A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT)
Abstract
1. Introduction
2. Materials and Methods
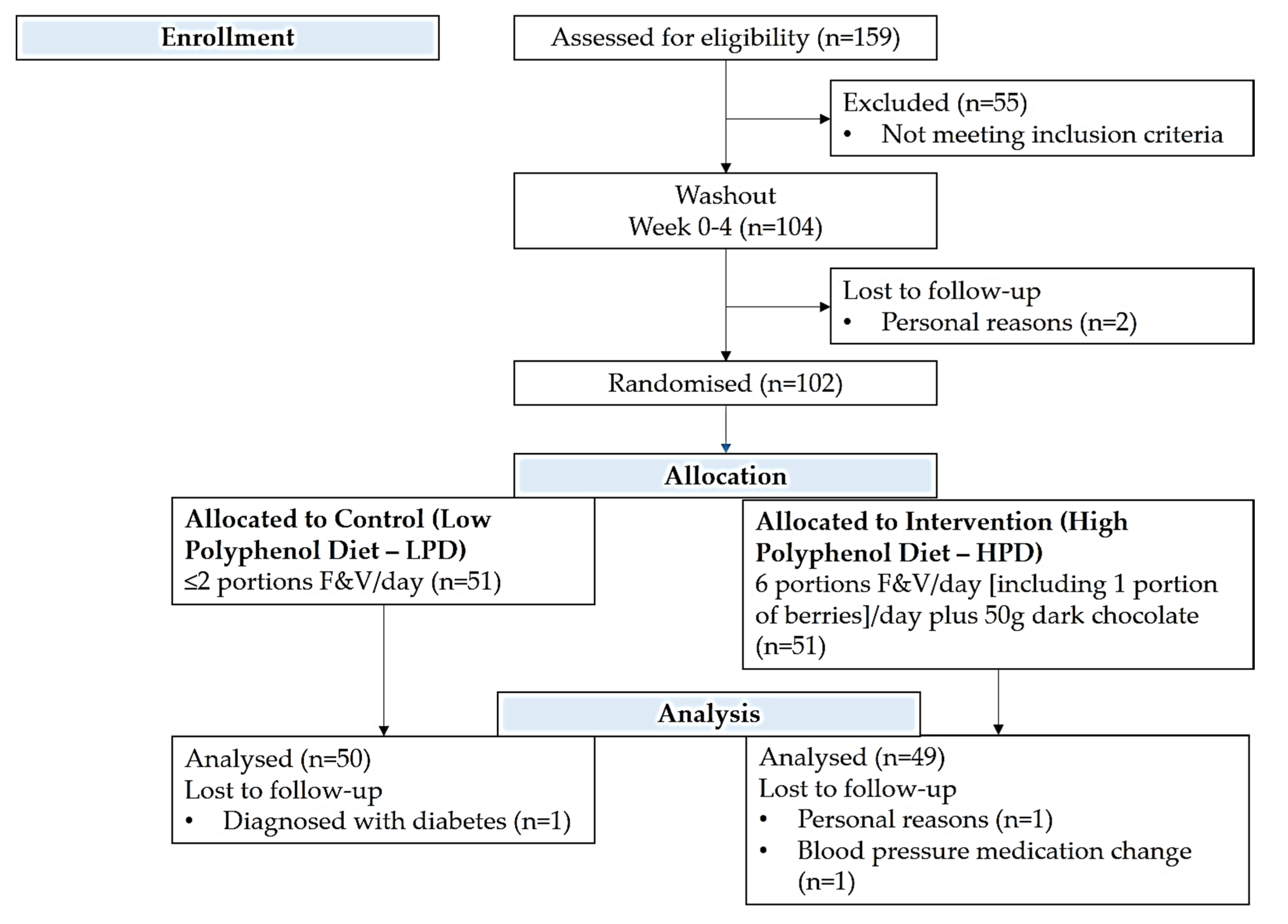
2.1. Setting and Study Population
2.2. Dietary Intervention
2.3. Other Lifestyle Parameters
2.4. Anthropometric, Clinical and Biochemical Assessments
2.5. Psychological Well-Being, Self-Esteem and Body Image
2.6. Mental and Physical Health
2.7. Statistical Analyses
3. Results
3.1. General Results
3.2. Changes in Aspects of Psychological Well-Being
3.3. Changes in Self-Esteem and Body Image Perception
3.4. Changes in Health-Related Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The World Health Organization. The World Health Report 2001—Mental Health: New Understanding, New Hope. 2001. Available online: https://www.who.int/whr/2001/en/ (accessed on 10 December 2019).
- The World Health Organization. The Global Burden of Disease: 2004 Update. 2004. Available online: https://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/ (accessed on 10 December 2019).
- Correll, C.U.; Solmi, M.; Veronese, N.; Bortolato, B.; Rosson, S.; Santonastaso, P.; Thapa-Chhetri, N.; Fornaro, M.; Gallicchio, D.; Collantoni, E. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017, 16, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.; Hancock, K.J.; Kisely, S. Cancer and mental illness. In Comorbidity of Mental and Physical Disorders; Karger Publishers: Basel, Switzerland, 2015; Volume 179, pp. 88–98. [Google Scholar]
- Vigo, D.; Thornicroft, G.; Atun, R. Estimating the true global burden of mental illness. Lancet Psychiatry 2016, 3, 171–178. [Google Scholar] [CrossRef]
- O'Neill, S.; Rooney, N. Mental health in Northern Ireland: An urgent situation. Lancet Psychiatry 2018, 5, 965–966. [Google Scholar] [CrossRef]
- Ljungberg, T.; Bondza, E.; Lethin, C. Evidence of the importance of dietary habits regarding depressive symptoms and depression. Int. J. Environ. Res. Public Health 2020, 17, 1616. [Google Scholar] [CrossRef] [PubMed]
- Velten, J.; Bieda, A.; Scholten, S.; Wannemüller, A.; Margraf, J. Lifestyle choices and mental health: A longitudinal survey with German and Chinese students. BMC Public Health 2018, 18, 632. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Kim, E.J.; Kim, A.; Lee, H.J.; Choi, H.J.; Yang, S.J. Nutritional factors affecting mental health. Clin. Nutr. Res. 2016, 5, 143–152. [Google Scholar] [CrossRef]
- Saghafian, F.; Malmir, H.; Saneei, P.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Fruit and vegetable consumption and risk of depression: Accumulative evidence from an updated systematic review and meta-analysis of epidemiological studies. Br. J. Nutr. 2018, 119, 1087–1101. [Google Scholar] [CrossRef]
- Jacka, F.N.; O'Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Smith, D.F. Benefits of flavanol-rich cocoa-derived products for mental well-being: A review. J. Funct. Foods 2013, 5, 10–15. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Potì, F.; Santi, D.; Spaggiari, G.; Zimetti, F.; Zanotti, I. Polyphenol health effects on cardiovascular and neurodegenerative disorders: A review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 351. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Knaze, V.; Zamora-Ros, R. Polyphenols: Dietary assessment and role in the prevention of cancers. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Martinoli, M. Considerations for the use of polyphenols as therapies in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.N.; Miller, M.G.; Fisher, D.R.; Bielinski, D.F.; Gilman, C.K.; Poulose, S.M.; Shukitt-Hale, B. Dietary supplementation with the polyphenol-rich açaí pulps (Euterpe oleracea Mart. and Euterpe precatoria Mart.) improves cognition in aged rats and attenuates inflammatory signaling in BV-2 microglial cells. Nutr. Neurosci. 2017, 20, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Bensalem, J.; Dudonné, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Layé, S.; Lafenetre, P.; Pallet, V. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018, 7, e19. [Google Scholar] [CrossRef]
- Travica, N.; D'Cunha, N.M.; Naumovski, N.; Kent, K.; Mellor, D.D.; Firth, J.; Georgousopoulou, E.N.; Dean, O.M.; Loughman, A.; Jacka, F. The effect of blueberry interventions on cognitive performance and mood: A systematic review of randomized controlled trials. Brain Behav. Immun. 2020, 85, 96–105. [Google Scholar] [CrossRef]
- Philip, P.; Sagaspe, P.; Taillard, J.; Mandon, C.; Constans, J.; Pourtau, L.; Pouchieu, C.; Angelino, D.; Mena, P.; Martini, D. Acute intake of a grape and blueberry polyphenol-rich extract ameliorates cognitive performance in healthy young adults during a sustained cognitive effort. Antioxidants 2019, 8, 650. [Google Scholar] [CrossRef]
- Vauzour, D. Polyphenols and brain health. OCL 2017, 24, A202. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Daglia, M.; Braidy, N.; Nabavi, S.F. Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutr. Neurosci. 2017, 20, 180–194. [Google Scholar] [CrossRef]
- Sureda, A.; Tejada, S. Polyphenols and depression: From chemistry to medicine. Curr. Pharm. Biotechnol. 2015, 16, 259–264. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods, Release 3; US Department of Agriculture: Washington, DC, USA, 2011.
- Rizzoli, R.; Stevenson, J.C.; Bauer, J.M.; van Loon, L.J.; Walrand, S.; Kanis, J.A.; Cooper, C.; Brandi, M.L.; Diez-Perez, A.; Reginster, J.Y.; et al. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: A consensus statement from the European society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO). Maturitas 2014, 79, 122–132. [Google Scholar] [CrossRef]
- Rooney, C.; McKinley, M.C.; Woodside, J.V. The potential role of fruit and vegetables in aspects of psychological well-being: A review of the literature and future directions. Proc. Nutr. Soc. 2013, 72, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Jesus, M.; Silva, T.; Cagigal, C.; Martins, V.; Silva, C. Dietary patterns: A New therapeutic approach for depression? Curr. Pharm. Biotechnol. 2019, 20, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, M.; Wei, Y.; Sun, L.; Zhang, J.; Zhang, H.; Li, B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Butterworth, P. Does reverse causality explain the relationship between diet and depression? J. Affect. Disord. 2015, 175, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Noad, R.L.; Rooney, C.; McCall, D.; Young, I.S.; McCance, D.; McKinley, M.C.; Woodside, J.V.; McKeown, P.P. Beneficial effect of a polyphenol-rich diet on cardiovascular risk: A randomised control trial. Heart 2016, 102, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- The National Health Service (NHS). 5 A Day Portion Sizes. 2018. Available online: https://www.nhs.uk/live-well/eat-well/5-a-day-portion-sizes/ (accessed on 2 May 2020).
- Besson, H.; Brage, S.; Jakes, R.W.; Ekelund, U.; Wareham, N.J. Estimating physical activity energy expenditure, sedentary time, and physical activity intensity by self-report in adults. Am. J. Clin. Nutr. 2010, 91, 106–114. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Vuilleumier, J.; Keck, E. Fluorometric assay of vitamin C in biological materials using a centrifugal analyser with fluorescence attachment. J. Micronutr. Anal. 1989, 5, 25–34. [Google Scholar]
- Craft, N.E. Carotenoid reversed-phase high-performance liquid chromatography methods: Reference compendium. Methods Enzymol. 1992, 213, 185–205. [Google Scholar]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck depression inventory-II. Psychol. Corp. 1996, 78, 490–498. [Google Scholar]
- Antony, M.M.; Bieling, P.J.; Cox, B.J.; Enns, M.W.; Swinson, R.P. Psychometric properties of the 42-item and 21-item versions of the depression anxiety stress scales in clinical groups and a community sample. Psychol. Assess. 1998, 10, 176–181. [Google Scholar] [CrossRef]
- Rosenberg, M. Society and the Adolescent Self-Image; Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- Alessandri, G.; Vecchione, M.; Eisenberg, N.; Łaguna, M. On the factor structure of the rosenberg (1965) general self-esteem scale. Psychol. Assess. 2015, 27, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Cash, T.F. MBSRQ Users™ Manual; Old Dominion University Press: Norfolk, VA, USA, 2000. [Google Scholar]
- Ware Jr, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- McHorney, C.A.; Ware Jr, J.E.; Raczek, A.E. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 1993, 31, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Sherbourne, C.D.; Mazel, R.M. The rand 36 item health survey 1.0. Health Econ. 1993, 2, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Sherbourne, C.D.; Mazel, R.M. User's Manual for the Medical Outcomes Study (Mos) Core Measures of Health-Related Quality of Life; Rand Corporation: Santa Monica, CA, USA, 1995. [Google Scholar]
- Button, K.; Kounali, D.; Thomas, L.; Wiles, N.; Peters, T.; Welton, N.; Ades, A.; Lewis, G. Minimal clinically important difference on the beck depression inventory-II according to the patient's perspective. Psychol. Med. 2015, 45, 3269–3279. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.G.d.; Teixeira, I.T.; Theodoro, H.; Branco, C.S. Dietary total antioxidant capacity as a preventive factor against depression in climacteric women. Dement. Neuropsychol. 2019, 13, 305–311. [Google Scholar] [CrossRef]
- Hintikka, J.; Tolmunen, T.; Honkalampi, K.; Haatainen, K.; Koivumaa-Honkanen, H.; Tanskanen, A.; Viinamäki, H. Daily tea drinking is associated with a low level of depressive symptoms in the finnish general population. Eur. J. Epidemiol. 2005, 20, 359–363. [Google Scholar] [CrossRef]
- Godos, J.; Castellano, S.; Ray, S.; Grosso, G.; Galvano, F. Dietary polyphenol intake and depression: Results from the mediterranean healthy eating, lifestyle and aging (meal) study. Molecules 2018, 23, 999. [Google Scholar] [CrossRef] [PubMed]
- Brody, S. High-dose ascorbic acid increases intercourse frequency and improves mood: A randomized controlled clinical trial. Biol. Psychiatry 2002, 52, 371–374. [Google Scholar] [CrossRef]
- Payne, M.E.; Steck, S.E.; George, R.R.; Steffens, D.C. Fruit, Vegetable, and antioxidant intakes are lower in older adults with depression. J. Acad. Nutr. Diet. 2012, 112, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Lua, P.; Wong, S. Dark chocolate consumption on anxiety, depression and health-related quality of life of patients with cancer: A randomised clinical investigation. Malays. J. Psychiatry 2012, 21, 1–11. [Google Scholar]
- Baruah, J.; Vasudevan, A. The vessels shaping mental health or illness. Open Neurol. J. 2019, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Page, A.C.; Hooke, G.R.; Morrison, D.L. Psychometric properties of the depression anxiety stress scales (DASS) in depressed clinical samples. Br. J. Clin. Psychol. 2007, 46, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Perkins-Porras, L.; Hilton, S.; Rink, E.; Cappuccio, F.P. Quality of life and self-rated health in relation to changes in fruit and vegetable intake and in plasma vitamins C and E in a randomised trial of behavioural and nutritional education counselling. Br. J. Nutr. 2004, 92, 177–184. [Google Scholar] [CrossRef][Green Version]
- Plaisted, C.S.; Lin, P.H.; Ard, J.D.; McClure, M.L.; Svetkey, L.P. The effects of dietary patterns on quality of life: A substudy of the dietary approaches to stop hypertension trial. J. Am. Diet. Assoc. 1999, 99, S84–S89. [Google Scholar] [CrossRef]
- Van de Mortel, T.F. Faking it: Social desirability response bias in self-report research. Aust. J. Adv. Nurs. 2008, 25, 40–48. [Google Scholar]
- García-Batista, Z.E.; Guerra-Peña, K.; Cano-Vindel, A.; Herrera-Martínez, S.X.; Medrano, L.A. Validity and reliability of the beck depression inventory (BDI-II) in general and hospital population of dominican republic. PLoS ONE 2018, 13, e0199750. [Google Scholar] [CrossRef]
- Osman, A.; Wong, J.L.; Bagge, C.L.; Freedenthal, S.; Gutierrez, P.M.; Lozano, G. The depression anxiety stress scales-21 (DASS-21): Further examination of dimensions, scale reliability, and correlates. J. Clin. Psychol. 2012, 68, 1322–1338. [Google Scholar] [CrossRef] [PubMed]
| Low Polyphenol (nmax = 50) | High Polyphenol (nmax = 49) | Between-Group Comparison p-Value * | |
|---|---|---|---|
| Age (years) | 55.6 ± 6.8 | 54.0 ± 7.0 | 0.25 |
| Sex (males, n (%)) | 30 (60.0) | 23 (46.9) | 0.23 |
| Education (years) | 13.9 (12.0, 16.8) | 13.6 (13.8, 15.8) | 0.57 |
| Body mass index (kg/m2) | 29.9 (26.9, 34.6) | 31.15 (27.7, 33.5) | 0.29 |
| Waist circumference (cm) | |||
| Male | 106.5 (98.0, 116.3) | 105.0 (98.0, 116.0) | 0.86 |
| Female | 94.0 (85.3, 108.8) | 96.0 (89.5, 108.5) | 0.56 |
| Current smoker n (%) | 5 (10.0) | 7 (14.3) | 0.55 |
| Use of antidepressants n (%) | 7 (14.0) | 8 (16.3) | 0.79 |
| Systolic blood pressure (mmHg) | 143.7 ± 6.6 | 143.6 ± 8.0 | 0.95 |
| Diastolic blood pressure (mmHg) | 86.9 ± 8.3 | 85.9 ± 7.1 | 0.55 |
| Total cholesterol (mmol/L) | 5.3 ± 1.2 | 5.7 ± 1.2 | 0.10 |
| HDL(mmol/L) | 1.4 ± 0.3 | 1.3 ± 0.4 | 0.58 |
| LDL(mmol/L) | 4.3 ± 1.3 | 4.6 ± 1.2 | 0.18 |
| Triglycerides (mmol/L) | 1.7 (1.6, 2.1) | 1.6 (1.5, 2.0) | 0.46 |
| Low Polyphenol Diet (nmax = 50) | High Polyphenol Diet (nmax = 49) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 0 1 | Week 12 | Median Change (IQR) 3 | Within Group (p Value) 4 | Week 0 1 | Week 12 | Median Change (IQR) 3 | Within Group (p Value) 4 | Between Group (p Value) 5 | |
| Dietary intake | |||||||||
| Fruits and vegetables intake (portions/day) | 2.68 ± 1.68) | 1.24 ± 0.56 | −1.44 (−1.87, −0.95) | <0.001 | 2.64 ± 1.70 | 6.73 ± 2.07 | 4.09 (3.45, 4.73) | <0.001 | <0.001 |
| Week 0 1 | Week 12 | Median Change (IQR) 3 | Within Group (p value) 4 | Week 0 1 | Week 12 | Median Change (IQR) 3 | Within Group (p value) 4 | Between Group (p value) 5 | |
| Berries (portions/day) | 0 (0.00, 0.00) | 0 (0.00, 0.00) | 0 (0.00, 0.00) | 0.69 | 0 (0.00, 0.00) | 1 (0.80, 1.25) | 1 (0.75, 1.17) | <0.001 | <0.001 |
| Dark chocolate (grams/day) | 0 (0.00, 0.00) | 0 (0.00, 0.00) | 0 (0.00, 0.00) | 0.18 | 0 (0.00, 0.00) | 50 (37.50, 50.00) | 50 (37.5, 50.0) | <0.001 | <0.001 |
| Micronutrient biomarkers | |||||||||
| Vitamin C (µmol/l) 6 | 44.3 (28.70, 61.90) | 34.2 (13.40, 49.50) | −7.60 (−26.50, 0.85) | <0.001 | 46.4 (31.70, 65.00) | 55.7 (43.10, 68.20) | 4.83 (−8.68, 20.75) | 0.1 | <0.001 |
| Total carotenoids (µmol/l) 6 | 1.09 (0.88, 1.43) | 1.09 (0.72, 1.35) | −0.01 (−0.03, 0.01) | 0.01 | 1.17 (0.96, 1.48) | 1.33 (1.06, 1.66) | 0.12 (−0.12, 0.40) | 0.07 | <0.001 |
| Lutein (µmol/l) 6 | 0.15 (0.12, 0.22) | 0.14 (0.11, 0.20) | −0.01 (−0.03, 0.01) | 0.08 | 0.14 (0.11, 0.20) | 0.20 (0.15, 0.26) | 0.04 (0.01, 0.07) | <0.001 | <0.001 |
| Zeaxanthin (µmol/l) 6 | 0.04 (0.03, 0.05) | 0.03 (0.03, 0.05) | −0.00 (−0.01, 0.01) | 0.3 | 0.04 (0.03, 0.05) | 0.04 (0.04, 0.06) | 0 (−0.00, 0.01) | 0.01 | 0.01 |
| β-cryptoxanthin (µmol/l) 6 | 0.06 (0.04, 0.07) | 0.05 (0.03, 0.08) | −0.01 (−0.02, 0.01) | 0.049 | 0.06 (0.04, 0.09) | 0.07 (0.05, 0.09) | 0.01 (−0.01, 0.03) | 0.03 | <0.001 |
| α-carotene (µmol/l) 6 | 0.12 (0.09, 0.15) | 0.1 (0.08, 0.15) | −0.01 (−0.04, 0.01) | 0.06 | 0.13 (0.11, 0.18) | 0.15 (0.11, 0.20) | 0.01 (−0.02, 0.03) | 0.17 | 0.02 |
| β-carotene (µmol/l) 6 | 0.23 (0.16, 0.34) | 0.22 (0.13, 0.32) | −0.01 (−0.09, 0.03) | 0.07 | 0.27 (0.19, 0.44) | 0.3 (0.21, 0.41) | −0.00 (−0.06, 0.08) | 0.79 | 0.14 |
| Lycopene (µmol/l) 6 | 0.49 (0.38, 0.66) | 0.47 (0.33, 0.58) | −0.04 (−0.19, 0.09) | 0.07 | 0.5 (0.39, 0.61) | 0.56 (0.40, 0.68) | 0.05 (−0.14, 0.21) | 0.31 | 0.048 |
| Epicatechin (nmol/mg crt/L) 6 | 0.43 (0.17, 1.10) | 0.55 (0.26, 1.00) | 0.04 (−0.24, 0.32) | 0.37 | 0.64 (0.25, 0.97) | 1.73 (0.51, 4.20) | 0.89 (0.10, 3.57) | <0.001 | <0.001 |
| Week 0 1 | Week 12 | Mean Change (95% CI) 2 | Within Group (p value) 4 | Week 0 1 | Week 12 | Mean Change (95% CI) 2 | Within Group (p value) 4 | Between Group (p value) 5 | |
| Physical activity (MET hours/day) | |||||||||
| Sedentary activities | 8.9 ± 4.7 | 8.6 ± 4.6 | −0.3 (−1.0, 0.4) | 0.6 | 8.4 ± 4.7 | 8.2 ± 4.8 | −0.2 (−1.0, 0.7) | 0.72 | 0.77 |
| Moderate intensity | 3.7 ± 5.6 | 2.6 ± 4.7 | −1.1 (−2.3, 0.2) | 0.1 | 3.9 ± 5.4 | 3.0 ± 5.4 | −0.9 (−2.0, 0.2) | 0.049 | 0.78 |
| Vigorous intensity | 1.1 ± 4.5 | 0.2 ± 0.5 | −1.0 (−2.2, 0.3) | 0.33 | 0.3 ± 1.6 | 0.1 ± 0.5 | −0.2 (−0.6, 0.3) | 0.89 | 0.63 |
| Weight (kg) | 87.2 ± 19.1 | 87.0 ± 19.1 | −0.15 (−0.7, 0.4) | 0.56 | 88.0 ± 20.1 | 88.2 ± 20.4 | 0.20 (−0.22, 6.2) | 0.35 | 0.29 |
| Low Polyphenol Diet (nmax = 50) | High Polyphenol Diet (nmax = 49) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 0 1 | Week 12 | Median Change (IQR) 2 | Within Group Change (p Value) 3 | Week 0 1 | Week 12 | Median Change (IQR) 2 | Within Group Change (p Value) 3 | Between Group Change (p Value) 4 | |
| BDI-II * | 6.0 (2.0, 12.5) | 7.0 (2.0, 11.0) | 0.2 (−1.5, 1.9) | 0.98 | 6.0 (3.0, 12.0) | 2.0 (0.0, 6.0) | −3.4 (−5.4, −1.5) | <0.001 | 0.01 |
| DASS-21 ** | |||||||||
| Depression 5 | 2.0 (0.0, 12.0) | 6.0 (0.0, 10.5) | 0 (−2.0, 6.0) | 0.29 | 2.0 (0.0, 10.0) | 0.0 (0.0, 6.0) | 0 (−2.0, 0.0) | 0.53 | 0.56 |
| Anxiety 5 | 4.0 (2.0, 9.0) | 2.0 (0.0, 8.0) | 0.0 (−3.0, 3.0) | 0.86 | 4.0 (0.0, 10.0) | 2.0 (0.0, 6.0) | 0.0 (−2.0, 0.0) | 0.16 | 0.8 |
| Stress 5 | 7.0 (2.0, 12.5) | 8.0 (0.0, 16.0) | 0 (−2.0, 4.0) | 0.76 | 6.0 (2.0, 14.0) | 4.0 (0.0, 10.0) | −2.0 (−6.0, 2.0) | 0.05 | 0.14 |
| PANAS *** | |||||||||
| Positive affect | 29.9 (8.3) | 30.4 (9.8) | 0.5 (−1.5, 2.5) | 0.63 | 33.0 (6.8) | 35.2 (7.4) | 2.2 (0.3, 4.1) | 0.03 | 0.21 |
| Negative affect | 11.0 (10.0, 13.0) | 11.0 (10.0, 13.0) | 0.0 (−1.5, 1.5) | 0.56 | 12.0 (10.0, 15.0) | 10.0 (10.0, 14.0) | 0.0 (−1.0, 0.5) | 0.68 | 0.99 |
| Rosenberg Self-Esteem Score † | 26.0 (25.0, 28.0) | 26.0 (25.0, 28.0) | 0.0 (−2.0, 2.0) | 0.74 | 26.0 (25.0, 27.0) | 27.0 (24.0, 27.0) | 0.0 (−2.0, 2.0) | 0.68 | 0.53 |
| MBSRQ-AS †† | |||||||||
| Appearance Evaluation | 2.9 (2.4, 3.5) | 3.0 (2.4, 3.6) | 0.0 (−0.3, 0.4) | 0.27 | 3.0 (2.5, 3.4) | 3.1 (2.7, 3.6) | 0.1 (−0.3, 0.4) | 0.15 | 0.76 |
| Appearance Orientation | 3.0 (2.5, 3.7) | 3.0 (2.5, 3.7) | 0.0 (−0.3, 0.2) | 0.35 | 3.2 (2.8, 3.5) | 3.3 (2.9, 3.8) | 0.2 (−0.2, 0.4) | 0.16 | 0.1 |
| Body areas Satisfaction | 3.0 (2.7, 3.4) | 3.3 (2.4, 3.7) | 0.2 (−0.1, 0.4) | 0.03 | 3.1 (2.7, 3.6) | 3.3 (2.8, 3.8) | 0.2 (−0.1, 0.3) | 0.02 | 0.71 |
| Overweight Preoccupation | 2.3 (1.8, 2.8) | 2.4 (1.8, 2.8) | 0.0 (−0.3, 0.5) | 0.87 | 2.5 (1.8, 3.3) | 2.6 (1.8, 3.2) | 0.0 (−0.5, 0.3) | 0.45 | 0.72 |
| Self-classified Weight 1 | 4.0 (3.4, 4.0) | 4.0 (3.0, 4.0) | 0.0 (0.0, 0.0) | 0.08 | 4.0 (3.5, 4.0) | 4.0 (3.5, 4.0) | 0.0 (0.0, 0.0) | 0.43 | 0.5 |
| SF-36 ††† | |||||||||
| Physical Functioning | 90 (75.0, 97.5) | 90 (81.3, 100.0) | 0 (0.0, 10.0) | 0.07 | 95 (80.0, 100.0) | 95 (85.0, 100.0) | 0 (−5.0, 10.0) | 0.15 | 0.44 |
| Role limitations—physical health | 100 (37.5, 100.0) | 100 (37.5, 100.0) | 0 (0.0, 25.0) | 0.45 | 100 (75.0, 100.0) | 100 (100.0, 100.0) | 0 (0.0, 0.0) | <0.001 | 0.61 |
| Pain | 80 (47.5, 100.0) | 80 (46.3, 100.0) | 0 (−10.0, 10.0) | 0.64 | 90 (60.0, 90.0) | 90 (70.0, 100.0) | 0 (−10.0, 22.5) | 0.2 | 0.51 |
| General health | 65 (45.0, 75.0) | 60 (50.0, 75.0) | 0 (−10.0, 10.0) | 0.47 | 65 (50.0, 75.0) | 75 (65.0, 85.0) | 10 (−5.0, 20.0) | <0.001 | 0.03 |
| Physical health component | 210.5 (168.5, 223.1) | 200.9 (172.9, 217.2) | −6.4 (−17.0, 4.2) | 0.09 | 213.2 (189.6, 225.1) | 216.6 (201.4, 225.3) | 2.2 (−8.1, 15.4) | 0.2 | 0.04 |
| Role limitations—emotional health | 100 (100.0, 100.0) | 100 (100.0, 100.0) | 0 (0.0, 0.0) | 0.99 | 100 (100.0, 100.0) | 100 (100.0, 100.0) | 0 (0.0, 0.0) | 0.1 | 0.85 |
| Energy/fatigue | 55 (40.0, 72.5) | 60 (45.0, 70.0) | 5 (−5.0, 10.0) | 0.39 | 60 (50.0, 80.0) | 70 (60.0, 80.0) | 5 (0.0, 20.0) | <0.001 | 0.02 |
| Emotional well-being | 76 (64.0, 84.0) | 80 (62.0, 86.0) | 0 (−8.0, 8.0) | 0.73 | 80 (60.0, 88.0) | 84 (72.0, 92.0) | 4 (0.0, 16.0) | 0 | 0.01 |
| Social functioning | 100 (75.0, 100.0) | 100 (75.0, 100.0) | 0 (−6.3, 0.0) | 0.97 | 100 (75.0, 100.0) | 100 (100.0, 100.0) | 0 (0.0, 25.0) | 0.02 | 0.08 |
| Mental health component | 209.1 (176.8, 222.3) | 197.9 (175.0, 217.2) | −4.0 (−26.6, 8.1) | 0.04 | 208 (181.4, 226.0) | 218.3 (201.4, 226.8) | 1.9 (−6.9, 19.1) | 0.09 | 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontogianni, M.D.; Vijayakumar, A.; Rooney, C.; Noad, R.L.; Appleton, K.M.; McCarthy, D.; Donnelly, M.; Young, I.S.; McKinley, M.C.; McKeown, P.P.; et al. A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT). Nutrients 2020, 12, 2445. https://doi.org/10.3390/nu12082445
Kontogianni MD, Vijayakumar A, Rooney C, Noad RL, Appleton KM, McCarthy D, Donnelly M, Young IS, McKinley MC, McKeown PP, et al. A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT). Nutrients. 2020; 12(8):2445. https://doi.org/10.3390/nu12082445
Chicago/Turabian StyleKontogianni, Meropi D., Aswathy Vijayakumar, Ciara Rooney, Rebecca L. Noad, Katherine M. Appleton, Danielle McCarthy, Michael Donnelly, Ian S. Young, Michelle C. McKinley, Pascal P. McKeown, and et al. 2020. "A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT)" Nutrients 12, no. 8: 2445. https://doi.org/10.3390/nu12082445
APA StyleKontogianni, M. D., Vijayakumar, A., Rooney, C., Noad, R. L., Appleton, K. M., McCarthy, D., Donnelly, M., Young, I. S., McKinley, M. C., McKeown, P. P., & Woodside, J. V. (2020). A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT). Nutrients, 12(8), 2445. https://doi.org/10.3390/nu12082445






