The Potential of Spent Coffee Grounds in Functional Food Development
Abstract
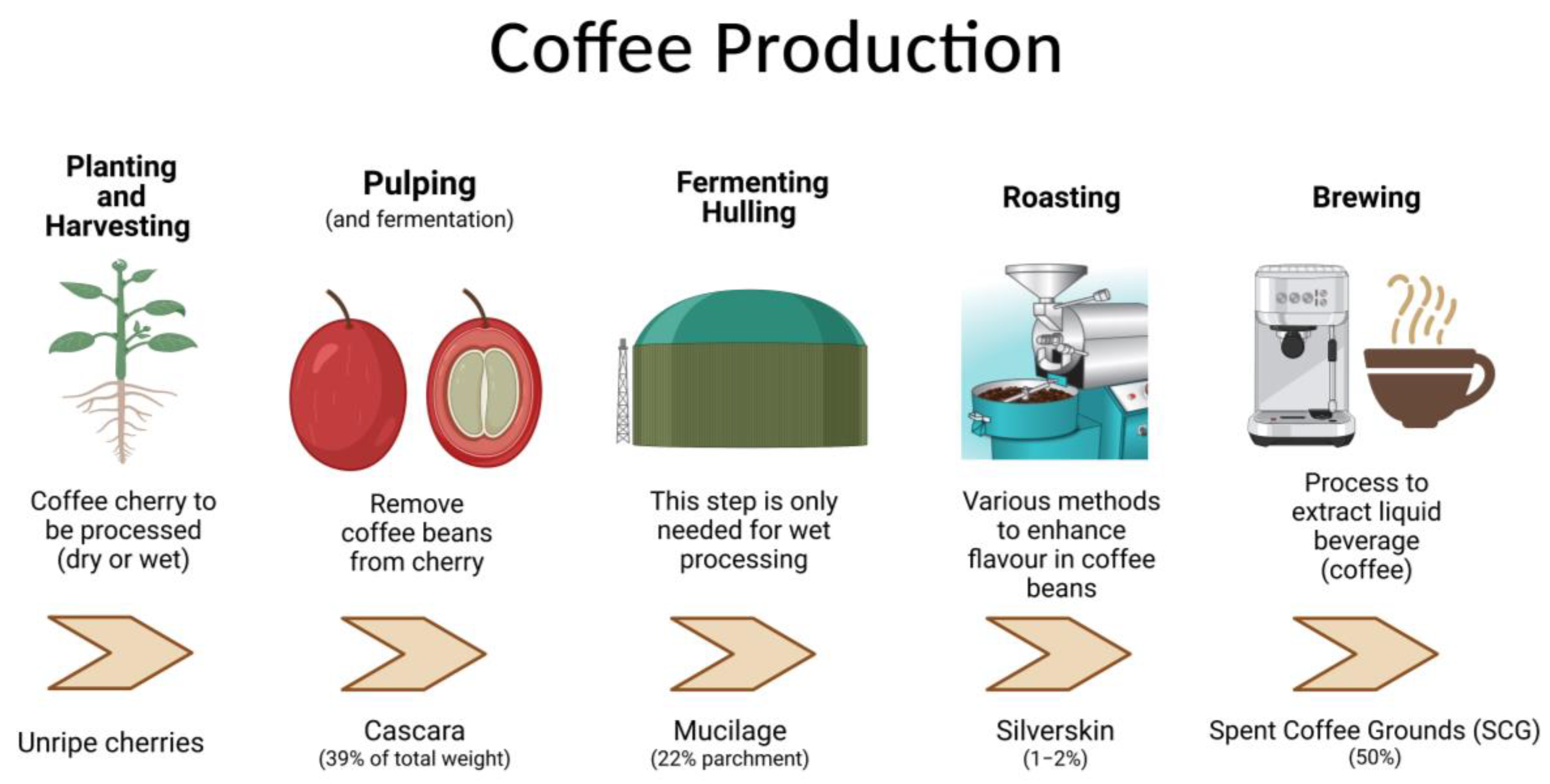
1. Coffee Waste Products—From Farm to Landfill
2. Bioactive Ingredients of SCG
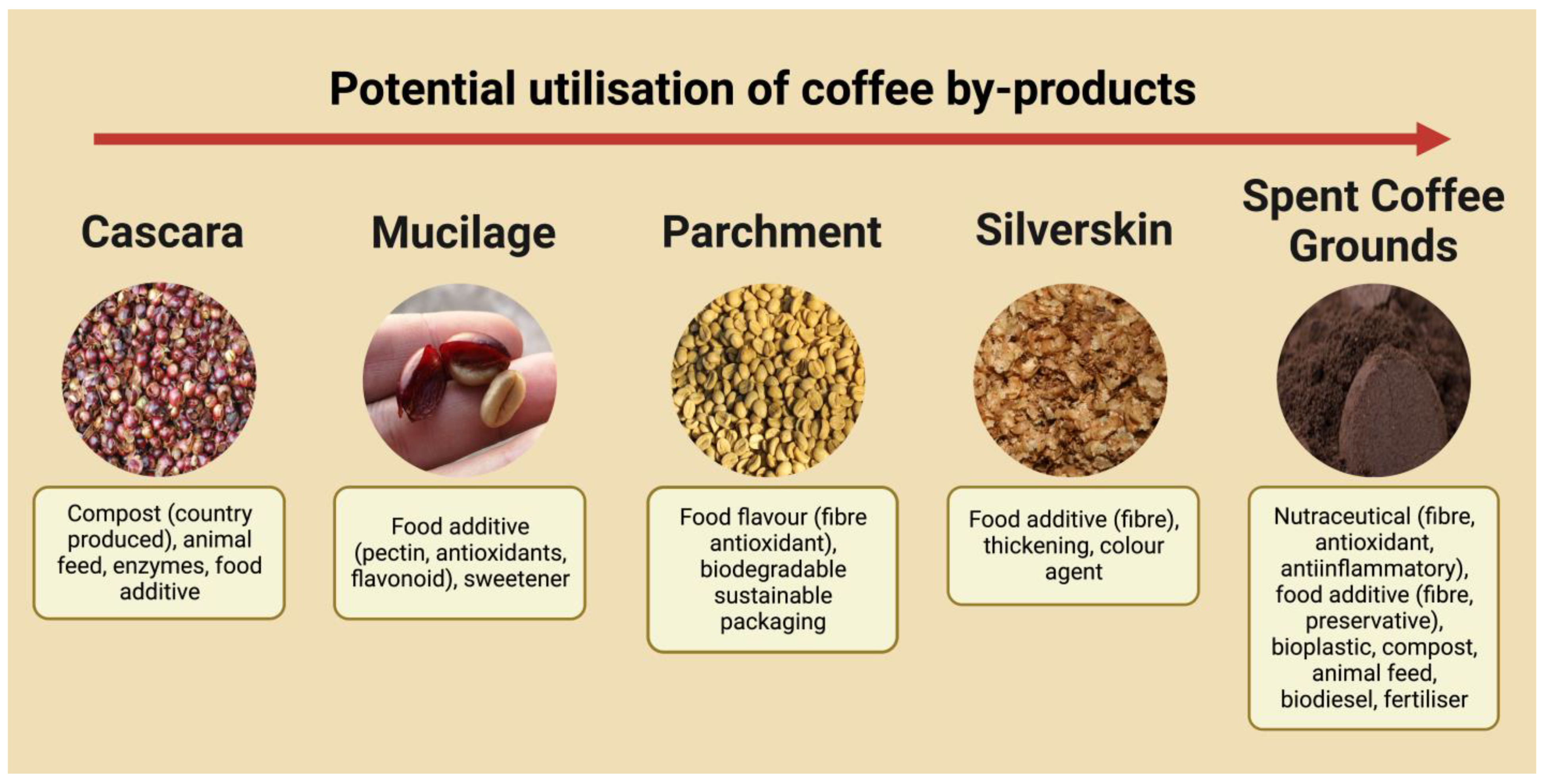
3. Value-Adding to SCG Outside the Health Industry
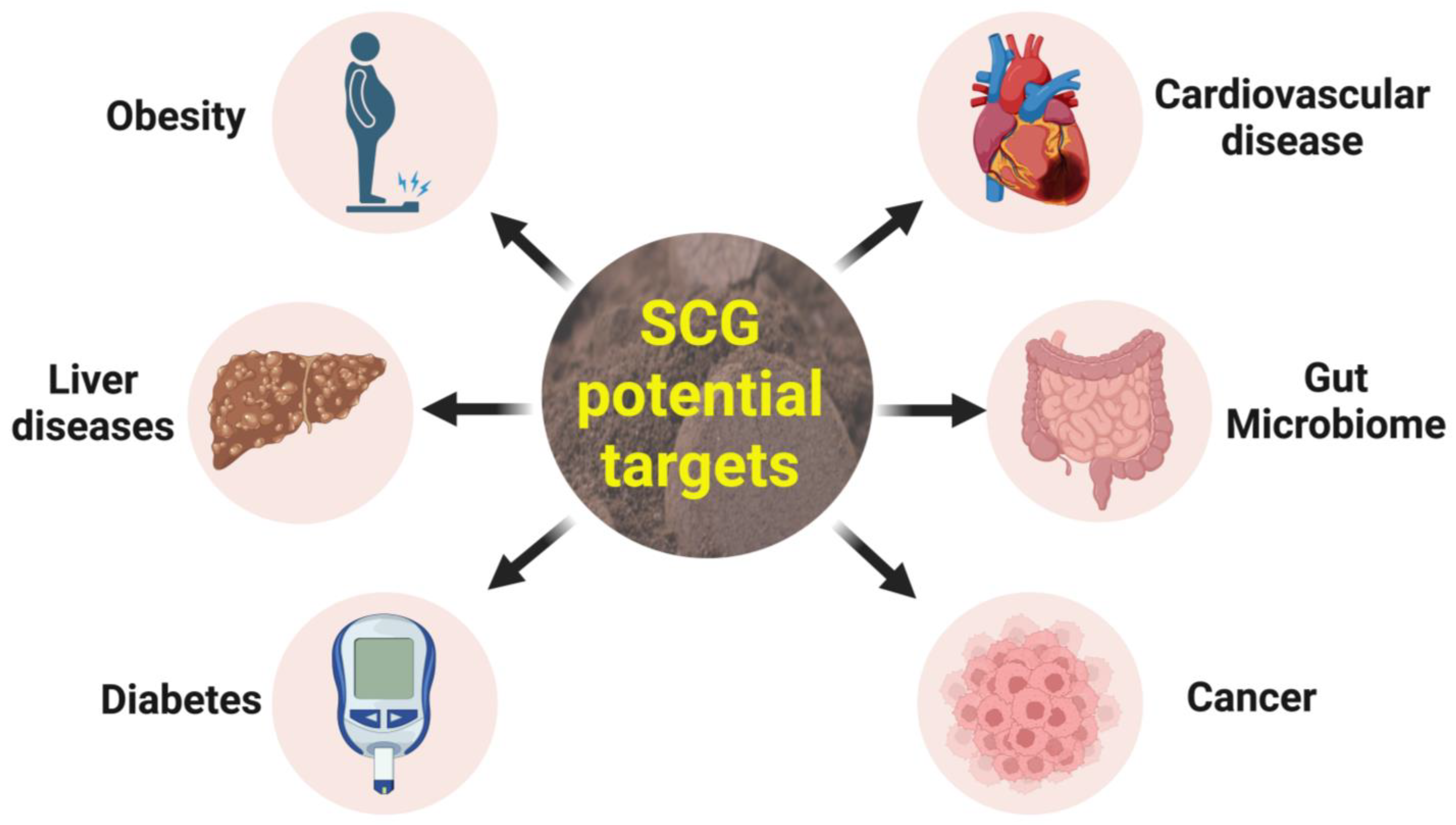
4. Health Benefits of SCG
5. Health Benefits of Compounds in SCG
5.1. Chlorogenic Acids (CGA)
5.2. Caffeine
5.3. Trigonelline
5.4. Cafestol and Kahweol
5.5. Melanoidins
6. Conclusions, Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maksud, H.; Ali Syahban, A. The function of a coffee shop as a social cultural entity. In Trends and Innovations in Food Science; Yehia, E.-S., Ed.; IntechOpen: London, UK, 2022; p. 103852. [Google Scholar] [CrossRef]
- Samper, L.F.; Quiñones-Ruiz, X.F. Towards a balanced sustainability vision for the coffee industry. Resources 2017, 6, 17. [Google Scholar] [CrossRef]
- Shahbandeh, M. Coffee Market: Worldwide Production 2003/04–2020/21. 2022. Available online: https://www.statista.com/statistics/263311/worldwide-production-of-coffee/ (accessed on 26 November 2022).
- Murthy, P.S.; Madhava Naidu, M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recy. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Soloway, B. In Brazil’s Coffee Industry, Some Workers Face ‘Conditions Analogous to Slavery’. Foreign Policy 13 April 2016. Available online: https://foreignpolicy.com/2016/04/13/in-brazils-coffee-industry-some-workers-face-conditions-analogous-to-slavery/ (accessed on 26 November 2022).
- Pines, L. Brazil’s Economy: Foreign Trade Figures Reveal Why They’re a Major Global Player. Commodity.com 7 April 2021. Available online: https://commodity.com/data/brazil/ (accessed on 26 November 2022).
- Ozbun, T. Brazil: Coffee Production 2010–2020. Statista: 2022. Available online: https://www.statista.com/statistics/806275/production-coffee-volume-brazil/ (accessed on 26 November 2022).
- United Nation Environment Programme. Coffee, Environmental Degradation and Smallholder Livelihoods. Available online: https://www.unep.org/resources/newsletter/coffee-environmental-degradation-and-smallholder-livelihoods (accessed on 26 November 2022).
- Moore, V. The Environmental Impact of Coffee Production: What’s Your Coffee Costing the Planet? Available online: https://www.sustainablebusinesstoolkit.com/environmental-impact-coffee-trade/ (accessed on 26 November 2022).
- Varcho, A.L. 2.2 A Bitter Brew-Coffee Production, Deforestation, Soil Erosion and Water Contamination. Available online: https://ohiostate.pressbooks.pub/sciencebites/chapter/a-bitter-brew-coffee-production-deforestation-soil-erosion-and-water-contamination/ (accessed on 26 November 2022).
- Chanakya, H.N.; De Alwis, A.A.P. Environmental issues and management in primary coffee processing. Process Saf. Environ. Prot. 2004, 82, 291–300. [Google Scholar] [CrossRef]
- de Queiroz, V.T.; Azevedo, M.M.; da Silva Quadros, I.P.; Costa, A.V.; do Amaral, A.A.; dos Santos, G.M.A.D.A.; Juvanhol, R.S.; de Almeida Telles, L.A.; dos Santos, A.R. Environmental risk assessment for sustainable pesticide use in coffee production. J. Contam. Hydrol. 2018, 219, 18–27. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Mello, F.V.C.; Thode Filho, S.; Carpes, R.M.; Honório, J.G.; Marques, M.R.C.; Felzenszwalb, I.; Ferraz, E.R.A. Impacts of discarded coffee waste on human and environmental health. Ecotoxicol. Environ. Saf. 2017, 141, 30–36. [Google Scholar] [CrossRef]
- Dattatraya Saratale, G.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Kant Bhatia, S.; Atabani, A.E.; Mulone, V.; et al. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Integrated strategies for water removal and lipid extraction from coffee industry residues. Sustain. Energy Technol. Assess. 2018, 29, 26–35. [Google Scholar] [CrossRef]
- Gemechu, G.E.; Mulualem, T. The blooming of coffee industry: Its waste problem and utilization through management option: A review. J. Biol. Chem. Res. 2020, 37, 36–46. [Google Scholar]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chapter Three—Chemical composition and health properties of coffee and coffee by-products. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 91, pp. 65–96. [Google Scholar] [CrossRef]
- Durán-Aranguren, D.D.; Robledo, S.; Gomez-Restrepo, E.; Arboleda Valencia, J.W.; Tarazona, N.A. Scientometric overview of coffee by-products and their applications. Molecules 2021, 26, 7605. [Google Scholar] [CrossRef]
- Ridder, M. Major Coffee Importing Countries Worldwide 2020. Statista: 2022. Available online: https://www.statista.com/statistics/1096400/main-import-countries-for-coffee-worldwide/ (accessed on 26 November 2022).
- Misachi, J. Which States Grow Coffee? 2019. Available online: https://www.worldatlas.com/articles/which-states-grow-coffee.html (accessed on 26 November 2022).
- Knoema DataHub. United States of America—Green Coffee Production Quantity. 2021. Available online: https://knoema.com/atlas/United-States-of-America/topics/Agriculture/Crops-Production-Quantity-tonnes/Coffee-production (accessed on 10 January 2023).
- Bai, C.L.; Liu, L.Y.; Guo, J.L.; Zeng, L.X.; Guo, Y. Microplastics in take-out food: Are we over taking it? Environ. Res. 2022, 215, 114390. [Google Scholar] [CrossRef]
- Ekvall, M.T.; Lundqvist, M.; Kelpsiene, E.; Šileikis, E.; Gunnarsson, S.B.; Cedervall, T. Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products. Nanoscale Adv. 2019, 1, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- PlanetArk. Coffee Go Ground; Planet Ark Environmental Foundation: Sydney, Australia, 2016; Available online: https://planetark.org/newsroom/archive/1059 (accessed on 29 November 2022).
- Cervera-Mata, A.; Navarro-Alarcón, M.; Delgado, G.; Pastoriza, S.; Montilla-Gómez, J.; Llopis, J.; Sánchez-González, C.; Rufián-Henares, J. Spent coffee grounds improve the nutritional value in elements of lettuce (Lactuca sativa L.) and are an ecological alternative to inorganic fertilizers. Food Chem. 2019, 282, 1–8. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; Del Castillo, M.D. Applications of compounds from coffee processing by-products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]
- Stylianou, M.; Agapiou, A.; Omirou, M.; Vyrides, I.; Ioannides, I.M.; Maratheftis, G.; Fasoula, D. Converting environmental risks to benefits by using spent coffee grounds (SCG) as a valuable resource. Environ. Sci. Pollut. Res. Int. 2018, 25, 35776–35790. [Google Scholar] [CrossRef]
- Hu, G.L.; Wang, X.; Zhang, L.; Qiu, M.H. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. 2019, 10, 3113–3126. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Hu, G.; Hong, D.; Guo, T.; Li, J.; Li, Z.; Qiu, M. Review on factors affecting coffee volatiles: From seed to cup. J. Sci. Food Agric. 2022, 102, 1341–1352. [Google Scholar] [CrossRef]
- Claassen, L.; Rinderknecht, M.; Porth, T.; Röhnisch, J.; Seren, H.Y.; Scharinger, A.; Gottstein, V.; Noack, D.; Schwarz, S.; Winkler, G.; et al. Cold brew coffee—Pilot studies on definition, extraction, consumer preference, chemical characterization and microbiological hazards. Foods 2021, 10, 865. [Google Scholar] [CrossRef]
- Moreira, A.S.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Rodríguez Casas, A.; Del Castillo, M.D. Interest of coffee melanoidins as sustainable healthier food ingredients. Front. Nutr. 2021, 8, 730343. [Google Scholar] [CrossRef] [PubMed]
- Schouten, M.A.; Tappi, S.; Romani, S. Acrylamide in coffee: Formation and possible mitigation strategies—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3807–3821. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, A.S.; de Oliveira, D.M.; Walling, E.; Babin, A.; Hersant, G.; Vaneeckhaute, C.; Dumont, M.-J.; Rodrigue, D. Spent coffee grounds characterization and reuse in composting and soil amendment. Waste 2022, 1, 2–20. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.E.; Socha, K. Influence of various factors on caffeine content in coffee brews. Foods 2021, 10, 1208. [Google Scholar] [CrossRef]
- Fuller, M.; Rao, N.Z. The effect of time, roasting temperature, and grind size on caffeine and chlorogenic acid concentrations in cold brew coffee. Sci. Rep. 2017, 7, 17979. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, G.; Guerrini, L.; Masella, P.; Bellumori, M.; Daluiso, S.; Parenti, A.; Innocenti, M. What kind of coffee do you drink? An investigation on effects of eight different extraction methods. Food Res. Int. 2019, 116, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, N.; Pataquiva, L.; Osorio, C.; Moreno, F.L.M.; Ruiz, R.Y. Effect of grinding, extraction time and type of coffee on the physicochemical and flavour characteristics of cold brew coffee. Sci. Rep. 2019, 9, 8440. [Google Scholar] [CrossRef]
- Batali, M.E.; Lim, L.X.; Liang, J.; Yeager, S.E.; Thompson, A.N.; Han, J.; Ristenpart, W.D.; Guinard, J.X. Sensory analysis of full immersion coffee: Cold brew is more floral, and less bitter, sour, and rubbery than hot brew. Foods 2022, 11, 2440. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, S.; Scortichini, S.; Fiorini, D.; Sagratini, G.; Vittori, S.; Neiens, S.D.; Steinhaus, M.; Zheljazkov, V.D.; Maggi, F.; Caprioli, G. Characterization of odor-active compounds, polyphenols, and fatty acids in coffee silverskin. Molecules 2020, 25, 2993. [Google Scholar] [CrossRef]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef]
- Batista, M.; Ávila, A.F.; Franca, A.S.; Oliveira, L.S. Polysaccharide-rich fraction of spent coffee grounds as promising biomaterial for films fabrication. Carbohydr. Polym. 2020, 233, 115851. [Google Scholar] [CrossRef]
- Coelho, G.O.; Batista, M.J.A.; Ávila, A.F.; Franca, A.S.; Oliveira, L.S. Development and characterization of biopolymeric films of galactomannans recovered from spent coffee grounds. J. Food Eng. 2021, 289, 110083. [Google Scholar] [CrossRef]
- Australian Government. Plastics; Department of Agriculture, Water and the Environment: 2022. Available online: https://www.awe.gov.au/environment/protection/waste/plastics-and-packaging (accessed on 26 November 2022).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; COM 650 Final; European Commission: Comission Work Programme 2018. 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52017DC0650&from=en (accessed on 29 November 2022).
- Fortune Business Insights. The Global Green Packaging Market Size was USD 258.35 Billion in 2020. The Market is Projected to Grow from USD 267.83 Billion in 2021 to USD 385.34 Billion by 2028; Fortune Business Insight: 2022. Available online: https://www.fortunebusinessinsights.com/green-packaging-market-105113 (accessed on 29 November 2022).
- Gouws, S.; Muller, M. Valorization of products from grounded-coffee beans. Sci. Rep. 2021, 11, 20445. [Google Scholar] [CrossRef] [PubMed]
- Whiitelaw, A. Record-High Fertiliser Prices in Australia Could Disrupt Food Supplies. The Guardian 12 November 2021. Available online: https://www.theguardian.com/australia-news/2021/nov/12/record-high-fertiliser-prices-in-australia-could-disrupt-food-supplies (accessed on 29 November 2022).
- Cervera-Mata, A.; Delgado, G.; Fernández-Arteaga, A.; Fornasier, F.; Mondini, C. Spent coffee grounds by-products and their influence on soil C–N dynamics. J. Environ. Manag. 2022, 302, 114075. [Google Scholar] [CrossRef]
- Santos, C.; Fonseca, J.; Aires, A.; Coutinho, J.; Trindade, H. Effect of different rates of spent coffee grounds (SCG) on composting process, gaseous emissions and quality of end-product. Waste Manag. 2017, 59, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Price, G.W. Evaluation of three composting systems for the management of spent coffee grounds. Bioresour. Technol. 2011, 102, 7966–7974. [Google Scholar] [CrossRef] [PubMed]
- Hanc, A.; Hrebeckova, T.; Grasserova, A.; Cajthaml, T. Conversion of spent coffee grounds into vermicompost. Bioresour. Technol. 2021, 341, 125925. [Google Scholar] [CrossRef]
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-value molecules recovery and biofuels production from spent coffee grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- United Nations Climate Change. The Paris Agreement; United Nations Climate Change: Bonn, Germany, 2022; Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 26 November 2022).
- Rocha, M.V.; de Matos, L.J.; Lima, L.P.; Figueiredo, P.M.; Lucena, I.L.; Fernandes, F.A.; Gonçalves, L.R. Ultrasound-assisted production of biodiesel and ethanol from spent coffee grounds. Bioresour. Technol. 2014, 167, 343–348. [Google Scholar] [CrossRef]
- Massaya, J.; Prates Pereira, A.; Mills-Lamptey, B.; Benjamin, J.; Chuck, C.J. Conceptualization of a spent coffee grounds biorefinery: A review of existing valorisation approaches. Food Bioprod. Process. 2019, 118, 149–166. [Google Scholar] [CrossRef]
- Yang, L.; Mahmood, N.; Corscadden, K.; Xu, C.; He, Q. Production of crude bio-oil via direct liquefaction of spent K-Cups. Biomass Bioenergy 2016, 95, 354–363. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef]
- Vu, D.C.; Vu, Q.T.; Huynh, L.; Lin, C.-H.; Alvarez, S.; Vo, X.T.; Nguyen, T.H.D. Evaluation of fatty acids, phenolics and bioactivities of spent coffee grounds prepared from Vietnamese coffee. Int. J. Food Prop. 2021, 24, 1548–1558. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; del Castillo, M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A review of coffee by-products including leaf, flower, cherry, husk, silver skin, and spent grounds as novel foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- Gaston, S. 12 Delicious Food Recipes with Coffee Grouds in Them. 2021. Available online: https://www.roastycoffee.com/recipes-with-ground-coffee/ (accessed on 26 November 2022).
- Castaldo, L.; Lombardi, S.; Gaspari, A.; Rubino, M.; Izzo, L.; Narváez, A.; Ritieni, A.; Grosso, M. In vitro bioaccessibility and antioxidant activity of polyphenolic compounds from spent coffee grounds-enriched cookies. Foods 2021, 10, 1837. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT Food Sci. Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Gaille, B. 18 Food Additives Industry Statistics and Trends. 2018. Available online: https://brandongaille.com/18-food-additives-industry-statistics-and-trends/#:~:text=If%20all%20possible%20additives%20are%20figured%20into%20the,their%20foods%20be%20free%20of%20any%20artificial%20additives (accessed on 26 November 2022).
- Franca, A.S.; Oliveira, L.S. Potential uses of spent coffee grounds in the food industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef] [PubMed]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee chlorogenic acids incorporation for bioactivity enhancement of foods: A review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The wastes of coffee bean processing for utilization in food: A review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Okur, I.; Soyler, B.; Sezer, P.; Oztop, M.H.; Alpas, H. Improving the recovery of phenolic compounds from spent coffee grounds (SCG) by environmentally friendly extraction techniques. Molecules 2021, 26, 613. [Google Scholar] [CrossRef] [PubMed]
- Bouhlal, F.; Aqil, Y.; Chamkhi, I.; Belmaghraoui, W.; Labjar, N.; Hajjaji, S.E.; Benabdellah, G.A.; Aurag, J.; Lotfi, E.M.; Mahi, M.E. GC-MS analysis, phenolic compounds quantification, antioxidant, and antibacterial activities of the hydro-alcoholic extract of spent coffee grounds. J. Biol. Active Prod. Nat. 2020, 10, 325–337. [Google Scholar] [CrossRef]
- Coelho, J.P.; Robalo, M.P.; Boyadzhieva, S.; Stateva, R.P. Microwave-assisted extraction of phenolic compounds from spent coffee grounds. Process optimization applying design of experiments. Molecules 2021, 26, 7320. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran Jeganathan, P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Leow, Y.; Yew, P.Y.M.; Chee, P.L.; Loh, X.J.; Kai, D. Recycling of spent coffee grounds for useful extracts and green composites. RSC Adv. 2021, 11, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Jin Cho, E.; Gyo Lee, Y.; Song, Y.; Nguyen, D.-T.; Bae, H.-J. An integrated process for conversion of spent coffee grounds into value-added materials. Bioresour. Technol. 2022, 346, 126618. [Google Scholar] [CrossRef]
- Lauberts, M.; Mierina, I.; Pals, M.; Latheef, M.A.; Shishkin, A. Spent coffee grounds valorization in biorefinery context to obtain valuable products using different extraction approaches and solvents. Plants 2023, 12, 30. [Google Scholar] [CrossRef]
- Bhandarkar, N.S.; Mouatt, P.; Goncalves, P.; Thomas, T.; Brown, L.; Panchal, S.K. Modulation of gut microbiota by spent coffee grounds attenuates diet-induced metabolic syndrome in rats. FASEB J. 2020, 34, 4783–4797. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of coffee consumption on the gut microbiota: A human volunteer study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef]
- Nakayama, T.; Oishi, K. Influence of coffee (Coffea arabica) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiol. Lett. 2013, 343, 161–168. [Google Scholar] [CrossRef]
- Nehlig, A. Effects of coffee on the gastro-intestinal tract: A narrative review and literature update. Nutrients 2022, 14, 399. [Google Scholar] [CrossRef]
- González, S.; Salazar, N.; Ruiz-Saavedra, S.; Gómez-Martín, M.; de Los Reyes-Gavilán, C.G.; Gueimonde, M. Long-term coffee consumption is associated with fecal microbial composition in humans. Nutrients 2020, 12, 1287. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Fernández-Arteaga, A.; Luzón, G.; Jiménez-Hernández, N.; D’Auria, G.; Francino, M.P.; Rufián-Henares, J.Á. Spent coffee grounds extract, rich in mannooligosaccharides, promotes a healthier gut microbial community in a dose-dependent manner. J. Agric. Food Chem. 2019, 67, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Arreguín-Campos, A.; Cruz-Medrano, M.A.; Del Castillo Bilbao, M.D. Spent coffee (Coffea arabica L.) grounds promote satiety and attenuate energy intake: A pilot study. J. Food Biochem. 2020, 44, e13204. [Google Scholar] [CrossRef] [PubMed]
- Oseguera-Castro, K.Y.; Madrid, J.A.; Martínez Madrid, M.J.; García, O.P.; Del Castillo, M.D.; Campos-Vega, R. Antioxidant dietary fiber isolated from spent coffee (Coffea arabica L.) grounds improves chronotype and circadian locomotor activity in young adults. Food Funct. 2019, 10, 4546–4556. [Google Scholar] [CrossRef]
- López-Barrera, D.M.; Vázquez-Sánchez, K.; Loarca-Piña, M.G.; Campos-Vega, R. Spent coffee grounds, an innovative source of colonic fermentable compounds, inhibit inflammatory mediators in vitro. Food Chem. 2016, 212, 282–290. [Google Scholar] [CrossRef]
- Oliveira Batista, J.; Car Cordeiro, C.; Klososki, S.J.; Mongruel Eleutério Dos Santos, C.; Leão, G.M.C.; Pimentel, T.C.; Rosset, M. Spent coffee grounds improve the nutritional value and technological properties of gluten-free cookies. J. Culin. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, identification and quantification of polyphenols from spent coffee grounds by chromatographic methods and chemometric analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef]
- Angeloni, S.; Nzekoue, F.K.; Navarini, L.; Sagratini, G.; Torregiani, E.; Vittori, S.; Caprioli, G. An analytical method for the simultaneous quantification of 30 bioactive compounds in spent coffee ground by HPLC-MS/MS. J. Mass Spectrom. 2020, 55, e4519. [Google Scholar] [CrossRef]
- Kozuma, K.; Tsuchiya, S.; Kohori, J.; Hase, T.; Tokimitsu, I. Antihypertensive effect of green coffee bean extract on mildly hypertensive subjects. Hypertens. Res. 2005, 28, 711–718. [Google Scholar] [CrossRef]
- Watanabe, T.; Arai, Y.; Mitsui, Y.; Kusaura, T.; Okawa, W.; Kajihara, Y.; Saito, I. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Lara-Guzmán, O.J.; Álvarez, R.; Muñoz-Durango, K. Changes in the plasma lipidome of healthy subjects after coffee consumption reveal potential cardiovascular benefits: A randomized controlled trial. Free Radic. Biol. Med. 2021, 176, 345–355. [Google Scholar] [CrossRef]
- Suzuki, A.; Nomura, T.; Jokura, H.; Kitamura, N.; Saiki, A.; Fujii, A. Chlorogenic acid-enriched green coffee bean extract affects arterial stiffness assessed by the cardio-ankle vascular index in healthy men: A pilot study. Int. J. Food Sci. Nutr. 2019, 70, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga, L.Y.; Aceves-de la Mora, M.C.A.; González-Ortiz, M.; Ramos-Núñez, J.L.; Martínez-Abundis, E. Effect of chlorogenic acid administration on glycemic control, insulin secretion, and insulin sensitivity in patients with impaired glucose tolerance. J. Med. Food 2018, 21, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Roshan, H.; Nikpayam, O.; Sedaghat, M.; Sohrab, G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: A randomised clinical trial. Br. J. Nutr. 2018, 119, 250–258. [Google Scholar] [CrossRef]
- Narita, Y.; Iwai, K.; Fukunaga, T.; Nakagiri, O. Inhibitory activity of chlorogenic acids in decaffeinated green coffee beans against porcine pancreas lipase and effect of a decaffeinated green coffee bean extract on an emulsion of olive oil. Biosci. Biotechnol. Biochem. 2012, 76, 2329–2331. [Google Scholar] [CrossRef]
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet-induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2019, 62, 78–88. [Google Scholar] [CrossRef]
- Zhong, Y.; Ding, Y.; Li, L.; Ge, M.; Ban, G.; Yang, H.; Dai, J.; Zhang, L. Effects and mechanism of chlorogenic acid on weight loss. Curr. Pharm. Biotechnol. 2020, 21, 1099–1106. [Google Scholar] [CrossRef]
- Postuma, R.B.; Anang, J.; Pelletier, A.; Joseph, L.; Moscovich, M.; Grimes, D.; Furtado, S.; Munhoz, R.P.; Appel-Cresswell, S.; Moro, A.; et al. Caffeine as symptomatic treatment for Parkinson disease (Café-PD): A randomized trial. Neurology 2017, 89, 1795–1803. [Google Scholar] [CrossRef]
- Walters, E.R.; Lesk, V.E. Time of day and caffeine influence some neuropsychological tests in the elderly. Psychol. Assess. 2015, 27, 161–168. [Google Scholar] [CrossRef]
- Sherman, S.M.; Buckley, T.P.; Baena, E.; Ryan, L. Caffeine enhances memory performance in young adults during their non-optimal time of day. Front. Psychol. 2016, 7, 1764. [Google Scholar] [CrossRef] [PubMed]
- Fahanik-Babaei, J.; Baluchnejadmojarad, T.; Nikbakht, F.; Roghani, M. Trigonelline protects hippocampus against intracerebral Aβ(1-40) as a model of Alzheimer’s disease in the rat: Insights into underlying mechanisms. Metab. Brain Dis. 2019, 34, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.M.; Yang, X.; Kuboyama, T.; Tohda, C. Trigonelline recovers memory function in Alzheimer’s disease model mice: Evidence of brain penetration and target molecule. Sci. Rep. 2020, 10, 16424. [Google Scholar] [CrossRef]
- Chowdhury, A.A.; Gawali, N.B.; Munshi, R.; Juvekar, A.R. Trigonelline insulates against oxidative stress, proinflammatory cytokines and restores BDNF levels in lipopolysaccharide induced cognitive impairment in adult mice. Metab. Brain Dis. 2018, 33, 681–691. [Google Scholar] [CrossRef]
- Liu, L.; Du, X.; Zhang, Z.; Zhou, J. Trigonelline inhibits caspase 3 to protect β cells apoptosis in streptozotocin-induced type 1 diabetic mice. Eur. J. Pharmacol. 2018, 836, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hamadi, S.A. Effect of trigonelline and ethanol extract of Iraqi Fenugreek seeds on oxidative stress in alloxan diabetic rabbits. J. Assoc. Arab Univ. Basic Appl. Sci. 2012, 12, 23–26. [Google Scholar] [CrossRef]
- Tohda, C.; Nakamura, N.; Komatsu, K.; Hattori, M. Trigonelline-induced neurite outgrowth in human neuroblastoma SK-N-SH cells. Biol. Pharm. Bull. 1999, 22, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Rufián-Henares, J.A.; Morales, F.J. Effect of in vitro enzymatic digestion on antioxidant activity of coffee melanoidins and fractions. J. Agric. Food Chem. 2007, 55, 10016–10021. [Google Scholar] [CrossRef] [PubMed]
- Rufián-Henares, J.A.; de la Cueva, S.P. Antimicrobial activity of coffee melanoidins—A study of their metal-chelating properties. J. Agric. Food Chem. 2009, 57, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Sauer, T.; Raithel, M.; Kressel, J.; Münch, G.; Pischetsrieder, M. Activation of the transcription factor Nrf2 in macrophages, Caco-2 cells and intact human gut tissue by Maillard reaction products and coffee. Amino Acids 2013, 44, 1427–1439. [Google Scholar] [CrossRef]
- Reichardt, N.; Gniechwitz, D.; Steinhart, H.; Bunzel, M.; Blaut, M. Characterization of high molecular weight coffee fractions and their fermentation by human intestinal microbiota. Mol. Nutr. Food Res. 2009, 53, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Xu, M.; Qiu, Y.; Liao, M.; Zhang, Q.; Yang, L.; Zheng, G. Chlorogenic acid and caffeine combination attenuates adipogenesis by regulating fat metabolism and inhibiting adipocyte differentiation in 3T3-L1 cells. J. Food Biochem. 2021, 45, e13795. [Google Scholar] [CrossRef] [PubMed]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.W.; Wong, C.N.; Pin, W.K.; Wong, M.H.; Kwok, C.Y.; Chan, R.Y.; Yu, P.H.; Chan, S.W. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother. Res. 2013, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk. Med. Hypotheses 2005, 64, 848–853. [Google Scholar] [CrossRef]
- Pérez-Nájera, V.C.; Gutiérrez-Uribe, J.A.; Antunes-Ricardo, M.; Hidalgo-Figueroa, S.; Del-Toro-Sánchez, C.L.; Salazar-Olivo, L.A.; Lugo-Cervantes, E. Smilax aristolochiifolia root extract and its compounds chlorogenic acid and astilbin inhibit the activity of α-amylase and α-glucosidase enzymes. Evid. Based Complement. Alternat. Med. 2018, 2018, 6247306. [Google Scholar] [CrossRef]
- Cappelletti, S.; Piacentino, D.; Sani, G.; Aromatario, M. Caffeine: Cognitive and physical performance enhancer or psychoactive drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Military Nutrition Research. 2. Pharmacology of caffeine. In Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations; National Academies Press (US): Washington, DC, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK223808/ (accessed on 29 November 2022).
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, caffeine, and health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galán, A.; Prieto, A. Caffeine’s vascular mechanisms of action. Int. J. Vasc. Med. 2010, 2010, 834060. [Google Scholar] [CrossRef]
- Nehlig, A.; Daval, J.L.; Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Fallon, S.J.; Davis, T.; Ankrett, S.; Munro, G.; Christopher, G.; Coulthard, E. Caffeine and attentional control: Improved and impaired performance in healthy older adults and Parkinson’s disease according to task demands. Psychopharmacology 2022, 239, 605–619. [Google Scholar] [CrossRef]
- Munoz, D.G.; Fujioka, S. Caffeine and Parkinson disease: A possible diagnostic and pathogenic breakthrough. Neurology 2018, 90, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, J.F. Caffeine and Parkinson’s disease: Multiple benefits and emerging mechanisms. Front. Neurosci. 2020, 14, 602697. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.W.; Abbott, R.D.; Petrovitch, H.; Morens, D.M.; Grandinetti, A.; Tung, K.H.; Tanner, C.M.; Masaki, K.H.; Blanchette, P.L.; Curb, J.D.; et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 2000, 283, 2674–2679. [Google Scholar] [CrossRef]
- Postuma, R.B.; Lang, A.E.; Munhoz, R.P.; Charland, K.; Pelletier, A.; Moscovich, M.; Filla, L.; Zanatta, D.; Rios Romenets, S.; Altman, R.; et al. Caffeine for treatment of Parkinson disease: A randomized controlled trial. Neurology 2012, 79, 651–658. [Google Scholar] [CrossRef]
- Wilson, P.W.; Bloom, H.L. Caffeine consumption and cardiovascular risks: Little cause for concern. J. Am. Heart Assoc. 2016, 5, e003089. [Google Scholar] [CrossRef]
- Zulli, A.; Smith, R.M.; Kubatka, P.; Novak, J.; Uehara, Y.; Loftus, H.; Qaradakhi, T.; Pohanka, M.; Kobyliak, N.; Zagatina, A.; et al. Caffeine and cardiovascular diseases: Critical review of current research. Eur. J. Nutr. 2016, 55, 1331–1343. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef]
- Saimaiti, A.; Zhou, D.-D.; Li, J.; Xiong, R.-G.; Gan, R.-Y.; Huang, S.-Y.; Shang, A.; Zhao, C.-N.; Li, H.-Y.; Li, H.-B. Dietary sources, health benefits, and risks of caffeine. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, N.; Sharififar, F.; Pournamdari, M.; Ansari, M. A review on biosynthesis, analytical techniques, and pharmacological activities of trigonelline as a plant alkaloid. J. Diet. Suppl. 2018, 15, 207–222. [Google Scholar] [CrossRef]
- Belayneh, A.; Molla, F. The effect of coffee on pharmacokinetic properties of drugs: A review. Biomed. Res. Int. 2020, 2020, 7909703. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.C. Chapter 44—Fenugreek: Multiple Health Benefits. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 599–617. [Google Scholar] [CrossRef]
- Koshiro, Y.; Zheng, X.-Q.; Wang, M.-L.; Nagai, C.; Ashihara, H. Changes in content and biosynthetic activity of caffeine and trigonelline during growth and ripening of Coffea arabica and Coffea canephora fruits. Plant Sci. 2006, 171, 242–250. [Google Scholar] [CrossRef]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaź, P. Neuroprotective effects of coffee bioactive compounds: A review. Int. J. Mol. Sci. 2020, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Aging. What Causes Alzheimer’s Disease? National Institute of Health: Bethesda, MD, USA, 2019. Available online: https://www.nia.nih.gov/health/what-causes-alzheimers-disease (accessed on 27 November 2022).
- Makowska, J.; Szczesny, D.; Lichucka, A.; Giełdoń, A.; Chmurzyński, L.; Kaliszan, R. Preliminary studies on trigonelline as potential anti-Alzheimer disease agent: Determination by hydrophilic interaction liquid chromatography and modeling of interactions with β-amyloid. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 968, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Oestreich-Janzen, S. 3.25—Chemistry of Coffee. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 1085–1117. [Google Scholar] [CrossRef]
- Finotello, C.; Forzato, C.; Gasparini, A.; Mammi, S.; Navarini, L.; Schievano, E. NMR quantification of 16-O-methylcafestol and kahweol in Coffea canephora var. robusta beans from different geographical origins. Food Cont. 2017, 75, 62–69. [Google Scholar] [CrossRef]
- Gunning, Y.; Defernez, M.; Watson, A.D.; Beadman, N.; Colquhoun, I.J.; Le Gall, G.; Philo, M.; Garwood, H.; Williamson, D.; Davis, A.P.; et al. 16-O-methylcafestol is present in ground roast Arabica coffees: Implications for authenticity testing. Food Chem. 2018, 248, 52–60. [Google Scholar] [CrossRef]
- Urgert, R.; Katan, M.B. The cholesterol-raising factor from coffee beans. Annu. Rev. Nutr. 1997, 17, 305–324. [Google Scholar] [CrossRef]
- Thelle, D.S.; Arnesen, E.; Førde, O.H. The Tromsø heart study. Does coffee raise serum cholesterol? N. Engl. J. Med. 1983, 308, 1454–1457. [Google Scholar] [CrossRef]
- De Roos, B.; Meyboom, S.; Kosmeijer-Schuil, T.G.; Katan, M.B. Absorption and urinary excretion of the coffee diterpenes cafestol and kahweol in healthy ileostomy volunteers. J. Intern. Med. 1998, 244, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Izumi, K.; Natsagdorj, A.; Naito, R.; Makino, T.; Kadomoto, S.; Hiratsuka, K.; Shigehara, K.; Kadono, Y.; Narimoto, K.; et al. Coffee diterpenes kahweol acetate and cafestol synergistically inhibit the proliferation and migration of prostate cancer cells. Prostate 2019, 79, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Motohashi, O.; Nakayama, N.; Nishimura, K.; Kasajima, R.; Miyagi, Y.; Shiozawa, M.; Yoshioka, E.; Suzuki, M.; Washimi, K.; et al. The clinicopathological significance of angiogenesis in hindgut neuroendocrine tumors obtained via an endoscopic procedure. Diagn. Pathol. 2016, 11, 128. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and kahweol: A review on their bioactivities and pharmacological properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef]
- Mellbye, F.B.; Jeppesen, P.B.; Hermansen, K.; Gregersen, S. Cafestol, a bioactive substance in coffee, stimulates insulin secretion and increases glucose uptake in muscle cells: Studies in vitro. J. Nat. Prod. 2015, 78, 2447–2451. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, K.S.; Jeong, H.G. Suppressive effects of the kahweol and cafestol on cyclooxygenase-2 expression in macrophages. FEBS Lett. 2004, 569, 321–326. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Qian, H.; Yao, W.-R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Fogliano, V.; Morales, F.J. Estimation of dietary intake of melanoidins from coffee and bread. Food Funct. 2011, 2, 117–123. [Google Scholar] [CrossRef]
- Bekedam, E.K.; Loots, M.J.; Schols, H.A.; Van Boekel, M.A.; Smit, G. Roasting effects on formation mechanisms of coffee brew melanoidins. J. Agric. Food Chem. 2008, 56, 7138–7145. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Rajakaruna, S.; Pastoriza, S.; Paliy, O.; Ángel Rufián-Henares, J. Bioactivity of food melanoidins is mediated by gut microbiota. Food Chem. 2020, 316, 126309. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, J.Á.; Ames, J.M.; Leake, D.S. Antioxidant activity and protective effects of green and dark coffee components against human low density lipoprotein oxidation. Eur. Food Res. Technol. 2008, 227, 1017–1024. [Google Scholar] [CrossRef]
- O’Brien, J.; Morrissey, P.A. Metal ion complexation by products of the Maillard reaction. Food Chem. 1997, 58, 17–27. [Google Scholar] [CrossRef]
- Daglia, M.; Papetti, A.; Aceti, C.; Sordelli, B.; Gregotti, C.; Gazzani, G. Isolation of high molecular weight components and contribution to the protective activity of coffee against lipid peroxidation in a rat liver microsome system. J. Agric. Food Chem. 2008, 56, 11653–11660. [Google Scholar] [CrossRef]
- Morales, F.J.; Somoza, V.; Fogliano, V. Physiological relevance of dietary melanoidins. Amino Acids 2012, 42, 1097–1109. [Google Scholar] [CrossRef]
- Brown, L.; Caligiuri, S.P.B.; Brown, D.; Pierce, G.N. Clinical trials using functional foods provide unique challenges. J. Funct. Foods 2018, 45, 233–238. [Google Scholar] [CrossRef]
| Compound | Type of Study | Findings |
|---|---|---|
| Chlorogenic acid | Human | Regulated blood pressure [89,90]; improved insulin secretion, uptake of glucose by intestinal cells [91]; improved insulin secretion [91]; improved dyslipidaemia and endothelial function [92,93]; improved fasting glucose in patients with impaired glucose tolerance [94,95]; body weight reduction and waist circumference reduction [95] |
| In vitro | Improved lipase reaction [96] | |
| Animal | Reduced accumulation of fat in the liver and reduced blood lipids [97,98]; improved body weight and reduced visceral fat [97] | |
| Caffeine | Human | Improved cognitive health in patients with degenerative disease [99]; better performance in tests in age-related cognitive impairment [100]; enhanced memory and cognitive performance in young adults [101] |
| Trigonelline | Animal | Improved specific neuron function [102]; improved memory in Alzheimer-induced mice [103]; suppressed oxidative stress and inflammation in the brain [102,104]; reduced blood glucose and improved lipid in metabolically ill animals [105,106] |
| In vitro | Promoted regeneration of neuronal network by neurite outgrowth [107] | |
| Melanoidins | In vitro | Antioxidant activity [108]; antibacterial activity against Gram-negative and Gram-positive bacteria [109]; antioxidant activity and activation of other gene-protective mechanisms in different cell lines [110] |
| Ex vivo | Antioxidant activity and activation of other gene-protective mechanisms in human gut tissue [110]; fermentation of gut bacteria, activation of antioxidant pathways and modulation of gut bacteria population [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bevilacqua, E.; Cruzat, V.; Singh, I.; Rose’Meyer, R.B.; Panchal, S.K.; Brown, L. The Potential of Spent Coffee Grounds in Functional Food Development. Nutrients 2023, 15, 994. https://doi.org/10.3390/nu15040994
Bevilacqua E, Cruzat V, Singh I, Rose’Meyer RB, Panchal SK, Brown L. The Potential of Spent Coffee Grounds in Functional Food Development. Nutrients. 2023; 15(4):994. https://doi.org/10.3390/nu15040994
Chicago/Turabian StyleBevilacqua, Elza, Vinicius Cruzat, Indu Singh, Roselyn B. Rose’Meyer, Sunil K. Panchal, and Lindsay Brown. 2023. "The Potential of Spent Coffee Grounds in Functional Food Development" Nutrients 15, no. 4: 994. https://doi.org/10.3390/nu15040994
APA StyleBevilacqua, E., Cruzat, V., Singh, I., Rose’Meyer, R. B., Panchal, S. K., & Brown, L. (2023). The Potential of Spent Coffee Grounds in Functional Food Development. Nutrients, 15(4), 994. https://doi.org/10.3390/nu15040994