Comparative Effect of Bergamot Polyphenolic Fraction and Red Yeast Rice Extract in Rats Fed a Hyperlipidemic Diet: Role of Antioxidant Properties and PCSK9 Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.1.1. Red Yeast Rice (RYR)
2.1.2. Preparation of BPF
2.1.3. High-Pressure Liquid Chromatography (HPLC) Analysis
2.2. Animal Studies
2.3. Blood Measurements
2.4. PCSK9 Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Michos, E.D.; McEvoy, J.W.; Blumenthal, R.S. Lipid Management for the Prevention of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2019, 381, 1557–1567. [Google Scholar] [CrossRef]
- Trinder, M.; Francis, G.A.; Brunham, L.R. Association of Monogenic vs. Polygenic Hypercholesterolemia with Risk of Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2020, 5, 390–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gencer, B.; Marston, N.A.; Im, K.; Cannon, C.P.; Sever, P.; Keech, A.; Braunwald, E.; Giugliano, R.P.; Sabatine, M.S. Efficacy and safety of lowering LDL cholesterol in older patients: A systematic review and meta-analysis of randomised controlled trials. Lancet 2020, 396, 1637–1643. [Google Scholar] [CrossRef]
- Lahera, V.; Goicoechea, M.; de Vinuesa, S.G.; Miana, M.; de las Heras, N.; Cachofeiro, V.; Luño, J. Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: Beneficial effects of statins. Curr. Med. Chem. 2007, 14, 243–248. [Google Scholar] [CrossRef]
- Drexel, H.; Coats, A.J.S.; Spoletini, I.; Bilato, C.; Mollace, V.; Perrone Filardi, P.; Rosano, G.M.C. An expert opinion paper on statin adherence and implementation of new lipid-lowering medications by the ESC Working Group on Cardiovascular Pharmacotherapy: Barriers to be overcome. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 115–121. [Google Scholar] [CrossRef]
- Sirtori, C.R. The pharmacology of statins. Pharmacol. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef]
- Castilla-Guerra, L.; Del Carmen Fernandez-Moreno, M.; Colmenero-Camacho, M.A. Statins in Stroke Prevention: Present and Future. Curr. Pharm. Des. 2016, 22, 4638–4644. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Stępień, K.M.; Tomaszewska, J.; Czuczwar, S.J. Statin-induced myopathies. Pharmacol. Rep. 2011, 63, 859–866. [Google Scholar] [CrossRef]
- Sakamoto, K.; Kimura, J. Mechanism of statin-induced rhabdomyolysis. J. Pharmacol. Sci. 2013, 123, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissen, S.E.; Stroes, E.; Dent-Acosta, R.E.; Rosenson, R.S.; Lehman, S.J.; Sattar, N.; Preiss, D.; Bruckert, E.; Ceška, R.; Lepor, N.; et al. Efficacy and Tolerability of Evolocumab vs Ezetimibe in Patients with Muscle-Related Statin Intolerance: The GAUSS-3 Randomized Clinical Trial. JAMA 2016, 315, 1580–1590. [Google Scholar] [CrossRef]
- Pohjola-Sintonen, S.; Julkunen, H. Muscle-related adverse effects of statins. Duodecim 2014, 130, 1622–1627. [Google Scholar]
- Ward, N.C.; Watt, G.F.; Eckel, R.H. Statin Toxicity Mechanistic Insights and Clinical Implications. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. Hypoglycemic and Hypolipemic Effects of a New Lecithin Formulation of Bergamot Polyphenolic Fraction: A Double Blind, Randomized, Placebo-Controlled Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef]
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolino, V.; Russo, V.; Janda, E.; Ragusa, S.; et al. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int. J. Cardiol. 2013, 170, 140–145. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Banach, M. Red Yeast Rice for Hypercholesterolemia. Methodist Debakey Cardiovasc. J. 2019, 15, 192–199. [Google Scholar] [CrossRef]
- Wang, T.J.; Lien, A.S.; Chen, J.L.; Lin, C.H.; Yang, Y.S.; Yang, S.H. A Randomized Clinical Efficacy Trial of Red Yeast Rice (Monascus pilosus) Against Hyperlipidemia. Am. J. Chin. Med. 2019, 47, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Karl, M.; Santini, A. Red Yeast Rice. Foods 2017, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Burke, F.M. Red yeast rice for the treatment of dyslipidemia. Curr. Atheroscler. Rep. 2015, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. 2018, 16, e05368. [Google Scholar] [CrossRef] [Green Version]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef] [PubMed]
- European Communities (EC). Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Off J Eur Communities 1986, L358, 1–28. [Google Scholar]
- Musolino, V.; Gliozzi, M.; Carresi, C.; Maiuolo, J.; Mollace, R.; Bosco, F.; Scarano, F.; Scicchitano, M.; Maretta, A.; Palma, E.; et al. Lipid-lowering effect of bergamot polyphenolic fraction: Role of pancreatic cholesterol ester hydrolase. J. Biol. Regul. Homeost. Agents 2017, 31, 1087–1093. [Google Scholar] [PubMed]
- Musolino, V.; Gliozzi, M.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; Scicchitano, M.; et al. The effect of bergamot polyphenolic fraction on lipid transfer protein system and vascular oxidative stress in a rat model of hyperlipemia. Lipids Health Dis. 2019, 18, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J.; et al. Bergamot Polyphenols Improve Dyslipidemia and Pathophysiological Features in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 2565. [Google Scholar] [CrossRef]
- Di Donna, L.; De Luca, G.; Mazzotti, F.; Napoli, A.; Salerno, R.; Taverna, D.; Sindona, G. Statin-like principles of bergamot fruit (Citrus bergamia): Isolation of 3-hydroxymethylglutaryl flavonoid glycosides. J. Nat. Prod. 2009, 72, 1352–1354. [Google Scholar] [CrossRef]
- Melendez, Q.M.; Krishnaji, S.T.; Wooten, C.J.; Lopez, D. Hypercholesterolemia: The role of PCSK9. Arch. Biochem. Biophys. 2017, 625–626, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Musolino, V.; Bosco, F.; Scicchitano, M.; Scarano, F.; Nucera, S.; Zito, M.C.; Ruga, S.; Carresi, C.; Macrì, R.; et al. Cholesterol homeostasis: Researching a dialogue between the brain and peripheral tissues. Pharmacol. Res. 2021, 163, 105215. [Google Scholar] [CrossRef]
- Cammisotto, V.; Pastori, D.; Nocella, C.; Bartimoccia, S.; Castellani, V.; Marchese, C.; Scavalli, A.S.; Ettorre, E.; Viceconte, N.; Violi, F.; et al. PCSK9 Regulates Nox2-Mediated Platelet Activation via CD36 Receptor in Patients with Atrial Fibrillation. Antioxidants 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidah, N.G.; Awan, Z.; Chrétien, M.; Mbikay, M. PCSK9: A key modulator of cardiovascular health. Circ. Res. 2014, 114, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Pedersen, T.R.; Park, J.G.; De Ferrari, G.M.; Gaciong, Z.A.; Ceska, R.; Toth, K.; Gouni-Berthold, I.; Lopez-Miranda, J.; Schiele, F.; et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: A prespecified secondary analysis of the FOURIER trial. Lancet 2017, 390, 1962–1971. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Hegele, R.A.; Fazio, S.; Cannon, C.P. The Evolving Future of PCSK9 Inhibitors. J. Am. Coll. Cardiol. 2018, 72, 314–329. [Google Scholar] [CrossRef] [PubMed]
- German, C.A.; Shapiro, M.D. Small Interfering RNA Therapeutic Inclisiran: A New Approach to Targeting PCSK9. BioDrugs 2020, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis 2015, 243, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Carrier, B.; Wen, S.; Zigouras, S.; Browne, R.W.; Li, Z.; Patel, M.S.; Williamson, D.L.; Rideout, T.C. Alpha-Lipoic Acid Reduces LDL-Particle Number and PCSK9 Concentrations in High-Fat Fed Obese Zucker Rats. PLoS ONE 2014, 9, e90863. [Google Scholar] [CrossRef] [Green Version]
- Santulli, G.; Jankauskas, S.S.; Gambardella, J. Inclisiran: A new milestone on the PCSK9 road to tackle cardiovascular risk. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 11–12. [Google Scholar] [CrossRef]
- Lin, Y.K.; Yeh, C.T.; Kuo, K.T.; Yadav, V.K.; Fong, I.K.; Kounis, N.J.; Hu, P.; Hung, M.Y. Pterostilbene Increases LDL Metabolism in HL-1 Cardiomyocytes by Modulating the PCSK9/HNF1α/SREBP2/LDLR Signaling Cascade, Upregulating Epigenetic hsa-miR-335 and hsa-miR-6825, and LDL Receptor Expression. Antioxidants 2021, 10, 1280. [Google Scholar] [CrossRef]
- Wang, D.; Yang, X.; Chen, Y.; Gong, K.; Yu, M.; Gao, Y.; Wu, X.; Hu, H.; Liao, C.; Han, J.; et al. Ascorbic acid enhances low-density lipoprotein receptor expression by suppressing proprotein convertase subtilisin/kexin 9 expression. J. Biol. Chem. 2020, 295, 15870–15882. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.B.; Wang, X.; Santulli, G. Functional role of gut microbiota and PCSK9 in the pathogenesis of diabetes mellitus and cardiovascular disease. Atherosclerosis 2019, 289, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Mulinacci, N.; Cecchi, L.; Bellumori, M.; Bollati, C.; Bartolomei, M.; Franchini, C.; Clodoveo, M.L.; Corbo, F.; Arnoldi, A. Virgin Olive Oil Extracts Reduce Oxidative Stress and Modulate Cholesterol Metabolism: Comparison between Oils Obtained with Traditional and Innovative Processes. Antioxidants 2020, 9, 798. [Google Scholar] [CrossRef]
- Taylor, B.A.; Thompson, P.D. Statins and Their Effect on PCSK9-Impact and Clinical Relevance. Curr. Atheroscler. Rep. 2016, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, L.; Fasano, T.; Bellocchio, A.; Bocchi, L.; Sallo, R.; Fresa, R.; Colangeli, I.; Cantafora, A.; Calandra, S.; Bertolini, S. Effect of ezetimibe coadministered with statins in genotype-confirmed heterozygous FH patients. Atherosclerosis 2007, 194, e116–e122. [Google Scholar] [CrossRef] [PubMed]
- Dounousi, E.; Tellis, C.; Pavlakou, P.; Duni, A.; Liakopoulos, V.; Mark, P.B.; Papagianni, A.; Tselepis, A.D. Association between PCSK9 Levels and Markers of Inflammation, Oxidative Stress, and Endothelial Dysfunction in a Population of Nondialysis Chronic Kidney Disease Patients. Oxid. Med. Cell. Longev. 2021, 2021, 6677012. [Google Scholar] [CrossRef] [PubMed]
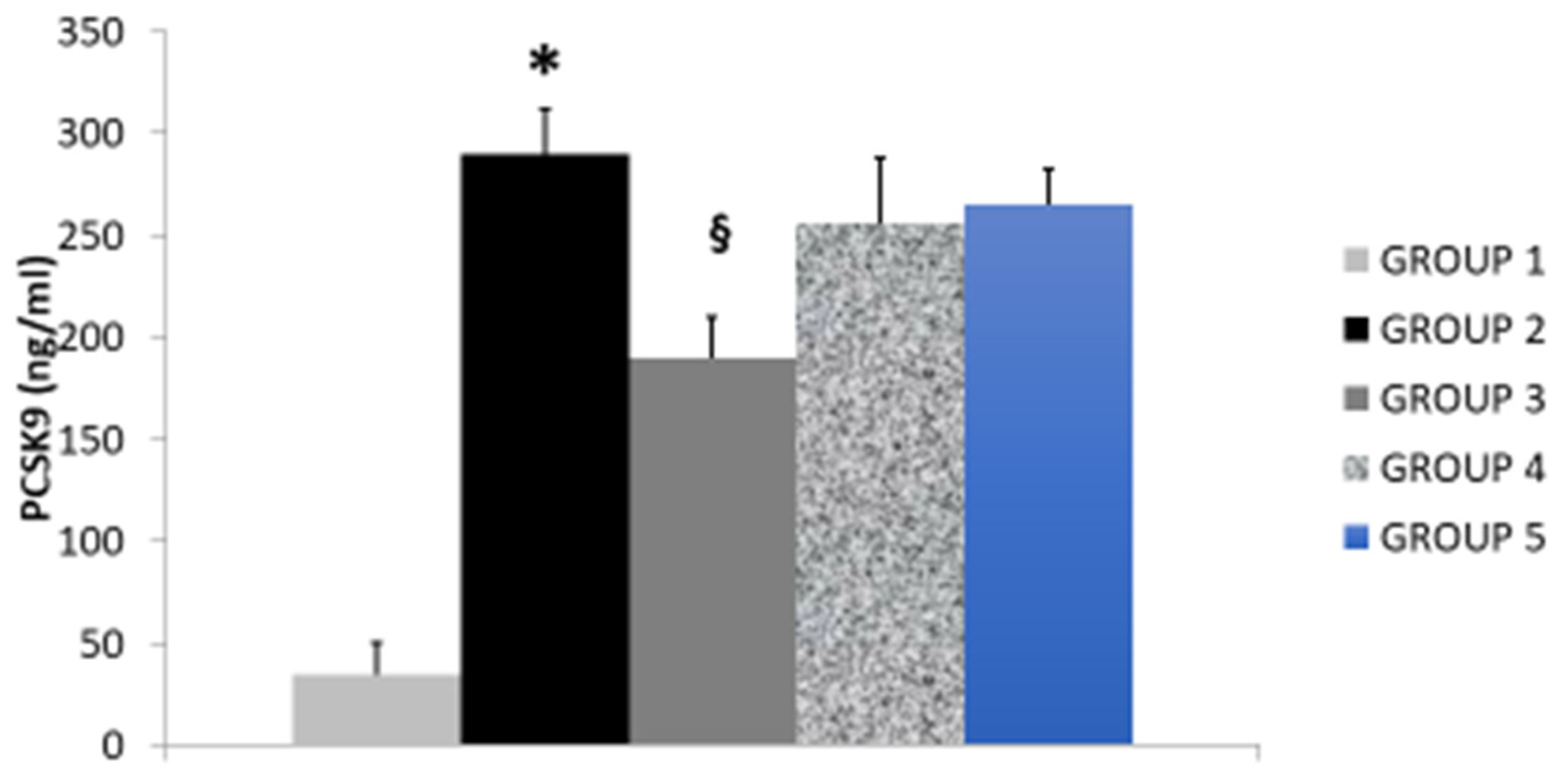
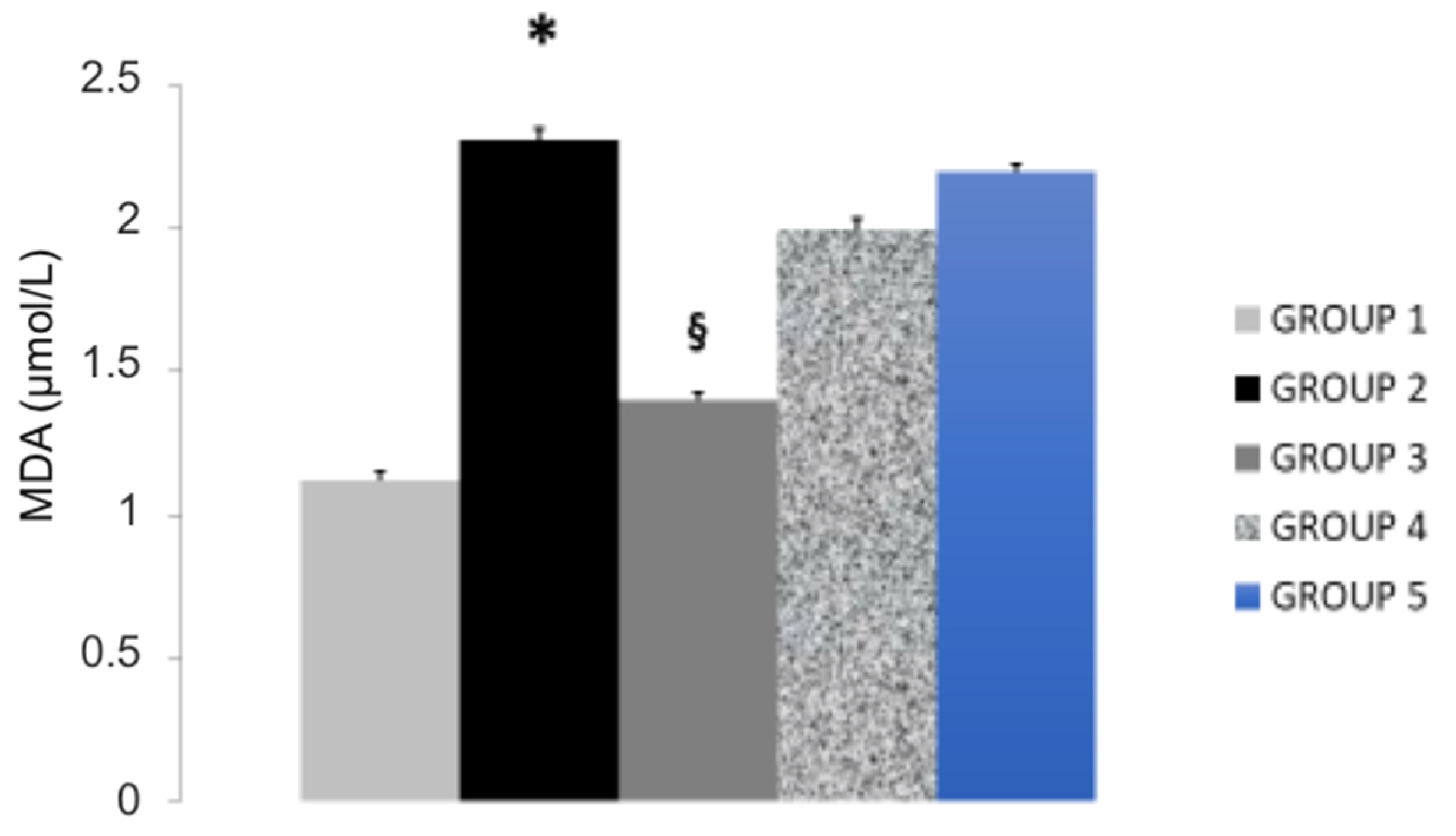
| Study Groups | Serum T-CHOL (mg/dL) | Serum LDL-C (mg/dL) | Serum HDL-C (mg/dL) | Serum Triglycerides (mg/dL) | Serum Paraoxonase (nmol/mL/min) | Glutathione Peroxidase (U/mL) |
|---|---|---|---|---|---|---|
| Group 1 | ||||||
| Standard diet (n = 10) | 110 ± 12 | 34 ± 6 | 41 ± 6 | 145 ± 16 | 85 ± 6 | 186 ± 5 |
| Group 2 | ||||||
| Hyperlipidemic diet (n = 10) | 196 ± 14 * | 117 ± 10 * | 32 ± 8 * | 235 ± 18 * | 132 ± 8 * | 175 ± 4 * |
| Group 3 | ||||||
| Hyperlipidemic diet + BPF 10 mg/Kg (n = 10) | 154 ± 12 § | 73 ± 8 § | 46 ± 7 § | 175 ± 15 § | 102 ± 10 § | 214 ± 4 § |
| Group 4 | ||||||
| Hyperlipidemic diet + RYR (3 mg/Kg) (n = 10) | 164 ± 14 § | 83 ± 7 § | 38 ± 5 | 215 ± 19 | 106 ± 9 § | 192 ± 5 |
| Group 5 | ||||||
| Hyperlipidemic diet + RYR (1 mg/Kg) (n = 10) | 176 ± 14 § | 94 ± 11 § | 36 ± 6 | 222 ± 16 | 116 ± 5 § | 194 ± 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mollace, R.; Macrì, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Fini, M.; Volterrani, M.; Mollace, V. Comparative Effect of Bergamot Polyphenolic Fraction and Red Yeast Rice Extract in Rats Fed a Hyperlipidemic Diet: Role of Antioxidant Properties and PCSK9 Expression. Nutrients 2022, 14, 477. https://doi.org/10.3390/nu14030477
Mollace R, Macrì R, Tavernese A, Gliozzi M, Musolino V, Carresi C, Maiuolo J, Fini M, Volterrani M, Mollace V. Comparative Effect of Bergamot Polyphenolic Fraction and Red Yeast Rice Extract in Rats Fed a Hyperlipidemic Diet: Role of Antioxidant Properties and PCSK9 Expression. Nutrients. 2022; 14(3):477. https://doi.org/10.3390/nu14030477
Chicago/Turabian StyleMollace, Rocco, Roberta Macrì, Annamaria Tavernese, Micaela Gliozzi, Vincenzo Musolino, Cristina Carresi, Jessica Maiuolo, Massimo Fini, Maurizio Volterrani, and Vincenzo Mollace. 2022. "Comparative Effect of Bergamot Polyphenolic Fraction and Red Yeast Rice Extract in Rats Fed a Hyperlipidemic Diet: Role of Antioxidant Properties and PCSK9 Expression" Nutrients 14, no. 3: 477. https://doi.org/10.3390/nu14030477
APA StyleMollace, R., Macrì, R., Tavernese, A., Gliozzi, M., Musolino, V., Carresi, C., Maiuolo, J., Fini, M., Volterrani, M., & Mollace, V. (2022). Comparative Effect of Bergamot Polyphenolic Fraction and Red Yeast Rice Extract in Rats Fed a Hyperlipidemic Diet: Role of Antioxidant Properties and PCSK9 Expression. Nutrients, 14(3), 477. https://doi.org/10.3390/nu14030477








