |
| No | 2623 (34.73) | |||
| Eggs | Yes | 3003 (29.10) | 12.987 | <0.001 |
| No | 1291 (32.29) | |||
| Beans–nuts | Yes | 2023 (27.47) | 45.962 | <0.001 |
| No | 2271 (32.66) | |||
| Milk | Yes | 1586 (28.37) | 11.436 | 0.001 |
| No | 2708 (31.03) |
| Variables | Cognitive Impairment | χ2 | p | |
|---|---|---|---|---|
| Dietary Diversity Scores | ≤2 | 1086 (39.63) | 260.706 a 256.236 b | <0.001 |
| 3 | 748 (33.51) | |||
| 4 | 851 (31.17) | |||
| 5 | 757 (26.74) | |||
| 6 | 546 (24.39) | |||
| 7 | 306 (19.79) | |||
| Dietary Patterns | DP1 | 1132 (33.47) | 219.698 a 157.422 b | <0.001 |
| DP2 | 1100 (37.68) | |||
| DP3 | 1003 (29.91) | |||
| DP4 | 1059 (22.71) |
| Variables | Crude Model | Adjusted Model | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Vegetables | 0.36 | (0.32, 0.40) *** | 0.79 | (0.72, 0.87) *** |
| Fruits | 0.64 | (0.60, 0.69) *** | 0.51 | (0.45, 0.58) *** |
| Red Meat | 0.63 | (0.58, 0.69) *** | 0.70 | (0.64, 0.78) *** |
| Fish | 0.62 | (0.57, 0.66) *** | 0.74 | (0.68, 0.81) *** |
| Eggs | 0.86 | (0.80, 0.93) *** | 0.99 | (0.90, 1.09) |
| Beans–nuts | 0.78 | (0.73, 0.84) *** | 0.85 | (0.78, 0.93) |
| Milk | 0.88 | (0.82, 0.95) *** | 0.91 | (0.83, 1.00) * |
| Variables | Crude Model | Adjusted Model | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Dietary Diversity Scores (ref = ≤ 2) | ||||
| 3 | 0.77 | (0.68, 0.86) *** | 0.79 | (0.69, 0.91) ** |
| 4 | 0.69 | (0.62, 0.77) *** | 0.75 | (0.65, 0.86) *** |
| 5 | 0.56 | (0.50, 0.62) *** | 0.66 | (0.57, 0.75) *** |
| 6 | 0.49 | (0.43, 0.56) *** | 0.59 | (0.51, 0.69) *** |
| 7 | 0.38 | (0.33, 0.44) *** | 0.54 | (0.45, 0.65) *** |
| Dietary Patterns (ref = DP1) | ||||
| DP2 | 1.20 | (1.08, 1.33) *** | 1.24 | (1.09, 1.40) *** |
| DP3 | 0.85 | (0.77, 0.94) *** | 0.91 | (0.81, 1.03) |
| DP4 | 0.58 | (0.53, 0.65) *** | 0.79 | (0.69, 0.89) *** |
| Variable | DDS | DP | Psychological Balance | Cognitive Impairment |
|---|---|---|---|---|
| DDS | 1 | |||
| DP | 0.862 ** | 1 | ||
| Psychological Balance | 0.177 ** | 0.150 ** | 1 | |
| Cognitive Impairment | −0.142 ** | −0.105 ** | −0.233 ** | 1 |
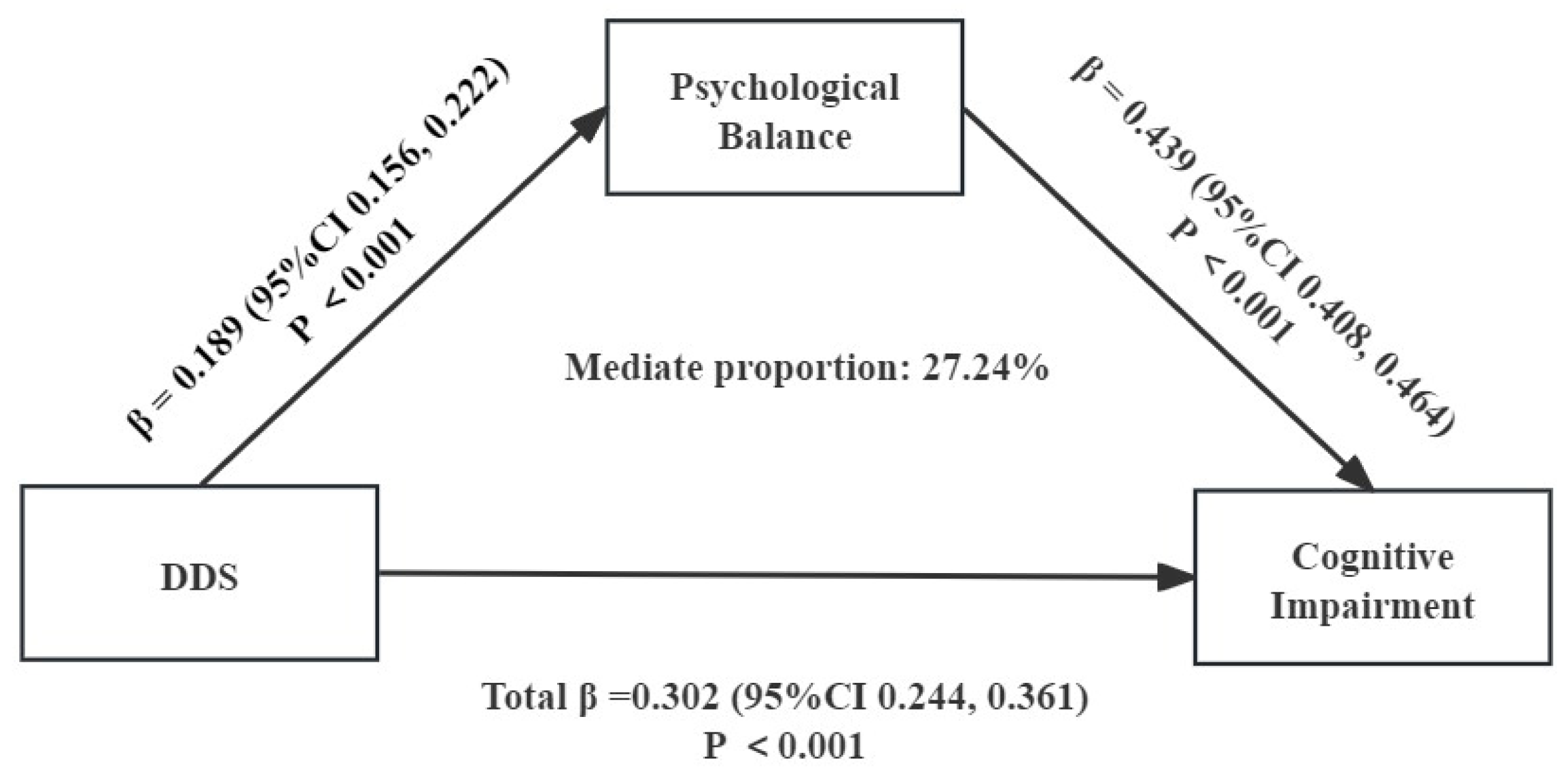
| Effect | BootSE | BootLLCI | BootULCI | Percentage of Total Effect | |
|---|---|---|---|---|---|
| Total effect | 0.302 | 0.030 | 0.244 | 0.361 | 100% |
| Direct effect | 0.220 | 0.029 | 0.163 | 0.277 | 73.79% |
| Dietary diversity scores → psychological balance → cognitive impairment | 0.082 | 0.008 | 0.066 | 0.099 | 27.24% |
| Effect | BootSE | BootLLCI | BootULCI | Percentage of Total Effect | |
|---|---|---|---|---|---|
| Total effect | 0.227 | 0.044 | 0.141 | 0.314 | 100% |
| Direct effect | 0.134 | 0.043 | 0.050 | 0.218 | 59.04% |
| Dietary patterns → psychological balance → cognitive impairment | 0.093 | 0.012 | 0.070 | 0.116 | 41.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, L.; Wen, X.; Liu, X. The Mediating Role of Psychological Balance on the Effects of Dietary Behavior on Cognitive Impairment in Chinese Elderly. Nutrients 2024, 16, 908. https://doi.org/10.3390/nu16060908
Chen Y, Zhang L, Wen X, Liu X. The Mediating Role of Psychological Balance on the Effects of Dietary Behavior on Cognitive Impairment in Chinese Elderly. Nutrients. 2024; 16(6):908. https://doi.org/10.3390/nu16060908
Chicago/Turabian StyleChen, Yating, Lingling Zhang, Xiaotong Wen, and Xiaojun Liu. 2024. "The Mediating Role of Psychological Balance on the Effects of Dietary Behavior on Cognitive Impairment in Chinese Elderly" Nutrients 16, no. 6: 908. https://doi.org/10.3390/nu16060908
APA StyleChen, Y., Zhang, L., Wen, X., & Liu, X. (2024). The Mediating Role of Psychological Balance on the Effects of Dietary Behavior on Cognitive Impairment in Chinese Elderly. Nutrients, 16(6), 908. https://doi.org/10.3390/nu16060908






