The (Poly)phenol-Carbohydrate Combination for Diabetes: Where Do We Stand?
Abstract
Author Contributions
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas 2021. 10th Edition. Available online: https://diabetesatlas.org/ (accessed on 22 December 2022).
- Matos, A.M.; Macedo, M.P.; Rauter, A.P. Bridging type 2 diabetes and Alzheimer’s disease: Assembling the puzzle pieces in the quest for the molecules with therapeutic and preventive potential. Med. Res. Rev. 2018, 38, 261–324. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Palacios, O.M.; Kramer, M.; Maki, K.C. Diet and prevention of type 2 diabetes mellitus: Beyond weight loss and exercise. Expert Rev. Endocrinol. Metab. 2019, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Paule, A.; Aragonès, G.; Barbosa, M.; Caddeo, C.; Debs, E.; Dinkova, R.; Eckert, G.P.; Fontana, A.; Gebrayel, P.; et al. Recent advances in research of polyphenols: Effects on microbiota, metabolism and health. Mol. Nutr. Food Res. 2022, 66, e2100670. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-mediated gut microbiota modulation: Toward prebiotics and further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
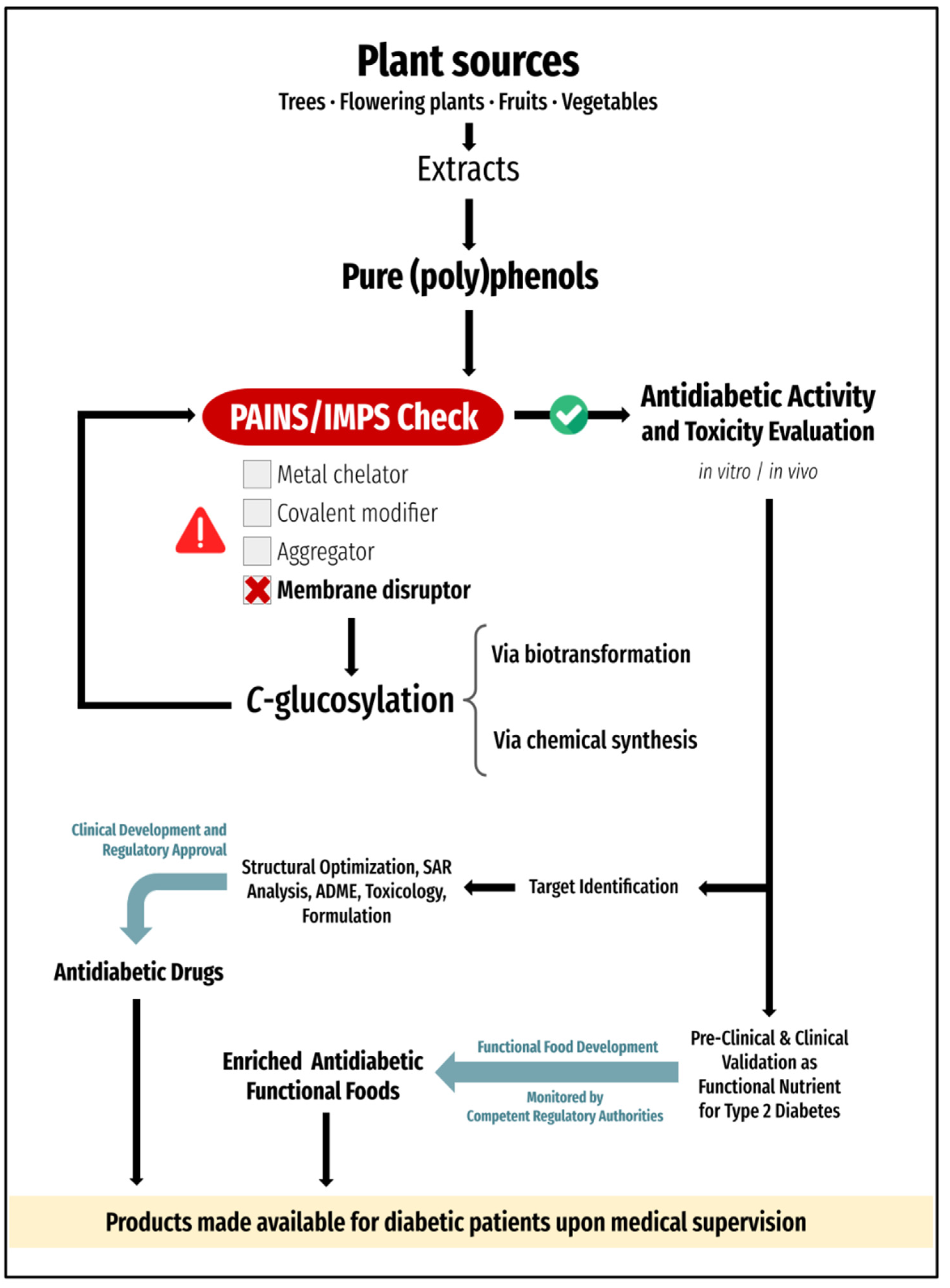
- Serina, J.J.C.; Castilho, P.C.M.F. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Crit. Rev. Food Sci. Nutr. 2022, 62, 8355–8387. [Google Scholar] [CrossRef]
- Guerreiro, I.; Ferreira-Pêgo, C.; Carregosa, D.; Santos, C.N.; Menezes, R.; Fernandes, A.S.; Costa, J.G. Polyphenols and their metabolites in renal diseases: An overview. Foods 2022, 11, 1060. [Google Scholar] [CrossRef]
- Menezes, R.; Matafome, P.; Freitas, M.; García-Conesa, M.-T. Updated information of the effects of (poly)phenols against type-2 diabetes mellitus in humans: Reinforcing the recommendations for future research. Nutrients 2022, 14, 3563. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of Pan Assay Interference Compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef]
- Bisson, J.; McAlpine, J.B.; Friesen, J.B.; Chen, S.N.; Graham, J.; Pauli, G.F. Can invalid bioactives undermine natural product-based drug discovery? J. Med. Chem. 2016, 59, 1671–1690. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
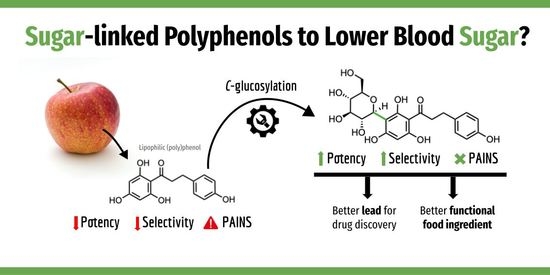
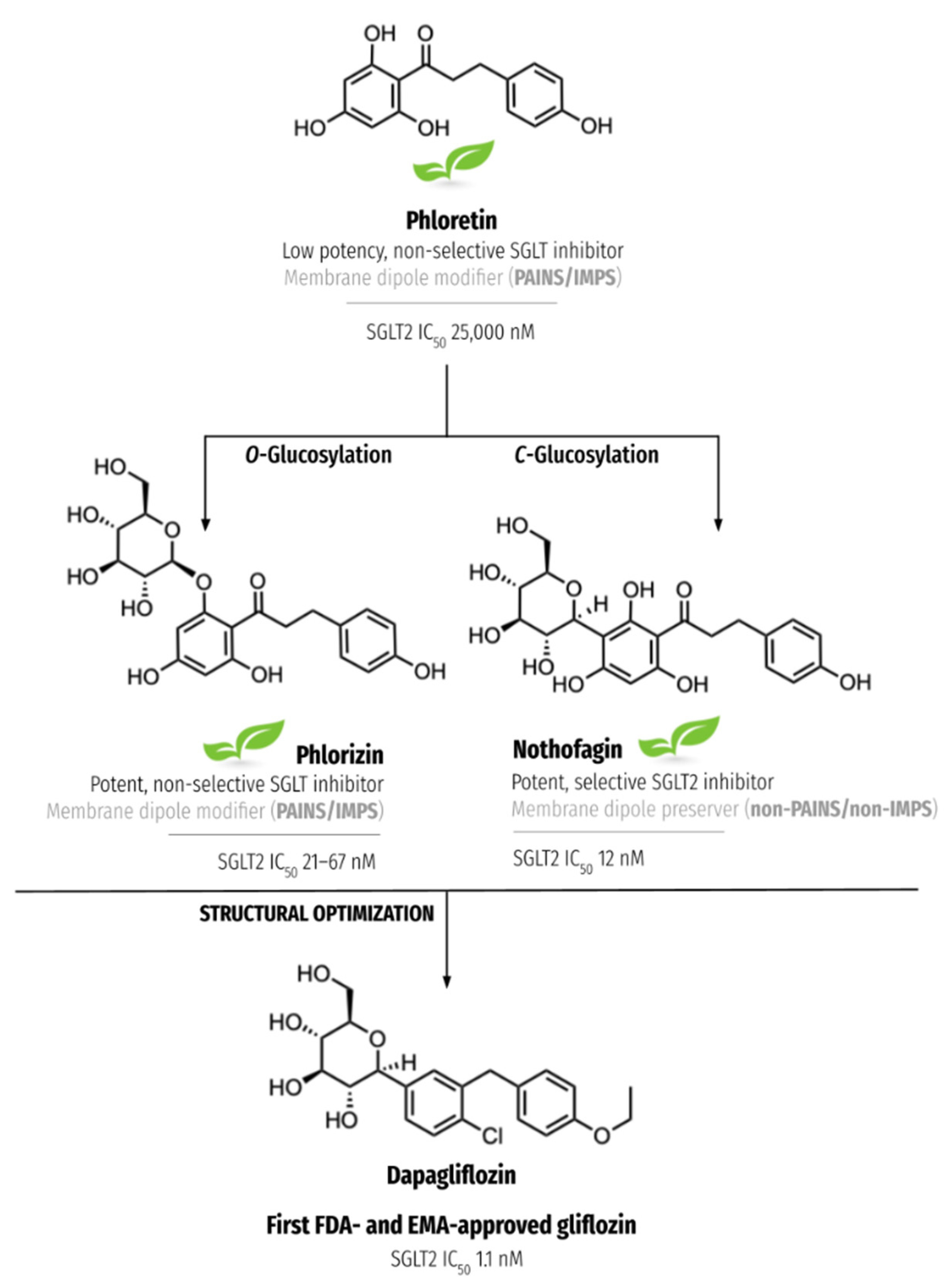
- Matos, A.M.; Blázquez-Sánchez, M.T.; Sousa, C.; Oliveira, M.C.; Almeida, R.F.M.; Rauter, A.P. C-Glucosylation as a tool for the prevention of PAINS-induced membrane dipole potential alterations. Sci. Rep. 2021, 11, 4443. [Google Scholar] [CrossRef] [PubMed]
- Ostroumova, O.S.; Efimova, S.S.; Schagina, L.V. Phloretin-induced reduction in dipole potential of sterol-containing bilayers. J. Membr. Biol. 2013, 246, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Cheng, K.H.; Huang, J. Assess the nature of cholesterol-lipid interactions through the chemical potential of cholesterol in phosphatidylcholine bilayers. Proc. Natl. Acad. Sci. USA 2007, 104, 5372–5377. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.R.; Vila-Viçosa, D.; Machuqueiro, M.; Marques, A.P.; Dore, T.M.; Rauter, A.P. Targeting type 2 diabetes with C-glucosyl dihydrochalcones as selective sodium glucose co-transporter 2 (SGLT2) inhibitors: Synthesis and biological evaluation. J. Med. Chem. 2017, 60, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Ellsworth, B.A.; Nirschl, A.A.; McCann, P.J.; Patel, M.; Girotra, R.N.; Wu, G.; Sher, P.M.; Morrison, E.P.; Biller, S.A.; et al. Discovery of dapagliflozin: A potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 2008, 51, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B. Feeling Nature’s PAINS: Natural products, natural product drugs, and Pan Assay Interference Compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Velander, P.; Wu, L.; Hildreth, S.B.; Vogelaar, N.J.; Mukhopadhyay, B.; Helm, R.F.; Zhang, S.; Xu, B. Catechol-containing compounds are a broad class of protein aggregation inhibitors: Redox state is a key determinant of the inhibitory activities. Pharmacol. Res. 2022, 184, 106409. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.M.; Cristóvão, J.S.; Yashunsky, D.V.; Nifantiev, N.E.; Viana, A.S.; Gomes, C.M.; Rauter, A.P. Synthesis and effects of flavonoid structure variation on amyloid-β aggregation. Pure Appl. Chem. 2017, 89, 1305–1320. [Google Scholar] [CrossRef]
- Jesus, A.R.; Dias, C.; Matos, A.M.; Almeida, R.F.M.; Viana, A.S.; Marcelo, F.; Ribeiro, R.T.; Macedo, M.P.; Airoldi, C.; Nicotra, F.; et al. Exploiting the therapeutic potential of 8-β-D-glucopyranosylgenistein: Synthesis, antidiabetic activity, and molecular interaction with islet amyloid polypeptide and amyloid β-peptide (1–42). J. Med. Chem. 2014, 57, 9463–9472. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Ramkumar, K.M.; Xu, B. Vitexin restores pancreatic β-cell function and insulin signaling through Nrf2 and NF-κB signaling pathways. Eur. J. Pharmacol. 2020, 888, 173606. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Zhao, Y.; Qiang, G.; Yang, X.; Xu, C.; Chen, X.; Liu, C.; Wang, X.; Zhang, L.; Du, G. Puerarin mitigates diabetic hepatic steatosis and fibrosis by inhibiting TGF-β signaling pathway activation in type 2 diabetic rats. Oxid. Med. Cell. Longev. 2018, 2018, 4545321. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, S.; Wang, X.; Gao, P.; Zhang, S.; Mo, Y.; Zhao, D.; Dai, L. Pharmacodynamic interactions between puerarin and metformin in type-2 diabetic rats. Molecules 2022, 27, 7197. [Google Scholar] [CrossRef]
- Panidthananon, W.; Chaowasku, T.; Sritularak, B.; Likhitwitayawuid, K. A new benzophenone C-glucoside and other constituents of Pseuduvaria fragrans and their α-glucosidase inhibitory activity. Molecules 2018, 23, 1600. [Google Scholar] [CrossRef]
- Matos, A.M.; Man, T.; Idrissi, I.; Souza, C.C.; Mead, E.; Dunbar, C.; Wolak, J.; Oliveira, M.C.; Evans, D.; Grayson, J.; et al. Discovery of N-methylpiperazinyl flavones as a novel class of compounds with therapeutic potential against Alzheimer’s disease: Synthesis, binding affinity towards amyloid β oligomers (Aβo) and ability to disrupt Aβo-PrPC interactions. Pure Appl. Chem. 2019, 91, 1107–1136. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Uchiyama, H.; Kadota, K.; Tozuka, Y. Designing amorphous formulations of polyphenols with naringenin by spray-drying for enhancing solubility and permeability. Adv. Powder Technol. 2022, 33, 103627. [Google Scholar] [CrossRef]
- Nadim, M.; Auriol, D.; Lamerant-Fayel, N.; Lefèvre, F.; Dubanet, L.; Redziniak, G.; Kieda, C.; Grillon, C. Improvement of polyphenol properties upon glucosylation in a UV-induced skin cell ageing model. Int. J. Cosmet. Sci. 2014, 36, 579–587. [Google Scholar] [CrossRef]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of flavonoid O-glycoside, C-glycoside and their aglycones on antioxidant capacity and metabolism during in vitro digestion and in vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef]
- Matos, A.M.; Calado, P.; Washburn, W.; Rauter, A.P. Recent advances on SGLT2 inhibitors: Synthetic approaches, therapeutic benefits and adverse events. In Successful Drug Discovery; Fischer, J., Klein, C., Childers, W., Eds.; Wiley-VCH GmbH: Weinheim, Germany, 2021; Volume 5, pp. 111–155. [Google Scholar]
- Zhao, Y.Y.; Fan, Y.; Wang, M.; Wang, J.; Cheng, J.X.; Zou, J.B.; Zhang, X.F.; Shi, Y.J.; Guo, D.Y. Studies on pharmacokinetic properties and absorption mechanism of phloretin: In vivo and in vitro. Biomed. Pharmacother. 2020, 132, 110809. [Google Scholar] [CrossRef]
- Bebernitz, G. Sodium–Glucose Cotransporters. In Comprehensive Medicinal Chemistry III; Elsevier Special Collection in Chemistry, Molecular Sciences and Chemistry Engeneering; Elsevier: Amsterdam, The Netherlands, 2017; Volume 7, pp. 291–511. [Google Scholar]
- Liao, H.; Ma, J.; Yao, H.; Liu, X.-W. Recent progress of C-glycosylation methods in the total synthesis of natural products and pharmaceuticals. Org. Biomol. Chem. 2018, 16, 1791–1806. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.; Matos, A.M.; Rauter, A.P. Chemical approaches towards neurodegenerative disease prevention: The role of coupling sugars to phenolic biomolecular entities. In Coupling and Decoupling of Diverse Molecular Units in Glycosciences, 1st ed.; Witczak, Z.J., Bielski, R., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 167–194. [Google Scholar]
- Gao, H.-Y.; Liu, Y.; Tan, F.-T.; Zhu, L.-W.; Jia, K.-Z.; Tang, Y.-J. The advances and challenges in enzymatic C-glycosylation of flavonoids in plants. Curr. Pharm. Des. 2022, 28, 1466–1479. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.-J.; Zhang, W. Role of dietary nutrients in the modulation of gut microbiota: A narrative review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.; Borges-Cunha, M.; Pimentel-Nunes, P. Microbiota modulation in patients with metabolic syndrome. Nutrients 2022, 14, 4490. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Matos, A.M.; Menezes, R. The (Poly)phenol-Carbohydrate Combination for Diabetes: Where Do We Stand? Nutrients 2023, 15, 996. https://doi.org/10.3390/nu15040996
de Matos AM, Menezes R. The (Poly)phenol-Carbohydrate Combination for Diabetes: Where Do We Stand? Nutrients. 2023; 15(4):996. https://doi.org/10.3390/nu15040996
Chicago/Turabian Stylede Matos, Ana Marta, and Regina Menezes. 2023. "The (Poly)phenol-Carbohydrate Combination for Diabetes: Where Do We Stand?" Nutrients 15, no. 4: 996. https://doi.org/10.3390/nu15040996
APA Stylede Matos, A. M., & Menezes, R. (2023). The (Poly)phenol-Carbohydrate Combination for Diabetes: Where Do We Stand? Nutrients, 15(4), 996. https://doi.org/10.3390/nu15040996