A Narrative Review on Maternal Choline Intake and Liver Function of the Fetus and the Infant; Implications for Research, Policy, and Practice
Abstract
1. Introduction
2. Choline Sources in the Diet
3. Choline Physiology and Relation to Liver Function
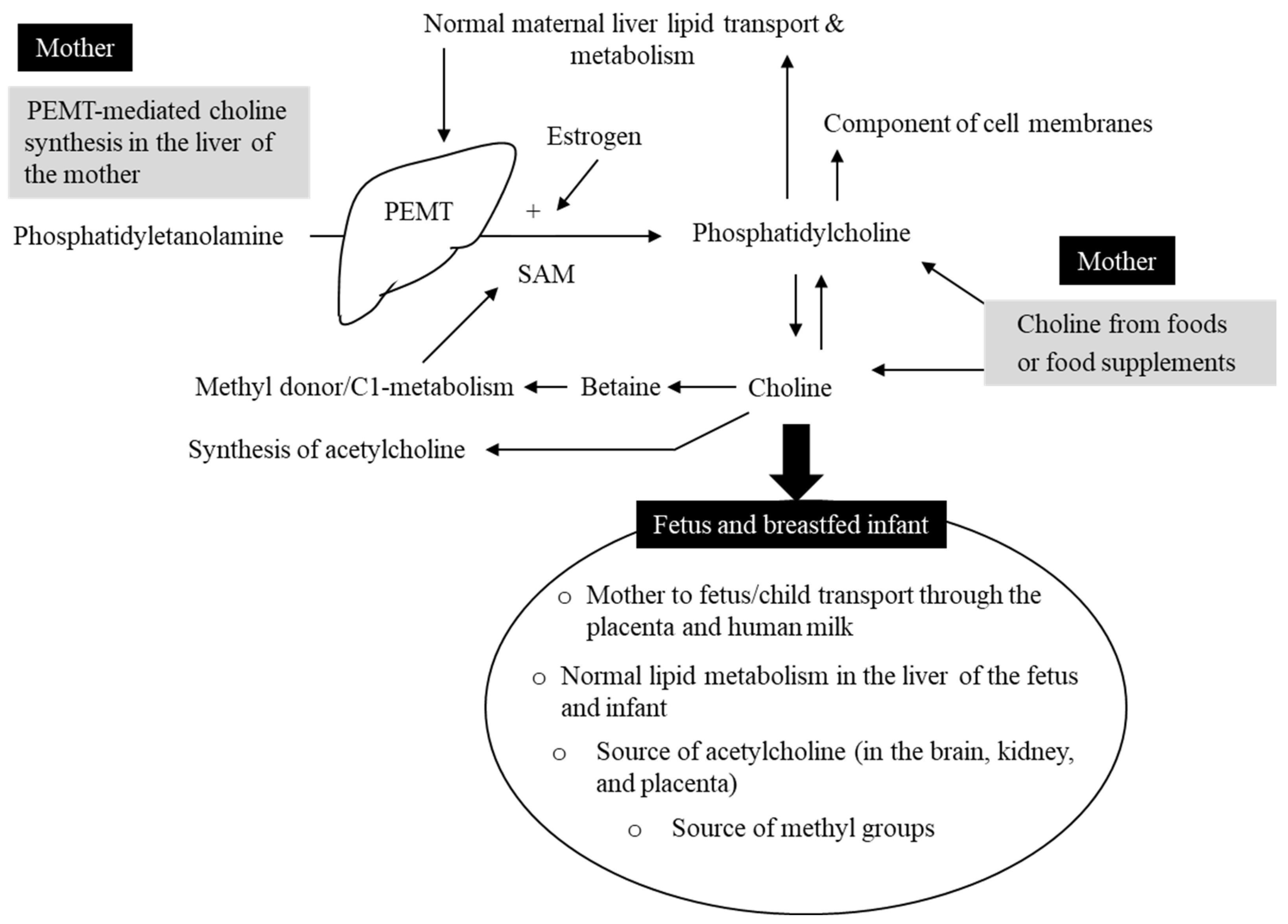
4. Source of Fetal and Neonatal Choline
5. Choline Homeostasis during Pregnancy and Lactation
6. Dietary Choline Deficiency and Fatty Liver in the Mother and the Offspring
7. Modifiers of Choline Requirements during Pregnancy and Lactation
8. Implications for Future Research
9. Implications for Policy and Practice
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Press: Washington, DC, USA, 1998; pp. 390–422. [Google Scholar]
- Zeisel, S.H.; Da Costa, K.; Franklin, P.D.; Alexander, E.A.; Lamont, J.T.; Sheard, N.F.; Beiser, A. Choline, an essential nutrient for humans. FASEB J. 1991, 5, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies NDA. Dietary reference values for choline. EFSA J. 2016, 14, 4484. [Google Scholar] [CrossRef]
- Hess, J.M.; Comeau, M.E.; Swanson, K.; Burbank, M. Modeling ovo-vegetarian, lacto-vegetarian, pescatarian, and vegan USDA food patterns and assessing nutrient adequacy for lactation among adult females. Curr. Dev. Nutr. 2023, 7, 102034. [Google Scholar] [CrossRef]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary choline intake: Current state of knowledge across the life cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.M.; da Costa, K.A.; Galanko, J.; Sha, W.; Stephenson, B.; Vick, J.; Zeisel, S.H. Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am. J. Clin. Nutr. 2010, 92, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Wiedeman, A.M.; Whitfield, K.C.; March, K.M.; Chen, N.N.; Kroeun, H.; Sokhoing, L.; Sophonneary, P.; Dyer, R.A.; Xu, Z.; Kitts, D.D.; et al. Concentrations of water-soluble forms of choline in human milk from lactating women in Canada and Cambodia. Nutrients 2018, 10, 381. [Google Scholar] [CrossRef]
- Best, C.H.; Hershey, J.M.; Huntsman, M.E. The effect of lecithine on fat deposition in the liver of the normal rat. J. Physiol. 1932, 75, 56–66. [Google Scholar] [CrossRef]
- Best, C.H.; Channon, H.J.; Ridout, J.H. Choline and the dietary production of fatty livers. J. Physiol. 1934, 81, 409–421. [Google Scholar] [CrossRef]
- Best, C.H.; Huntsman, M.E. The effects of the components of lecithine upon deposition of fat in the liver. J. Physiol. 1932, 75, 405–412. [Google Scholar] [CrossRef]
- Best, C.H.; Huntsman, M.E. The effect of choline on the liver fat of rats in various states of nutrition. J. Physiol. 1935, 83, 255–274. [Google Scholar] [CrossRef]
- Newberne, P.M.; de Camargo, J.L.V.; Clark, A.J. Choline deficiency, partial hepatectomy, and liver tumors in rats and mice. Toxicol. Pathol. 1982, 10, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Cantrill, E.; Mehta, I.; Farrell, G.C. Impaired expression of microsomal cytochrome P450 2C11 in choline-deficient rat liver during the development of cirrhosis. J. Pharmacol. Exp. Ther. 1992, 261, 373–380. [Google Scholar]
- Griffith, W.H.; Wade, N.J. Choline metabolism. I. The occurrence and prevention of hemorrhagic degeneration in young rats on a low choline diet. Nutr. Rev. 1974, 32, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.; Williams, L. Influence of dietary fat on fatty livers of choline-deficient rats. J. Nutr. 1977, 107, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, P.; Goldblatt, H. Choline as a member of the vitamin B(2) complex. J. Exp. Med. 1940, 72, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Handler, P.; Bernheim, F. Choline deficiency in the hamster. Proc. Soc. Exp. Biol. Med. 1949, 72, 569–571. [Google Scholar] [CrossRef]
- Asai, J. Histological and electron microscopic investigation of the liver in the choline deficient guinea pig. Nagoya J. Med. Sci. 1965, 28, 81–100. [Google Scholar]
- Blair, R.; Newsome, F. Involvement of water-soluble vitamins in diseases of swine. J. Anim. Sci. 1985, 60, 1508–1517. [Google Scholar] [CrossRef]
- Best, C.H.; Ferguson, G.C.; Hershey, J.M. Choline and liver fat in diabetic dogs. J. Physiol. 1933, 79, 94–102. [Google Scholar] [CrossRef]
- Wilgram, G.F.; Lucas, C.C.; Best, C.H. Kwashiorkor type of fatty liver in primates. J. Exp. Med. 1958, 108, 361–370. [Google Scholar] [CrossRef]
- Hoffbauer, F.W.; Zaki, F.G. Choline deficiency in baboon and rat compared. Arch. Pathol. 1965, 79, 364–369. [Google Scholar]
- Patek, A.J.; Bowry, S.; Hayes, K.C. Cirrhosis of choline deficiency in the Rhesus Monkey. Possible role of dietary cholesterol (38541). Proc. Soc. Exp. Biol. Med. 1975, 148, 370–374. [Google Scholar] [CrossRef]
- Buchman, A.L.; Dubin, M.D.; Moukarzel, A.A.; Jenden, D.J.; Roch, M.; Rice, K.M.; Gornbein, J.; Ament, M.E. Choline deficiency: A cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology 1995, 22, 1399–1403. [Google Scholar] [PubMed]
- Buchman, A.L.; Ament, M.E.; Sohel, M.; Dubin, M.; Jenden, D.J.; Roch, M.; Pownall, H.; Farley, W.; Awal, M.; Ahn, C. Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: Proof of a human choline requirement: A placebo-controlled trial. J. Parenter. Enter. Nutr. 2001, 25, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, A.K.; Farber, E. Liver biochemical pathology of choline deficiency and of methyl group deficiency: A new orientation and assessment. Histol. Histopathol. 1995, 10, 457–462. [Google Scholar] [PubMed]
- Li, Z.; Vance, D.E. Thematic Review Series: Glycerolipids. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008, 49, 1187–1194. [Google Scholar] [CrossRef]
- Yao, Z.M.; Vance, D.E. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 1988, 263, 2998–3004. [Google Scholar] [CrossRef]
- Yao, Z.; Vance, D.E. Reduction in VLDL, but not HDL, in plasma of rats deficient in choline. Biochem. Cell Biol. 1990, 68, 552–558. [Google Scholar] [CrossRef]
- Cole, L.K.; Vance, J.E.; Vance, D.E. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim. Biophys. Act. Mol. Cell Biol. Lipids 2012, 1821, 754–761. [Google Scholar] [CrossRef]
- Chalvardjian, A. Mode of action of choline. V. Sequential changes in hepatic and serum lipids of choline-deficient rats. Can. J. Biochem. 1970, 48, 1234–1240. [Google Scholar] [CrossRef]
- Robins, S.J.; Armstrong, M.J. Biliary Lecithin Secretion. II. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Gastroenterology 1976, 70, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Wells, I.C.; Buckley, J.M. Lipid composition of Bile from choline deficient rats. Exp. Biol. Med. 1965, 119, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Shields, D.J.; Altarejos, J.Y.; Wang, X.; Agellon, L.B.; Vance, D.E. Molecular dissection of the S-adenosylmethionine-binding site of phosphatidylethanolamine N-methyltransferase. J. Biol. Chem. 2003, 278, 35826–35836. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Sichler, A.; Ecker, J.; Laschinger, M.; Liebisch, G.; Höring, M.; Basic, M.; Bleich, A.; Zhang, X.-J.; Kübelsbeck, L.; et al. Gut microbiota promote liver regeneration through hepatic membrane phospholipid biosynthesis. J. Hepatol. 2023, 78, 820–835. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Sano, M.; Isozaki, M. Studies on phospholipid metabolism in choline deficient fatty liver. J. Biochem. 1969, 65, 85–91. [Google Scholar] [CrossRef]
- Johnson, B.C.; James, M.F. Choline Deficiency in the Baby Pig. J. Nutr. 1948, 36, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Miller, J.W.; da Costa, K.-A.; Nadeau, M.; Smith, D.; Selhub, J.; Zeisel, S.H.; Mason, J.B. Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J. Nutr. 1994, 124, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.M.; Dacosta, K.A.; Kwock, L.; Stewart, P.W.; Lu, T.-S.; Stabler, S.P.; Allen, R.H.; Zeisel, S.H. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am. J. Clin. Nutr. 2007, 85, 1275–1285. [Google Scholar] [CrossRef]
- Resseguie, M.; Song, J.; Niculescu, M.D.; Costa, K.-A.; Randall, T.A.; Zeisel, S.H. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007, 21, 2622–2632. [Google Scholar] [CrossRef]
- Griffith, W.H. The relation of the age, weight and sex of young rats to the occurrence of hemprrhagic degeneration on a low choline diet. J. Nutr. 1940, 19, 437–448. [Google Scholar] [CrossRef]
- Gwee, M.C.E.; Sim, M.K. Changes in the concentration of free choline and cephalin-N-methyltransferase activity of the rat material and foetal liver and placeta during gestation and of the maternal and neonatal liver in the early postpartum period. Clin. Exp. Pharmacol. Physiol. 1979, 6, 259–265. [Google Scholar] [CrossRef]
- Gwee, M.C.E.; Sim, M.K. Free choline concentration and cephalin-N-methyltransferase activity in the maternal and foetal liver and placenta of pregnant rats. Clin. Exp. Pharmacol. Physiol. 1978, 5, 649–653. [Google Scholar] [CrossRef]
- Welsch, F.; Wenger, W.C.; Stedman, D.B. Choline metabolism in placenta: Evidence for the biosynthesis of phosphatidylcholine in microsomes via the methylation pathway. Placenta 1981, 2, 211–221. [Google Scholar] [CrossRef]
- Sweiry, J.H.; Yudilevich, D.L. Characterization of choline transport at maternal and fetal interfaces of the perfused guinea-pig placenta. J. Physiol. 1985, 366, 251–266. [Google Scholar] [CrossRef]
- Sweiry, J.H.; Page, K.R.; Dacke, C.G.; Abramovich, D.R.; Yudilevich, D.L. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human placenta by rapid paired-tracer dilution: Studies with calcium and choline. J. Dev. Physiol. 1986, 8, 435–445. [Google Scholar]
- Welsch, F. Studies on accumulation and metabolic fate of [N-Me3H]choline in human term placenta fragments. Biochem. Pharmacol. 1976, 25, 1021–1030. [Google Scholar] [CrossRef]
- Taesuwan, S.; McDougall, M.Q.; Malysheva, O.V.; Bender, E.; Nevins, J.E.H.; Devapatla, S.; Vidavalur, R.; Caudill, M.A.; Klatt, K.C. Choline metabolome response to prenatal choline supplementation across pregnancy: A randomized controlled trial. FASEB J. 2021, 35, e22063. [Google Scholar] [CrossRef]
- Garner, S.C.; Mar, M.H.; Zeisel, S.H. Choline distribution and metabolism in pregnant rats and fetuses are influenced by the choline content of the maternal diet. J. Nutr. 1995, 125, 2851–2858. [Google Scholar]
- Goss, K.C.W.; Goss, V.M.; Townsend, J.P.; Koster, G.; Clark, H.W.; Postle, A.D. Postnatal adaptations of phosphatidylcholine metabolism in extremely preterm infants: Implications for choline and PUFA metabolism. Am. J. Clin. Nutr. 2020, 112, 1438–1447. [Google Scholar] [CrossRef]
- McMahon, K.E.; Farrell, P.M. Measurement of free choline concentrations in maternal and neonatal blood by micropyrolysis gas chromatography. Clin. Chim. Acta 1985, 149, 1–12. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Epstein, M.F.; Wurtman, R.J. Elevated choline concentration in neonatal plasma. Life Sci. 1980, 26, 1827–1831. [Google Scholar] [CrossRef] [PubMed]
- Molloy, A.M.; Mills, J.L.; Cox, C.; Daly, S.F.; Conley, M.; Brody, L.C.; Kirke, P.N.; Scott, J.M.; Ueland, P.M. Choline and homocysteine interrelations in umbilical cord and maternal plasma at delivery. Am. J. Clin. Nutr. 2005, 82, 836–842. [Google Scholar] [CrossRef]
- Ilcol, Y.O.; Uncu, G.; Ulus, I.H. Free and Phospholipid-Bound Choline Concentrations in Serum during Pregnancy, after Delivery and in Newborns. Arch. Physiol. Biochem. 2002, 110, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Holmes-McNary, M.Q.; Cheng, W.L.; Mar, M.H.; Fussell, S.; Zeisel, S.H. Choline and choline esters in human and rat milk and in infant formulas. Am. J. Clin. Nutr. 1996, 64, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Ozarda, Y.; Cansev, M.; Ulus, I.H. Relations of Human Breastmilk Choline Content with Maternal Hormonal Status. Breastfeed. Med. 2014, 9, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Ilcol, Y.; Ozbek, R.; Hamurtekin, E.; Ulus, I. Choline status in newborns, infants, children, breast-feeding women, breast-fed infants and human breast milk. J. Nutr. Biochem. 2005, 16, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Mar, M.H.; Zhou, Z.; A Da Costa, K. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J. Nutr. 1995, 125, 3049–3054. [Google Scholar] [CrossRef] [PubMed]
- Fernàndez-Roig, S.; Cavallé-Busquets, P.; Fernandez-Ballart, J.D.; Ballesteros, M.; Berrocal-Zaragoza, M.I.; Salat-Batlle, J.; Ueland, P.M.; Murphy, M.M. Low folate status enhances pregnancy changes in plasma betaine and dimethylglycine concentrations and the association between betaine and homocysteine. Am. J. Clin. Nutr. 2013, 97, 1252–1259. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Devapatla, S.; Pressman, E.; Vermeylen, F.; Stabler, S.P.; Allen, R.H.; et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am. J. Clin. Nutr. 2012, 95, 1060–1071. [Google Scholar] [CrossRef]
- Borglin, N.E. On the Metabolism of Choline in Pregnancy. Acta Obstet. Gynecol. Scand. 1956, 35, 424–439. [Google Scholar] [CrossRef]
- Ensminger, M.E.; Bowland, J.P.; Cunha, T.J. Observations on the Thiamine, Riboflavin, and Choline Needs of Sows for Reproduction. J. Anim. Sci. 1947, 6, 409–423. [Google Scholar] [CrossRef]
- Ensminger, R.W.; Colby, R.W.; Cunha, T.J. Effect of certain B-complex vitamins on gestation and lactation in swine. Stn. Circ. 1951, 134, 1–35. [Google Scholar]
- Meader, R.D. Livers of choline-deficient pregnant and fetal rats. Anat. Rec. 1965, 153, 407–419. [Google Scholar] [CrossRef]
- Peet, M.; Sampson, M.M. Loss of Contractility of the Uterus and Partial Atrophy of the Uterus and Ovaries in Albino Rats Fed Choline-deficient Diets. Science 1948, 107, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Chmurzynska, A.; Seremak-Mrozikiewicz, A.; Malinowska, A.M.; Różycka, A.; Radziejewska, A.; KurzawiŃska, G.; Barlik, M.; Wolski, H.; Drews, K. Associations between folate and choline intake, homocysteine metabolism, and genetic polymorphism of MTHFR, BHMT and PEMT in healthy pregnant Polish women. Nutr. Diet. 2020, 77, 368–372. [Google Scholar] [CrossRef]
- Klatt, K.C.; McDougall, M.Q.; Malysheva, O.V.; Taesuwan, S.; Loinard-González, A.P.; Nevins, J.E.H.; Beckman, K.; Bhawal, R.; Anderson, E.; Zhang, S.; et al. Prenatal choline supplementation improves biomarkers of maternal docosahexaenoic acid (DHA) status among pregnant participants consuming supplemental DHA: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 116, 820–832. [Google Scholar] [CrossRef]
- van Wijk, N.; Balvers, M.; Cansev, M.; Maher, T.J.; Sijben, J.W.C.; Broersen, L.M. Dietary Crude Lecithin Increases Systemic Availability of Dietary Docosahexaenoic Acid with Combined Intake in Rats. Lipids 2016, 51, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Loinard-González, A.P.; Malysheva, O.V.; Klatt, K.C.; Caudill, M.A. Genetic Variants in One-Carbon Metabolism and Their Effects on DHA Biomarkers in Pregnant Women: A Post-Hoc Analysis. Nutrients 2022, 14, 3801. [Google Scholar] [CrossRef]
- Obeid, R.; Derbyshire, E.; Schön, C. Association between maternal choline, foetal brain development and child neurocognition; systematic review and meta-analysis of human studies. Adv. Nutr. Int. Rev. J. 2022, 13, 2445–2457. [Google Scholar] [CrossRef]
- Ko, H.H.; Yoshida, E. Acute Fatty Liver of Pregnancy. Can. J. Gastroenterol. 2006, 20, 25–30. [Google Scholar] [CrossRef]
- Sarkar, M.; Grab, J.; Dodge, J.L.; Gunderson, E.P.; Rubin, J.; Irani, R.A.; Cedars, M.; Terrault, N. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J. Hepatol. 2020, 73, 516–522. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, B.J.; Koo, J.N.; Norwitz, E.R.; Oh, I.H.; Kim, S.M.; Kim, S.Y.; Kim, G.M.; Kwak, S.H.; Kim, W.; et al. Nonalcoholic fatty liver disease is a risk factor for large-for-gestational-age birthweight. PLoS ONE 2019, 14, e0221400. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.P.; Heinemann, M.; Man, E.B. The lipids of serum in pregnancy. J. Clin. Investig. 1951, 30, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Ryckman, K.; Spracklen, C.; Smith, C.; Robinson, J.; Saftlas, A. Maternal lipid levels during pregnancy and gestational diabetes: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 643–651. [Google Scholar] [CrossRef] [PubMed]
- E Louck, L.; Cara, K.C.; Klatt, K.; Wallace, T.C.; Chung, M. The relationship of circulating choline and choline-related metabolite levels with health outcomes: A scoping review of genome-wide association studies and Mendelian Randomization studies. Adv. Nutr. Int. Rev. J. 2024, 15, 100164. [Google Scholar] [CrossRef]
- Wu, P.; Ye, Y.; Yang, X.; Huang, Y.; Ye, Y.; Lai, Y.; Ouyang, J.; Wu, L.; Xu, J.; Yuan, J.; et al. Liver biomarkers, lipid metabolites, and risk of gestational diabetes mellitus in a prospective study among Chinese pregnant women. BMC Med. 2023, 1, 150. [Google Scholar] [CrossRef]
- Wilgram, G.F.; Hartroft, W.S.; Best, C.H. Dietary Choline and the Maintenance of the Cardiovascular System in Rats. BMJ 1954, 2, 1–5. [Google Scholar] [CrossRef][Green Version]
- Blomhoff, R.; Andersen, R.; Arnesen, E.K.; Christensen, J.J.; Eneroth, H.; Erkkola, M.; Gudanaviciene, I.; Halldorsson, T.I.; Høyer-Lund, A.; Lemming, E.W.; et al. Nordic Nutrition Recommendations; Choline; Nordic Council of Ministers: Copenhagen, Denmark, 2023; pp. 184–185. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens). Choline and contribution to normal liver function of the foetus and exclusively breastfed infants: Evaluation of a health claim pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2023, 21, e08115. [Google Scholar] [CrossRef]
- Roeren, M.; Kordowski, A.; Sina, C.; Smollich, M. Inadequate Choline Intake in Pregnant Women in Germany. Nutrients 2022, 14, 4862. [Google Scholar] [CrossRef]
- Wallace, T.C.; Fulgoni, V.L., III. Assessment of total choline intakes in the United States. J. Am. Coll. Nutr. 2016, 35, 108–112. [Google Scholar] [CrossRef]
| Risk Factor | Explanation |
|---|---|
| Men (vs. women) | Men are more susceptible to fatty liver under low choline intake because PEMT gene expression is induced by estrogen in women (higher PEMT-mediated choline production in women than in men) |
| Postmenopausal women (vs. pre-menopausal) | Young women have higher endogenous production of choline due to the effect of estrogen on PEMT |
| Newborns and infants | Liver PEMT activity is low at birth and the demands for choline are higher than in adults |
| Pregnancy | High demands compared to non-pregnant women and active transfer of choline to the fetus can deplete choline from the liver of the mother and predispose her for choline deficiency (e.g., fatty liver) |
| Lactation | High excretion of choline derivatives into breastmilk may deplete choline from mother’s liver and predispose her for choline deficiency (e.g., fatty liver) |
| Low vitamin B12 or folate intake or MTHFR 677 TT genotype | Adequate folate and vitamin B12 support choline endogenous production by providing S-adenosylmethionine needed for PEMT enzyme |
| High-fat diet | Triglycerides accumulate in the liver if choline intake is not proportional to fat content in the diet. Adult Wister rats fed a choline-deficient and fat-rich (40%) diet developed fatty infiltration of the liver within 21 days [11]. Supplementing the high-fat diet with 50–70 mg of choline daily reduced fat content in the liver of the animals that were previously fed a choline-deficient diet. Choline also prevented further accumulation of fat in the liver under continuous high-fat diet [11]. These results suggest that dietary fat intake could determine choline requirements |
| High sugar intake, toxins such as alcohol | These factors decrease the ability of the liver to metabolize fats and enhance fatty liver |
| Polymorphisms in PEMT gene (e.g., PEMT rs7946) | Carriers of some genotypes could have lower PEMT activity, implying higher requirements for dietary sources of choline |
| A plant-based diet | Adherence to a lacto-vegetarian or a vegan diet provides up to 50% lower dietary intake of choline compared to the adequate intake levels for pregnant and lactating women [4]. In addition, low choline and B12 intake in the same time can challenge one-carbon metabolism (e.g., hyperhomocysteinemia). Women adhering to a vegetarian or a vegan diet are at risk of insufficient choline intake and a target group for choline supplementation |
| The duration of a low-choline diet | A choline-deficient diet (e.g., contains 50 mg/d of choline) can cause liver damage (elevated liver enzymes) within 3 weeks in male subjects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obeid, R.; Schön, C.; Derbyshire, E.; Jiang, X.; Mellott, T.J.; Blusztajn, J.K.; Zeisel, S.H. A Narrative Review on Maternal Choline Intake and Liver Function of the Fetus and the Infant; Implications for Research, Policy, and Practice. Nutrients 2024, 16, 260. https://doi.org/10.3390/nu16020260
Obeid R, Schön C, Derbyshire E, Jiang X, Mellott TJ, Blusztajn JK, Zeisel SH. A Narrative Review on Maternal Choline Intake and Liver Function of the Fetus and the Infant; Implications for Research, Policy, and Practice. Nutrients. 2024; 16(2):260. https://doi.org/10.3390/nu16020260
Chicago/Turabian StyleObeid, Rima, Christiane Schön, Emma Derbyshire, Xinyin Jiang, Tiffany J. Mellott, Jan Krzysztof Blusztajn, and Steven H. Zeisel. 2024. "A Narrative Review on Maternal Choline Intake and Liver Function of the Fetus and the Infant; Implications for Research, Policy, and Practice" Nutrients 16, no. 2: 260. https://doi.org/10.3390/nu16020260
APA StyleObeid, R., Schön, C., Derbyshire, E., Jiang, X., Mellott, T. J., Blusztajn, J. K., & Zeisel, S. H. (2024). A Narrative Review on Maternal Choline Intake and Liver Function of the Fetus and the Infant; Implications for Research, Policy, and Practice. Nutrients, 16(2), 260. https://doi.org/10.3390/nu16020260









