Influence of CLOCK Gene Variants on Weight Response after Bariatric Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. DNA Extraction and Genotyping of Samples
2.2. Statistical Analyses
3. Results
3.1. Case Control Association Study
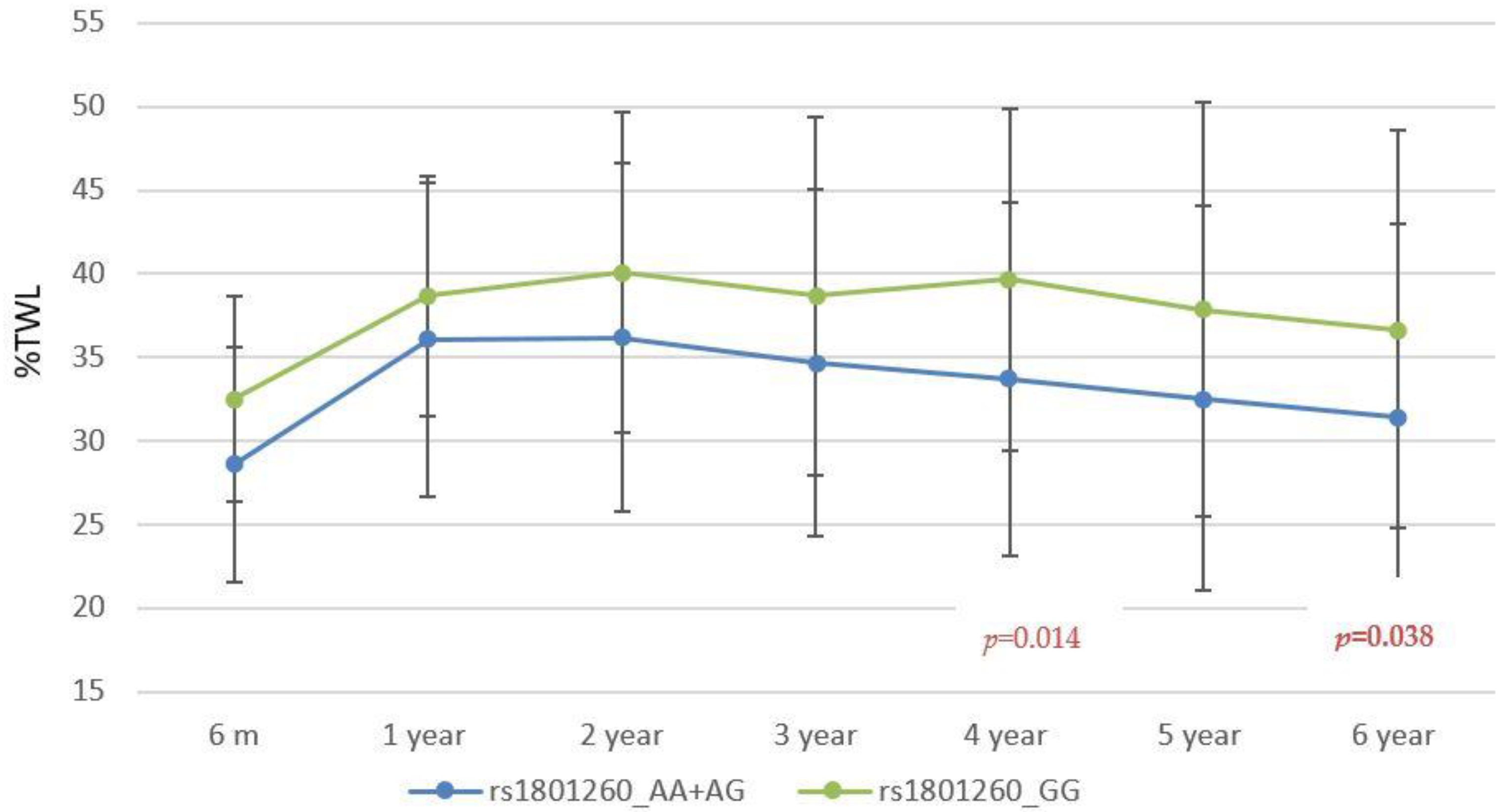
3.2. Association Study with Weight Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant nice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sookoian, S.; Gemma, C.; Fernández Gianotti, T.; Burgueño, A.; Castaño, G.; Pirola, C.J. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am. J. Clin. Nutr. 2008, 87, 1606–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, E.M.; Carter, A.M.; Grant, P.J. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int. J. Obes. 2008, 32, 658–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Lozano, T.; Vidal, J.; De Hollanda, A.; Canteras, M.; Garaulet, M.; Izquierdo-Pulido, M. Evening chronotype associates with obesity in severely obese subjects: Interaction with CLOCK 3111T/C. Int. J. Obes. 2016, 40, 1550–1557. [Google Scholar] [CrossRef]
- Garaulet, M.; Madrid, J.A. Chronobiology, genetics and metabolic syndrome. Curr. Opin. Lipidol. 2009, 20, 127–134. [Google Scholar] [CrossRef]
- Garaulet, M.; Gómez-Abellán, P. Timing of food intake and obesity: A novel association. Physiol. Behav. 2014, 134, 44–50. [Google Scholar] [CrossRef]
- Lago-Sampedro, A.; Ho-Plagaro, A.; Garcia-Serrano, S.; Santiago-Fernandez, C.; Rodríguez-Díaz, C.; Lopez-Gómez, C.; Martín-Reyes, F.; Ruiz-Aldea, G.; Alcaín-Martínez, G.; Gonzalo, M.; et al. Oleic acid restores the rhythmicity of the disrupted circadian rhythm found in gastrointestinal explants from patients with morbid obesity. Clin. Nutr. 2021, 40, 4324–4333. [Google Scholar] [CrossRef]
- Garaulet, M.; Corbalán, M.D.; Madrid, J.A.; Morales, E.; Baraza, J.C.; Lee, Y.C.; Ordovas, J.M. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int. J. Obes. 2010, 34, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Murube, M.C.; Borregon-Rivilla, E.; Colmenarejo, G.; Aguilar-Aguilar, E.; Martínez, J.A.; De Molina, A.R.; Reglero, G.; Loria-Kohen, V. Polymorphism of CLOCK Gene rs3749474 as a Modulator of the Circadian Evening Carbohydrate Intake Impact on Nutritional Status in an Adult Sample. Nutrients 2020, 12, 1142. [Google Scholar] [CrossRef] [Green Version]
- Loria-Kohen, V.; Espinosa-Salinas, I.; Marcos-Pasero, H.; Lourenço-Nogueira, T.; Herranz, J.; Molina, S.; Reglero, G.; de Molina, A.R. Polymorphism in the CLOCK gene may influence the effect of fat intake reduction on weight loss. Nutrition 2016, 32, 453–460. [Google Scholar] [CrossRef]
- Ruiz-Lozano, T.; Vidal, J.; de Hollanda, A.; Scheer, F.A.J.L.; Garaulet, M.; Izquierdo-Pulido, M. Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clin. Nutr. 2016, 35, 1308–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrego-Ellacuría, M.; Barabash, A.; Larrad-Sainz, A.; Hernández-Nuñez, G.M.; Matía-Martín, P.; Pérez-Ferre, N.; Marcuello, C.; Sánchez-Pernaute, A.; Torres, A.J.; Calle-Pascual, A.L.; et al. Weight Regain Outcomes after Bariatric Surgery in the Long-term Follow-up: Role of Preoperative Factors. Obes. Surg. 2021, 31, 3947–3955. [Google Scholar] [CrossRef] [PubMed]
- King, W.C.; Hinerman, A.S.; Belle, S.H.; Wahed, A.S.; Courcoulas, A.P. Comparison of the Performance of Common Measures of Weight Regain after Bariatric Surgery for Association with Clinical Outcomes. JAMA 2018, 320, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Home—SNP—NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/ (accessed on 22 November 2021).
- Solé, X.; Guinó, E.; Valls, J.; Iniesta, R.; Moreno, V. SNPStats: A web tool for the analysis of association studies. Bioinformatics 2006, 22, 1928–1929. [Google Scholar] [CrossRef] [Green Version]
- de Vries, L.; Van den Broecke, C.; Decruyeneare, A.; Van Nieuwenhove, Y. A SIMPLE Performance Assessment of Bariatric Procedures and Post-operative Weight Regain. J. Gastrointest. Surg. 2022, 26, 542–549. [Google Scholar] [CrossRef]
- Bonouvrie, D.S.; Uittenbogaart, M.; Luijten, A.A.P.M.; van Dielen, F.M.H.; Leclercq, W.K.G. Lack of Standard Definitions of Primary and Secondary (Non)responders after Primary Gastric Bypass and Gastric Sleeve: A Systematic Review. Obes. Surg. 2019, 29, 691–697. [Google Scholar] [CrossRef]
- Goodarzi, M.O. Genetics of obesity: What genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018, 6, 223–236. [Google Scholar] [CrossRef]
- Loos, R.J. The genetics of adiposity. Curr. Opin. Genet. Dev. 2018, 50, 86–95. [Google Scholar] [CrossRef]
- Vettori, A.; Pompucci, G.; Paolini, B.; Del Ciondolo, I.; Bressan, S.; Dundar, M.; Kenanoglu, S.; Unfer, V.; Bertelli, M.; Project, G. Genetic background, nutrition and obesity: A review. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1751–1761. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Guan, D.; Lazar, M. Interconnections between circadian clocks and metabolism. J. Clin. Investig. 2021, 131, e148278. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Salinas, I.; San-Cristobal, R.; Colmenarejo, G.; Loria-Kohen, V.; Molina, S.; Reglero, G.; de Molina, A.R.; Martinez, J.A. Polymorphic appetite effects on waist circumference depend on RS3749474 clock gene variant. Nutrients 2020, 12, 1846. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rios, A.; Gomez-Delgado, F.J.; Garaulet, M.; Alcala-Diaz, J.F.; Delgado-Lista, F.J.; Marín, C.; Rangel-Zuñiga, O.A.; Rodriguez-Cantalejo, F.; Gomez-Luna, P.; Ordovas, J.M.; et al. Beneficial effect of CLOCK gene polymorphism rs1801260 in combination with low-fat diet on insulin metabolism in the patients with metabolic syndrome. Chronobiol. Int. 2014, 31, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Brethauer, S.A.; Kim, J.; el Chaar, M.; Papasavas, P.; Eisenberg, D.; Rogers, A.; Ballem, N.; Kligman, M.; Kothari, S. Standardized outcomes reporting in metabolic and bariatric surgery. Surg. Obes. Relat. Dis. 2015, 11, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Ibarzabal, A.; Moizé, V.; Pané, A.; Andreu, A.; Molero, J.; de Hollanda, A.; Flores, L.; Ortega, E.; Lacy, A.; et al. Ten-year outcomes after Roux-en-Y gastric bypass and sleeve gastrectomy: An observational nonrandomized cohort study. Surg. Obes. Relat. Dis. 2019, 15, 382–388. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Johnson, E.; Coleman, K.J.; Herrinton, L.J.; Courcoulas, A.P.; Fisher, D.; Li, R.A.; Theis, M.K.; Liu, L.; Fraser, J.R.; et al. Weight Outcomes of Sleeve Gastrectomy and Gastric Bypass Compared to Nonsurgical Treatment. Ann. Surg. 2021, 274, e1269–e1276. [Google Scholar] [CrossRef]
- Ciudin, A.; Fidilio, E.; Gutiérrez-Carrasquilla, L.; Caixàs, A.; Vilarrasa, N.; Pellitero, S.; Simó-Servat, A.; Vilallonga, R.; Ruiz, A.; de la Fuente, M.; et al. A Clinical-Genetic Score for Predicting Weight Loss after Bariatric Surgery: The OBEGEN Study. J. Pers. Med. 2021, 11, 1040. [Google Scholar] [CrossRef]
- Cooper, T.C.; Simmons, E.B.; Webb, K.; Burns, J.L.; Kushner, R.F. Trends in Weight Regain Following Roux-en-Y Gastric Bypass (RYGB) Bariatric Surgery. Obes. Surg. 2015, 25, 1474–1481. [Google Scholar] [CrossRef]


| SNP | Genotype | Controls n (%) | Cases n (%) |
|---|---|---|---|
| rs3749474 | C/C * | 100 (44.2%) | 142 (37.9%) |
| C/T | 101 (44.7%) | 152 (40.5%) | |
| T/T | 25 (11.1%) | 81 (21.6%) | |
| rs1801260 | A/A * | 115 (50.2%) | 202 (53.9%) |
| A/G | 90 (39.3%) | 157 (41.9%) | |
| G/G | 24 (10.5%) | 16 (4.3%) | |
| rs4580704 | G/G * | 30 (13.1%) | 52 (13.9%) |
| G/C | 105 (45.9%) | 169 (45.1%) | |
| C/C | 94 (41%) | 154 (41.1%) |
| Variable | Value |
|---|---|
| Age, in years | 44.79 ± 11.99 |
| BMI_00, kg/m2 | 44.87 ± 6.59 |
| Female gender, n (%) | 259 (69) |
| T2D, n (%) | 134 (35.7) |
| HTN, n (%) | 182 (49) |
| %TWL_nadir, | 38.79 ± 9.84 |
| %EWL_nadir, | 91.19 ± 23.69 |
| %TWL_6y | 31.67 ± 11.62 |
| %EWL_6y | 74.08 ± 26.89 |
| %EWL > 50%, n (%) | 311 (82.93) |
| %TWL > 20%, n (%) | 317 (84.53) |
| %WR_MWL, median (IQR) | 15.76 (7.99–28.69) |
| SNP | Genotype | n | %TWL_nadir | %TWL_6y | %WR_MWL |
|---|---|---|---|---|---|
| rs3749474 | C/C | 142 | 39.48 ± 0.86 | 32.26 ± 0.97 | 20.04 ± 1.55 |
| C/T | 152 | 38.11 ± 0.78 | 31.45 ± 0.93 | 19.24 ± 1.31 | |
| T/T | 81 | 38.84 ± 1.07 | 31.03 ± 1.35 | 22.35 ± 2.41 | |
| rs1801260 | A/A/ | 202 | 38.87 ± 0.67 | 31.22 ± 0.83 | 21.63 ± 1.36 |
| A/G | 157 | 38.31 ± 0.82 | 31.73 ± 0.9 | 18.95 ± 1.37 | |
| G/G | 16 | 42.43 ± 2.31 | 36.7 ± 2.97 | 14.88 ± 3.61 | |
| rs4580704 | G/G | 52 | 39.09 ± 1.22 | 30.65 ± 1.5 | 20.39 ± 1.5 |
| G/C | 169 | 39.48 ± 0.8 | 32.56 ± 0.9 | 19.29 ± 1.43 | |
| C/C | 154 | 37.93 ± 0.77 | 31.03 ± 0.95 | 22.73 ± 2.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrego-Ellacuría, M.; Barabash, A.; Matía-Martín, P.; Sánchez-Pernaute, A.; Torres, A.J.; Calle-Pascual, A.L.; Rubio-Herrera, M.A. Influence of CLOCK Gene Variants on Weight Response after Bariatric Surgery. Nutrients 2022, 14, 3472. https://doi.org/10.3390/nu14173472
Torrego-Ellacuría M, Barabash A, Matía-Martín P, Sánchez-Pernaute A, Torres AJ, Calle-Pascual AL, Rubio-Herrera MA. Influence of CLOCK Gene Variants on Weight Response after Bariatric Surgery. Nutrients. 2022; 14(17):3472. https://doi.org/10.3390/nu14173472
Chicago/Turabian StyleTorrego-Ellacuría, Macarena, Ana Barabash, Pilar Matía-Martín, Andrés Sánchez-Pernaute, Antonio J. Torres, Alfonso L. Calle-Pascual, and Miguel A. Rubio-Herrera. 2022. "Influence of CLOCK Gene Variants on Weight Response after Bariatric Surgery" Nutrients 14, no. 17: 3472. https://doi.org/10.3390/nu14173472
APA StyleTorrego-Ellacuría, M., Barabash, A., Matía-Martín, P., Sánchez-Pernaute, A., Torres, A. J., Calle-Pascual, A. L., & Rubio-Herrera, M. A. (2022). Influence of CLOCK Gene Variants on Weight Response after Bariatric Surgery. Nutrients, 14(17), 3472. https://doi.org/10.3390/nu14173472






