Muscle Protein Synthesis and Whole-Body Protein Turnover Responses to Ingesting Essential Amino Acids, Intact Protein, and Protein-Containing Mixed Meals with Considerations for Energy Deficit
Abstract
1. Introduction
2. Considerations for the Application of MPS Measures
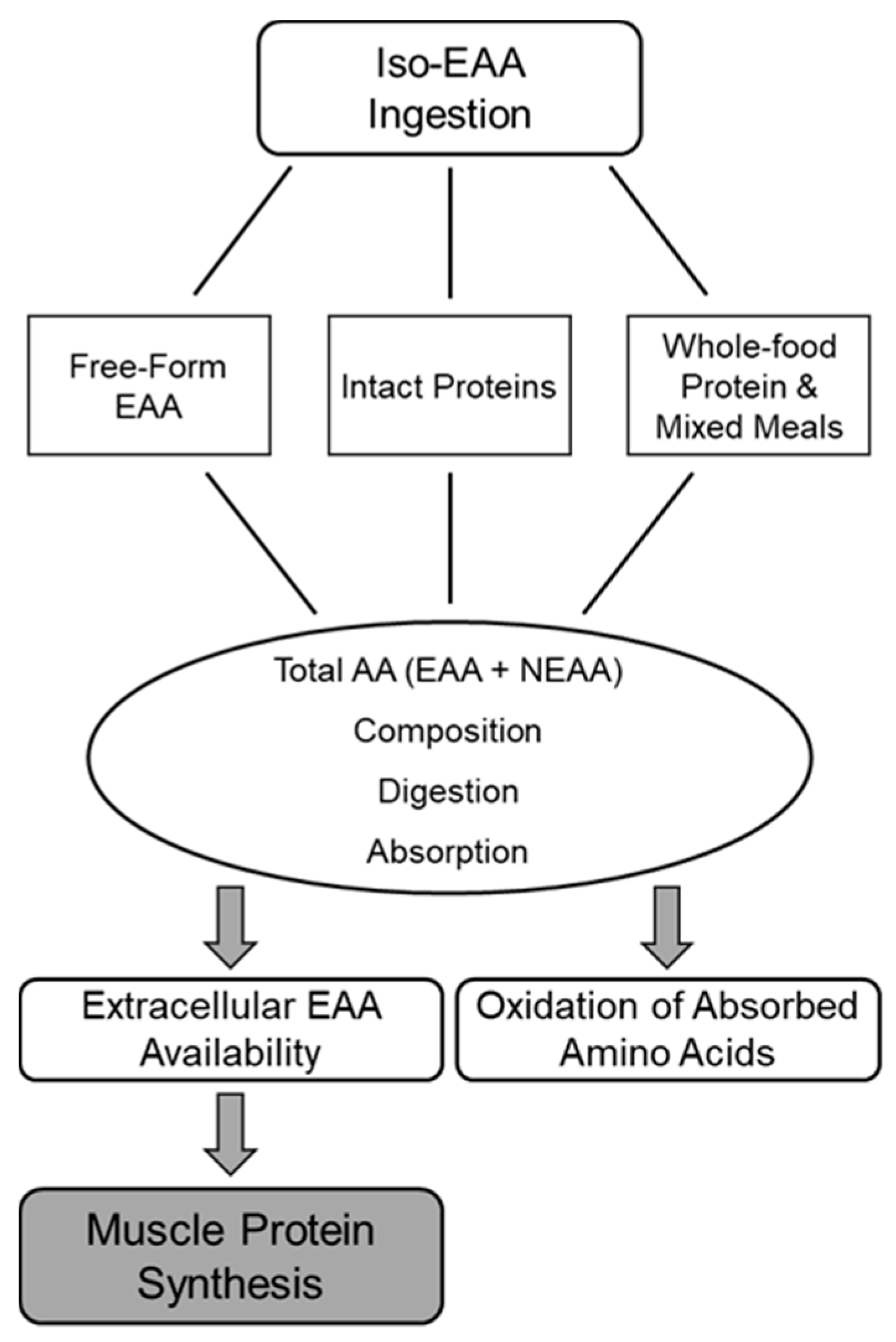
3. Supplemental EAA and Protein Feeding Format Effects on MPS
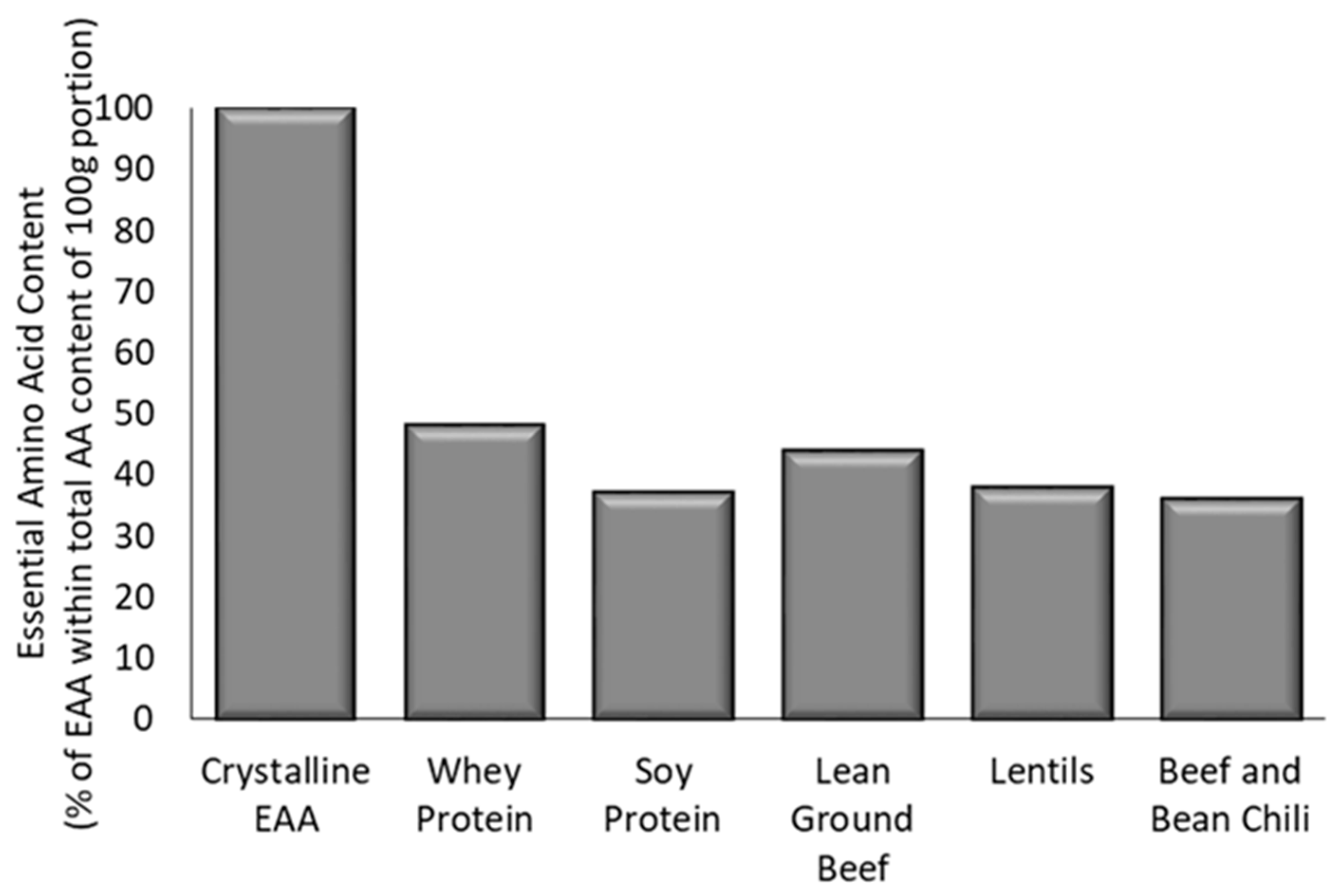
3.1. Total Amino Acid Content and Composition
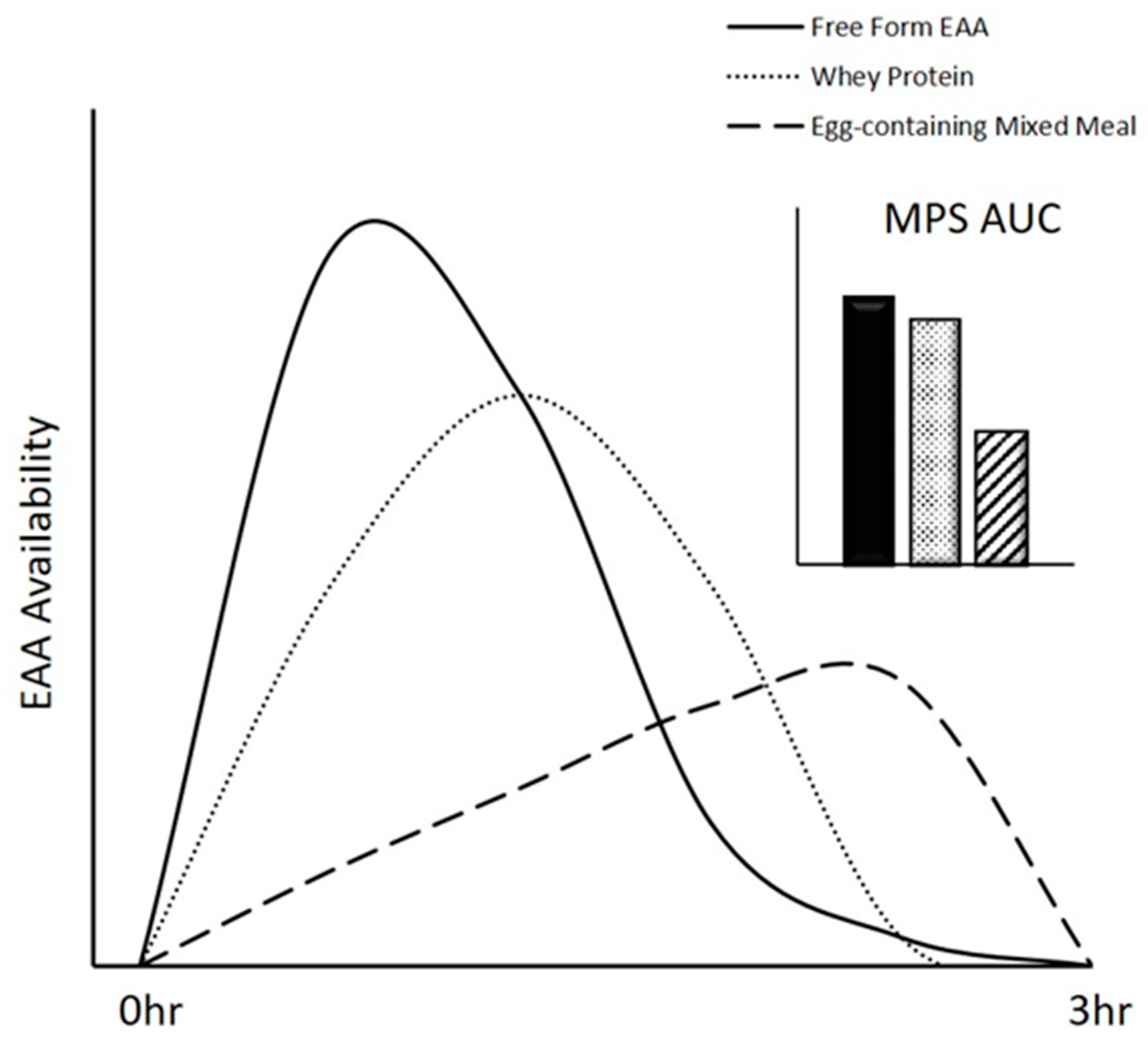
3.2. Digestion and Absorption
4. The Importance of Measuring MPS and Whole-Body Protein Turnover Together
5. Maximizing MPS and Whole-Body Protein Status during Energy Deficit
6. Knowledge Gaps Surrounding Protein and EAA Formats to Support Muscle Protein Synthesis and Whole-Body Protein Balance under Energy Deficit
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, S.L.; Tipton, K.D.; Chinkes, D.L.; Wolf, S.E.; Wolfe, R.R. Independent and combined effects of amino acids and glucose after resistance exercise. Med. Sci. Sports Exerc. 2003, 35, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Gurkin, B.E.; Matin, S.; Wolfe, R.R. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J. Nutr. Biochem. 1999, 10, 89–95. [Google Scholar] [CrossRef]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Burd, N.A.; Breen, L.; Rerecich, T.; Yang, Y.; Hector, A.J.; Baker, S.K.; Phillips, S.M. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2013, 38, 120–125. [Google Scholar] [CrossRef]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef]
- Holwerda, A.M.; Paulussen, K.J.M.; Overkamp, M.; Goessens, J.P.B.; Kramer, I.F.; Wodzig, W.; Verdijk, L.B.; van Loon, L.J.C. Dose-Dependent Increases in Whole-Body Net Protein Balance and Dietary Protein-Derived Amino Acid Incorporation into Myofibrillar Protein During Recovery from Resistance Exercise in Older Men. J. Nutr. 2019, 149, 221–230. [Google Scholar] [CrossRef]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef]
- Yang, Y.; Churchward-Venne, T.A.; Burd, N.A.; Breen, L.; Tarnopolsky, M.A.; Phillips, S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. 2012, 9, 57. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Austin, K.G.; Lieberman, H.R.; Askew, E.W. Efficacy and safety of protein supplements for U.S. Armed Forces personnel: Consensus statement. J. Nutr. 2013, 143, 1811S–1814S. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.M.; Carbone, J.W.; Berryman, C.E.; Carrigan, C.T.; Murphy, N.E.; Ferrando, A.A.; Young, A.J.; Pasiakos, S.M. Severe energy deficit at high altitude inhibits skeletal muscle mTORC1-mediated anabolic signaling without increased ubiquitin proteasome activity. FASEB J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.M.; Murphy, N.E.; Martini, S.; Gundersen, Y.; Castellani, J.W.; Karl, J.P.; Carrigan, C.T.; Teien, H.K.; Madslien, E.H.; Montain, S.J.; et al. Effects of Supplemental Energy on Protein Balance during 4-d Arctic Military Training. Med. Sci. Sports Exerc. 2016, 48, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Cao, J.J.; Margolis, L.M.; Sauter, E.R.; Whigham, L.D.; McClung, J.P.; Rood, J.C.; Carbone, J.W.; Combs, G.F., Jr.; Young, A.J. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: A randomized controlled trial. FASEB J. 2013, 27, 3837–3847. [Google Scholar] [CrossRef]
- Hector, A.J.; Marcotte, G.R.; Churchward-Venne, T.A.; Murphy, C.H.; Breen, L.; von Allmen, M.; Baker, S.K.; Phillips, S.M. Whey protein supplementation preserves postprandial myofibrillar protein synthesis during short-term energy restriction in overweight and obese adults. J. Nutr. 2015, 145, 246–252. [Google Scholar] [CrossRef]
- Areta, J.L.; Burke, L.M.; Camera, D.M.; West, D.W.; Crawshay, S.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; Hawley, J.A.; Coffey, V.G. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E989–E997. [Google Scholar] [CrossRef]
- Knapik, J.; Meredith, C.; Jones, B.; Fielding, R.; Young, V.; Evans, W. Leucine metabolism during fasting and exercise. J. Appl. Physiol. 1991, 70, 43–47. [Google Scholar] [CrossRef]
- Nair, K.S.; Woolf, P.D.; Welle, S.L.; Matthews, D.E. Leucine, glucose, and energy metabolism after 3 days of fasting in healthy human subjects. Am. J. Clin. Nutr. 1987, 46, 557–562. [Google Scholar] [CrossRef]
- Tsalikian, E.; Howard, C.; Gerich, J.E.; Haymond, M.W. Increased leucine flux in short-term fasted human subjects: Evidence for increased proteolysis. Am. J. Physiol. 1984, 247, E323–E327. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; Young, A.J.; Karl, J.P.; Kenefick, R.W.; Margolis, L.M.; Cole, R.E.; Carbone, J.W.; Lieberman, H.R.; Kim, I.Y.; Ferrando, A.A.; et al. Severe negative energy balance during 21 d at high altitude decreases fat-free mass regardless of dietary protein intake: A randomized controlled trial. FASEB. J. 2018, 32, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. Regulation of muscle protein by amino acids. J. Nutr. 2002, 132, 3219S–3224S. [Google Scholar] [CrossRef] [PubMed]
- Bohe, J.; Low, A.; Wolfe, R.R.; Rennie, M.J. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: A dose-response study. J. Physiol. 2003, 552, 315–324. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef]
- Carbone, J.W.; McClung, J.P.; Pasiakos, S.M. Recent Advances in the Characterization of Skeletal Muscle and Whole-Body Protein Responses to Dietary Protein and Exercise during Negative Energy Balance. Adv. Nutr. 2019, 10, 70–79. [Google Scholar] [CrossRef]
- Trommelen, J.; Betz, M.W.; van Loon, L.J.C. The Muscle Protein Synthetic Response to Meal Ingestion Following Resistance-Type Exercise. Sports Med. 2019, 49, 185–197. [Google Scholar] [CrossRef]
- Burd, N.A.; McKenna, C.F.; Salvador, A.F.; Paulussen, K.J.M.; Moore, D.R. Dietary Protein Quantity, Quality, and Exercise Are Key to Healthy Living: A Muscle-Centric Perspective Across the Lifespan. Front. Nutr. 2019, 6, 83. [Google Scholar] [CrossRef]
- Vliet, S.V.; Beals, J.W.; Martinez, I.G.; Skinner, S.K.; Burd, N.A. Achieving Optimal Post-Exercise Muscle Protein Remodeling in Physically Active Adults through Whole Food Consumption. Nutrients 2018, 10, 224. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Witard, O.C. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc. Nutr. Soc. 2018, 77, 20–31. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Chinkes, D.L. Isotope Tracers in Metabolic Research, 2nd ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Tipton, K.D.; Hamilton, D.L.; Gallagher, I.J. Assessing the Role of Muscle Protein Breakdown in Response to Nutrition and Exercise in Humans. Sports Med. (Auckland, N.Z.) 2018, 48, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Phillips, S.; Vechin, F.C.; Ugrinowitsch, C. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med. (Auckland, N.Z.) 2015, 45, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Churchward-Venne, T.A.; West, D.W.; Burd, N.A.; Breen, L.; Baker, S.K.; Phillips, S.M. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J. Appl. Physiol. (Bethesda, Md. 1985) 2012, 113, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; West, D.W.; Staples, A.W.; Atherton, P.J.; Baker, J.M.; Moore, D.R.; Holwerda, A.M.; Parise, G.; Rennie, M.J.; Baker, S.K.; et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS ONE 2010, 5, e12033. [Google Scholar] [CrossRef]
- West, D.W.D.; Kujbida, G.W.; Moore, D.R.; Atherton, P.; Burd, N.A.; Padzik, J.P.; De Lisio, M.; Tang, J.E.; Parise, G.; Rennie, M.J.; et al. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J. Physiol. 2009, 587, 5239–5247. [Google Scholar] [CrossRef]
- Mayhew, D.L.; Kim, J.-S.; Cross, J.M.; Ferrando, A.A.; Bamman, M.M. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J. Appl. Physiol. (Bethesda, Md. 1985) 2009, 107, 1655–1662. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Churchward-Venne, T.A.; Parise, G.; Bellamy, L.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Acute Post-Exercise Myofibrillar Protein Synthesis Is Not Correlated with Resistance Training-Induced Muscle Hypertrophy in Young Men. PLoS ONE 2014, 9, e89431. [Google Scholar] [CrossRef]
- Damas, F.; Phillips, S.M.; Libardi, C.A.; Vechin, F.C.; Lixandrão, M.E.; Jannig, P.R.; Costa, L.A.; Bacurau, A.V.; Snijders, T.; Parise, G.; et al. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J. Physiol. 2016, 594, 5209–5222. [Google Scholar] [CrossRef]
- Damas, F.; Libardi, C.A.; Ugrinowitsch, C. The development of skeletal muscle hypertrophy through resistance training: The role of muscle damage and muscle protein synthesis. Eur. J. Appl. Physiol. 2018, 118, 485–500. [Google Scholar] [CrossRef]
- Burd, N.A.; De Lisio, M. Skeletal muscle remodeling: Interconnections between stem cells and protein turnover. Exerc. Sport Sci. Rev. 2017, 45, 187–191. [Google Scholar] [CrossRef]
- Biolo, G.; Fleming, R.Y.; Maggi, S.P.; Nguyen, T.T.; Herndon, D.N.; Wolfe, R.R. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J. Clin. Endocrinol. Metab. 2002, 87, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Deutz, N.E.P.; Wolfe, R.R. Update on maximal anabolic response to dietary protein. Clin. Nutr. (Edinb., Scotl.) 2018, 37, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Reeds, P.J. Of flux and flooding: The advantages and problems of different isotopic methods for quantifying protein turnover in vivo: II. Methods based on the incorporation of a tracer. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.J.; McNurlan, M.A.; Essén, P.; Wernerman, J. Measurement of tissue protein synthesis rates in vivo: A critical analysis of contrasting methods. Am. J. Physiol. 1994, 266, E287–E297. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Patterson, B.W.; Mittendorfer, B. Human muscle protein turnover—Why is it so variable? J. Appl. Physiol. (Bethesda, Md. 1985) 2011, 110, 480–491. [Google Scholar] [CrossRef]
- Rennie, M.J.; Tipton, K.D. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu. Rev. Nutr. 2000, 20, 457–483. [Google Scholar] [CrossRef]
- Gropper, S.S.; Acosta, P.B. Effect of simultaneous ingestion of L-amino acids and whole protein on plasma amino acid and urea nitrogen concentrations in humans. JPEN J. Parenter. Enter. Nutr. 1991, 15, 48–53. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Dietary Protein Quality Evaluation in Human Nutrition. In Proceedings of the Report of an FAO Expert Consultation, Auckland, New Zealand, 31 March–2 April 2011; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Fry, C.S.; Borack, M.S.; Cope, M.B.; Mukherjea, R. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J. Nutr. 2013, 143, 410–416. [Google Scholar] [CrossRef]
- Borack, M.S.; Reidy, P.T.; Husaini, S.H.; Markofski, M.M.; Deer, R.R.; Richison, A.B.; Lambert, B.S.; Cope, M.B.; Mukherjea, R.; Jennings, K. Soy-Dairy Protein Blend or Whey Protein Isolate Ingestion Induces Similar Postexercise Muscle Mechanistic Target of Rapamycin Complex 1 Signaling and Protein Synthesis Responses in Older Men–4. J. Nutr. 2016, 146, 2468–2475. [Google Scholar] [CrossRef]
- Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Timmerman, K.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J. Nutr. 2010, 140, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Burd, N.A.; Mitchell, C.J.; West, D.W.; Philp, A.; Marcotte, G.R.; Baker, S.K.; Baar, K.; Phillips, S.M. Supplementation of a suboptimal protein dose with leucine or essential amino acids: Effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J. Physiol. 2012, 590, 2751–2765. [Google Scholar] [CrossRef] [PubMed]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef]
- Areta, J.L.; Burke, L.M.; Ross, M.L.; Camera, D.M.; West, D.W.; Broad, E.M.; Jeacocke, N.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 2013, 591, 2319–2331. [Google Scholar] [CrossRef]
- Atherton, P.J.; Etheridge, T.; Watt, P.W.; Wilkinson, D.; Selby, A.; Rankin, D.; Smith, K.; Rennie, M.J. Muscle full effect after oral protein: Time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am. J. Clin. Nutr. 2010, 92, 1080–1088. [Google Scholar] [CrossRef]
- Bohé, J.; Low, J.A.; Wolfe, R.R.; Rennie, M.J. Rapid report: Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J. Physiol. 2001, 532, 575–579. [Google Scholar] [CrossRef]
- Burd, N.A.; Gorissen, S.H.; Van Vliet, S.; Snijders, T.; Van Loon, L.J. Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 828–836. [Google Scholar] [CrossRef]
- Owen, O.E.; Smalley, K.J.; D’Alessio, D.A.; Mozzoli, M.A.; Dawson, E.K. Protein, fat, and carbohydrate requirements during starvation: Anaplerosis and cataplerosis. Am. J. Clin. Nutr. 1998, 68, 12–34. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Park, S.; Kim, I.Y.; Moughan, P.J.; Ferrando, A.A. Advances in stable isotope tracer methodology part 2: New thoughts about an “old” method-measurement of whole body protein synthesis and breakdown in the fed state. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2020, 68, 11–15. [Google Scholar] [CrossRef]
- Kumar, V.; Atherton, P.; Smith, K.; Rennie, M.J. Human muscle protein synthesis and breakdown during and after exercise. J. Appl. Physiol. (Bethesda, Md. 1985) 2009, 106, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Wagenmakers, A.J.; Manders, R.J.; Zorenc, A.H.; Senden, J.M.; Gorselink, M.; Keizer, H.A.; van Loon, L.J. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E645–E653. [Google Scholar] [CrossRef] [PubMed]
- Beelen, M.; Tieland, M.; Gijsen, A.P.; Vandereyt, H.; Kies, A.K.; Kuipers, H.; Saris, W.H.M.; Koopman, R.; van Loon, L.J.C. Coingestion of Carbohydrate and Protein Hydrolysate Stimulates Muscle Protein Synthesis during Exercise in Young Men, with No Further Increase during Subsequent Overnight Recovery. J. Nutr. 2008, 138, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Schutzler, S.; Schrader, A.; Spencer, H.J.; Azhar, G.; Ferrando, A.A.; Wolfe, R.R. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E73–E80. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Shin, Y.-A.; Schutzler, S.E.; Azhar, G.; Wolfe, R.R.; Ferrando, A.A. Quality of meal protein determines anabolic response in older adults. Clin. Nutr. 2018, 37, 2076–2083. [Google Scholar] [CrossRef]
- Park, S.; Church, D.D.; Azhar, G.; Schutzler, S.E.; Ferrando, A.A.; Wolfe, R.R. Anabolic response to essential amino acid plus whey protein composition is greater than whey protein alone in young healthy adults. J. Int. Soc. Sports Nutr. 2020, 17, 9. [Google Scholar] [CrossRef]
- Murphy, N.E.; Carrigan, C.T.; Philip Karl, J.; Pasiakos, S.M.; Margolis, L.M. Threshold of Energy Deficit and Lower-Body Performance Declines in Military Personnel: A Meta-Regression. Sports Med. (Auckland, N.Z.) 2018, 48, 2169–2178. [Google Scholar] [CrossRef]
- Friedl, K.E.; Moore, R.J.; Martinez-Lopez, L.E.; Vogel, J.A.; Askew, E.W.; Marchitelli, L.J.; Hoyt, R.W.; Gordon, C.C. Lower limit of body fat in healthy active men. J. Appl. Physiol. (Bethesda, Md. 1985) 1994, 77, 933–940. [Google Scholar] [CrossRef]
- Margolis, L.M.; Berryman, C.E.; Carbone, J.W.; Carrigan, C.T.; Murphy, N.E.; Ferrando, A.A.; Young, A.J.; Pasiakos, S.M. Anabolic signaling responses to exercise and recovery whey protein are suppressed at high altitude. FASEB J. 2018, 32, 909.6. [Google Scholar]
- Gwin, J.A.; Church, D.D.; Hatch-McChesney, A.; Howard, E.E.; Carrigan, C.T.; Murphy, N.E.; Wilson, M.A.; Margolis, L.M.; Carbone, J.W.; Wolfe, R.R.; et al. Effects of high versus standard essential amino acid intakes on whole-body protein turnover and mixed muscle protein synthesis during energy deficit: A randomized, crossover study. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Vislocky, L.M.; Carbone, J.W.; Altieri, N.; Konopelski, K.; Freake, H.C.; Anderson, J.M.; Ferrando, A.A.; Wolfe, R.R.; Rodriguez, N.R. Acute Energy Deprivation Affects Skeletal Muscle Protein Synthesis and Associated Intracellular Signaling Proteins in Physically Active Adults, 2. J. Nutr. 2010, 140, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.J.; McGlory, C.; Damas, F.; Mazara, N.; Baker, S.K.; Phillips, S.M. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. 2017, 32, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Smith, G.I.; Shah, K.; Mittendorfer, B. Effect of weight loss on the rate of muscle protein synthesis during fasted and fed conditions in obese older adults. Obesity 2012, 20, 1780–1786. [Google Scholar] [CrossRef]
- Campbell, W.W.; Haub, M.D.; Wolfe, R.R.; Ferrando, A.A.; Sullivan, D.H.; Apolzan, J.W.; Iglay, H.B. Resistance training preserves fat-free mass without impacting changes in protein metabolism after weight loss in older women. Obesity 2009, 17, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. The 2017 Sir David P Cuthbertson lecture. Amino acids and muscle protein metabolism in critical care. Clin. Nutr. 2018, 37, 1093–1100. [Google Scholar] [CrossRef]
- Pasiakos, S.M. Nutritional Requirements for Sustaining Health and Performance During Exposure to Extreme Environments. Annu. Rev. Nutr. 2020. [Google Scholar] [CrossRef]
- Hirsch, E.S.; Kramer, F.M.; Meiselman, H.L. Effects of food attributes and feeding environment on acceptance, consumption and body weight: Lessons learned in a twenty-year program of military ration research: US Army Research (Part 2). Appetite 2005, 44, 33–45. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwin, J.A.; Church, D.D.; Wolfe, R.R.; Ferrando, A.A.; Pasiakos, S.M. Muscle Protein Synthesis and Whole-Body Protein Turnover Responses to Ingesting Essential Amino Acids, Intact Protein, and Protein-Containing Mixed Meals with Considerations for Energy Deficit. Nutrients 2020, 12, 2457. https://doi.org/10.3390/nu12082457
Gwin JA, Church DD, Wolfe RR, Ferrando AA, Pasiakos SM. Muscle Protein Synthesis and Whole-Body Protein Turnover Responses to Ingesting Essential Amino Acids, Intact Protein, and Protein-Containing Mixed Meals with Considerations for Energy Deficit. Nutrients. 2020; 12(8):2457. https://doi.org/10.3390/nu12082457
Chicago/Turabian StyleGwin, Jess A., David D. Church, Robert R. Wolfe, Arny A. Ferrando, and Stefan M. Pasiakos. 2020. "Muscle Protein Synthesis and Whole-Body Protein Turnover Responses to Ingesting Essential Amino Acids, Intact Protein, and Protein-Containing Mixed Meals with Considerations for Energy Deficit" Nutrients 12, no. 8: 2457. https://doi.org/10.3390/nu12082457
APA StyleGwin, J. A., Church, D. D., Wolfe, R. R., Ferrando, A. A., & Pasiakos, S. M. (2020). Muscle Protein Synthesis and Whole-Body Protein Turnover Responses to Ingesting Essential Amino Acids, Intact Protein, and Protein-Containing Mixed Meals with Considerations for Energy Deficit. Nutrients, 12(8), 2457. https://doi.org/10.3390/nu12082457





