Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review
Abstract
1. Introduction
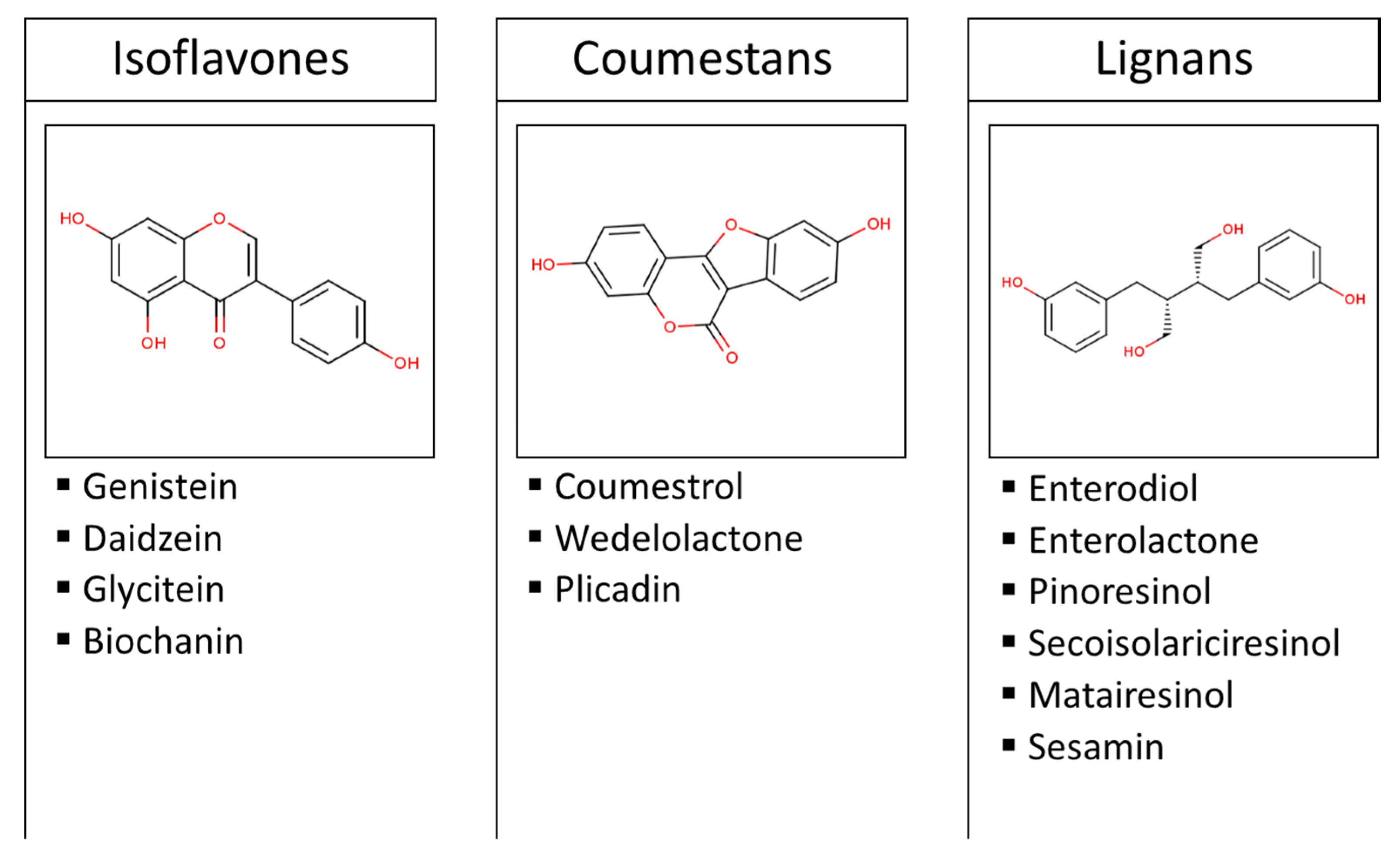
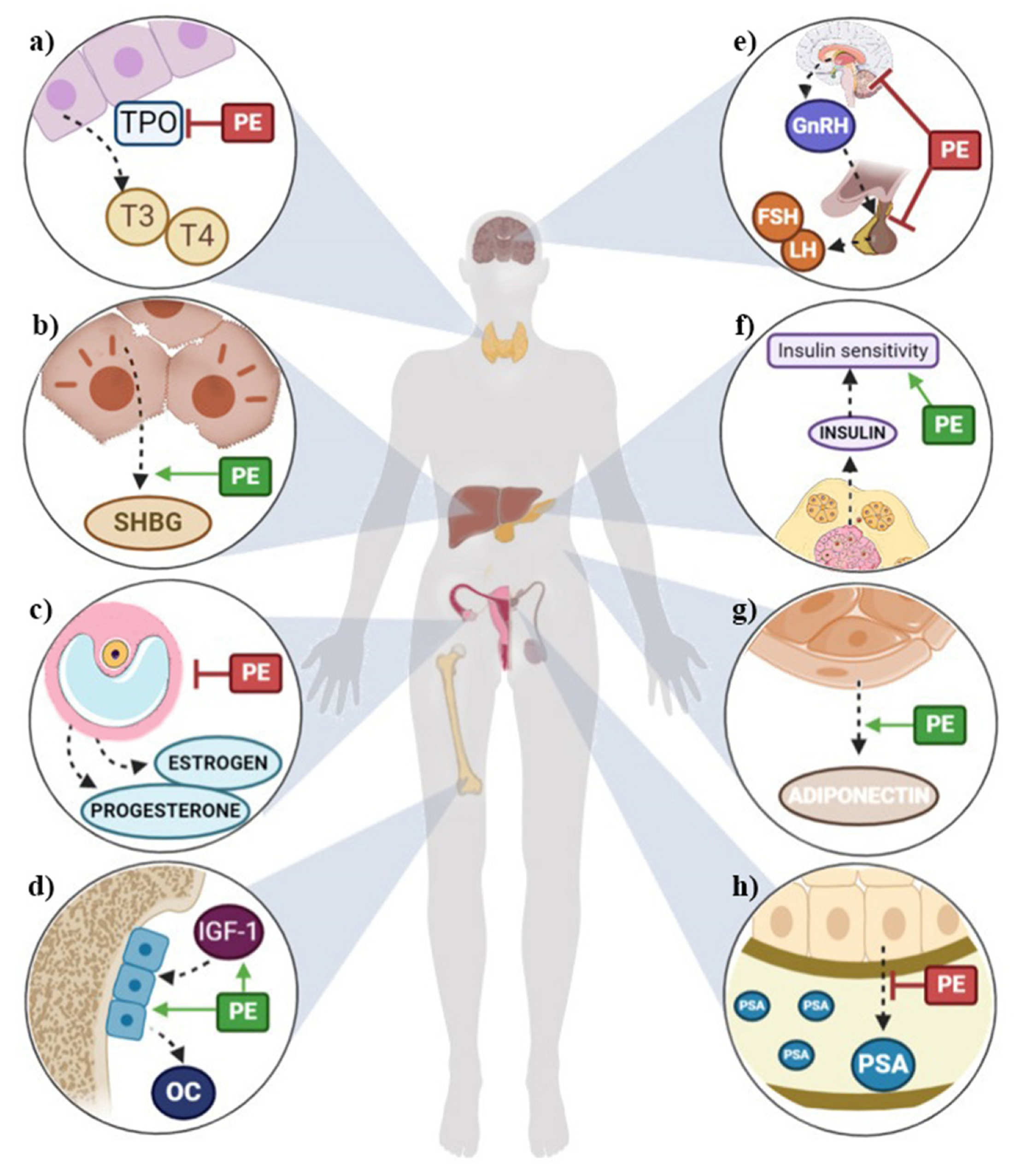
2. Effects of Phytoestrogen Intake on Sex Hormones
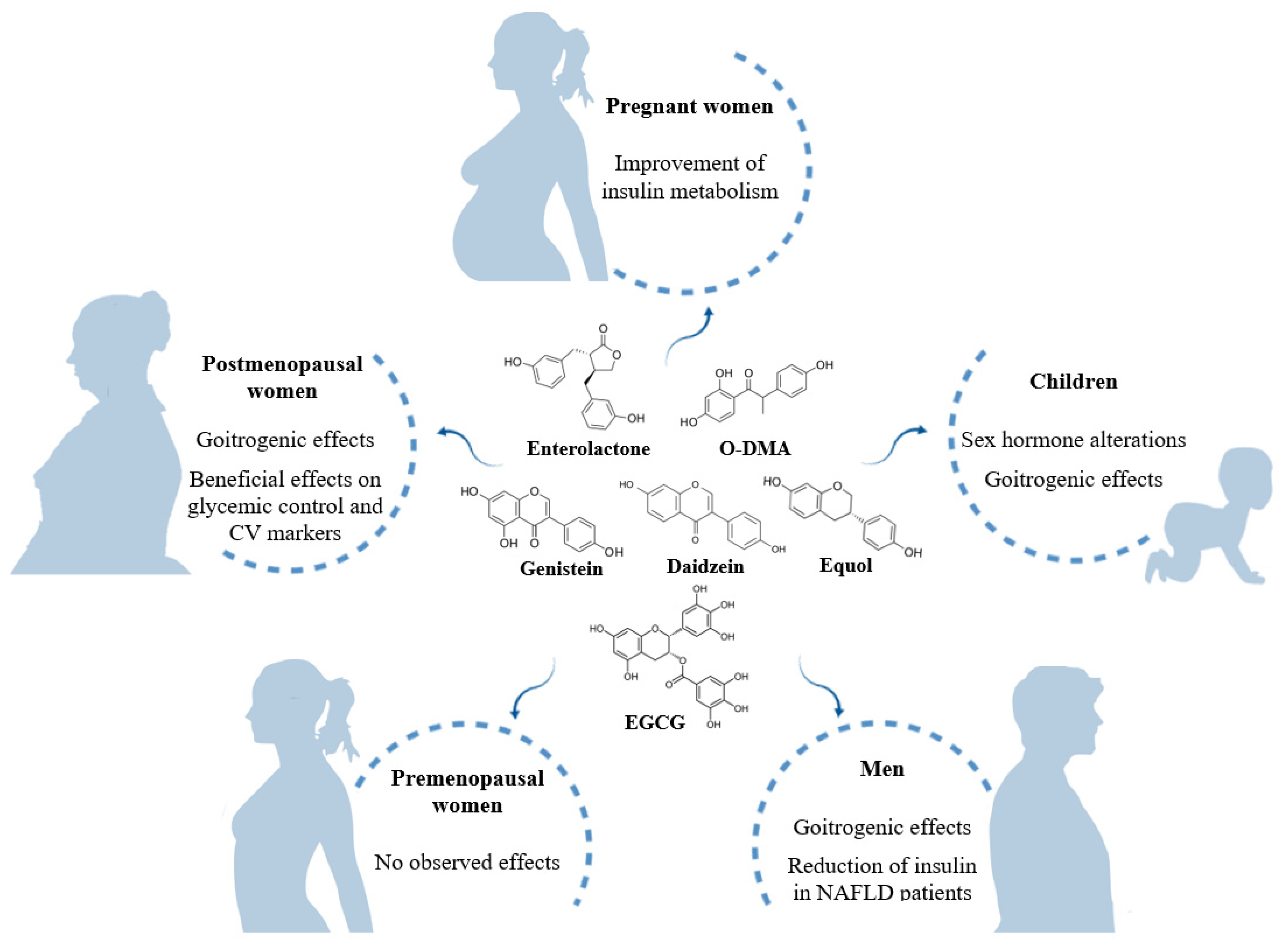
2.1. Pregnancy
2.2. Children
2.3. Men
2.4. Premenopausal Women
2.5. Postmenopausal Women
3. Effect of Phytoestrogen Intake on Thyroid Hormones
3.1. Pregnant Women
3.2. Children
3.3. Men
3.4. Premenopausal Women
3.5. Postmenopausal Women
4. Effect of Phytoestrogen Intake on Cardiometabolic Risk-Related Hormones
4.1. Pregnancy
4.2. Adults
4.3. Postmenopausal Women
5. Effect of Phytoestrogen Intake on Hormones Related to Stress Response
6. Effect of Phytoestrogen Intake on Hormones Related to Bone Remodeling
6.1. Children
6.2. Premenopausal Women
6.3. Postmenopausal Women
7. Effect of Phytoestrogen Intake on Insulin-Like Growth Factors
7.1. Premenopausal Women
7.2. Postmenopausal Women
7.3. Men
8. Conclusions
9. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Viggiani, M.T.; Polimeno, L.; Di Leo, A.; Barone, M. Phytoestrogens: Dietary intake, bioavailability, and protective mechanisms against colorectal neoproliferative lesions. Nutrients 2019, 11, 1709. [Google Scholar] [CrossRef] [PubMed]
- Bennetau-Pelissero, C. Risks and benefits of phytoestrogens: Where are we now. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Kuhnle, G.G.C.; Mulligan, A.A.; Touillaud, M.; Slimani, N.; Romieu, I.; Powell, N.; Tumino, R.; et al. Dietary intakes and food sources of phytoestrogens in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24-hour dietary recall cohort. Eur. J. Clin. Nutr. 2012, 66, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Bedell, S.; Nachtigall, M.; Naftolin, F. The pros and cons of plant estrogens for menopause. J. Steroid Biochem. Mol. Biol. 2014, 139, 225–236. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Hallund, J.; Talbot, D.; Schroot, J.; Williams, C.M.; Bugel, S.; Cassidy, A. Absorption of isoflavones in humans: Effects of food matrix and processing. J. Nutr. Biochem. 2006, 17, 257–264. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Hüser, S.; Guth, S.; Joost, H.G.; Soukup, S.T.; Köhrle, J.; Kreienbrock, L.; Diel, P.; Lachenmeier, D.W.; Eisenbrand, G.; Vollmer, G.; et al. Effects of isoflavones on breast tissue and the thyroid hormone system in humans: A comprehensive safety evaluation. Arch. Toxicol. 2018, 92, 2703–2748. [Google Scholar] [CrossRef]
- Desmawati, D.; Sulastri, D. Phytoestrogens and Their Health Effect. Maced. J. Med. Sci. 2019, 7, 495–499. [Google Scholar] [CrossRef]
- Bilancio, A.; Migliaccio, A. Phosphoinositide 3-Kinase Assay in Breast Cancer Cell Extracts. Methods Mol. Biol. 2014, 1204, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, A.; Castoria, G.; de Falco, A.; Bilancio, A.; Giovannelli, P.; Di Donato, M.; Marino, I.; Yamaguchi, H.; Appella, E.; Auricchio, F. Polyproline and Tat transduction peptides in the study of the rapid actions of steroid receptors. Steroids 2012, 77, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Giovannelli, P.; Cernera, G.; Di Santi, A.; Marino, I.; Bilancio, A.; Galasso, G.; Auricchio, F.; Migliaccio, A.; Castoria, G. Non-genomic androgen action regulates proliferative/migratory signaling in stromal cells. Front. Endocrinol. 2015, 5, 225. [Google Scholar] [CrossRef][Green Version]
- Nicholls, J.; Lasley, B.L.; Nakajima, S.T.; Setchell, K.D.R.; Schneeman, B.O. Effects of Soy Consumption on Gonadotropin Secretion and Acute Pituitary Responses to Gonadotropin-Releasing Hormone in Women. J. Nutr. 2002, 132, 708–714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jefferson, W.N. Adult Ovarian Function Can Be Affected by High Levels of Soy. J. Nutr. 2010, 140, 2322S–2325S. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.S.; Bongiovanni, B.; Bralley, J.A. Estrogen metabolism and the diet-cancer connection: Rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern. Med. Rev. 2002, 7, 112–129. [Google Scholar] [PubMed]
- Tamaya, T. Phytoestrogens and reproductive biology. Reprod. Med. Biol. 2005, 4, 225–229. [Google Scholar] [CrossRef]
- Duncan, A.M.; Underhill, K.E.; Xu, X.; LaValleur, J.; Phipps, W.R.; Kurzer, M.S. Modest hormonal effects of soy isoflavones in postmenopausal women. J. Clin. Endocrinol. Metab. 1999, 84, 3479–3484. [Google Scholar] [CrossRef]
- Verkasalo, P.K.; Appleby, P.N.; Davey, G.K.; Key, T.J. Soy milk intake and plasma sex hormones: A cross-sectional study in pre- and postmenopausal women (EPIC-Oxford). Nutr. Cancer 2001, 40, 79–86. [Google Scholar] [CrossRef]
- Wang, L.-Q. Mammalian phytoestrogens: Enterodiol and enterolactone. J. Chromatogr. B 2002, 777, 289–309. [Google Scholar] [CrossRef]
- Zhao, E.; Mu, Q. Phytoestrogen biological actions on mammalian reproductive system and cancer growth. Sci. Pharm. 2011, 79, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Duncan, A.M.; Merz, B.E.; Kurzer, M.S. Effects of soy isoflavones on estrogen and phytoestrogen metabolism in premenopausal women. Cancer Epidemiol. Biomark. Prev. 1998, 7, 1101–1108. [Google Scholar]
- Brown, B.D.; Thomas, W.; Hutchins, A.; Martini, M.C.; Slavin, J.L. Types of dietary fat and soy minimally affect hormones and biomarkers associated with breast cancer risk in premenopausal women. Nutr. Cancer 2002, 43, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Iwasa, S.; Shiraki, M.; Ueno, T.; Uchiyama, S.; Urata, K.; Sahashi, Y.; Shimizu, H. Associations among maternal soy intake, isoflavone levels in urine and blood samples, and maternal and umbilical hormone concentrations (Japan). Cancer Causes Control 2006, 17, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Nakamura, K.; Masue, T.; Sahashi, Y.; Ando, K.; Nagata, C. Soy intake and urinary sex hormone levels in preschool Japanese children. Am. J. Epidemiol. 2011, 173, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Todaka, E.; Sakurai, K.; Fukata, H.; Miyagawa, H.; Uzuki, M.; Omori, M.; Osada, H.; Ikezuki, Y.; Tsutsumi, O.; Iguchi, T.; et al. Fetal exposure to phytoestrogens—The difference in phytoestrogen status between mother and fetus. Environ. Res. 2005, 99, 195–203. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Yamada, T.; Wahala, K.; Watanabe, S. Maternal and neonatal phytoestrogens in Japanese women during birth. Am. J. Obstet. Gynecol. 1999, 180, 737–743. [Google Scholar] [CrossRef]
- Franke, A.A.; Lai, J.F.; Halm, B.M. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake. Arch. Biochem. Biophys. 2014, 559, 24–28. [Google Scholar] [CrossRef]
- Giampietro, P.G.; Bruno, G.; Furcolo, G.; Casati, A.; Brunetti, E.; Spadoni, G.L.; Galli, E. Soy protein formulas in children: No hormonal effects in long-term feeding. J. Pediatr. Endocrinol. Metab. 2004, 17, 191–196. [Google Scholar] [CrossRef]
- Cao, Y.; Calafat, A.M.; Doerge, D.R.; Umbach, D.M.; Bernbaum, J.C.; Twaddle, N.C.; Ye, X.; Rogan, W.J. Isoflavones in urine, saliva, and blood of infants: Data from a pilot study on the estrogenic activity of soy formula. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 223–234. [Google Scholar] [CrossRef]
- Adgent, M.A.; Umbach, D.M.; Zemel, B.S.; Kelly, A.; Schall, J.I.; Ford, E.G.; James, K.; Darge, K.; Botelho, J.C.; Vesper, H.W.; et al. A Longitudinal Study of Estrogen-Responsive Tissues and Hormone Concentrations in Infants Fed Soy Formula. J. Clin. Endocrinol. Metab. 2018, 103, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Morimoto, Y.; Novotny, R.; Nordt, F.J.; Stanczyk, F.Z.; Franke, A.A. Urinary sex steroid excretion levels during a soy intervention among young girls: A pilot study. Nutr. Cancer 2005, 52, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Inaba, S.; Kawakami, N.; Kakizoe, T.; Shimizu, H. Inverse association of soy product intake with serum androgen and estrogen concentrations in Japanese men. Nutr. Cancer 2000, 36, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fujimoto, K.; Chihara, Y.; Torimoto, K.; Yoneda, T.; Tanaka, N.; Hirayama, A.; Miyanaga, N.; Akaza, H.; Hirao, Y. Isoflavone supplements stimulated the production of serum equol and decreased the serum dihydrotestosterone levels in healthy male volunteers. Prostate Cancer Prostatic Dis. 2009, 12, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Applegate, C.C.; Rowles, J.L.; Ranard, K.M.; Jeon, S.; Erdman, J.W. Soy consumption and the risk of prostate cancer: An updated systematic review and meta-analysis. Nutrients 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Dalais, F.S.; Meliala, A.; Wattanapenpaiboon, N.; Frydenberg, M.; Suter, D.A.I.; Thomson, W.K.; Wahlqvist, M.L. Effects of a diet rich in phytoestrogens on prostate-specific antigen and sex hormones in men diagnosed with prostate cancer. Urology 2004, 64, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Banerjee, M.; Sarkar, F.H.; Djuric, Z.; Pollak, M.N.; Doerge, D.; Fontana, J.; Chinni, S.; Davis, J.; Forman, J.; et al. Soy Isoflavones in the Treatment of Prostate Cancer. Nutr. Cancer 2003, 47, 111–117. [Google Scholar] [CrossRef]
- Pendleton, J.M.; Tan, W.W.; Anai, S.; Chang, M.; Hou, W.; Shiverick, K.T.; Rosser, C.J. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer 2008, 8, 132. [Google Scholar] [CrossRef]
- Hamilton-Reeves, J.M.; Banerjee, S.; Banerjee, S.K.; Holzbeierlein, J.M.; Thrasher, J.B.; Kambhampati, S.; Keighley, J.; Van Veldhuizen, P. Short-Term Soy Isoflavone Intervention in Patients with Localized Prostate Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial. PLoS ONE 2013, 8, e68331. [Google Scholar] [CrossRef]
- Fischer, L.; Mahoney, C.; Jeffcoat, A.R.; Koch, M.A.; Thomas, B.F.; Valentine, J.L.; Stinchcombe, T.; Boan, J.; Crowell, J.A.; Zeisel, S.H. Clinical characteristics and pharmacokinetics of purified soy isoflavones: Multiple-dose administration to men with prostate neoplasia. Nutr. Cancer 2004, 48, 160–170. [Google Scholar] [CrossRef]
- Bylund, A.; Lundin, E.; Zhang, J.X.; Nordin, A.; Kaaks, R.; Stenman, U.H.; Åman, P.; Adlercreutz, H.; Nilsson, T.K.; Hallmans, G.; et al. Randomised controlled short-term intervention pilot study on rye bran bread in prostate cancer. Eur. J. Cancer Prev. 2003, 12, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Bosland, M.C. The role of steroid hormones in prostate carcinogenesis. J. Natl. Cancer Inst. Monogr. 2000, 2000, 39–66. [Google Scholar] [CrossRef] [PubMed]
- Shaneyfelt, T.; Husein, R.; Bubley, G.; Mantzoros, C.S. Hormonal predictors of prostate cancer: A meta-analysis. J. Clin. Oncol. 2000, 18, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Reeves, J.M.; Vazquez, G.; Duval, S.J.; Phipps, W.R.; Kurzer, M.S.; Messina, M.J. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: Results of a meta-analysis. Fertil. Steril. 2010, 94, 997–1007. [Google Scholar] [CrossRef]
- Ho, S.M.; Lee, M.T.; Lam, H.M.; Leung, Y.K. Estrogens and Prostate Cancer: Etiology, Mediators, Prevention, and Management. Endocrinol. Metab. Clin. N. Am. 2011, 40, 591–614. [Google Scholar] [CrossRef]
- Hamilton-Reeves, J.; Rebello, S.; Thomas, W.; Slaton, J.; Kurzer, M. Soy protein isolate increases urinary estrogens and the ratio of 2:16alfa-hydroxyesterone in men at high risk of prostate cancer. J. Nutr. 2007, 137, 2258–2263. [Google Scholar] [CrossRef]
- Lu, L.J.; Anderson, K.E.; Grady, J.J.; Nagamani, M. Effects of soya consumption for one month on steroid hormones in premenopausal women: Implications for breast cancer risk reduction. Cancer Epidemiol. Biomark. Prev. 1996, 5, 63–70. [Google Scholar]
- Lu, L.J.; Cree, M.; Josyula, S.; Nagamani, M.; Grady, J.J.; Anderson, K.E. Increased urinary excretion of 2-hydroxyestrone but not 16α- hydroxyestrone in premenopausal women during a soya diet containing isoflavones. Cancer Res. 2000, 60, 1299–1305. [Google Scholar]
- Lu, L.J.; Anderson, K.E.; Grady, J.J.; Nagamani, M. Effects of an isoflavone-free soy diet on ovarian hormones in premenopausal women. J. Clin. Endocrinol. Metab. 2001, 86, 3045–3052. [Google Scholar] [CrossRef]
- Maskarinec, G.; Beckford, F.; Morimoto, Y.; Franke, A.A.; Stanczyk, F.Z. Association of estrogen measurements in serum and urine of premenopausal women. Biomark. Med. 2015, 9, 417–424. [Google Scholar] [CrossRef]
- Kapiszewska, M.; Miskiewicz, M.; Ellison, P.T.; Thune, I.; Jasienska, G. High tea consumption diminishes salivary 17β-estradiol concentration in Polish women. Br. J. Nutr. 2006, 95, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Bingham, S.; Setchell, K. Biological effects of isoflavones in young women: Importance of the chemical composition of soyabean products. Br. J. Nutr. 1995, 74, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Romualdi, D.; Costantini, B.; Campagna, G.; Lanzone, A.; Guido, M. Is there a role for soy isoflavones in the therapeutic approach to polycystic ovary syndrome? Results from a pilot study. Fertil. Steril. 2008, 90, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.S.; Park, S.Y.; Kim, M.G.; Yim, C.H.; Yoon, H.K.; Han, K.O. Marked individual variation in isoflavone metabolism after a soy challenge can modulate the skeletal effect of isoflavones in premenopausal women. J. Korean Med. Sci. 2009, 24, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Williams, A.E.; Inouye, J.S.; Stanczyk, F.Z.; Franke, A.A. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol. Biomark. Prev. 2002, 11, 195–201. [Google Scholar]
- Martini, M.C.; Dancisak, B.B.; Haggans, C.J.; Thomas, W.; Slavin, J.L. Effects of soy intake on sex hormone metabolism in premenopausal women. Nutr. Cancer 1999, 34, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Tamai, Y.; Wada, K.; Nakamura, K.; Hayashi, M.; Takeda, N.; Yasuda, K.; Nagata, C. Associations of intakes of fat, dietary fiber, soy isoflavones, and alcohol with levels of sex hormones and prolactin in premenopausal Japanese women. Cancer Causes Control 2012, 23, 683–689. [Google Scholar] [CrossRef]
- Maskarinec, G.; Ollberding, N.J.; Conroy, S.M.; Morimoto, Y.; Pagano, I.S.; Franke, A.A.; Gentzschein, E.; Stanczyk, F.Z. Estrogen levels in nipple aspirate fluid and serum during a randomized soy trial. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1815–1821. [Google Scholar] [CrossRef]
- Morimoto, Y.; Conroy, S.M.; Pagano, I.S.; Isaki, M.; Franke, A.A.; Nordt, F.J.; Maskarinec, G. Urinary estrogen metabolites during a randomized soy trial. Nutr. Cancer 2012, 64, 307–314. [Google Scholar] [CrossRef]
- Duncan, A.M.; Merz-Demlow, B.E.; Xu, X.; Phipps, W.R.; Kurzer, M.S. Premenopausal equol excretors show plasma hormone profiles associated with lowered risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 2000, 9, 581–586. [Google Scholar]
- Setchell, K.D.R.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Phipps, W.R.; Martini, M.C.; Lampe, J.W.; Slavin, J.L.; Kurzer, M.S. Effect of flax seed ingestion on the menstrual cycle. J. Clin. Endocrinol. Metab. 1993, 77, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Haggans, C.J.; Travelli, E.J.; Thomas, W.; Martini, M.C.; Slavin, J.L. The effect of flaxseed and wheat bran consumption on urinary estrogen metabolites in premenopausal women. Cancer Epidemiol. Biomark. Prev. 2000, 9, 719–725. [Google Scholar]
- Cassidy, A.; Bingham, S.; Setchell, K.D. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am. J. Clin. Nutr. 1994, 60, 333–340. [Google Scholar] [CrossRef]
- Maskarinec, G.; Franke, A.A.; Williams, A.E.; Hebshi, S.; Oshiro, C.; Murphy, S.; Stanczyk, F.Z. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1736–1744. [Google Scholar] [CrossRef]
- Nagata, C.; Takatsuka, N.; Inaba, S.; Kawakami, N.; Shimizu, H. Effect of Soymilk Consumption on Serum Estrogen Concentrations in Premenopausal Japanese Women. J. Natl. Cancer Inst. 1998, 90, 1830–1835. [Google Scholar] [CrossRef]
- Watanabe, S.; Terashima, K.; Sato, Y.; Arai, S.; Eboshida, A. Effects of isoflavone supplement on healthy women. BioFactors 2000, 12, 233–241. [Google Scholar] [CrossRef]
- Kumar, N.B.; Cantor, A.; Allen, K.; Riccardi, D.; Cox, C.E. The specific role of isoflavones on estrogen metabolism in premenopausal women. Cancer 2002, 94, 1166–1174. [Google Scholar] [CrossRef]
- Nelson, H.D. Menopause. Lancet 2008, 371, 760–770. [Google Scholar] [CrossRef]
- Llaneza, P.; González, C.; Fernández-Iñarrea, J.; Alonso, A.; Díaz, F.; Pérez-López, F.R. Soy isoflavones improve insulin sensitivity without changing serum leptin among postmenopausal women. Climacteric 2012, 15, 611–620. [Google Scholar] [CrossRef]
- Villa, P.; Costantini, B.; Suriano, R.; Perri, C.; Macrì, F.; Ricciardi, L.; Panunzi, S.; Lanzone, A. The differential effect of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women: Relationship with the metabolic status. J. Clin. Endocrinol. Metab. 2009, 94, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.; Amar, I.D.; Bottoni, C.; Cipolla, C.; Dinoi, G.; Moruzzi, M.C.; Scambia, G.; Lanzone, A. The impact of combined nutraceutical supplementation on quality of life and metabolic changes during the menopausal transition: A pilot randomized trial. Arch. Gynecol. Obstet. 2017, 296, 791–801. [Google Scholar] [CrossRef]
- Rosa Lima, S.M.R.; Bernardo, B.F.A.; Yamada, S.S.; Reis, B.F.; Da Silva, G.M.D.; Longo Galvão, M.A. Effects of Glycine max (L.) Merr. soy isoflavone vaginal gel on epithelium morphology and estrogen receptor expression in postmenopausal women: A 12-week, randomized, double-blind, placebo-controlled trial. Maturitas 2014, 78, 205–211. [Google Scholar] [CrossRef]
- Tousen, Y.; Ezaki, J.; Fujii, Y.; Ueno, T.; Nishimuta, M.; Ishimi, Y. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: A pilot randomized, placebo-controlled trial. Menopause 2011, 18, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Oka, J.; Higuchi, M.; Tabata, I.; Toda, T.; Fujioka, M.; Fuku, N.; Teramoto, T.; Okuhira, T.; Ueno, T.; et al. Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: A randomized placebo-controlled trial. Metabolism 2006, 55, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Oka, J.; Tabata, I.; Higuchi, M.; Toda, T.; Fuku, N.; Ezaki, J.; Sugiyama, F.; Uchiyama, S.; Yamada, K.; et al. Effects of isoflavone and exercise on BMD and fat mass in postmenopausal Japanese women: A 1-year randomized placebo-controlled trial. J. Bone Miner. Res. 2006, 21, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qin, L.; Shi, Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: A 24-month randomized, double-blind and placebo-controlled trial. J. Bone Miner. Res. 2007, 22, 1072–1079. [Google Scholar] [CrossRef]
- Spence, L.A.; Lipscomb, E.R.; Cadogan, J.; Martin, B.; Wastney, M.E.; Peacock, M.; Weaver, C.M. The effect of soy protein and soy isoflavones on calcium metabolism in postmenopausal women: A randomized crossover study. Am. J. Clin. Nutr. 2005, 81, 916–922. [Google Scholar] [CrossRef]
- Reed, S.D.; Newton, K.M.; LaCroix, A.Z.; Grothaus, L.C.; Grieco, V.S.; Ehrlich, K. Vaginal, endometrial, and reproductive Hormone Findings: Randomized, placebo-controlled trial of black cohosh, multibotanical Herbs, and dietary soy for vasomotor symptoms: The Herbal Alternatives for Menopause (HALT) Study. Menopause 2008, 15, 51–58. [Google Scholar] [CrossRef]
- Evans, M.; Elliott, J.G.; Sharma, P.; Berman, R.; Guthrie, N. The effect of synthetic genistein on menopause symptom management in healthy postmenopausal women: A multi-center, randomized, placebo-controlled study. Maturitas 2011, 68, 189–196. [Google Scholar] [CrossRef]
- Wu, W.-H.; Kang, Y.-P.; Wang, N.-H.; Jou, H.-J.; Wang, T.-A. Sesame Ingestion Affects Sex Hormones, Antioxidant Status, and Blood Lipids in Postmenopausal Women. J. Nutr. 2006, 136, 1270–1275. [Google Scholar] [CrossRef]
- Foth, D.; Nawroth, F. Effect of soy supplementation on endogenous hormones in postmenopausal women. Gynecol. Obstet. Investig. 2003, 55, 135–138. [Google Scholar] [CrossRef]
- Baird, D.D.; Umbach, D.M.; Lansdell, L.; Hughes, L.; Setchell, K.D.; Weinberg, R.; Haney, F.; Wilcox, J.; Mclachian, J.A. Dietary Intervention Study to Assess Estrogenicity of Dietary Soy Among Postmenopausal Women. J. Clin. Endocrinol. Metab. 1995, 80, 1685–1690. [Google Scholar]
- Murray, M.J.; Meyer, W.R.; Lessey, B.A.; Oi, R.H.; DeWire, R.E.; Fritz, M.A. Soy protein isolate with isoflavones does not prevent estradiol-induced endometrial hyperplasia in postmenopausal women: A pilot trial. Menopause 2003, 10, 456–464. [Google Scholar] [CrossRef]
- Wu, A.H.; Stanczyk, F.Z.; Martinez, C.; Tseng, C.C.; Hendrich, S.; Murphy, P.; Chaikittisilpa, S.; Stram, D.O.; Pike, M.C. A controlled 2-mo dietary fat reduction and soy food supplementation study in postmenopausal women. Am. J. Clin. Nutr. 2005, 81, 1133–1141. [Google Scholar] [CrossRef][Green Version]
- Lee, C.C.; Bloem, C.J.; Kasa-Vubu, J.Z.; Liang, L.-J. Effect of oral phytoestrogen on androgenicity and insulin sensitivity in postmenopausal women. Diabetes Obes. Metab. 2012, 14, 315–319. [Google Scholar] [CrossRef]
- Goldin, B.R.; Brauner, E.; Adlercreutz, H.; Ausman, L.M.; Lichtenstein, A.H. Hormonal response to diets high in soy or animal protein without and with isoflavones in moderately hypercholesterolemic subjects. Nutr. Cancer 2005, 51, 1–6. [Google Scholar] [CrossRef]
- Lucas, E.A.; Wild, R.D.; Hammond, L.J.; Khalil, D.A.; Juma, S.; Daggy, B.P.; Stoecker, B.J.; Arjmandi, B.H. Flaxseed improves lipid profile without altering biomarkers of bone metabolism in postmenopausal women. J. Clin. Endocrinol. Metab. 2002, 87, 1527–1532. [Google Scholar] [CrossRef]
- Rashid, A.; Khurshid, R.; Latif, A.; Ahmad, N.; Aftab, L. Role of phytoestrogen in suppressing bone turnover in a group of postmenopausal women. J. Ayub Med. Coll. Abbottabad 2010, 22, 201–204. [Google Scholar]
- Rios, D.R.A.; Rodrigues, E.T.; Cardoso, A.P.Z.; Montes, M.B.A.; Franceschini, S.A.; Toloi, M.R.T. Lack of effects of isoflavones on the lipid profile of Brazilian postmenopausal women. Nutrition 2008, 24, 1153–1158. [Google Scholar] [CrossRef]
- Wu, A.H.; Spicer, D.; Stanczyk, F.Z.; Tseng, C.C.; Yang, C.S.; Pike, M.C. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev. Res. 2012, 5, 393–402. [Google Scholar] [CrossRef]
- Morabito, N.; Crisafulli, A.; Vergara, C.; Gaudio, A.; Lasco, A.; Frisina, N.; D’Anna, R.; Corrado, F.; Pizzoleo, M.A.; Cincotta, M.; et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: A randomized double-blind placebo-controlled study. J. Bone Miner. Res. 2002, 17, 1904–1912. [Google Scholar] [CrossRef]
- Levis, S.; Strickman-Stein, N.; Ganjei-Azar, P.; Xu, P.; Doerge, D.R.; Krischer, J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Arch. Intern. Med. 2011, 171, 1363–1369. [Google Scholar] [CrossRef]
- Pino, A.M.; Valladares, L.E.; Palma, M.A.; Mancilla, A.M.; Yáñez, M.; Albala, C. Dietary isoflavones affect sex hormone-binding globulin levels in postmenopausal women. J. Clin. Endocrinol. Metab. 2000, 85, 2797–2800. [Google Scholar] [CrossRef]
- Persky, V.W.; Turyk, M.E.; Wang, L.; Freels, S.; Chatterton, R., Jr.; Barnes, S.; Erdman, J., Jr.; Sepkovic, D.W.; Bradlow, H.L.; Potter, S. Effect of Soy Protein on Endogenous Hormones in Postmenopausal Women. Am. J. Clin. Nutr. 2002, 75, 145–153. [Google Scholar] [CrossRef]
- Oh, H.Y.; Kim, S.S.; Chung, H.Y.; Yoon, S. Isoflavone supplements exert hormonal and antioxidant effects in postmenopausal Korean women with diabetic retinopathy. J. Med. Food 2005, 8, 1–7. [Google Scholar] [CrossRef]
- Uesugi, S.; Watanabe, S.; Ishiwata, N.; Uehara, M.; Ouchi, K. Effects of isoflavone supplements on bone metabolic markers and climacteric symptoms in Japanese women. BioFactors 2004, 22, 221–228. [Google Scholar] [CrossRef]
- Sapbamrer, R.; Visavarungroj, N.; Suttajit, M. Effects of dietary traditional fermented soybean on reproductive hormones, lipids, and glucose among postmenopausal women in northern thailand. Asia Pac. J. Clin. Nutr. 2013, 22, 222–228. [Google Scholar] [CrossRef]
- Hutchins, A.M.; Martini, M.C.; Olson, B.A.; Thomas, W.; Joanne, L.; Slavin, J.L. Flaxseed Consumption Influences Endogenous Hormone Concentrations in Postmenopausal Women. Nutr. Cancer 2009, 5581, 37–41. [Google Scholar] [CrossRef]
- Sturgeon, S.R.; Heersink, J.L.; Volpe, S.L.; Bertone-Johnson, E.R.; Puleo, E.; Stanczyk, F.Z.; Sabelawski, S.; Wähälä, K.; Kurzer, M.S.; Bigelow, C. Effect of dietary flaxseed on serum levels of estrogens and androgens in postmenopausal women. Nutr. Cancer 2008, 60, 612–618. [Google Scholar] [CrossRef]
- Törmälä, R.; Appt, S.; Clarkson, T.B.; Mueck, A.O.; Seeger, H.; Mikkola, T.S.; Ylikorkala, O. Impact of soy supplementation on sex steroids and vascular inflammation markers in postmenopausal women using tibolone: Role of equol production capability. Climacteric 2008, 11, 409–415. [Google Scholar] [CrossRef]
- Low, Y.L.; Taylor, J.I.; Grace, P.B.; Dowsett, M.; Scollen, S.; Dunning, A.M.; Mulligan, A.A.; Welch, A.A.; Luben, R.N.; Khaw, K.T.; et al. Phytoestrogen exposure correlation with plasma estradiol in postmenopausal women in European Prospective Investigation of Cancer and Nutrition-Norfolk may involve diet-gene interactions. Cancer Epidemiol. Biomark. Prev. 2005, 14, 213–220. [Google Scholar]
- Lambert, M.N.T.; Hu, L.M.; Jeppesen, P.B. A systematic review and meta-analysis of the effects of isoflavone formulations against estrogen-deficient bone resorption in peri- and postmenopausal women. Am. J. Clin. Nutr. 2017, 106, 801–811. [Google Scholar] [CrossRef]
- Haggans, C.J.; Hutchins, A.M.; Olson, B.A.; Thomas, W.; Martini, M.C.; Slavin, J.L. Effect of flaxseed consumption on urinary estrogen metabolites in postmenopausal women. Nutr. Cancer 1999, 33, 188–195. [Google Scholar] [CrossRef]
- Brooks, J.D.; Ward, W.E.; Lewis, J.E.; Hilditch, J.; Nickell, L.; Wong, E.; Thompson, L.U. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy 1-3. Am. J. Clin. Nutr. 2004, 79, 318–325. [Google Scholar] [CrossRef]
- Sturgeon, S.R.; Volpe, S.L.; Puleo, E.; Bertone-Johnson, E.R.; Heersink, J.; Sabelawski, S.; Wahala, K.; Bigelow, C.; Kurzer, M.S. Effect of flaxseed consumption on urinary levels of estrogen metabolites in postmenopausal women. Nutr. Cancer 2010, 62, 175–180. [Google Scholar] [CrossRef]
- Scambia, G.; Mango, D.; Signorile, P.G.; Angeli, R.A.; Palena, C.; Gallo, D.; Bombardelli, E.; Morazzoni, P.; Riva, A.; Mancuso, S. Clinical effects of a standardized soy extract in postmenopausal women: A pilot study. Menopause 2000, 7, 105–111. [Google Scholar] [CrossRef]
- Sosvorová, L.; Mikšátková, P.; Bičíková, M.; Kaňová, N.; Lapčík, O. The presence of monoiodinated derivates of daidzein and genistein in human urine and its effect on thyroid gland function. Food Chem. Toxicol. 2012, 50, 2774–2779. [Google Scholar] [CrossRef]
- Jayagopal, V.; Albertazzi, P.; Kilpatrick, E.S.; Howarth, E.M.; Jennings, P.E.; Hepburn, D.A.; Atkin, S.L. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care 2002, 25, 1709–1714. [Google Scholar] [CrossRef]
- Crisafulli, A.; Altavilla, D.; Marini, H.; Bitto, A.; Cucinotta, D.; Frisina, N.; Corrado, F.; D’Anna, R.; Squadrito, G.; Adamo, E.B.; et al. Effects of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women. Menopause 2005, 12, 186–192. [Google Scholar] [CrossRef]
- Basaria, S.; Wisniewski, A.; Dupree, K.; Bruno, T.; Song, M.; Yao, F.; Ojumu, A.; John, M.; Dobs, A.S. Effect of High-Dose Isoflavones on Cognition, Quality of life, Androgens, and Lipoprotein in Post-Menopausal Women. J. Endocrinol. Investig. 2009, 32, 150–155. [Google Scholar] [CrossRef]
- Baber, R.J.; Templeman, C.; Morton, T.; Kelly, G.E.; West, L. Randomized placebo-controlled trial of an isoflavone supplement and menopausal symptoms in women. Climacteric 1999, 2, 85–92. [Google Scholar] [CrossRef]
- Kapoor, R.; Ronnenberg, A.; Puleo, E.; Chatterton, R.T.; Dorgan, J.F.; Seeram, N.P.; Sturgeon, S.R. Effects of Pomegranate Juice on Hormonal Biomarkers of Breast Cancer Risk. Nutr. Cancer 2015, 67, 1113–1119. [Google Scholar] [CrossRef]
- Monroe, K.R.; Murphy, S.P.; Henderson, B.E.; Kolonel, L.N.; Stanczyk, F.Z.; Adlercreutz, H.; Pike, M.C. Dietary fiber intake and endogenous serum hormone levels in naturally postmenopausal Mexican American women: The multiethnic cohort study. Nutr. Cancer 2007, 58, 127–135. [Google Scholar] [CrossRef]
- Low, Y.L.; Dunning, A.M.; Dowsett, M.; Folkerd, E.; Doody, D.; Taylor, J.; Bhaniani, A.; Luben, R.; Khaw, K.T.; Wareham, N.J.; et al. Phytoestrogen exposure is associated with circulating sex hormone levels in postmenopausal women and interact with ESR1 and NR1I2 gene variants. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1009–1016. [Google Scholar] [CrossRef]
- Wu, A.H.; Stanczyk, F.Z.; Seow, A.; Lee, H.P.; Yu, M.C. Soy intake and other lifestyle determinants of serum estrogen levels among postmenopausal Chinese women in Singapore. Cancer Epidemiol. Biomark. Prev. 2002, 11, 844–851. [Google Scholar]
- Messina, M.; Redmond, G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: A review of the relevant literature. Thyroid 2006, 16, 249–258. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Manuchehri, A.M.; Thatcher, N.J.; Rigby, A.S.; Chapman, T.; Kilpatrick, E.S.; Atkin, S.L. The effect of soy phytoestrogen supplementation on thyroid status and cardiovascular risk markers in patients with subclinical hypothyroidism: A randomized, double-blind, crossover study. J. Clin. Endocrinol. Metab. 2011, 96, 1442–1449. [Google Scholar] [CrossRef]
- Li, J.; Teng, X.; Wang, W.; Chen, Y.; Yu, X.; Wang, S.; Li, J.; Zhu, L.; Li, C.; Fan, C.; et al. Effects of dietary soy intake on maternal thyroid functions and serum anti-thyroperoxidase antibody level during early pregnancy. J. Med. Food 2011, 14, 543–550. [Google Scholar] [CrossRef]
- Milerová, J.; Čeřovská, J.; Zamrazil, V.; Bílek, R.; Lapčík, O.; Hampl, R. Actual levels of soy phytoestrogens in children correlate with thyroid laboratory parameters. Clin. Chem. Lab. Med. 2006, 44, 171–174. [Google Scholar] [CrossRef]
- Conrad, S.C.; Chiu, H.; Silverman, B.L. Soy formula complicates management of congenital hypothyroidism. Arch. Dis. Child. 2004, 89, 37–40. [Google Scholar] [CrossRef]
- Zung, A.; Shachar, S.; Zadik, Z.; Kerem, Z. Soy-derived isoflavones treatment in children with hypercholesterolemia: A pilot study. J. Pediatr. Endocrinol. Metab. 2010, 23, 133–141. [Google Scholar] [CrossRef]
- Hampl, R.; Ostatnikova, D.; Celec, P.; Putz, Z.; Lapcík, O.; Matucha, P. Short-term Effect of Soy Consumption on Thyroid Hormone Levels and Correlation with Phytoestrogen Level in Healthy Subjects. Endocr. Regul. 2008, 42, 53–61. [Google Scholar]
- Sathyapalan, T.; Rigby, A.S.; Bhasin, S.; Thatcher, N.J.; Kilpatrick, E.S.; Atkin, S.L. Effect of soy in men with type 2 diabetes mellitus and subclinical hypogonadism: A randomized controlled study. J. Clin. Endocrinol. Metab. 2017, 102, 425–433. [Google Scholar] [CrossRef]
- Zhou, Y.; Alekel, D.L.; Dixon, P.M.; Messina, M.; Reddy, M.B. The effect of soy food intake on mineral status in premenopausal women. J. Women’s Health 2011, 20, 771–780. [Google Scholar] [CrossRef]
- Duncan, A.M.; Merz, B.E.; Xu, X.; Nagel, T.C.; Phipps, W.R.; Kurzer, M.S. Soy Isoflavones Exert Modest Hormonal Effects in Premenopausal Women. J. Clin. Endocrinol. Metab. 1999, 84, 192–197. [Google Scholar] [CrossRef]
- Mittal, N.; Hota, D.; Dutta, P.; Bhansali, A.; Suri, V.; Aggarwal, N.; Marwah, R.K.; Chakrabarti, A. Evaluation of effect of isoflavone on thyroid economy & autoimmunity in oophorectomised women: A randomised, double-blind, placebo-controlled trial. Indian J. Med. Res. 2011, 133, 633–640. [Google Scholar]
- Khaodhiar, L.; Ricciotti, H.A.; Li, L.; Pan, W.; Schickel, M.; Zhou, J.; Blackburn, G.L. Daidzein-rich isoflavone aglycones are potentially effective in reducing hot flashes in menopausal women. Menopause 2008, 15, 125–132. [Google Scholar] [CrossRef]
- Ryan-Borchers, T.; Chew, B.; Park, J.S.; Mcguire, M.; Fournier, L.; Beerman, K. Effects of Dietary and Supplemental Forms of Isoflavones on Thyroid Function in Healthy Postmenopausal Women. Top. Clin. Nutr. 2007, 23, 13–22. [Google Scholar] [CrossRef]
- Pop, E.A.; Fischer, L.M.; Coan, A.D.; Gitzinger, M.; Nakamura, J.; Zeisel, S.H. Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis, and estrogenic outcomes in healthy postmenopausal women: A phase I clinical trial. Menopause 2008, 15, 684–692. [Google Scholar] [CrossRef]
- Bitto, A.; Polito, F.; Atteritano, M.; Altavilla, D.; Mazzaferro, S.; Marini, H.; Adamo, E.B.; D’Anna, R.; Granese, R.; Corrado, F.; et al. Genistein aglycone does not affect thyroid function: Results from a three-year, randomized, double-blind, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2010, 95, 3067–3072. [Google Scholar] [CrossRef]
- Alekel, D.L.; Genschel, U.; Koehler, K.J.; Hofmann, H.; Van Loan, M.D.; Beer, B.S.; Hanson, L.N.; Peterson, C.T.; Kurzer, M.S. Soy Isoflavones for Reducing Bone Loss Study: Effects of a 3-year trial on hormones, adverse events, and endometrial thickness in postmenopausal women. Menopause 2015, 22, 185–197. [Google Scholar] [CrossRef]
- Steinberg, F.M.; Murray, M.J.; Lewis, R.D.; Cramer, M.A.; Amato, P.; Young, R.L.; Barnes, S.; Konzelmann, K.L.; Fischer, J.G.; Ellis, K.J.; et al. Clinical outcomes of a 2-y soy isoflavone supplementation in menopausal women. Am. J. Clin. Nutr. 2011, 93, 356–367. [Google Scholar] [CrossRef]
- Liu, Y.; Vu, V.; Sweeney, G. Examining the Potential of Developing and Implementing Use of Adiponectin-Targeted Therapeutics for Metabolic and Cardiovascular Diseases. Front. Endocrinol. Lausanne 2019, 10, 842. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. Lausanne 2013, 4, 71. [Google Scholar] [CrossRef]
- Su, X.; Peng, D. Adipokines as novel biomarkers of cardio-metabolic disorders. Clin. Chim. Acta 2020, 507, 31–38. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Tokudome, T.; Otani, K.; Miyazato, M.; Kangawa, K. Ghrelin and the heart. Peptides 2019, 111, 42–46. [Google Scholar] [CrossRef]
- Shi, L.; Ryan, H.H.; Jones, E.; Moore Simas, T.A.; Lichtenstein, A.H.; Sun, Q.; Hayman, L.L. Urinary Isoflavone Concentrations Are Inversely Associated with Cardiometabolic Risk Markers in Pregnant U.S. Women. J. Nutr. 2014, 144, 344–351. [Google Scholar] [CrossRef]
- Van der Schouw, Y.T.; Sampson, L.; Willett, W.C.; Rimm, E.B. The Usual Intake of Lignans but Not That of Isoflavones May Be Related to Cardiovascular Risk Factors in U.S. Men. J. Nutr. 2005, 135, 260–266. [Google Scholar] [CrossRef]
- Rohrmann, S.; Shvetsov, Y.B.; Morimoto, Y.; Wilkens, L.R.; Monroe, K.R.; Le Marchand, L.; Franke, A.A.; Kolonel, L.N.; Maskarinec, G. Self-reported dietary flavonoid intake and serum markers of inflammation: The multiethnic cohort. Cancer Causes Control 2018, 29, 601–607. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Ryan, M.F.; Gibney, E.R.; Brennan, L.; Roche, H.M.; Reilly, M.P. Dietary isoflavone intake is associated with evoked responses to inflammatory cardiometabolic stimuli and improved glucose homeostasis in healthy volunteers. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 996–1003. [Google Scholar] [CrossRef]
- Reverri, E.J.; Lasalle, C.D.; Franke, A.A.; Steinberg, F.M. Soy provides modest benefits on endothelial function without affecting inflammatory biomarkers in adults at cardiometabolic risk. Mol. Nutr. Food Res. 2015, 59, 323–333. [Google Scholar] [CrossRef]
- Qin, Y.; Shu, F.; Zeng, Y.; Meng, X.; Wang, B.; Diao, L.; Wang, L.; Wan, J.; Zhu, J.; Wang, J.; et al. Daidzein Supplementation Decreases Serum Triglyceride and Uric Acid Concentrations in Hypercholesterolemic Adults with the Effect on Triglycerides Being Greater in Those with the GA Compared with the GG Genotype of ESR-β RsaI. J. Nutr. 2014, 144, 49–54. [Google Scholar] [CrossRef]
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A.; Shimatsu, A.; Inaguma, A.; Ueno, T.; Uchiyama, S.; et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. Oxf. 2013, 78, 365–372. [Google Scholar] [CrossRef]
- Amanat, S.; Eftekhari, M.H.; Fararouei, M.; Bagheri Lankarani, K.; Massoumi, S.J. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: A randomized, controlled trial. Clin. Nutr. 2018, 37, 1210–1215. [Google Scholar] [CrossRef]
- Vrieling, A.; Rookus, M.A.; Kampman, E.; Bonfrer, J.M.G.; Korse, C.M.; Van Doorn, J.; Lampe, J.W.; Cats, A.; Witteman, B.J.M.; van Leeuwen, F.E.; et al. Isolated Isoflavones Do Not Affect the Circulating Insulin-Like Growth Factor System in Men at Increased Colorectal Cancer Risk. J. Nutr. 2007, 137, 379–383. [Google Scholar] [CrossRef][Green Version]
- Maskarinec, G.; Oum, R.; Chaptman, A.K.; Ognjanovic, S. Inflammatory markers in a randomised soya intervention among men. Br. J. Nutr. 2009, 101, 1740–1744. [Google Scholar] [CrossRef]
- Kreijkamp-Kaspers, S.; Kok, L.; Bots, M.L.; Grobbee, D.E.; Van Der Schouw, Y.T. Dietary phytoestrogens and vascular function in postmenopausal women: A cross-sectional study. J. Hypertens. 2004, 22, 1381–1388. [Google Scholar] [CrossRef]
- Morisset, A.S.; Lemieux, S.; Veilleux, A.; Bergeron, J.; John Weisnagel, S.; Tchernof, A. Impact of a lignan-rich diet on adiposity and insulin sensitivity in post-menopausal women. Br. J. Nutr. 2009, 102, 195–200. [Google Scholar] [CrossRef]
- Atteritano, M.; Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Mazzaferro, S.; D’Anna, R.; Cannata, M.L.; Gaudio, A.; et al. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: A two-year randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2007, 92, 3068–3075. [Google Scholar] [CrossRef]
- Marini, H.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; Frisina, N.; et al. Efficacy of genistein aglycone on some cardiovascular risk factors and homocysteine levels: A follow-up study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 332–340. [Google Scholar] [CrossRef]
- Irace, C.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; Arcoraci, V.; Minutoli, L.; Di Benedetto, A.; Di Vieste, G.; et al. Genistein and endothelial function in postmenopausal women with metabolic syndrome. Eur. J. Clin. Investig. 2013, 43, 1025–1031. [Google Scholar] [CrossRef]
- Squadrito, F.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; D’Anna, R.; Arcoraci, V.; Burnett, B.P.; Minutoli, L.; et al. Genistein in the metabolic syndrome: Results of a randomized clinical trial. J. Clin. Endocrinol. Metab. 2013, 98, 3366–3374. [Google Scholar] [CrossRef]
- Braxas, H.; Rafraf, M.; Karimi Hasanabad, S.; Asghari Jafarabadi, M. Effectiveness of Genistein Supplementation on Metabolic Factors and Antioxidant Status in Postmenopausal Women with Type 2 Diabetes Mellitus. Can. J. Diabetes 2019, 43, 490–497. [Google Scholar] [CrossRef]
- Hall, W.L.; Vafeiadou, K.; Hallund, J.; Bugel, S.; Reimann, M.; Koebnick, C.; Zunft, H.J.F.; Ferrari, M.; Branca, F.; Dadd, T.; et al. Soy-isoflavone-enriched foods and markers of lipid and glucose metabolism in postmenopausal women: Interactions with genotype and equol production. Am. J. Clin. Nutr. 2006, 83, 592–600. [Google Scholar] [CrossRef]
- Nadadur, M.; Stanczyk, F.Z.; Tseng, C.C.; Kim, L.; Wu, A.H. The Effect of Reduced Dietary Fat and Soy Supplementation on Circulating Adipocytokines in Postmenopausal Women: A Randomized Controlled 2-Month Trial. Nutr. Cancer 2016, 68, 554–559. [Google Scholar] [CrossRef]
- Matvienko, O.A.; Alekel, D.L.; Genschel, U.; Ritland, L.; Van Loan, M.D.; Koehler, K.J. Appetitive hormones, but not isoflavone tablets, influence overall and central adiposity in healthy postmenopausal women. Menopause 2010, 17, 594–601. [Google Scholar] [CrossRef]
- Llaneza, P.; González, C.; Fernandez-Iñarrea, J.; Alonso, A.; Diaz, F.; Arnott, I.; Ferrer-Barriendos, J. Soy isoflavones, diet and physical exercise modify serum cytokines in healthy obese postmenopausal women. Phytomedicine 2011, 18, 245–250. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Aye, M.; Rigby, A.S.; Thatcher, N.J.; Dargham, S.R.; Kilpatrick, E.S.; Atkin, S.L. Soy isoflavones improve cardiovascular disease risk markers in women during the early menopause. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 691–697. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Shaw, N.S.; Tsai, K.S.; Chen, C.Y. The hypoglycemic effects of soy isoflavones on postmenopausal women. J. Women’s Health 2004, 13, 1080–1086. [Google Scholar] [CrossRef]
- Charles, C.; Yuskavage, J.; Carlson, O.; John, M.; Tagalicud, A.S.; Maggio, M.; Muller, D.C.; Egan, J.; Basaria, S. Effects of high-dose isoflavones on metabolic and inflammatory markers in healthy postmenopausal women. Menopause 2009, 16, 395–400. [Google Scholar] [CrossRef]
- Christie, D.R.; Grant, J.; Darnell, B.E.; Chapman, V.R.; Gastaldelli, A.; Sites, C.K. Metabolic effects of soy supplementation in postmenopausal Caucasian and African American women: A randomized, placebo-controlled trial. Am. J. Obstet. Gynecol. 2010, 203, 153.e1–153.e9. [Google Scholar] [CrossRef]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef]
- Chiang, S.S.; Pan, T.M. Beneficial effects of phytoestrogens and their metabolites produced by intestinal microflora on bone health. Appl. Microbiol. Biotechnol. 2013, 97, 1489–1500. [Google Scholar] [CrossRef]
- Seibel, M.J. Biochemical markers of bone remodeling. Endocrinol. Metab. Clin. N. Am. 2003, 32, 83–113. [Google Scholar] [CrossRef]
- Goltzman, D. Physiology of Parathyroid Hormone. Endocrinol. Metab. Clin. N. Am. 2018, 47, 743–758. [Google Scholar] [CrossRef]
- Wangen, K.E.; Duncan, A.M.; Merz-Demlow, B.E.; Xu, X.; Marcus, R.; Phipps, W.R.; Kurzer, M.S. Effects of soy isoflavones on markers of bone turnover in premenopausal and postmenopausal women. J. Clin. Endocrinol. Metab. 2000, 85, 3043–3048. [Google Scholar] [CrossRef]
- Zittermann, A.; Geppert, J.; Baier, S.; Zehn, N.; Gouni-Berthold, I.; Berthold, H.K.; Reinsberg, J.; Stehle, P. Short-term effects of high soy supplementation on sex hormones, bone markers, and lipid parameters in young female adults. Eur. J. Nutr. 2004, 43, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The Pathophysiology and Treatment of Osteoporosis. Clin. Ther. 2015, 37, 1837–1850. [Google Scholar] [CrossRef] [PubMed]
- Chiechi, L.M.; Secreto, G.; D’Amore, M.; Fanelli, M.; Venturelli, E.; Cantatore, F.; Valerio, T.; Laselva, G.; Loizzi, P. Efficacy of a soy rich diet in preventing postmenopausal osteoporosis: The Menfis randomized trial. Maturitas 2002, 42, 295–300. [Google Scholar] [CrossRef]
- Scheiber, M.D.; Liu, J.H.; Subbiah, M.T.R.; Rebar, R.W.; Setchell, K.D.R. Dietary Inclusion of Whole Soy Foods Results in Significant Reductions in Clinical Risk Factors for Osteoporosis and Cardiovascular Disease in Normal Postmenopausal Women. Menopause 2001, 8, 384–392. [Google Scholar] [CrossRef]
- Ye, Y.B.; Tang, X.Y.; Verbruggen, M.A.; Su, Y.X. Soy isoflavones attenuate bone loss in early postmenopausal Chinese women: A single-blind randomized, placebo-controlled trial. Eur. J. Nutr. 2006, 45, 327–334. [Google Scholar] [CrossRef]
- Roudsari, A.H.; Tahbaz, F.; Hossein-Nezhad, A.; Arjmandi, B.; Larijani, B.; Kimiagar, S.M. Assessment of soy phytoestrogens’ effects on bone turnover indicators in menopausal women with osteopenia in Iran: A before and after clinical trial. Nutr. J. 2005, 4, 3–7. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef]
- Uesugi, T.; Fukui, Y.; Yamori, Y. Beneficial Effects of Soybean Isoflavone Supplementation on Bone Metabolism and Serum Lipids in Postmenopausal Japanese Women: A Four-Week Study. J. Am. Coll. Nutr. 2002, 21, 97–102. [Google Scholar] [CrossRef]
- Gambacciani, M.; Ciaponi, M.; Cappagli, B.; Piaggesi, L.; Genazzani, A.R. Effects of combined low dose of the isoflavone derivative ipriflavone and estrogen replacement on bone mineral density and metabolism in postmenopausal women. Maturitas 1997, 28, 75–81. [Google Scholar] [CrossRef]
- Weaver, C.M.; Martin, B.R.; Jackson, G.S.; McCabe, G.P.; Nolan, J.R.; McCabe, L.D.; Barnes, S.; Reinwald, S.; Boris, M.E.; Peacock, M. Antiresorptive effects of phytoestrogen supplements compared with estradiol or risedronate in postmenopausal women using 41Ca methodology. J. Clin. Endocrinol. Metab. 2009, 94, 3798–3805. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.; Briongos, L.S.; Ruiz-Mambrilla, M.; Velasco, E.A.; Linares, L.; Cuellar, L.; Olmos, J.M.; De Luis, D.; Dueñas-Laita, A.; Pérez-Castrillón, J.L. The Effect of Genistein Supplementation on Vitamin D Levels and Bone Turnover Markers during the Summer in Healthy Postmenopausal Women: Role of Genotypes of Isoflavone Metabolism. Lifestyle Genom. 2017, 10, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Vupadhyayula, P.M.; Gallagher, J.C.; Templin, T.; Logsdon, S.M.; Smith, L.M. Effects of soy protein isolate on bone mineral density and physical performance indices in postmenopausal women—A 2-year randomized, double-blind, placebo-controlled trial. Menopause 2009, 16, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: A randomized trial. Ann. Intern. Med. 2007, 146, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Yeung, S.S.C.; Kung, A.W.C. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J. Clin. Endocrinol. Metab. 2001, 86, 5217–5221. [Google Scholar] [CrossRef]
- Bahamonde, M.; Misra, M. Potential applications for rhIGF-I: Bone disease and IGFI. Growth Horm. IGF Res. 2020, 52, 101317. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Minireview: IGF, insulin, and cancer. Endocrinology 2011, 152, 2546–2551. [Google Scholar] [CrossRef]
- Scarth, J.P. Modulation of the growth hormone-insulin-like growth factor (GH-IGF) axis by pharmaceutical, nutraceutical and environmental xenobiotics: An emerging role for xenobiotic-metabolizing enzymes and the transcription factors regulating their expression. A rev. Xenobiotica 2006, 36, 119–218. [Google Scholar] [CrossRef]
- Campbell, M.J.; Woodside, J.V.; Honour, J.W.; Morton, M.S.; Leathem, A.J.C. Effect of red clover-derived isoflavone supplementation on insulin-like growth factor, lipid and antioxidant status in healthy female volunteers: A pilot study. Eur. J. Clin. Nutr. 2004, 58, 173–179. [Google Scholar] [CrossRef]
- Nagata, C.; Shimizu, H.; Takami, R.; Hayashi, M.; Takeda, N.; Yasuda, K. Dietary soy and fats in relation to serum insulin-like growth factor-1 and insulin-like growth factor-binding protein-3 levels in premenopausal Japanese women. Nutr. Cancer 2003, 45, 185–189. [Google Scholar] [CrossRef]
- Takata, Y.; Maskarinec, G.; Rinaldi, S.; Kaaks, R.; Nagata, C. Serum insulin-like growth factor-I levels among women in Hawaii and Japan with different levels of tofu intake. Nutr. Cancer 2006, 56, 136–142. [Google Scholar] [CrossRef]
- Vrieling, A.; Rookus, M.A.; Kampman, E.; Bonfrer, J.M.G.; Bosma, A.; Cats, A.; Van Doorn, J.; Korse, C.M.; Witteman, B.J.M.; Van Leeuwen, F.E.; et al. No effect of red clover-derived isoflavone intervention on the insulin-like growth factor system in women at increased risk of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2585–2593. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arjmandi, B.H.; Khalil, D.A.; Smith, B.J.; Lucas, E.A.; Juma, S.; Payton, M.E.; Wild, R.A. Soy protein has a greater effect on bone in postmenopausal women not on hormone replacement therapy, as evidenced by reducing bone resorption and urinary calcium excretion. J. Clin. Endocrinol. Metab. 2003, 88, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Probst-Hensch, N.M.; Wang, H.; Goh, V.H.H.; Seow, A.; Lee, H.P.; Yu, M.C. Determinants of circulating insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations in a cohort of Singapore men and women. Cancer Epidemiol. Biomark. Prev. 2003, 12, 739–746. [Google Scholar] [CrossRef]
- Rowlands, M.A.; Gunnell, D.; Harris, R.; Vatten, L.J.; Holly, J.M.P.; Martin, R.M. Circulating insulin-like growth factor peptides and prostate cancer risk: A systematic review and meta-analysis. Int. J. Cancer 2009, 124, 2416–2429. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-López, I.; Yago-Aragón, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, S. Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review. Nutrients 2020, 12, 2456. https://doi.org/10.3390/nu12082456
Domínguez-López I, Yago-Aragón M, Salas-Huetos A, Tresserra-Rimbau A, Hurtado-Barroso S. Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review. Nutrients. 2020; 12(8):2456. https://doi.org/10.3390/nu12082456
Chicago/Turabian StyleDomínguez-López, Inés, Maria Yago-Aragón, Albert Salas-Huetos, Anna Tresserra-Rimbau, and Sara Hurtado-Barroso. 2020. "Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review" Nutrients 12, no. 8: 2456. https://doi.org/10.3390/nu12082456
APA StyleDomínguez-López, I., Yago-Aragón, M., Salas-Huetos, A., Tresserra-Rimbau, A., & Hurtado-Barroso, S. (2020). Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review. Nutrients, 12(8), 2456. https://doi.org/10.3390/nu12082456






