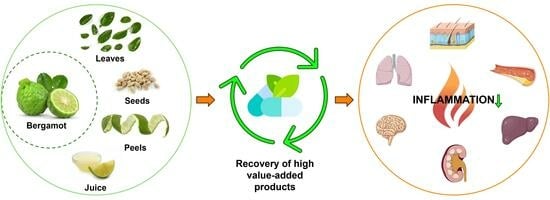
Bergamot Byproducts: A Sustainable Source to Counteract Inflammation
Abstract
1. Introduction
2. Inflammatory Process
3. Main Pathological Conditions Linked to Inflammation
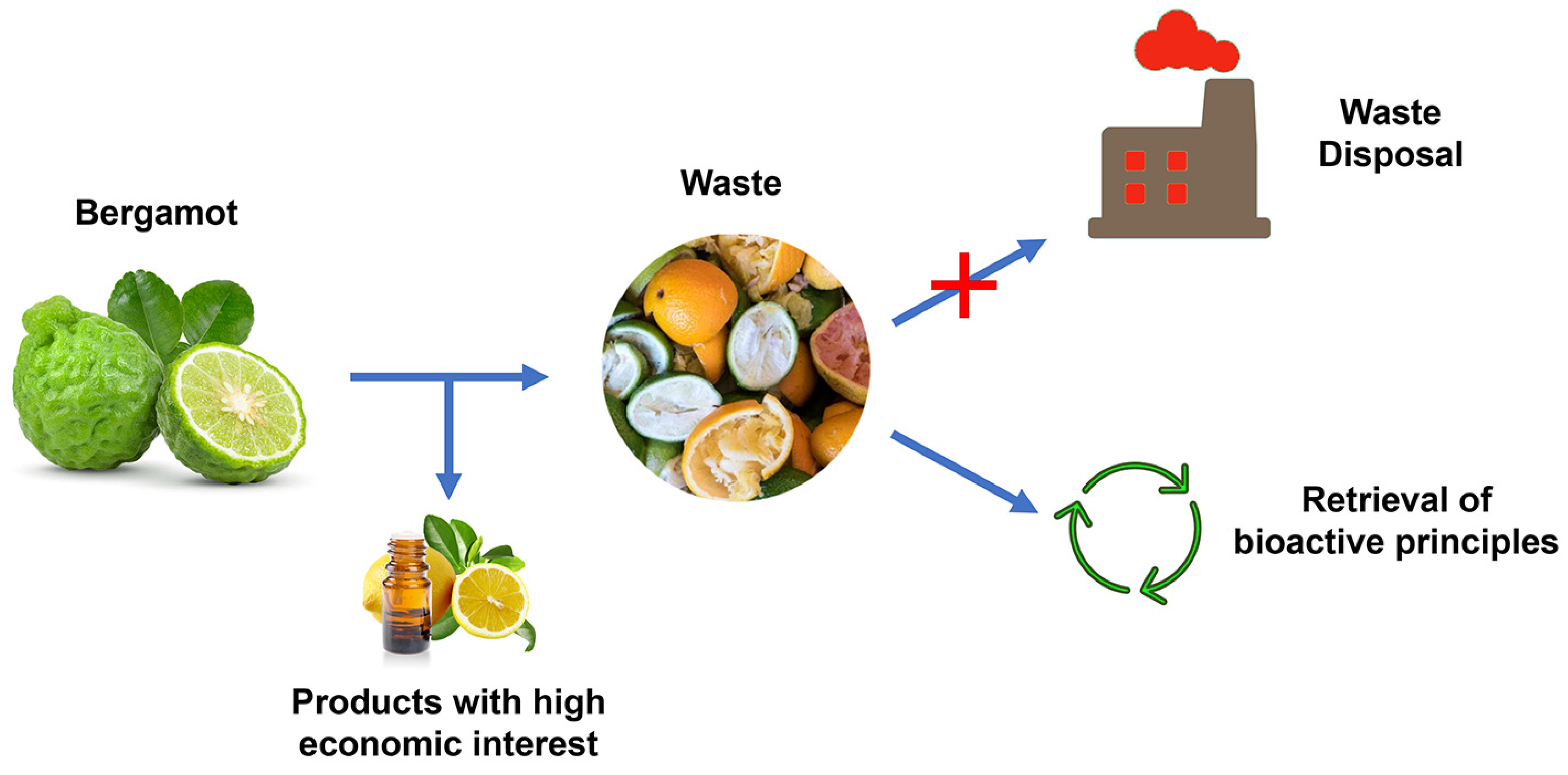
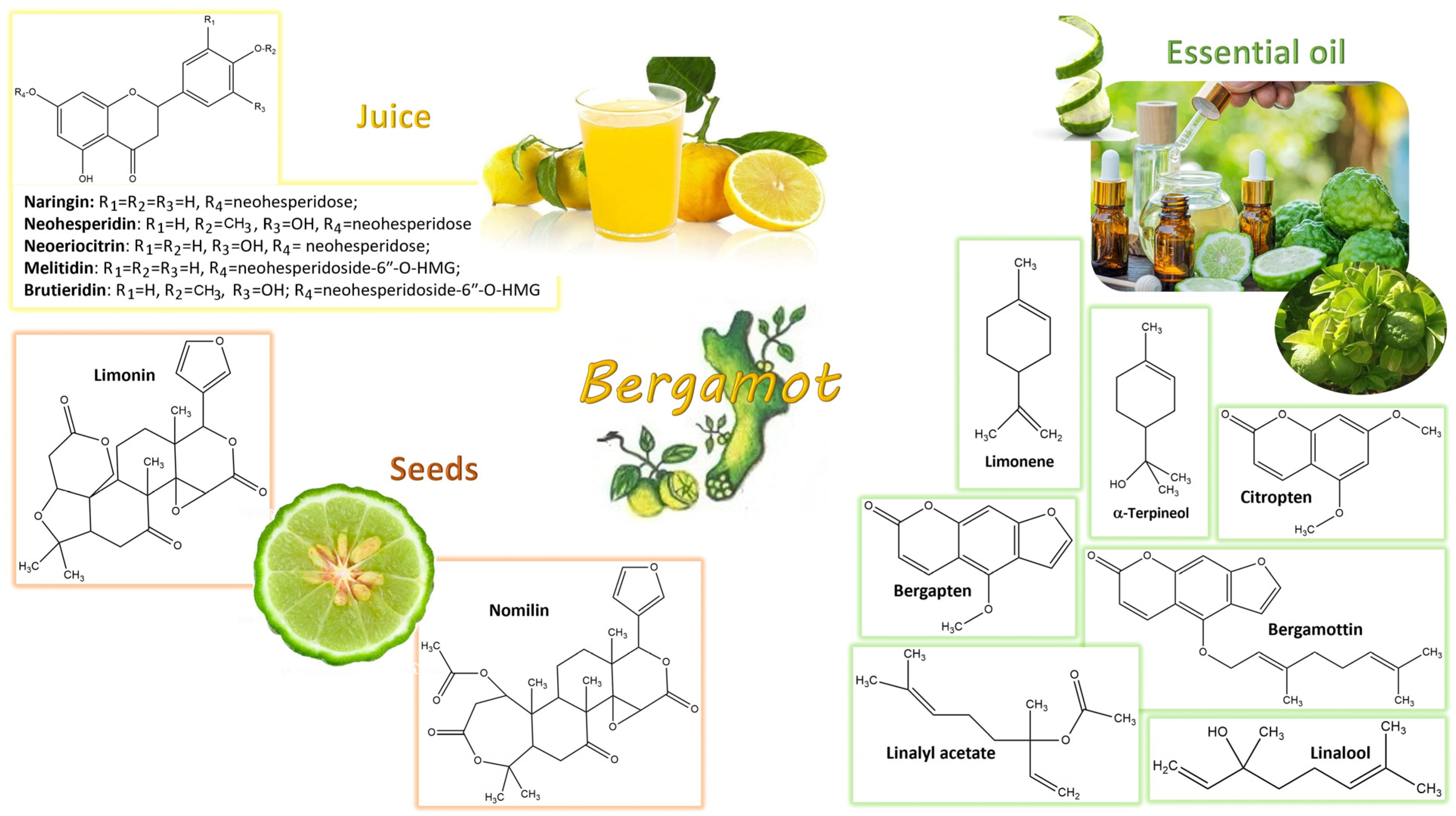
4. Bergamot Derivatives and Byproducts
5. Anti-Inflammatory Activity of Bergamot Derivatives and Byproducts
5.1. Bergamot Derivatives and Byproducts against Acute Inflammation
5.2. Bergamot Derivatives and Byproducts in Inflammatory-Based Respiratory Ailments
5.3. Bergamot Derivatives and Byproducts against Metabolic Syndrome
5.4. Bergamot Derivatives and Byproducts in Obesity and Overweight
5.5. Bergamot Derivatives and Byproducts in Liver Diseases
5.6. Bergamot Derivatives and Byproducts in Dyslipidemic Disorders
5.7. Bergamot Derivatives and Byproducts in Renal, Gynecological and Rectal Dysfynctions
5.8. Bergamot Derivatives and Byproducts in Neurological Disorders
5.9. Bergamot Derivatives and Byproducts in Cancer
5.10. Bergamot Derivatives and Byproducts in Skin Disorders
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langgut, D. The citrus route revealed: From Southeast Asia into the Mediterranean. HortScience 2017, 52, 814–822. [Google Scholar] [CrossRef]
- Wang, R.X.; Zhou, M.; Ma, H.L.; Qiao, Y.B.; Li, Q.S. The Role of Chronic Inflammation in Various Diseases and Anti-inflammatory Therapies Containing Natural Products. ChemMedChem 2021, 16, 1576–1592. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Navarra, M.; Woodside, J.V.; Cantwell, M.M. Citrus fruits intake and oral cancer risk: A systematic review and meta-analysis. Pharmacol. Res. 2018, 133, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, C.; Navarra, M.; Calapai, F.; Squeri, R.; Gangemi, S.; Calapai, G. Clinical Pharmacology of Citrus bergamia: A Systematic Review. Phytother. Res. 2017, 31, 27–39. [Google Scholar] [CrossRef]
- Marino, A.; Paterniti, I.; Cordaro, M.; Morabito, R.; Campolo, M.; Navarra, M.; Esposito, E.; Cuzzocrea, S. Role of natural antioxidants and potential use of bergamot in treating rheumatoid arthritis. PharmaNutrition 2015, 3, 53–59. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Cirmi, S.; Calapai, G.; Ventura-Spagnolo, E.; Gangemi, S.; Navarra, M. Anti-Inflammatory Activity of Citrus bergamia Derivatives: Where Do We Stand? Molecules 2016, 21, 1273. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Russo, C.; Musumeci, L.; Navarra, M.; Lombardo, G.E. Oleacein Attenuates Lipopolysaccharide-Induced Inflammation in THP-1-Derived Macrophages by the Inhibition of TLR4/MyD88/NF-kappaB Pathway. Int. J. Mol. Sci. 2022, 23, 1206. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Abbate, F.; Maugeri, A.; Laura, R.; Levanti, M.; Navarra, M.; Cirmi, S.; Germana, A. Zebrafish as a Useful Model to Study Oxidative Stress-Linked Disorders: Focus on Flavonoids. Antioxidants 2021, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, D.; Wang, B.; Li, H.; Xiong, J.; Xu, S.; Chen, Q.; Tao, K.; Yang, X.; Zhu, Y.; et al. HMGB1 translocation and release mediate cigarette smoke-induced pulmonary inflammation in mice through a TLR4/MyD88-dependent signaling pathway. Mol. Biol. Cell 2017, 28, 201–209. [Google Scholar] [CrossRef]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Rossaint, J.; Margraf, A.; Zarbock, A. Role of Platelets in Leukocyte Recruitment and Resolution of Inflammation. Front. Immunol. 2018, 9, 2712. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Pierre, O.; Le Gall-Ianotto, C.; Lebonvallet, N.; Chernyshov, P.V.; Le Garrec, R.; Talagas, M. Basic mechanisms of itch. J. Allergy Clin. Immunol. 2023, 152, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, H.; Rosmarin, D.; Schon, M.P.; Stander, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef]
- Chung, K.F. p38 mitogen-activated protein kinase pathways in asthma and COPD. Chest 2011, 139, 1470–1479. [Google Scholar] [CrossRef]
- Calbet, M.; Ramis, I.; Calama, E.; Carreno, C.; Paris, S.; Maldonado, M.; Orellana, A.; Calaf, E.; Pauta, M.; De Alba, J.; et al. Novel Inhaled Pan-JAK Inhibitor, LAS194046, Reduces Allergen-Induced Airway Inflammation, Late Asthmatic Response, and pSTAT Activation in Brown Norway Rats. J. Pharmacol. Exp. Ther. 2019, 370, 137–147. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, C.; Wang, W.; Jin, R.; Li, Q.; Ge, Q.; Guan, Y.; Zhang, Y. Prostaglandins E2 signal mediated by receptor subtype EP2 promotes IgE production in vivo and contributes to asthma development. Sci. Rep. 2016, 6, 20505. [Google Scholar] [CrossRef]
- Luczak, E.; Wieczfinska, J.; Sokolowska, M.; Pniewska, E.; Luczynska, D.; Pawliczak, R. Troglitazone, a PPAR-gamma agonist, decreases LTC(4) concentration in mononuclear cells in patients with asthma. Pharmacol. Rep. 2017, 69, 1315–1321. [Google Scholar] [CrossRef]
- Devchand, P.R.; Keller, H.; Peters, J.M.; Vazquez, M.; Gonzalez, F.J.; Wahli, W. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature 1996, 384, 39–43. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Musumeci, L.; De Sarro, G.; Cirmi, S.; Navarra, M. Inflammation and Obesity: The Pharmacological Role of Flavonoids in the Zebrafish Model. Int. J. Mol. Sci. 2023, 24, 2899. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Jorge, A.S.B.; Andrade, J.M.O.; Paraiso, A.F.; Jorge, G.C.B.; Silveira, C.M.; de Souza, L.R.; Santos, E.P.; Guimaraes, A.L.S.; Santos, S.H.S.; De-Paula, A.M.B. Body mass index and the visceral adipose tissue expression of IL-6 and TNF-alpha are associated with the morphological severity of non-alcoholic fatty liver disease in individuals with class III obesity. Obes. Res. Clin. Pract. 2018, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Montanari, N.R.; Ramirez, R.; Aggarwal, A.; van Buuren, N.; Doukas, M.; Moon, C.; Turner, S.; Diehl, L.; Li, L.; Debes, J.D.; et al. Multi-parametric analysis of human livers reveals variation in intrahepatic inflammation across phases of chronic hepatitis B infection. J. Hepatol. 2022, 77, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendia, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kang, E.; Han, J.S. HM-chromanone attenuates TNF-alpha-mediated inflammation and insulin resistance by controlling JNK activation and NF-kappaB pathway in 3T3-L1 adipocytes. Eur. J. Pharmacol. 2022, 921, 174884. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cirmi, S.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. A flavonoid-rich extract of orange juice reduced oxidative stress in an experimental model of inflammatory bowel disease. J. Funct. Foods 2017, 30, 168–178. [Google Scholar] [CrossRef]
- Plotkin, J.D.; Elias, M.G.; Dellinger, A.L.; Kepley, C.L. NF-kappaB inhibitors that prevent foam cell formation and atherosclerotic plaque accumulation. Nanomedicine 2017, 13, 2037–2048. [Google Scholar] [CrossRef]
- Lu, N.; Cheng, W.; Liu, D.; Liu, G.; Cui, C.; Feng, C.; Wang, X. NLRP3-Mediated Inflammation in Atherosclerosis and Associated Therapeutics. Front. Cell Dev. Biol. 2022, 10, 823387. [Google Scholar] [CrossRef]
- Lainampetch, J.; Panprathip, P.; Phosat, C.; Chumpathat, N.; Prangthip, P.; Soonthornworasiri, N.; Puduang, S.; Wechjakwen, N.; Kwanbunjan, K. Association of Tumor Necrosis Factor Alpha, Interleukin 6, and C-Reactive Protein with the Risk of Developing Type 2 Diabetes: A Retrospective Cohort Study of Rural Thais. J. Diabetes Res. 2019, 2019, 9051929. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Morris, D.R.; Smith, S.; Moxon, J.V.; Golledge, J. Systematic Review and Meta-Analysis of the Association Between C-Reactive Protein and Major Cardiovascular Events in Patients with Peripheral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 220–233. [Google Scholar] [CrossRef]
- Adolph, T.E.; Meyer, M.; Schwarzler, J.; Mayr, L.; Grabherr, F.; Tilg, H. The metabolic nature of inflammatory bowel diseases. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Randazzo, B.; Russo, C.; Musumeci, L.; Maugeri, A.; Montalbano, G.; Guerrera, M.C.; Lombardo, G.E.; Levanti, M. Anti-inflammatory effect of a flavonoid-rich extract of orange juice in adult zebrafish subjected to Vibrio anguillarum-induced enteritis. Nat. Prod. Res. 2021, 35, 5350–5353. [Google Scholar] [CrossRef]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Calvello, R.; Cavallo, P.; Dragone, T.; Carofiglio, V.; Panaro, M.A. Modulation of NF-kappaB activation by resveratrol in LPS treated human intestinal cells results in downregulation of PGE2 production and COX-2 expression. Toxicol. Vitro 2012, 26, 1122–1128. [Google Scholar] [CrossRef]
- Bruschetta, G.; Fazio, E.; Cravana, C.; Ferlazzo, A.M. Effects of partial versus complete separation after weaning on plasma serotonin, tryptophan and pituitary-adrenal pattern of Anglo-Arabian foals. Livest. Sci. 2017, 198, 157–161. [Google Scholar] [CrossRef]
- Bruschetta, G.; Medica, P.; Fazio, E.; Cravana, C.; Ferlazzo, A.M. The effect of training sessions and feeding regimes on neuromodulator role of serotonin, tryptophan, and β-endorphin of horses. J. Vet. Behav. 2018, 23, 82–86. [Google Scholar] [CrossRef]
- Wang, M.; Xu, H.; Chong Lee Shin, O.L.; Li, L.; Gao, H.; Zhao, Z.; Zhu, F.; Zhu, H.; Liang, W.; Qian, K.; et al. Compound alpha-keto acid tablet supplementation alleviates chronic kidney disease progression via inhibition of the NF-kB and MAPK pathways. J. Transl. Med. 2019, 17, 122. [Google Scholar] [CrossRef]
- Milo, R.; Korczyn, A.D.; Manouchehri, N.; Stuve, O. The temporal and causal relationship between inflammation and neurodegeneration in multiple sclerosis. Mult. Scler. 2020, 26, 876–886. [Google Scholar] [CrossRef]
- Song, L.; Pei, L.; Yao, S.; Wu, Y.; Shang, Y. NLRP3 Inflammasome in Neurological Diseases, from Functions to Therapies. Front. Cell Neurosci. 2017, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Filaly, H.E.; Outlioua, A.; Medyouf, H.; Guessous, F.; Akarid, K. Targeting IL-1beta in patients with advanced Helicobacter pylori infection: A potential therapy for gastric cancer. Future Microbiol. 2022, 17, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Cirmi, S.; Minciullo, P.L.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Citrus fruits and inflammaging: A systematic review. Phytochem. Rev. 2019, 18, 1025–1049. [Google Scholar] [CrossRef]
- Mannucci, C.; Calapai, F.; Cardia, L.; Inferrera, G.; D’Arena, G.; Di Pietro, M.; Navarra, M.; Gangemi, S.; Ventura Spagnolo, E.; Calapai, G. Clinical Pharmacology of Citrus aurantium and Citrus sinensis for the Treatment of Anxiety. Evid. Based Complement. Altern. Med. 2018, 2018, 3624094. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, A.; Germanò, M.P. Citrus × bergamia Risso & Poiteau Botanical classification, Morphology and Anatomy. In Citrus bergamia: Bergamot and Its Derivatives; CRC Press: Boca Raton, FL, USA, 2014; pp. 9–11. [Google Scholar]
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Giuffre, A.M. Bergamot (Citrus bergamia, Risso): The Effects of Cultivar and Harvest Date on Functional Properties of Juice and Cloudy Juice. Antioxidants 2019, 8, 221. [Google Scholar] [CrossRef]
- Gonzalez-Mas, M.C.; Rambla, J.L.; Lopez-Gresa, M.P.; Blazquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Cirmi, S.; Bisignano, C.; Mandalari, G.; Navarra, M. Anti-infective potential of Citrus bergamia Risso et Poiteau (bergamot) derivatives: A systematic review. Phytother. Res. 2016, 30, 1404–1411. [Google Scholar] [CrossRef]
- Navarra, M.; Ferlazzo, N.; Cirmi, S.; Trapasso, E.; Bramanti, P.; Lombardo, G.E.; Minciullo, P.L.; Calapai, G.; Gangemi, S. Effects of bergamot essential oil and its extractive fractions on SH-SY5Y human neuroblastoma cell growth. J. Pharm. Pharmacol. 2015, 67, 1042–1053. [Google Scholar] [CrossRef]
- Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Russo, C.; Gangemi, S.; Calapai, G.; Cirmi, S.; Navarra, M. Bergamottin and 5-Geranyloxy-7-methoxycoumarin Cooperate in the Cytotoxic Effect of Citrus bergamia (Bergamot) Essential Oil in Human Neuroblastoma SH-SY5Y Cell Line. Toxins 2021, 13, 275. [Google Scholar] [CrossRef]
- Gattuso, A.; Piscopo, A.; Romeo, R.; De Bruno, A.; Poiana, M. Recovery of Bioactive Compounds from Calabrian Bergamot Citrus Waste: Selection of Best Green Extraction. Agriculture 2023, 13, 1095. [Google Scholar] [CrossRef]
- Salerno, R.; Casale, F.; Calandruccio, C.; Procopio, A. Characterization of flavonoids in Citrus bergamia (Bergamot) polyphenolic fraction by liquid chromatography–high resolution mass spectrometry (LC/HRMS). PharmaNutrition 2016, 4, S1–S7. [Google Scholar]
- Musumeci, L.; Maugeri, A.; Russo, C.; Lombardo, G.E.; Cirmi, S.; Navarra, M. Citrus Flavonoids and Autoimmune Diseases: A Systematic Review of Clinical Studies. Curr. Med. Chem. 2023, 30, 2191–2204. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, E.; Pizzimenti, F.; Ferlazzo, A.; Giofre, S.V.; Iannazzo, D.; Piperno, A.; Romeo, R.; Chiacchio, M.A.; Mastino, A.; Macchi, B. Antiviral activity of seed extract from Citrus bergamia towards human retroviruses. Bioorg Med. Chem. 2011, 19, 2084–2089. [Google Scholar] [CrossRef]
- Kirbaslar, Ş.İ.; Kirbaslar, F.G. Composition of Turkish mandarin and bergamot leaf oils. J. Essent. Oil Res. 2006, 18, 318–327. [Google Scholar] [CrossRef]
- Maugeri, A.; Ferlazzo, N.; De Luca, L.; Gitto, R.; Navarra, M. The link between the AMPK/SIRT1 axis and a flavonoid-rich extract of Citrus bergamia juice: A cell-free, in silico, and in vitro study. Phytother. Res. 2019, 33, 1805–1814. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Fusco, R.; D’Amico, R.; Peditto, M.; Oteri, G.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. Treatment with a Flavonoid-Rich Fraction of Bergamot Juice Improved Lipopolysaccharide-Induced Periodontitis in Rats. Front. Pharmacol. 2018, 9, 1563. [Google Scholar] [CrossRef]
- Lombardo, G.E.; Cirmi, S.; Musumeci, L.; Pergolizzi, S.; Maugeri, A.; Russo, C.; Mannucci, C.; Calapai, G.; Navarra, M. Mechanisms Underlying the Anti-Inflammatory Activity of Bergamot Essential Oil and Its Antinociceptive Effects. Plants 2020, 9, 704. [Google Scholar] [CrossRef]
- Feng, S.; Xu, G.; Fu, Y.; Ding, Q.; Shi, Y. Exploring the Mechanism of Bergamot Essential Oil against Asthma Based on Network Pharmacology and Experimental Verification. ACS Omega 2023, 8, 10202–10213. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Yang, W.; Qi, X.; Lan, L.; Luo, L.; Yin, Z. Bergapten prevents lipopolysaccharide-induced inflammation in RAW264.7 cells through suppressing JAK/STAT activation and ROS production and increases the survival rate of mice after LPS challenge. Int. Immunopharmacol. 2017, 48, 159–168. [Google Scholar] [CrossRef]
- Jiang, Y.; Nguyen, T.V.; Jin, J.; Yu, Z.N.; Song, C.H.; Chai, O.H. Bergapten ameliorates combined allergic rhinitis and asthma syndrome after PM2.5 exposure by balancing Treg/Th17 expression and suppressing STAT3 and MAPK activation in a mouse model. Biomed. Pharmacother. 2023, 164, 114959. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Mol, M.; Garcia, J.L.; Correa, C.; Raucci, A.; Mancinelli, L.; Mazzotta, S.; Fumagalli, L.; Trunfio, G.; et al. Analytical Profile and Antioxidant and Anti-Inflammatory Activities of the Enriched Polyphenol Fractions Isolated from Bergamot Fruit and Leave. Antioxidants 2021, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Della Vedova, L.; Gado, F.; Vieira, T.A.; Grandini, N.A.; Palacio, T.L.N.; Siqueira, J.S.; Carini, M.; Bombardelli, E.; Correa, C.R.; Aldini, G.; et al. Chemical, Nutritional and Biological Evaluation of a Sustainable and Scalable Complex of Phytochemicals from Bergamot By-Products. Molecules 2023, 28, 2964. [Google Scholar] [CrossRef] [PubMed]
- Palacio, T.L.N.; Siqueira, J.S.; de Paula, B.H.; Rego, R.M.P.; Vieira, T.A.; Baron, G.; Altomare, A.; Ferron, A.J.T.; Aldini, G.; Kano, H.T.; et al. Bergamot (Citrus bergamia) leaf extract improves metabolic, antioxidant and anti-inflammatory activity in skeletal muscles in a metabolic syndrome experimental model. Int. J. Food Sci. Nutr. 2023, 74, 64–71. [Google Scholar] [CrossRef]
- De Leo, M.; Piragine, E.; Pirone, A.; Braca, A.; Pistelli, L.; Calderone, V.; Miragliotta, V.; Testai, L. Protective Effects of Bergamot (Citrus bergamia Risso & Poiteau) Juice in Rats Fed with High-Fat Diet. Planta Med. 2020, 86, 180–189. [Google Scholar] [CrossRef]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. [Google Scholar] [CrossRef]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef]
- Di Folco, U.; Pollakova, D.; De Falco, D.; Nardone, M.R.; Tubili, F.; Tubili, C. Effects of a nutraceutical multicompound including bergamot (Citrus bergamia Risso) juice on metabolic syndrome: A pilot study. Mediterr. J. Nutr. Metab. 2018, 11, 119–126. [Google Scholar] [CrossRef]
- Upala, S.; Jaruvongvanich, V.; Riangwiwat, T.; Jaruvongvanich, S.; Sanguankeo, A. Association between Helicobacter pylori infection and metabolic syndrome: A systematic review and meta-analysis. J. Dig. Dis. 2016, 17, 433–440. [Google Scholar] [CrossRef]
- Filocamo, A.; Bisignano, C.; Ferlazzo, N.; Cirmi, S.; Mandalari, G.; Navarra, M. In vitro effect of bergamot (Citrus bergamia) juice against cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Complement. Altern. Med. 2015, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macri, R.; Ruga, S.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.S.; Nakandakare-Maia, E.T.; Vieira, T.A.; Palacio, T.L.N.; Grandini, N.A.; Belin, M.A.F.; Nai, G.A.; Moreto, F.; Altomare, A.; Baron, G. Effect of Bergamot Leaves (Citrus bergamia) in the Crosstalk between Adipose Tissue and Liver of Diet-Induced Obese Rats. Livers 2023, 3, 258–270. [Google Scholar] [CrossRef]
- Nakandakare-Maia, E.T.; Siqueira, J.S.; Ferron, A.J.T.; Vieira, T.A.; Palacio, T.L.N.; Grandini, N.A.; Garcia, J.L.; Belin, M.A.; Altomare, A.; Baron, G.; et al. Treatment with bergamot (Citrus bergamia) leaves extract attenuates leptin resistance in obese rats. Mol. Cell Endocrinol. 2023, 566–567, 111908. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Three-arm, placebo-controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phytotherapy Res. 2019, 33, 2094–2101. [Google Scholar] [CrossRef]
- Parafati, M.; Lascala, A.; La Russa, D.; Mignogna, C.; Trimboli, F.; Morittu, V.M.; Riillo, C.; Macirella, R.; Mollace, V.; Brunelli, E.; et al. Bergamot Polyphenols Boost Therapeutic Effects of the Diet on Non-Alcoholic Steatohepatitis (NASH) Induced by “Junk Food”: Evidence for Anti-Inflammatory Activity. Nutrients 2018, 10, 1604. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J.; et al. Bergamot Polyphenols Improve Dyslipidemia and Pathophysiological Features in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 2565. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef]
- Spigoni, V.; Mena, P.; Fantuzzi, F.; Tassotti, M.; Brighenti, F.; Bonadonna, R.C.; Del Rio, D.; Dei Cas, A. Bioavailability of Bergamot (Citrus bergamia) Flavanones and Biological Activity of Their Circulating Metabolites in Human Pro-Angiogenic Cells. Nutrients 2017, 9, 1328. [Google Scholar] [CrossRef]
- Fogacci, F.; Rizzoli, E.; Giovannini, M.; Bove, M.; D’Addato, S.; Borghi, C.; Cicero, A.F.G. Effect of Dietary Supplementation with Eufortyn((R)) Colesterolo Plus on Serum Lipids, Endothelial Reactivity, Indexes of Non-Alcoholic Fatty Liver Disease and Systemic Inflammation in Healthy Subjects with Polygenic Hypercholesterolemia: The ANEMONE Study. Nutrients 2022, 14, 2099. [Google Scholar] [CrossRef] [PubMed]
- Bonfigli, A.R.; Protic, O.; Olivieri, F.; Montesanto, A.; Malatesta, G.; Di Pillo, R.; Antonicelli, R. Effects of a novel nutraceutical combination (BruMeChol) in subjects with mild hypercholesterolemia: Study protocol of a randomized, double-blind, controlled trial. Trials 2020, 21, 616. [Google Scholar] [CrossRef] [PubMed]
- Dari, F.F.; Jaccob, A.A.; AL-Moziel, M.S. The potential protective effects of citrus bergamot extract against amikacin-induced nephrotoxicity in male albino rats. Toxicol. Environ. Health Sci. 2023, 15, 9–17. [Google Scholar] [CrossRef]
- Cirmi, S.; Maugeri, A.; Micali, A.; Marini, H.R.; Puzzolo, D.; Santoro, G.; Freni, J.; Squadrito, F.; Irrera, N.; Pallio, G.; et al. Cadmium-Induced Kidney Injury in Mice Is Counteracted by a Flavonoid-Rich Extract of Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, via the Enhancement of Different Defense Mechanisms. Biomedicines 2021, 9, 1797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Kong, F.; Zhao, L.; Yang, X.; Wu, W.; Zhang, L.; Ji, B.; Zhou, F. Essential oil, juice, and ethanol extract from bergamot confer improving effects against primary dysmenorrhea in rats. J. Food Biochem. 2021, 45, e13614. [Google Scholar] [CrossRef]
- Cafaro, D.; Celedon, F.; Sturiale, A.; Sinicropi, M.S. Innovative results in the treatment of inespecific anusitis-proctitis with the use of bergamot gel (Benebeo gel)®. Insights Clin. Cell. Immunol. 2019, 3, 020–024. [Google Scholar]
- Curro, M.; Risitano, R.; Ferlazzo, N.; Cirmi, S.; Gangemi, C.; Caccamo, D.; Ientile, R.; Navarra, M. Citrus bergamia Juice Extract Attenuates beta-Amyloid-Induced Pro-Inflammatory Activation of THP-1 Cells Through MAPK and AP-1 Pathways. Sci. Rep. 2016, 6, 20809. [Google Scholar] [CrossRef]
- Cui, Y.; Che, Y.; Wang, H. Bergamot essential oil attenuate aluminum-induced anxiety-like behavior through antioxidation, anti-inflammatory and GABA regulation in rats. Food Chem. Toxicol. 2020, 145, 111766. [Google Scholar] [CrossRef]
- Bruno, A.; Pandolfo, G.; Crucitti, M.; Cedro, C.; Zoccali, R.A.; Muscatello, M.R.A. Bergamot Polyphenolic Fraction Supplementation Improves Cognitive Functioning in Schizophrenia: Data From an 8-Week, Open-Label Pilot Study. J. Clin. Psychopharmacol. 2017, 37, 468–471. [Google Scholar] [CrossRef]
- Delle Monache, S.; Sanità, P.; Trapasso, E.; Ursino, M.R.; Dugo, P.; Russo, M.; Ferlazzo, N.; Calapai, G.; Angelucci, A.; Navarra, M. Mechanisms underlying the anti-tumoral effects of Citrus bergamia juice. PLoS ONE 2013, 8, e61484. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Cirmi, S.; Russo, M.; Trapasso, E.; Ursino, M.R.; Lombardo, G.E.; Gangemi, S.; Calapai, G.; Navarra, M. NF-kappaB mediates the antiproliferative and proapoptotic effects of bergamot juice in HepG2 cells. Life Sci. 2016, 146, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Visalli, G.; Ferlazzo, N.; Cirmi, S.; Campiglia, P.; Gangemi, S.; Di Pietro, A.; Calapai, G.; Navarra, M. Bergamot juice extract inhibits proliferation by inducing apoptosis in human colon cancer cells. Anticancer Agents Med. Chem. 2014, 14, 1402–1413. [Google Scholar] [CrossRef]
- Maugeri, A.; Russo, C.; Musumeci, L.; Lombardo, G.E.; De Sarro, G.; Barreca, D.; Cirmi, S.; Navarra, M. The Anticancer Effect of a Flavonoid-Rich Extract of Bergamot Juice in THP-1 Cells Engages the SIRT2/AKT/p53 Pathway. Pharmaceutics 2022, 14, 2168. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Maugeri, A.; De Luca, L.; Gitto, R.; Lombardo, G.E.; Musumeci, L.; De Sarro, G.; Cirmi, S.; Navarra, M. The SIRT2 Pathway Is Involved in the Antiproliferative Effect of Flavanones in Human Leukemia Monocytic THP-1 Cells. Biomedicines 2022, 10, 2383. [Google Scholar] [CrossRef] [PubMed]
- Navarra, M.; Ursino, M.R.; Ferlazzo, N.; Russo, M.; Schumacher, U.; Valentiner, U. Effect of Citrus bergamia juice on human neuroblastoma cells in vitro and in metastatic xenograft models. Fitoterapia 2014, 95, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Navarra, M.; Femia, A.P.; Romagnoli, A.; Tortora, K.; Luceri, C.; Cirmi, S.; Ferlazzo, N.; Caderni, G. A flavonoid-rich extract from bergamot juice prevents carcinogenesis in a genetic model of colorectal cancer, the Pirc rat (F344/NTac-Apc(am1137)). Eur. J. Nutr. 2020, 59, 885–894. [Google Scholar] [CrossRef]
- Han, X.; Beaumont, C.; Stevens, N. Chemical composition analysis and in vitro biological activities of ten essential oils in human skin cells. Biochim. Open 2017, 5, 1–7. [Google Scholar] [CrossRef]
- Sun, P.; Zhao, L.; Zhang, N.; Wang, C.; Wu, W.; Mehmood, A.; Zhang, L.; Ji, B.; Zhou, F. Essential Oil and Juice from Bergamot and Sweet Orange Improve Acne Vulgaris Caused by Excessive Androgen Secretion. Mediat. Inflamm. 2020, 2020, 8868107. [Google Scholar] [CrossRef]
- Albash, R.; Badawi, N.M.; Hamed, M.I.A.; Ragaie, M.H.; Mohammed, S.S.; Elbesh, R.M.; Darwish, K.M.; Lashkar, M.O.; Elhady, S.S.; Mosallam, S. Exploring the Synergistic Effect of Bergamot Essential Oil with Spironolactone Loaded Nano-Phytosomes for Treatment of Acne Vulgaris: In Vitro Optimization, In Silico Studies, and Clinical Evaluation. Pharmaceuticals 2023, 16, 128. [Google Scholar] [CrossRef]
- Cristiano, M.C.; d’Avanzo, N.; Mancuso, A.; Tarsitano, M.; Barone, A.; Torella, D.; Paolino, D.; Fresta, M. Ammonium Glycyrrhizinate and Bergamot Essential Oil Co-Loaded Ultradeformable Nanocarriers: An Effective Natural Nanomedicine for In Vivo Anti-Inflammatory Topical Therapies. Biomedicines 2022, 10, 1039. [Google Scholar] [CrossRef]
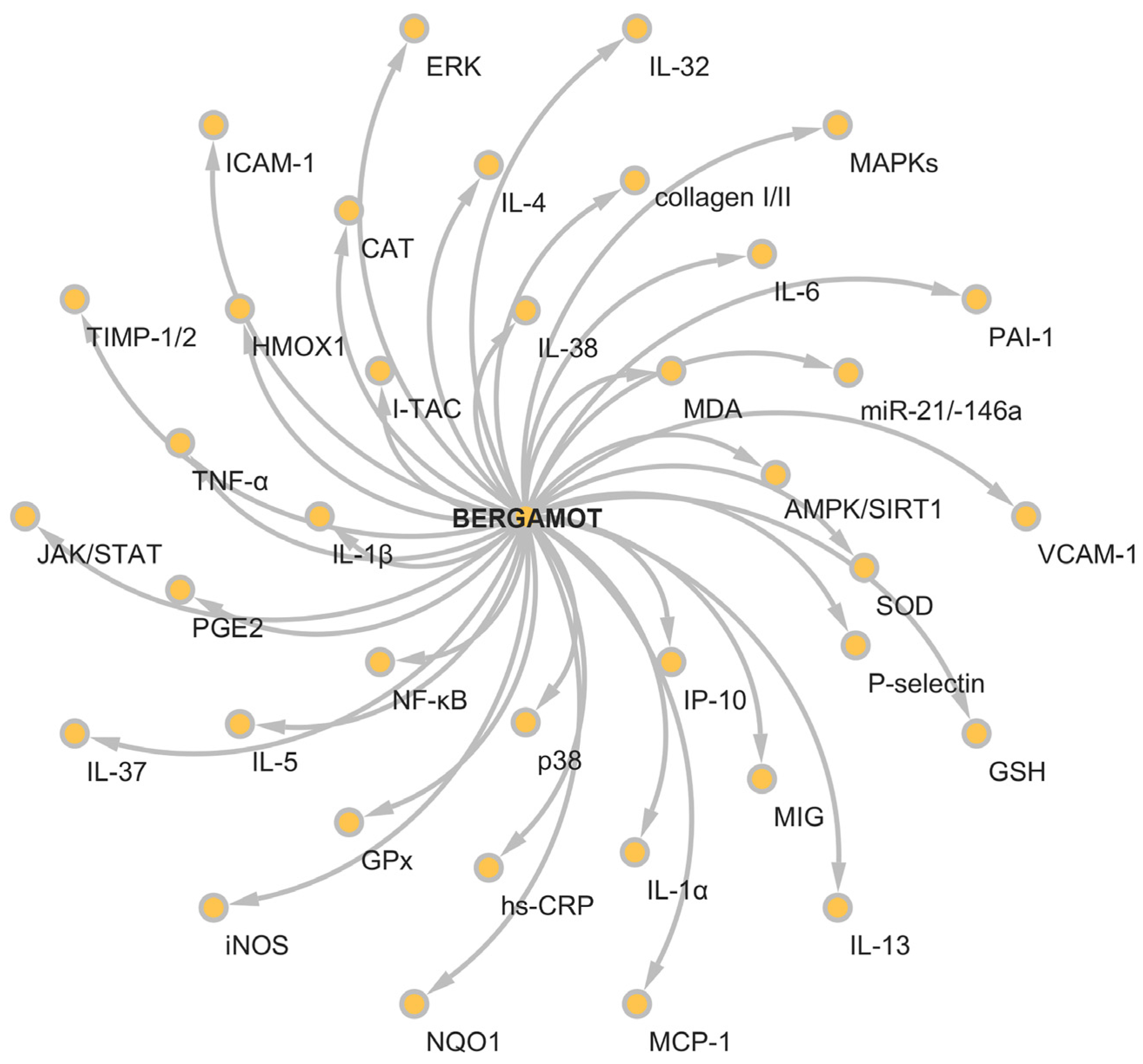
| Derivatives/Byproducts | Inflammation-Related Disease | Experimental Model | Inflammatory Biomarkers | References | |
|---|---|---|---|---|---|
| A flavonoid-rich extract of bergamot juice | Acute inflammation | In vitro | Leukemic monocytes THP-1 exposed to LPS | AMPK/SIRT1 axis | [67] |
| A flavonoid-rich extract of bergamot juice | Periodontitis | In vivo | LPS-induced gingival inflammation in rats | NF-κB, TNF-α, IL-1β, ICAM, P-selectin and myeloperoxidase | [68] |
| Bergamot essential oil fraction deprived of furocoumarins | Acute inflammation | Carrageenan-induced paw edema in rats | IL-1β, IL-6, TNF-α, nitrite/nitrate and PGE2 | [69] | |
| Derivatives/Byproducts | Inflammation- Related Disease | Experimental Model | Inflammatory Biomarkers | References | |
|---|---|---|---|---|---|
| Bergamot essential oil | Asthma | In vitro | MH-S cells exposed to LPS | IL-6 (Il6), IL-1β (Il1b), TNF-α (Tnf-alpha), MAPKs1,3,8,14, Jak2, Stat3, Ptgs2 and Ppara | [70] |
| In vivo | Ovalbumin-induced mice | IL-4 (Il4), IL-5 (Il5), IL-13 (Il13), IL-6, IL-1β, TNF-α and collagen deposition | |||
| Derivatives/Byproducts | Inflammation- Related Disease | Experimental Model | Inflammatory Biomarkers | References | |
|---|---|---|---|---|---|
| Enriched polyphenol fraction from bergamot fruit and leaves | Metabolic syndrome | In vitro | IL-1α-induced inflammation in R3/1 NF-κB cell line | NF-κB | [74] |
| Powder from bergamot juice | Metabolic syndrome | TNF-α-induced inflammation in R3/1 NF-κB cell line | NF-κB | [75] | |
| Bergamot leaf extract | Metabolic syndrome and impaired function of skeletal muscle | In vivo | Rats fed with high-sugar diet | TNF-α, IL-6 and IL-10 | [76] |
| Bergamot juice | Metabolic syndrome | High-fat-diet-induced steatosis in rats | IL-6 and TNF-α | [77] | |
| Nutraceutical multicompound including bergamot juice | Metabolic syndrome | Clinical trial | Untreated subjects with metabolic syndrome | CRP | [80] |
| Derivatives/Byproducts | Inflammation- Related Disease | Experimental Model | Inflammatory Biomarkers | References | |
|---|---|---|---|---|---|
| Bergamot leaf extract | Obesity | In vivo | Diet-induced obese rats | TNF-α, IL-6 | [85] |
| Bergamot leaf extract | Obesity | Obese rats fed with high-sugar and high-fat diet | TNF-α, IL-6, JAK2/STAT3 pathway and SOCS3 | [86] | |
| Nutraceutical containing a bergamot standardized flavonoid extract | Overweight | Clinical trial | Dyslipidemic overweight patients | hs-CRP and TNF-α | [87] |
| Derivatives/Byproducts | Inflammation- Related Disease | Experimental Model | Inflammatory Biomarkers | References | |
|---|---|---|---|---|---|
| Bergamot polyphenol fraction | Non-alcoholic steatohepatitis (NASH) | In vivo | Rats exposed to a cafeteria diet | Il-6 and Il-10 | [88] |
| Bergamot polyphenolic formulation | Non-alcoholic fatty liver disease (NAFLD) | Mice fed with a Western diet | JNK, p38, procollagen I and III | [89] | |
| Bergacyn (combination between bergamot polyphenolic fraction and Cynara cardunculus extract) | NAFLD | Clinical trial | Patients with NAFLD and type 2 diabetes | TNF-α | [90] |
| Derivatives/Byproducts | Inflammation-Related Disease | Experimental Model | Inflammatory Biomarkers | References | |
|---|---|---|---|---|---|
| Bergamot juice flavanones (i.e., hesperetin-7-O-glucuronide, hesperetin-3′-O-glucuronide, naringenin-7-O-glucuronide and naringenin-4′-O-glucuronide) | Endothelial dysfunction | In vitro | Stearate-induced inflammation in myeloid angiogenic cells. | Il-1b, Il-6, Il-8 and Tnf-alpha | [91] |
| Eufortyn® Colesterolo Plus (a nutraceutical containing a standardized bergamot polyphenolic fraction) | Hypercholesterolemia | Clinical trial | Patients with moderate hypercholesterolemia | hs-CRP and ER | [92] |
| BruMeChol™ (supplement composed by a mixture of flavonoids extracted from bergamot, olive polyphenols, plant sterols and vitamin K2) | Hypercholesterolemia | Patients with mild hypercholesterolemia | IL-6, IL-32, IL-37, IL-38, hs-CRP, miR-21 and miR-146a | [93] | |
| Derivatives/Byproducts | Inflammation- Related Disease | Experimental Model | Inflammatory Biomarkers | References | |
|---|---|---|---|---|---|
| Bergamot extract | Nephrotoxicity | In vivo | Amikacin-induced nephrotoxicity in rats | IL-6 | [94] |
| A flavonoid-rich extract of bergamot juice, alone or in association with curcumin and resveratrol | Nephrotoxicity | Cadmium-induced kidney damage in a murine model | Nos2, Il1b, Nrf2 and Nqo1 | [95] | |
| Bergamot essential oil, bergamot juice and ethanol extract of bergamot | Primary dysmenorrhea | Dysmenorrhea induced by estradiol benzoate and oxytocin in rats | PGF2α, PGE2 and iNOS | [96] | |
| Benebeo® gel (Bergamot oil) | Anitis/proctitis | Clinical trial | Patients with anitis/proctitis | Local bleeding and hyperemia | [97] |
| Derivatives/Byproducts | Inflammation- Related Disease | Experimental Model | Inflammatory Biomarkers | References | |
|---|---|---|---|---|---|
| Bergamot juice extract | Alzheimer’s disease | In vitro | THP-1 cells exposed to β-amyloid | IL-6 (Il-6), IL-1β (Il1b), NF-κB, AP-1 and MAPKs pathway | [98] |
| Bergamot essential oil | Anxiety | In vivo | Rats exposed to aluminum | IL-6, IL-1β and TNF-α | [99] |
| Derivatives/Byproducts | Inflammation- Related Disease | Experimental Model | Inflammatory Biomarkers | References | |
|---|---|---|---|---|---|
| Bergamot juice | Hepatocellular carcinoma | In vitro | HepG2 cells | NF-κB | [102] |
| Bergamot juice extract | Colorectal cancer | In vivo | Pirc rat (F344/NTac-Apcam1137) | Ptgs2, iNos, Il-1b, Il-6, Il-10 and Arg1 | [107] |
| Derivatives/Byproducts | Inflammation-Related Disease | Experimental Model | Inflammatory Biomarkers | References | |
|---|---|---|---|---|---|
| Bergamot essential oil | Chronic skin inflammation | In vitro | Primary fibroblasts stimulated with a mixture of IL-1β, TNF-α, IFNγ, bFGF, EGF and PDGF | MCP-1, VCAM-1, ICAM-1, IP-10, I-TAC, MIG, collagen I and III, PAI-1 and TIMP-1 and -2 | [108] |
| Bergamot essential oil and juice | Acne vulgaris | In vivo | Administration of compound pearl acne capsules on golden hamsters | IL-1α, IL-6, TNF-α, MMP-2 and MMP-9 | [109] |
| Encapsulation of bergamot essential oil into ammonium glycyrrhizinate-loaded nanoparticles | Skin inflammation | Clinical trial | Human volunteers subjected to methylnicotinate solution on specific skin sites | Erythema index | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, C.; Lombardo, G.E.; Bruschetta, G.; Rapisarda, A.; Maugeri, A.; Navarra, M. Bergamot Byproducts: A Sustainable Source to Counteract Inflammation. Nutrients 2024, 16, 259. https://doi.org/10.3390/nu16020259
Russo C, Lombardo GE, Bruschetta G, Rapisarda A, Maugeri A, Navarra M. Bergamot Byproducts: A Sustainable Source to Counteract Inflammation. Nutrients. 2024; 16(2):259. https://doi.org/10.3390/nu16020259
Chicago/Turabian StyleRusso, Caterina, Giovanni Enrico Lombardo, Giuseppe Bruschetta, Antonio Rapisarda, Alessandro Maugeri, and Michele Navarra. 2024. "Bergamot Byproducts: A Sustainable Source to Counteract Inflammation" Nutrients 16, no. 2: 259. https://doi.org/10.3390/nu16020259
APA StyleRusso, C., Lombardo, G. E., Bruschetta, G., Rapisarda, A., Maugeri, A., & Navarra, M. (2024). Bergamot Byproducts: A Sustainable Source to Counteract Inflammation. Nutrients, 16(2), 259. https://doi.org/10.3390/nu16020259