An Update Regarding the Bioactive Compound of Cereal By-Products: Health Benefits and Potential Applications
Abstract
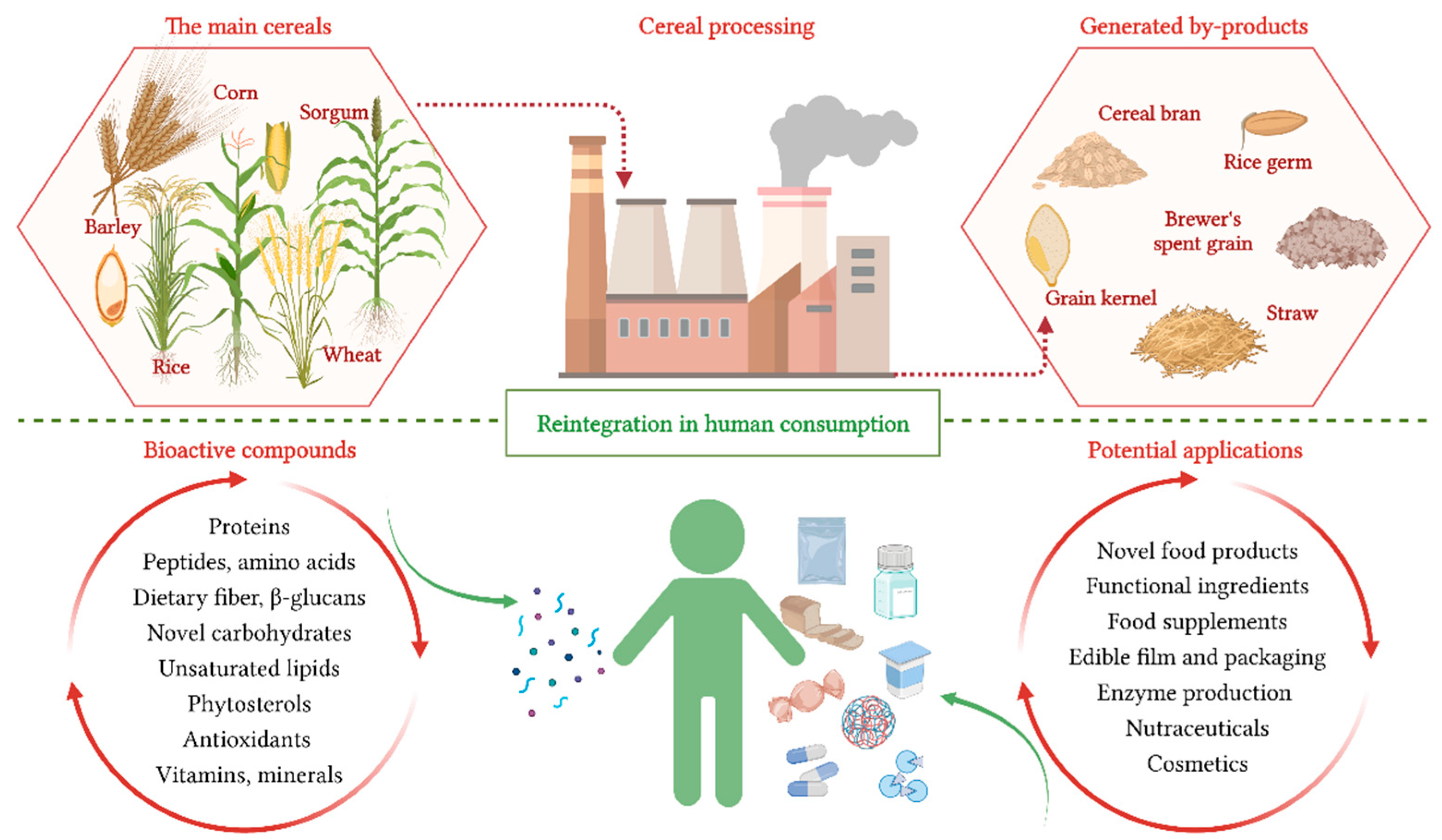
:1. Introduction
2. Bioactive Compounds from Cereal Wastes and By-Products
2.1. Carbohydrates
2.2. Proteins and Amino Acids from Cereal Waste and By-Products
2.3. Vitamins and Mineral Microelements from Cereal Wastes
2.4. Lipids from Cereal Wastes
| Cereal By-Product/Waste | Fatty Acid | Concentration (% of Total Lipids) | Extraction Methods | Functional Properties | Reference |
|---|---|---|---|---|---|
| Rice bran | Triacylglycerol | 60.12 | Solvent extraction (n-hexane) | Balanced fatty acid profile; Delicate flavor; High smoke point; High bioactive ingredient. | [92,98] |
| Polyunsaturated fatty acids | 40.73 | ||||
| Linoleic acid | 38.84 | ||||
| Oleic acid | 34.31 | ||||
| Palmitic acid | 19.87 | ||||
| Free fatty acids | 29.69 | ||||
| Diacylglycerol | 9.98 | ||||
| Monoacylglycerol | 0.21 | ||||
| γ-oryzanol | 18.53 | ||||
| Phytosterol | 22.40 | ||||
| Wheat germ | Linoleic acid | 57 | Solvent extraction (hexane) | Food ingredients with potential health benefits. | [93] |
| Palmitic acid | 17.5 | ||||
| Oleic acid | 15 | ||||
| Linolenic acid | 6 | ||||
| Total polyunsaturated fatty acids | 64.5–63.7 | ||||
| Brewer’s spent grain | Free fatty acids | 18 | Soxhlet acetone extraction; Hot water extraction; Sulfuric acid hydrolysis; Alkali extraction | Nutraceutical, pharmaceutical, and cosmetic properties. | [96] |
| Triglycerides | 67 | ||||
| Monoglycerides | 1.7 | ||||
| Diglycerides | 7.7 | ||||
| Steroid compounds | 5 | ||||
| Oat bran | Oleic acid | 44.09–46.68 | Subcritical butane extraction | Preventive effects on cardiovascular disease and development of atherosclerosis; Reducing body fat. | [99] |
| Linoleic acid | 32.54–32.88 | ||||
| Stearic acid | 1.71–1.89 | ||||
| Palmitic acid | 15.68–16.03 | ||||
| Corn germ | Palmitic acid | 11.57 | Pressing extraction | Commercial shortening replacement in food industries. | [100] |
| Stearic acid | 2.89 | ||||
| Oleic acid | 29.45 | ||||
| Linoleic acid | 54.31 | ||||
| Rye bran | Linoleic acid | 61.09 | Supercritical carbon dioxide extraction using response surface methodology | Food grade ingredient. | [101] |
| Palmitic acid | 13.74 | ||||
| Oleic acid | 13.65 | ||||
| Linolenic acid | 6.37 | ||||
| Corn waste | Palmitic acid | 23.0 | Solvent extraction analyzed by gas chromatography (Folch method) | Feed or pharmaceutical industry. | [102] |
| Stearic acid | 3.4 | ||||
| Oleic acid | 11.7 | ||||
| Linoleic acid | 52.9 | ||||
| α-Linolenic acid | 5.3 |
3. Compounds with Antioxidant Properties from Cereal By-Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siracusa, L.; Ruberto, G. Not only what is food is good—Polyphenols from edible and nonedible vegetable waste. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–21. [Google Scholar]
- Ng, H.S.; Kee, P.E.; Yim, H.S.; Chen, P.-T.; Wei, Y.-H.; Lan, J.C.-W. Recent advances on the sustainable approaches for conversion and reutilization of food wastes to valuable bioproducts. Bioresour. Technol. 2020, 302, 122889. [Google Scholar] [CrossRef]
- Patel, S.; Shukla, S. Fermentation of food wastes for generation of nutraceuticals and supplements. In Fermented Foods in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 707–734. [Google Scholar]
- Pop, C.; Suharoschi, R.; Pop, O.L. Dietary fiber and prebiotic compounds in fruits and vegetables food waste. Sustainability 2021, 13, 7219. [Google Scholar] [CrossRef]
- Schwan, R.F.; Ramos, C.L. Functional beverages from cereals. In Functional and Medicinal Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 351–379. [Google Scholar]
- Román, G.; Jackson, R.; Gadhia, R.; Román, A.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cei, L.; Sacchi, G.; Benedettelli, S.; Stefani, G.; Gagliardi, E.; Tosi, P.; Bocci, R. Health and nutrition studies related to cereal biodiversity: A participatory multi-actor literature review approach. Nutrients 2018, 10, 1207. [Google Scholar] [CrossRef] [Green Version]
- Akanbi, T.O.; Dare, K.O.; Aryee, A.N. High-Value Products from Cereal, Nuts, Fruits, and Vegetables Wastes. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma, and Fuels; Wiley: Hoboken, NJ, USA, 2019; pp. 347–368. [Google Scholar]
- Fărcaș, A.; Drețcanu, G.; Pop, T.D.; Enaru, B.; Socaci, S.; Diaconeasa, Z. Cereal Processing By-Products as Rich Sources of Phenolic Compounds and Their Potential Bioactivities. Nutrients 2021, 13, 3934. [Google Scholar] [CrossRef]
- Poutanen, K.S.; Kårlund, A.O.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N.; et al. Grains—A major source of sustainable protein for health. Nutr. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef]
- Ilhan-Ayisigi, E.; Budak, G.; Celiktas, M.S.; Sevimli-Gur, C.; Yesil-Celiktas, O. Anticancer activities of bioactive peptides derived from rice husk both in free and encapsulated form in chitosan. J. Ind. Eng. Chem. 2021, 103, 381–391. [Google Scholar] [CrossRef]
- Kyriakidou, Y.; Wood, C.; Ferrier, C.; Dolci, A.; Elliott, B. The effect of Omega-3 polyunsaturated fatty acid supplementation on exercise-induced muscle damage. J. Int. Soc. Sports Nutr. 2021, 18, 9. [Google Scholar] [CrossRef]
- Oliveira Godoy Ilha, A.; Sutti Nunes, V.; Silva Afonso, M.; Regina Nakandakare, E.; da Silva Ferreira, G.; de Paula Assis Bombo, R.; Rodrigues Giorgi, R.; Marcondes Machado, R.; Carlos Rocha Quintão, E.; Lottenberg, A.M. Phytosterols Supplementation Reduces Endothelin-1 Plasma Concentration in Moderately Hypercholesterolemic Individuals Independently of Their Cholesterol-Lowering Properties. Nutrients 2020, 12, 1507. [Google Scholar] [CrossRef]
- Arzami, A.N.; Ho, T.M.; Mikkonen, K.S. Valorization of cereal by-product hemicelluloses: Fractionation and purity considerations. Food Res. Int. 2022, 151, 110818. [Google Scholar] [CrossRef]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation Biotechnology Applied to Cereal Industry By-Products: Nutritional and Functional Insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Hu, X.; Lu, S.; Liao, X.; Song, Y.; Hu, X. Nanocellulose: A promising green treasure from food wastes to available food materials. Crit. Rev. Food Sci. Nutr. 2020, 62, 1–14. [Google Scholar] [CrossRef]
- Belc, N.; Mustatea, G.; Apostol, L.; Iorga, S.; Vlăduţ, V.-N.; Mosoiu, C. Cereal supply chain waste in the context of circular economy. In Proceedings of the E3S Web of Conferences, Villeurbanne, France, 20 August 2019; p. 03031. [Google Scholar]
- Zduńczyk, Z.; Flis, M.; Zieliński, H.; Wróblewska, M.; Antoszkiewicz, Z.; Juśkiewicz, J. In vitro antioxidant activities of barley, husked oat, naked oat, triticale, and buckwheat wastes and their influence on the growth and biomarkers of antioxidant status in rats. J. Agric. Food Chem. 2006, 54, 4168–4175. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Kumar, Y.; Malav, O.P.; Sazili, A.Q.; Domínguez, R.; Lorenzo, J.M. Microencapsulation as a Noble Technique for the Application of Bioactive Compounds in the Food Industry: A Comprehensive Review. Appl. Sci. 2022, 12, 1424. [Google Scholar] [CrossRef]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food Front. 2021, 2, 426–442. [Google Scholar] [CrossRef]
- Skendi, A.; Zinoviadou, K.G.; Papageorgiou, M.; Rocha, J.M. Advances on the Valorisation and Functionalization of By-Products and Wastes from Cereal-Based Processing Industry. Foods 2020, 9, 1243. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chapter 5—Wheat-based raw materials. In Wheat—An Exceptional Crop; Wieser, H., Koehler, P., Scherf, K.A., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 103–131. [Google Scholar]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Pathirannehelage, N.P.V.; Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Parchami, M.; Ferreira, J.A.; Taherzadeh, M.J. Starch and protein recovery from brewer’s spent grain using hydrothermal pretreatment and their conversion to edible filamentous fungi—A brewery biorefinery concept. Bioresour. Technol. 2021, 337, 125409. [Google Scholar] [CrossRef]
- Johansson, E.V.; Nilsson, A.C.; Östman, E.M.; Björck, I.M.E. Effects of indigestible carbohydrates in barley on glucose metabolism, appetite and voluntary food intake over 16 h in healthy adults. Nutr. J. 2013, 12, 46. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Dexter, J.E. Barley β-glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products—A Review. Food Res. Int. 2008, 41, 850–868. [Google Scholar] [CrossRef]
- Bastos, R.; Coelho, E.; Coimbra, M.A. 8-Arabinoxylans from cereal by-products: Insights into structural features, recovery, and applications. In Sustainable Recovery and Reutilization of Cereal Processing By-Products; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 227–251. [Google Scholar]
- Pérez-Flores, J.G.; Contreras-López, E.; Castañeda-Ovando, A.; Pérez-Moreno, F.; Aguilar-Arteaga, K.; Álvarez-Romero, G.A.; Téllez-Jurado, A. Physicochemical characterization of an arabinoxylan-rich fraction from brewers’ spent grain and its application as a release matrix for caffeine. Food Res. Int. 2019, 116, 1020–1030. [Google Scholar] [CrossRef]
- Hughes, J.; Grafenauer, S. Oat and Barley in the Food Supply and Use of Beta Glucan Health Claims. Nutrients 2021, 13, 2556. [Google Scholar] [CrossRef]
- Onipe, O.O.; Jideani, A.I.O.; Beswa, D. Composition and functionality of wheat bran and its application in some cereal food products. Int. J. Food Sci. Technol. 2015, 50, 2509–2518. [Google Scholar] [CrossRef]
- Malunga, L.N.; Izydorczyk, M.; Beta, T. Effect of water-extractable arabinoxylans from wheat aleurone and bran on lipid peroxidation and factors influencing their antioxidant capacity. Bioact. Carbohydr. Diet. Fibre 2017, 10, 20–26. [Google Scholar] [CrossRef]
- Boll, E.V.; Ekström, L.M.; Courtin, C.M.; Delcour, J.A.; Nilsson, A.C.; Björck, I.M.; Östman, E.M. Effects of wheat bran extract rich in arabinoxylan oligosaccharides and resistant starch on overnight glucose tolerance and markers of gut fermentation in healthy young adults. Eur. J. Nutr. 2016, 55, 1661–1670. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M. Beta glucan: A valuable functional ingredient in foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 201–212. [Google Scholar] [CrossRef]
- Brennan, C.S.; Cleary, L.J. The potential use of cereal (1→3,1→4)-β-d-glucans as functional food ingredients. J. Cereal Sci. 2005, 42, 1–13. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Deehan, E.C.; Zhang, Z.; Jin, M.; Baskota, N.; Perez-Muñoz, M.E.; Cole, J.; Tuncil, Y.E.; Seethaler, B.; Wang, T.; et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome 2020, 8, 118. [Google Scholar] [CrossRef]
- Lam, K.-L.; Chi-Keung Cheung, P. Non-digestible long chain beta-glucans as novel prebiotics. Bioact. Carbohydr. Diet. Fibre 2013, 2, 45–64. [Google Scholar] [CrossRef]
- Treimo, J.; Westereng, B.; Horn, S.J.; Forssell, P.; Robertson, J.A.; Faulds, C.B.; Waldron, K.W.; Buchert, J.; Eijsink, V.G.H. Enzymatic Solubilization of Brewers’ Spent Grain by Combined Action of Carbohydrases and Peptidases. J. Agric. Food Chem. 2009, 57, 3316–3324. [Google Scholar] [CrossRef]
- Chetrariu, A.; Dabija, A. Brewer’s Spent Grains: Possibilities of Valorization, a Review. Appl. Sci. 2020, 10, 5619. [Google Scholar] [CrossRef]
- Bai, F.-W.; Yang, S.; Ho, N.W.Y. 3.05-Fuel Ethanol Production From Lignocellulosic Biomass. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2019; pp. 49–65. [Google Scholar]
- Henrion, M.; Francey, C.; Lê, K.A.; Lamothe, L. Cereal B-Glucans: The Impact of Processing and How It Affects Physiological Responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef] [Green Version]
- Steiner, J.; Procopio, S.; Becker, T. Brewer’s spent grain: Source of value-added polysaccharides for the food industry in reference to the health claims. Eur. Food Res. Technol. 2015, 241, 303–315. [Google Scholar] [CrossRef]
- Koh, E.M.; Lee, E.K.; Song, J.; Kim, S.J.; Song, C.H.; Seo, Y.; Chae, C.H.; Jung, K.J. Anticancer activity and mechanism of action of fermented wheat germ extract against ovarian cancer. J. Food Biochem. 2018, 42, e12688. [Google Scholar] [CrossRef]
- Stevenson, L.; Phillips, F.; O’Sullivan, K.; Walton, J. Wheat bran: Its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef] [Green Version]
- Dapčević-Hadnađev, T.; Hadnađev, M.; Pojić, M. 2-The healthy components of cereal by-products and their functional properties. In Sustainable Recovery and Reutilization of Cereal Processing by-Products; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 27–61. [Google Scholar]
- Kamal-Eldin, A.; Lærke, H.N.; Knudsen, K.E.; Lampi, A.M.; Piironen, V.; Adlercreutz, H.; Katina, K.; Poutanen, K.; Man, P. Physical, microscopic and chemical characterisation of industrial rye and wheat brans from the Nordic countries. Food Nutr. Res. 2009, 53, 19412350. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, K.E.B.; Lærke, H.N. REVIEW: Rye Arabinoxylans: Molecular Structure, Physicochemical Properties and Physiological Effects in the Gastrointestinal Tract. Cereal Chem. 2010, 87, 353–362. [Google Scholar] [CrossRef]
- Prykhodko, O.; Sandberg, J.; Burleigh, S.; Björck, I.; Nilsson, A.; Fåk Hållenius, F. Impact of Rye Kernel-Based Evening Meal on Microbiota Composition of Young Healthy Lean Volunteers With an Emphasis on Their Hormonal and Appetite Regulations, and Blood Levels of Brain-Derived Neurotrophic Factor. Front. Nutr. 2018, 5, 45. [Google Scholar] [CrossRef]
- Johansson, L.; Tuomainen, P.; Anttila, H.; Rita, H.; Virkki, L. Effect of processing on the extractability of oat β-glucan. Food Chem. 2007, 105, 1439–1445. [Google Scholar] [CrossRef]
- Xu, D.; Feng, M.; Chu, Y.; Wang, S.; Shete, V.; Tuohy, K.M.; Liu, F.; Zhou, X.; Kamil, A.; Pan, D.; et al. The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared With Rice: A Randomized, Controlled Trial. Front. Immunol. 2021, 12, 787797. [Google Scholar] [CrossRef]
- Ciudad-Mulero, M.; Fernández-Ruiz, V.; Matallana-González, M.C.; Morales, P. Chapter Two—Dietary fiber sources and human benefits: The case study of cereal and pseudocereals. In Advances in Food and Nutrition Research; Ferreira, I.C.F.R., Barros, L., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 90, pp. 83–134. [Google Scholar]
- Ciecierska, A.; Drywień, M.E.; Hamulka, J.; Sadkowski, T. Nutraceutical functions of beta-glucans in human nutrition. Rocz. Panstw. Zakl. Hig. 2019, 70, 315–324. [Google Scholar] [CrossRef]
- Juhnevica-Radenkova, K.; Kviesis, J.; Moreno, D.A.; Seglina, D.; Vallejo, F.; Valdovska, A.; Radenkovs, V. Highly-Efficient Release of Ferulic Acid from Agro-Industrial By-Products via Enzymatic Hydrolysis with Cellulose-Degrading Enzymes: Part I–The Superiority of Hydrolytic Enzymes Versus Conventional Hydrolysis. Foods 2021, 10, 782. [Google Scholar] [CrossRef]
- Sandak, A.; Sandak, J.; Modzelewska, I. Manufacturing fit-for-purpose paper packaging containers with controlled biodegradation rate by optimizing addition of natural fillers. Cellulose 2019, 26, 2673–2688. [Google Scholar] [CrossRef] [Green Version]
- Gil-Chávez, J.; Gurikov, P.; Hu, X.; Meyer, R.; Reynolds, W.; Smirnova, I. Application of novel and technical lignins in food and pharmaceutical industries: Structure-function relationship and current challenges. Biomass Convers. Biorefinery 2021, 11, 2387–2403. [Google Scholar] [CrossRef]
- Schweiggert-Weisz, U.; Eisner, P.; Bader-Mittermaier, S.; Osen, R. Food proteins from plants and fungi. Curr. Opin. Food Sci. 2020, 32, 156–162. [Google Scholar] [CrossRef]
- Roth, M.; Jekle, M.; Becker, T. Opportunities for upcycling cereal byproducts with special focus on Distiller’s grains. Trends Food Sci. Technol. 2019, 91, 282–293. [Google Scholar] [CrossRef]
- Uraipong, C.; Zhao, J. Rice bran protein hydrolysates exhibit strong in vitro α-amylase, β-glucosidase and ACE-inhibition activities. J. Sci. Food Agric. 2016, 96, 1101–1110. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Castro-Jácome, T.P.; Alcántara-Quintana, L.E.; Montalvo-González, E.; Chacón-López, A.; Kalixto-Sánchez, M.A.; del Pilar Rivera, M.; López-García, U.M.; Tovar-Pérez, E.G. Skin-protective properties of peptide extracts produced from white sorghum grain kafirins. Ind. Crops Prod. 2021, 167, 113551. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Kaur, P.; Purewal, S.S.; Sandhu, K.S.; Kaur, M.; Salar, R.K. Millets: A cereal grain with potent antioxidants and health benefits. J. Food Meas. Charact. 2019, 13, 793–806. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Carvajal-Millan, E.; López-Ahumada, G.A.; Castro-Enriquez, D.D.; Barreras-Urbina, C.G.; Rodríguez-Felix, F. Prolamins from cereal by-products: Classification, extraction, characterization and its applications in micro- and nanofabrication. Trends Food Sci. Technol. 2019, 90, 111–132. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.; Ma, H.; Yagoub, A.E.; Yu, X.; Owusu, J.; Ma, H.; Qin, X. Antioxidant peptides from corn gluten meal: Orthogonal design evaluation. Food Chem. 2015, 187, 270–278. [Google Scholar] [CrossRef]
- Connolly, A.; Piggott, C.O.; FitzGerald, R.J. Characterisation of protein-rich isolates and antioxidative phenolic extracts from pale and black brewers’ spent grain. Int. J. Food Sci. Technol. 2013, 48, 1670–1681. [Google Scholar] [CrossRef]
- Yu, D.; Sun, Y.; Wang, W.; O’Keefe, S.F.; Neilson, A.P.; Feng, H.; Wang, Z.; Huang, H. Recovery of protein hydrolysates from brewer’s spent grain using enzyme and ultrasonication. Int. J. Food Sci. Technol. 2020, 55, 357–368. [Google Scholar] [CrossRef]
- Qin, F.; Johansen, A.Z.; Mussatto, S.I. Evaluation of different pretreatment strategies for protein extraction from brewer’s spent grains. Ind. Crops Prod. 2018, 125, 443–453. [Google Scholar] [CrossRef]
- He, Y.; Kuhn, D.D.; Ogejo, J.A.; O’Keefe, S.F.; Fraguas, C.F.; Wiersema, B.D.; Jin, Q.; Yu, D.; Huang, H. Wet fractionation process to produce high protein and high fiber products from brewer’s spent grain. Food Bioprod. Process. 2019, 117, 266–274. [Google Scholar] [CrossRef]
- Li, W.; Yang, H.; Coldea, T.E.; Zhao, H. Modification of structural and functional characteristics of brewer’s spent grain protein by ultrasound assisted extraction. LWT 2021, 139, 110582. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Viganó, J.; Castro, L.E.N.; Maciel-Silva, F.W.; Rostagno, M.A.; Mussatto, S.I.; Forster-Carneiro, T. Recovery of sugars and amino acids from brewers’ spent grains using subcritical water hydrolysis in a single and two sequential semi-continuous flow-through reactors. Food Res. Int. 2022, 157, 111470. [Google Scholar] [CrossRef]
- Rodríguez-Restrepo, Y.A.; Ferreira-Santos, P.; Orrego, C.E.; Teixeira, J.A.; Rocha, C.M.R. Valorization of rice by-products: Protein-phenolic based fractions with bioactive potential. J. Cereal Sci. 2020, 95, 103039. [Google Scholar] [CrossRef]
- Bedin, S.; Zanella, K.; Bragagnolo, N.; Taranto, O.P. Implication of Microwaves on the Extraction Process of Rice Bran Protein. Braz. J. Chem. Eng. 2019, 36, 1653–1665. [Google Scholar] [CrossRef] [Green Version]
- Phongthai, S.; Lim, S.-T.; Rawdkuen, S. Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties. J. Cereal Sci. 2016, 70, 146–154. [Google Scholar] [CrossRef]
- Prandi, B.; Faccini, A.; Lambertini, F.; Bencivenni, M.; Jorba, M.; Van Droogenbroek, B.; Bruggeman, G.; Schöber, J.; Petrusan, J.; Elst, K.; et al. Food wastes from agrifood industry as possible sources of proteins: A detailed molecular view on the composition of the nitrogen fraction, amino acid profile and racemisation degree of 39 food waste streams. Food Chem. 2019, 286, 567–575. [Google Scholar] [CrossRef]
- Alzuwaid, N.T.; Sissons, M.; Laddomada, B.; Fellows, C.M. Nutritional and functional properties of durum wheat bran protein concentrate. Cereal Chem. 2020, 97, 304–315. [Google Scholar] [CrossRef]
- Zhu, K.-X.; Zhou, H.-M.; Qian, H.-F. Proteins Extracted from Defatted Wheat Germ: Nutritional and Structural Properties. Cereal Chem. 2006, 83, 69–75. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Savoire, R.; Subra-Paternault, P.; Harscoat-Schiavo, C. Oil and protein recovery from corn germ: Extraction yield, composition and protein functionality. Food Bioprod. Process. 2020, 120, 131–142. [Google Scholar] [CrossRef]
- Guan, X.; Yao, H. Optimization of Viscozyme L-assisted extraction of oat bran protein using response surface methodology. Food Chem. 2008, 106, 345–351. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Brinch-Pedersen, H.; Borg, S.; Tauris, B.; Holm, P.B. Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J. Cereal Sci. 2007, 46, 308–326. [Google Scholar] [CrossRef]
- Galanakis, C.M. Sustainable Applications for the Valorization of Cereal Processing By-Products. Foods 2022, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Lan, Y.; Ohm, J.-B.; Gillespie, J.; Schwarz, P.; Chen, B. Physicochemical composition, fermentable sugars, free amino acids, phenolics, and minerals in brewers’ spent grains obtained from craft brewing operations. J. Cereal Sci. 2022, 104, 103413. [Google Scholar] [CrossRef]
- Tuncel, N.B.; Yılmaz, N.; Kocabıyık, H.; Uygur, A. The effect of infrared stabilized rice bran substitution on B vitamins, minerals and phytic acid content of pan breads: Part II. J. Cereal Sci. 2014, 59, 162–166. [Google Scholar] [CrossRef]
- Lech, M.; Labus, K. The methods of brewers’ spent grain treatment towards the recovery of valuable ingredients contained therein and comprehensive management of its residues. Chem. Eng. Res. Des. 2022, 183, 494–511. [Google Scholar] [CrossRef]
- Luithui, Y.; Baghya Nisha, R.; Meera, M.S. Cereal by-products as an important functional ingredient: Effect of processing. J. Food Sci. Technol. 2019, 56, 1–11. [Google Scholar] [CrossRef]
- Feizollahi, E.; Mirmahdi, R.S.; Zoghi, A.; Zijlstra, R.T.; Roopesh, M.S.; Vasanthan, T. Review of the beneficial and anti-nutritional qualities of phytic acid, and procedures for removing it from food products. Food Res. Int. 2021, 143, 110284. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Soliven, A. Lipophilic bioactive compounds in the oils recovered from cereal by-products. J. Sci. Food Agric. 2016, 96, 3256–3265. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Skendi, A. 1-Introduction to cereal processing and by-products. In Sustainable Recovery and Reutilization of Cereal Processing by-Products; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 1–25. [Google Scholar]
- Saad, N.; Ismail, N.; Mastuki, S.N.; Leong, S.W.; Chia, S.L.; Abdullah, C.A.C. Chapter 16—Rice bran oil main bioactive compounds and biological activities. In Multiple Biological Activities of Unconventional Seed Oils; Mariod, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 195–213. [Google Scholar]
- Xu, D.; Hao, J.; Wang, Z.; Liang, D.; Wang, J.; Ma, Y.; Zhang, M. Physicochemical properties, fatty acid compositions, bioactive compounds, antioxidant activity and thermal behavior of rice bran oil obtained with aqueous enzymatic extraction. LWT 2021, 149, 111817. [Google Scholar] [CrossRef]
- Meriles, S.P.; Penci, M.C.; Curet, S.; Boillereaux, L.; Ribotta, P.D. Effect of microwave and hot air treatment on enzyme activity, oil fraction quality and antioxidant activity of wheat germ. Food Chem. 2022, 386, 132760. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, J.; Ruan, S.; Lv, R.; Zhou, J.; Tian, J.; Cheng, H.; Xu, E.; Liu, D. A comprehensive review of cereal germ and its lipids: Chemical composition, multi-objective process and functional application. Food Chem. 2021, 362, 130066. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Mikes, F.; Bühler, S.; Matsakas, L. Valorization of Brewers’ Spent Grain for the Production of Lipids by Oleaginous Yeast. Molecules 2018, 23, 3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Río, J.C.; Prinsen, P.; Gutiérrez, A. Chemical composition of lipids in brewer’s spent grain: A promising source of valuable phytochemicals. J. Cereal Sci. 2013, 58, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Borsini, A.; Nicolaou, A.; Camacho-Muñoz, D.; Kendall, A.C.; Di Benedetto, M.G.; Giacobbe, J.; Su, K.P.; Pariante, C.M. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: Relevance for major depression and for human hippocampal neurogenesis. Mol. Psychiatry 2021, 26, 6773–6788. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Faiza, M.; Shi, L.; Wang, W.; Liu, N.; Wang, Y. The enhancement of rice bran oil quality through a novel moderate biorefining process. LWT 2021, 151, 112118. [Google Scholar] [CrossRef]
- Liu, J.; Jin, S.; Song, H.; Huang, K.; Li, S.; Guan, X.; Wang, Y. Effect of extrusion pretreatment on extraction, quality and antioxidant capacity of oat (Avena Sativa L.) bran oil. J. Cereal Sci. 2020, 95, 102972. [Google Scholar] [CrossRef]
- Zhao, M.; Lan, Y.; Cui, L.; Monono, E.; Rao, J.; Chen, B. Formation, characterization, and potential food application of rice bran wax oleogels: Expeller-pressed corn germ oil versus refined corn oil. Food Chem. 2020, 309, 125704. [Google Scholar] [CrossRef]
- Povilaitis, D.; Venskutonis, P.R. Optimization of supercritical carbon dioxide extraction of rye bran using response surface methodology and evaluation of extract properties. J. Supercrit. Fluids 2015, 100, 194–200. [Google Scholar] [CrossRef]
- Klempová, T.; Slaný, O.; Šišmiš, M.; Marcinčák, S.; Čertík, M. Dual production of polyunsaturated fatty acids and beta-carotene with Mucor wosnessenskii by the process of solid-state fermentation using agro-industrial waste. J. Biotechnol. 2020, 311, 1–11. [Google Scholar] [CrossRef]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Antioxidants versus Food Antioxidant Additives and Food Preservatives. Antioxidants 2019, 8, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Santos, L. Delivery systems for cosmetics-From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Janciauskiene, S. The beneficial effects of antioxidants in health and diseases. Chronic Obstr. Pulm. Dis. J. COPD Found. 2020, 7, 182. [Google Scholar] [CrossRef]
- Yang, C.S.; Ho, C.-T.; Zhang, J.; Wan, X.; Zhang, K.; Lim, J. Antioxidants: Differing meanings in food science and health science. J. Agric. Food Chem. 2018, 66, 3063–3068. [Google Scholar] [CrossRef]
- Socaci, S.A.; Rugină, D.O.; Diaconeasa, Z.M.; Pop, O.L.; Fărcaș, A.C.; Păucean, A.; Tofană, M.; Pintea, A. Antioxidant compounds recovered from food wastes. In Functional Food-Improve Health through Adequate Food; IntechOpen: London, UK, 2017. [Google Scholar]
- Dursun, D.; Dalgıç, A.C. Optimization of astaxanthin pigment bioprocessing by four different yeast species using wheat wastes. Biocatal. Agric. Biotechnol. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Machado, E.; Matumoto Pintro, P.T.; Ítavo, L.C.V.; Agustinho, B.C.; Daniel, J.L.P.; Santos, N.W.; Bragatto, J.M.; Ribeiro, M.G.; Zeoula, L.M. Reduction in lignin content and increase in the antioxidant capacity of corn and sugarcane silages treated with an enzymatic complex produced by white rot fungus. PLoS ONE 2020, 15, e0229141. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, S.; Conte, A.; Lecce, L.; Padalino, L.; Del Nobile, M.A. Supercritical carbon dioxide extraction of brewer’s spent grain. J. Supercrit. Fluids 2016, 107, 69–74. [Google Scholar] [CrossRef]
- Petrón, M.; Andrés, A.; Esteban, G.; Timón, M. Study of antioxidant activity and phenolic compounds of extracts obtained from different craft beer by-products. J. Cereal Sci. 2021, 98, 103162. [Google Scholar] [CrossRef]
- Walters, M.E.; Willmore, W.G.; Tsopmo, A. Antioxidant, physicochemical, and cellular secretion of glucagon-like peptide-1 properties of oat bran protein hydrolysates. Antioxidants 2020, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Burlini, I.; Lorenzo, B.H.; Grandini, A.; Vertuani, S.; Tacchini, M.; Sacchetti, G. Antioxidant and antimicrobial extracts obtained from agricultural by-products: Strategies for a sustainable recovery and future perspectives. Food Bioprod. Process. 2020, 124, 397–407. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, J.; Zhang, B.H.; Zhang, H. Antioxidant activities of the fractionated protein hydrolysates from heat stable defatted rice bran. Int. J. Food Sci. Technol. 2014, 49, 1330–1336. [Google Scholar] [CrossRef]
- Cheetangdee, N.; Benjakul, S. Antioxidant activities of rice bran protein hydrolysates in bulk oil and oil-in-water emulsion. J. Sci. Food Agric. 2015, 95, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, Z.; Aalim, H.; Limwachiranon, J.; Li, L.; Duan, Z.; Ren, G.; Luo, Z. Green recovery of phenolic compounds from rice byproduct (rice bran) using glycerol based on viscosity, conductivity and density. Int. J. Food Sci. Technol. 2019, 54, 1363–1371. [Google Scholar] [CrossRef]
- Görgüç, A.; Özer, P.; Yılmaz, F.M. Microwave-assisted enzymatic extraction of plant protein with antioxidant compounds from the food waste sesame bran: Comparative optimization study and identification of metabolomics using LC/Q-TOF/MS. J. Food Process. Preserv. 2020, 44, e14304. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Ueno, T.; Nogata, Y.; Hayakawa, M.; Koga, H.; Torimura, T. Wheat-bran autolytic peptides containing a branched-chain amino acid attenuate non-alcoholic steatohepatitis via the suppression of oxidative stress and the upregulation of AMPK/ACC in high-fat diet-fed mice. Int. J. Mol. Med. 2017, 39, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Mikucka, W.; Zielinska, M.; Bulkowska, K.; Witonska, I. Recovery of polyphenols from distillery stillage by microwave-assisted, ultrasound-assisted and conventional solid–liquid extraction. Sci. Rep. 2022, 12, 1–13. [Google Scholar]
- Călinoiu, L.F.; Vodnar, D.C. Thermal processing for the release of phenolic compounds from wheat and oat bran. Biomolecules 2019, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Zaky, A.A.; Abd El-Aty, A.; Ma, A.; Jia, Y. An overview on antioxidant peptides from rice bran proteins: Extraction, identification, and applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 1350–1362. [Google Scholar] [CrossRef]
- Stefanello, F.S.; Dos Santos, C.O.; Bochi, V.C.; Fruet, A.P.B.; Soquetta, M.B.; Dörr, A.C.; Nörnberg, J.L. Analysis of polyphenols in brewer’s spent grain and its comparison with corn silage and cereal brans commonly used for animal nutrition. Food Chem. 2018, 239, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Kottaras, P.; Koulianos, M.; Makris, D.P. Low-Transition temperature mixtures (LTTMs) made of bioorganic molecules: Enhanced extraction of antioxidant phenolics from industrial cereal solid wastes. Recycling 2017, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Li, F.; Bruckbauer, A.; Cao, Q.; Cui, X.; Wu, R.; Shi, H.; Xue, B.; Zemel, M.B. Interaction between leucine and phosphodiesterase 5 inhibition in modulating insulin sensitivity and lipid metabolism. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 227. [Google Scholar]
- Elhassan, F.; Suad, A.; Dahawi, F. Antimicrobial activities of six types of wheat bran. IOSR J. Appl. Chem. 2017, 10, 61–69. [Google Scholar] [CrossRef]
- Moreirinha, C.; Vilela, C.; Silva, N.H.C.S.; Pinto, R.J.B.; Almeida, A.; Rocha, M.A.M.; Coelho, E.; Coimbra, M.A.; Silvestre, A.J.D.; Freire, C.S.R. Antioxidant and antimicrobial films based on brewers spent grain arabinoxylans, nanocellulose and feruloylated compounds for active packaging. Food Hydrocoll. 2020, 108, 105836. [Google Scholar] [CrossRef]
- Mitrea, L.; Nemeş, S.A.; Szabo, K.; Teleky, B.E.; Vodnar, D.C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association With Neurological and Psychiatric Disorders. Front. Med. Lausanne 2022, 9, 813204. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; Connolly, A.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Phenolic-enriched fractions from brewers’ spent grain possess cellular antioxidant and immunomodulatory effects in cell culture model systems. J. Sci. Food Agric. 2014, 94, 1373–1379. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; Connolly, A.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Brewers’ spent grain (BSG) protein hydrolysates decrease hydrogen peroxide (H2O2)-induced oxidative stress and concanavalin-A (con-A) stimulated IFN-γ production in cell culture. Food Funct. 2013, 4, 1709–1716. [Google Scholar] [CrossRef]
| Compounds | By-Product | Concentration | Industrial Applications | Health Benefits | References |
|---|---|---|---|---|---|
| Residual undigested starch | BSG | 1.3–10% | Production of fungal biomass and ethanol; Development of prebiotic ingredients for the meat industry | Positive effects on metabolism regulate the fermentative processes in the colon and increase the levels of glucagon-like peptide-1, known for its anti-diabetic and anti-obesogenic features | [22,26,27,28] |
| Beta-glucans | Oat bran | 5.5% dry matter | Using supercritical carbon dioxide to remove the oat bran lipids can increase by more than 40% the beta-glucan level; Incorporated high molecular weight oat beta-glucan into milk to obtain calorie-reduced and cholesterol-lowering dairy products; Increase of beverage satiety capacity; Ingredient for wheat flour substitutes; Food hydrocolloids; Wound dressing products; Curing partial-thickness burns; A bone-substituting material; Novel prebiotics; Film-forming moisturizer; Skin and dermatological compositions; Cosmetic product; Animal and fish feed additives | Antioxidant and antiproliferative activities, regulate the glycemic index and blood sugar and reduce LDL cholesterol. Immune-modulating effects, prophylactic roles against colorectal cancer, prolong satiety and have prebiotic effects, facilitating the elimination of fecal matter and avoiding constipation problems Anti-inflammatory, skin-care effects | [22,28,29,47] |
| BSG | 0.36% dry matter | ||||
| Arabinoxylans | Different cereals bran | 10.9–26.0% of the bran dry matter | Food-thickening and stabilizing agents and films for the food industry (packaging materials); Controlled release of bioactive compounds. | Prebiotic effect, reduce the risk of metabolic disorders such as obesity, have the ability to regulate the postprandial glycemic response and stabilize cholesterol levels Minimizes the risk of developing diabetes and chronic heart disease Anticarcinogenic properties | [30,31,33,47] |
| Cellulose | Rye bran | 5.5–6.5% | Feed supplement Paper packaging containers | It facilitates the shortening of the intestinal transit time and also the elimination of possible carcinogens, which contributes to reducing the risk of developing colon cancer. | [22,47,53,55,56] |
| Wheat bran | 9.3–12.1% | ||||
| BSG | 15.1–25% | ||||
| Lignin | BSG | 7–28% | Food industry (dispersing, binding, and emulsifying agent), food supplement, animal feed and medicine, construction industry, cosmetic products, crop protection (lignin-based pesticides), printing ink | Anticarcinogenic, antimicrobial, and antioxidant properties, increase fecal bulk and stimulates intestinal transit, can undergo fermentation when exposed to colon microbiota, anti-hyperlipidemia and anti-obesogenic agent, protective activity against oxidative stress and inhibition of LDL oxidation | [22,33,47,53,57] |
| Wheat bran | 3.3–4.9% | ||||
| Corn bran | 10 g/kg |
| Cereal Waste | Protein/Amino Acids Quantity | Extraction Methods/Treatments | Extraction Efficiency/Yield | Properties/Applications/Other Observations | References |
|---|---|---|---|---|---|
| Brewers’ Spent Grain (BSG) | Protein: 23.10 g/100 g dw for pale BSG Protein: 26.93 g/100 g dw for black BSG | Sequential aqueous and alkaline (110 mM NaOH) extraction, followed by isoelectric precipitation (pH 3.8) | Pale BSG: 59% protein extraction yield Black BSG: 15% protein extraction yield | Protein-enriched isolates can be used as bioactive ingredients for incorporation into conventional and functional foods. | [67] |
| Protein: 23.4 g/100 g BSG dw | Enzymatic (Alcalase 2.4 L) and ultrasound-assisted enzymatic extraction (amplitude 40%, treatment time 10 min, pulse 5 s:3 s off) | 61.6% recovery for enzymatic treatments and 69.8% recovery for ultrasound enzymatic extraction | Ultrasound pretreatment increases the efficiency of protein separation, reduces enzyme loading, and decreases enzyme incubation time. | [68] | |
| Protein: 22.63 g/100 g defatted BSG | Acid pretreatment (one-step dilute acid pretreatment with the acid solution (11,400 mg H2SO4/g BSG) autoclaved at 121 °C for 1 h) Hydrothermal pretreatment (a. 60 °C/24 h, shaker incubator—250 rpm)/(b. 25 °C, 1.5 h) | Protein extraction efficiency 90% Protein extraction efficiency: 64–66% (a) and 43% (b) | Even though the acid treatment had a higher efficiency, a significant amount of carbohydrates and lignin was also solubilized together with protein; instead, the hydrothermal pretreatment had a better selectivity and is more environmentally friendly. | [69] | |
| Protein: 22.9 g/100 g defatted BSG | Sodium hydroxide treatment 5% (w/w) Alcalase treatment (20 μL/g dry BSG) Sodium bisulfite treatment (5% w/w) | Protein separation efficiency 81.8% Protein separation efficiency 83.7% Protein separation efficiency 68% | Enzymatic treatment proved to be the most effective and the resulting protein concentrate had also the highest lysine content (4.1%, w/w). | [70] | |
| Protein: 24.70 g/100 g dw | Sodium hydroxide (110 mM) and ultrasound treatment (power 250 W, duty cycle 60%, 20 min/25 °C) | Extraction yield of 86.16% and purity at 57.84% | Plant-based protein source to the food industry. Improved fat absorption capacity, emulsifying, and foaming properties. | [71] | |
| Amino acids: 43.62 mg/g−1 proteins | Subcritical water hydrolysis in a single reactor (120 min at 15 MPa, 5 mL water min, 80–180 °C, solid: fluid of 20 g−1 BSG) | The main amino acids of hydrolysate: tryptophan 215.55 µg mL−1, aspartic acid 123.35 µg mL−1, valine 64.35 µg mL−1, lysine 16.55 µg mL−1, and glycine 16.1 µg mL−1 | Applicability in the field of food and supplements production | [72] | |
| Rice bran defatted (RBD) | Soluble proteins: 8.23 g/100 RBD | Alkaline extraction of proteins and fractionation by the Osborne method | 55.8% of the total soluble proteins, of which 6.1%albumin, 4.5% globulin, and 43.5% glutelin. | Applicability in the field of food and supplements and cosmetics production. | [73] |
| Protein: 15.67 g/100 g RBD | Alkaline extraction (60 min, pH 11, 55 °C) Microwave-assisted extraction (120 s; pH 11, 55 °C) | Protein content of concentrated product 75.32% and extraction yield 12.85% Protein content of concentrated product 79.98% and extraction yield 15.68% | Comparing the two methods, the microwave-assisted one proved to be more efficient and environmentally friendly. Also, the microwaves did not affect the extracted rice bran proteins. | [74] | |
| Protein: 14.13% of concentrate product | Microwave-assisted extraction (1000 W of MW power, extraction time 90 s, solid to liquid ratio of 0.89 g rice bran/10 mL of distilled water) and response surface methodology | Protein content of concentrated product 71.27% and recovery yield 22.07% | Food industry—strong antioxidant activity. MAE is considered an environmentally friendly technique. | [75] | |
| Malted barley germs (MBG) | Protein: 29.1% on a dry matter basis | Amino acid profile by LC/fluorescence | Total amino acid 214 mg/g of which 35–40% are essential (leucine 15.7 mg/g, valine 13.5 mg/g, lysine 11.7 mg/g, and arginine 12.5 mg/g dw | Valuable source of good quality nitrogen fraction. Applicability in the field of food and supplements production. | [76] |
| Brewing cake | Protein: 30.4% on a dry matter basis | Amino acid profile by LC/fluorescence | Total amino acid content 238 mg/g of witch 35–40% are essential amino acids (leucine 18.0 mg/g), phenylalanine 14.6 mg/g, valine 13.3 mg/g, cysteine 11.3 mg/g, arginine 12.1 mg/g dw | Valuable source of good quality nitrogen fraction. Applicability in the field of food and supplements production. | [76] |
| Wheat bran (WB) | Protein: 17.2 g/100 g WB dw Total amino acids (AA): 12.5 g/100 g WB proteinTotal essential amino acids (EAA): of 4.28 g/100 g WB protein | Alkaline extraction (pH 9.5, 2 h, followed by isoelectric precipitation, pH 4.2) | Wheat bran concentrate (WBPC) protein content: 61% Protein recovery yield: 20.5–24.8% Total AA of WBPC 60.11 g/100 g), total EAA 22.79 g/100 g | WBPC showed excellent functional properties in terms of high solubility, good water, and fat absorption capacity. Balanced amino acid composition, high in essential amino acids, with good levels of lysine and threonine, and phenolic acids. | [77] |
| Defatted Wheat Germ (DWG) | Protein: 34.9% dw (albumin 34.5% globulin 15.6%, glutelin 10.6%, and prolamine 4.6%) | Alcaline extraction (pH 9.5 with 1 M NaOH, stirring 30 min, the supernatant was adjusted to pH 4.0 with 1.0 M HCl to precipitate the proteins, washed and adjusted to pH 7.0 using 0.1 M NaOH, then freeze-dried) | Isolate protein content 88.5%, recovery yield in the range of 24.0–37.0% | Significant level of essential amino acids. DWG can be considered a good vegetable protein supplement for cereal-based diets. | [78] |
| Defatted corn germ (DCG) | Protein: 12.48% fresh weight basis | Alkaline extraction of corn germ partially defatted by supercritical fluid extraction | Protein content of DCG concentrate 48.5% dry base reported Yield of protein extraction 21.3% | Good foaming capacity and stability | [79] |
| Defatted oat bran (DOB) | Protein: 17.6% | Enzyme-assisted extraction (Viscozyme L, pH 4.6, incubation time 2.8 h, and temperature 44 °C) | Extraction yield 56.2% | Applicability in the field of food and supplements production. | [80] |
| Cereal Waste | Antioxidant Compounds | Extraction Methods/Biotechnology | Extraction/ Production Yield | Antioxidant Activity | Application | References |
|---|---|---|---|---|---|---|
| Corn silage | Polyphenols | Enzymatic treatment | 412.83 mg GAE/100 g | 2961.6 μM (ABTS) | - | [111] |
| Brewers’ spent grain | Phenolic compounds | Supercritical carbon dioxide | 3 g mass of extract | 2% DPPH | [112] | |
| Polyphenols | Acidifies solution (pH 2,5–3) | 1.14 mg GAE/g | 8–13% | - | [113] | |
| Oat bran | Protein hydrolysates | Hydrolyzed with Flavourzyme (1), Papain (2), or Alcalase (3) | 89–93% | 627.17 (1); 682.90 (2); 652.67 (3) µM TE/g (ORAC) | - | [114] |
| Rice bran | Free phenols Bound phenols | Ultrasound-assisted extraction (65% ethanolic solution) Ultrasound-assisted alkaline hydrolysis | 17–20% | 275.1 (DPPH) IC50 (μg/mL) 38.01 (DPPH) IC50 (μg/mL) | Cosmetic formulation | [115] |
| Protein hydrolysates | Hydrolysate by Alcalase 2.4 L and Protease 500 G | 79.12% | 75–90% (DPPH) | - | [116] | |
| Protein hydrolysates | Protein enzyme-assisted extraction/hydrolysis | - | 2.8 μmol TE/g (DPPH) | Food additive | [117] | |
| Polyphenols | Glycerol extraction | 708.58 ± 12.36 mg GAE/100 g dw | 700.35 mg GAE/100 g | - | [118] | |
| Sesame bran | Phenols | Microwave-assisted enzymatic extraction | - | 1.94 µmol TE/g | Functional food ingredient | [119] |
| Wheat bran | Free phenols Bound phenols | Ultrasound-assisted extraction (65% ethanolic solution) Ultrasound-assisted alkaline hydrolysis | 17–20% | 1194.8 (DPPH) IC50 (μg/mL) 3.61 (DPPH) IC50 (μg/mL) | Cosmetic formulation | [115] |
| Peptides | HPLC purification | - | 3000–3300 μmol/L biological antioxidant potential (free radical analyzer system) | Antidiabetic compound | [120] | |
| Wheat and rye waste (distillery stillage) | Polyphenols | Conventional solid-liquid extraction (1) Ultrasound-assisted extraction (2) Microwave-assisted extraction (3) | 52–99% | 10.84 (1); 16.67 (2); 26.73 (3) μmol TE/g (ABTS) 10.84 (1); 2.95 (2); 5.57 (3) μmol TE/g (DPPH) 36.73 (1); 5.57 (2); 3.71 (3) μmol FeSO4/g (FRAP) | - | [121] |
| Wheat waste | Astaxanthin | Solid state fermentation | 17–109% | 90–95% of the antioxidant (DPPH) activity of astaxanthin from plant | - | [110] |
| Wheat and Oat Bran | Phenolic compounds | Ultrasound-assisted extraction | 25–50 mg GAE/100 g | 40–52% (DPPH) | - | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Pop, O.L.; Coldea, T.E.; Fogarasi, M.; Biriș-Dorhoi, E.S. An Update Regarding the Bioactive Compound of Cereal By-Products: Health Benefits and Potential Applications. Nutrients 2022, 14, 3470. https://doi.org/10.3390/nu14173470
Fărcaș AC, Socaci SA, Nemeș SA, Pop OL, Coldea TE, Fogarasi M, Biriș-Dorhoi ES. An Update Regarding the Bioactive Compound of Cereal By-Products: Health Benefits and Potential Applications. Nutrients. 2022; 14(17):3470. https://doi.org/10.3390/nu14173470
Chicago/Turabian StyleFărcaș, Anca Corina, Sonia Ancuța Socaci, Silvia Amalia Nemeș, Oana Lelia Pop, Teodora Emilia Coldea, Melinda Fogarasi, and Elena Suzana Biriș-Dorhoi. 2022. "An Update Regarding the Bioactive Compound of Cereal By-Products: Health Benefits and Potential Applications" Nutrients 14, no. 17: 3470. https://doi.org/10.3390/nu14173470
APA StyleFărcaș, A. C., Socaci, S. A., Nemeș, S. A., Pop, O. L., Coldea, T. E., Fogarasi, M., & Biriș-Dorhoi, E. S. (2022). An Update Regarding the Bioactive Compound of Cereal By-Products: Health Benefits and Potential Applications. Nutrients, 14(17), 3470. https://doi.org/10.3390/nu14173470












