Predicted Metabolic Pathway Distributions in Stool Bacteria in Very-Low-Birth-Weight Infants: Potential Relationships with NICU Faltered Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stool Sample Processing
3. Results
3.1. Demographics
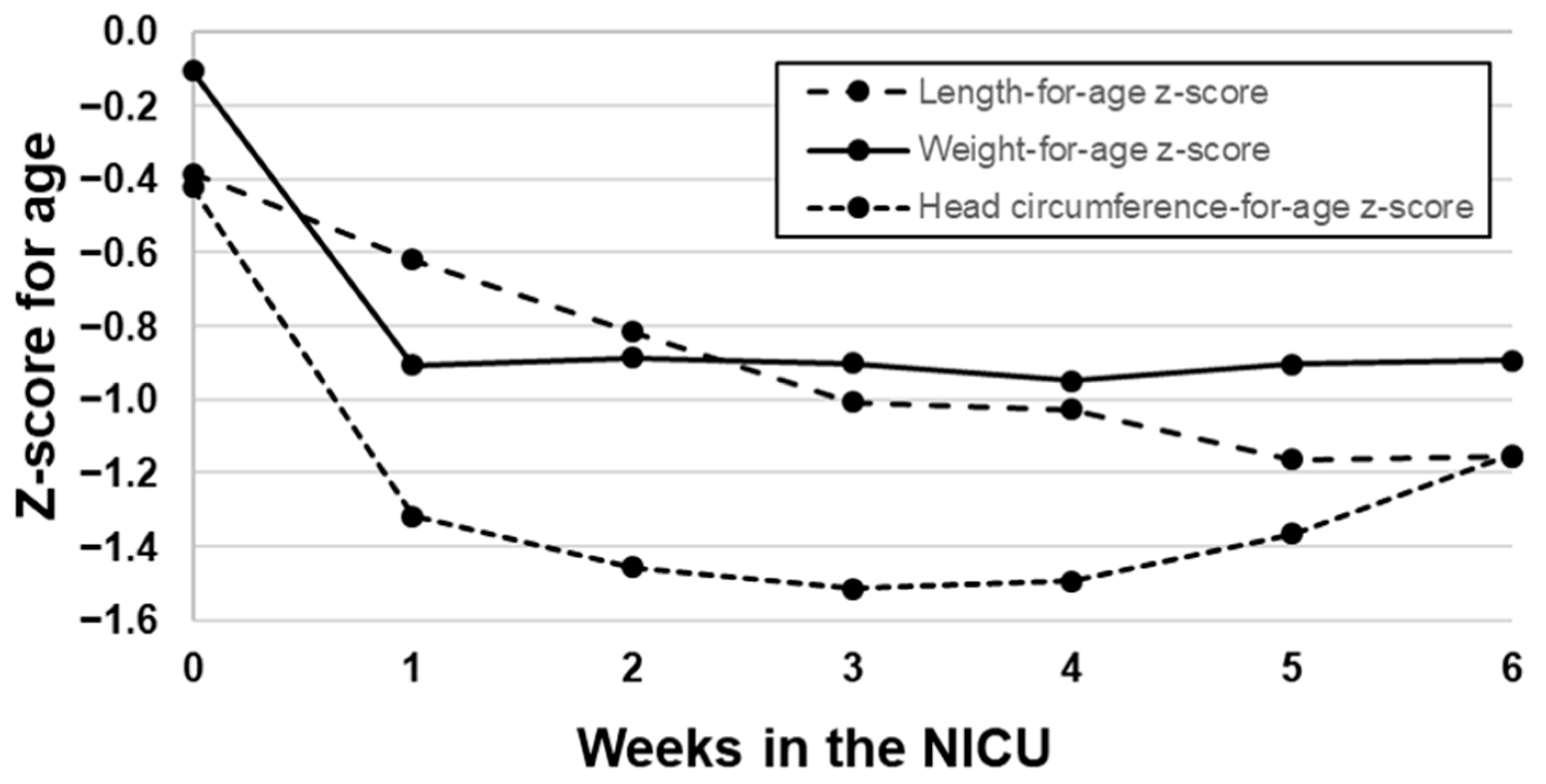
3.2. Growth of VLBW Infants
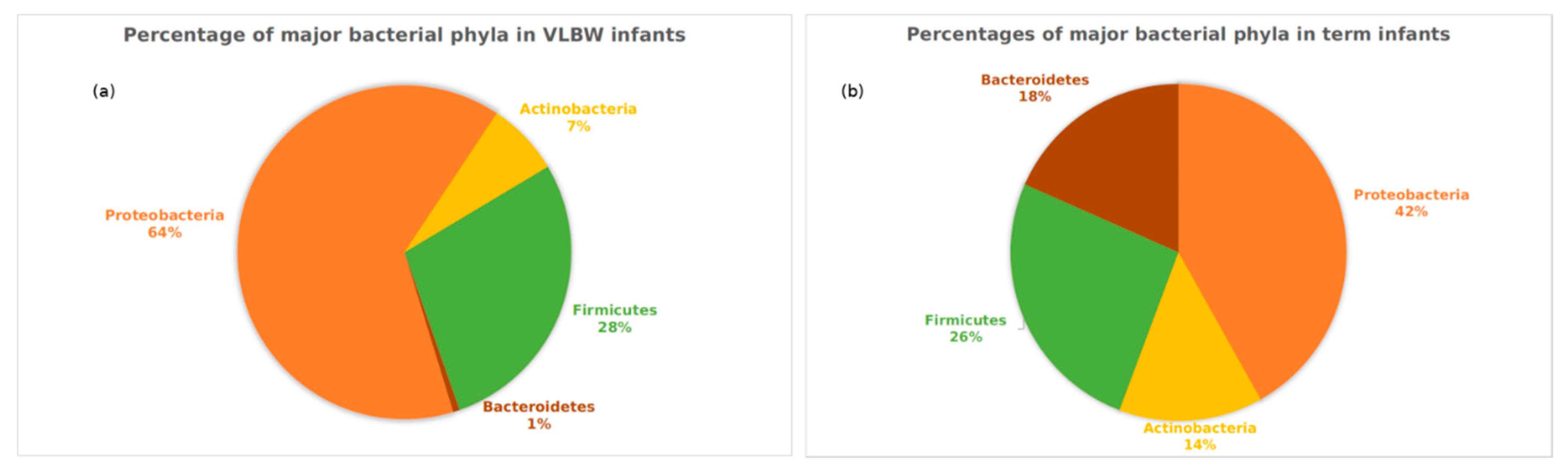
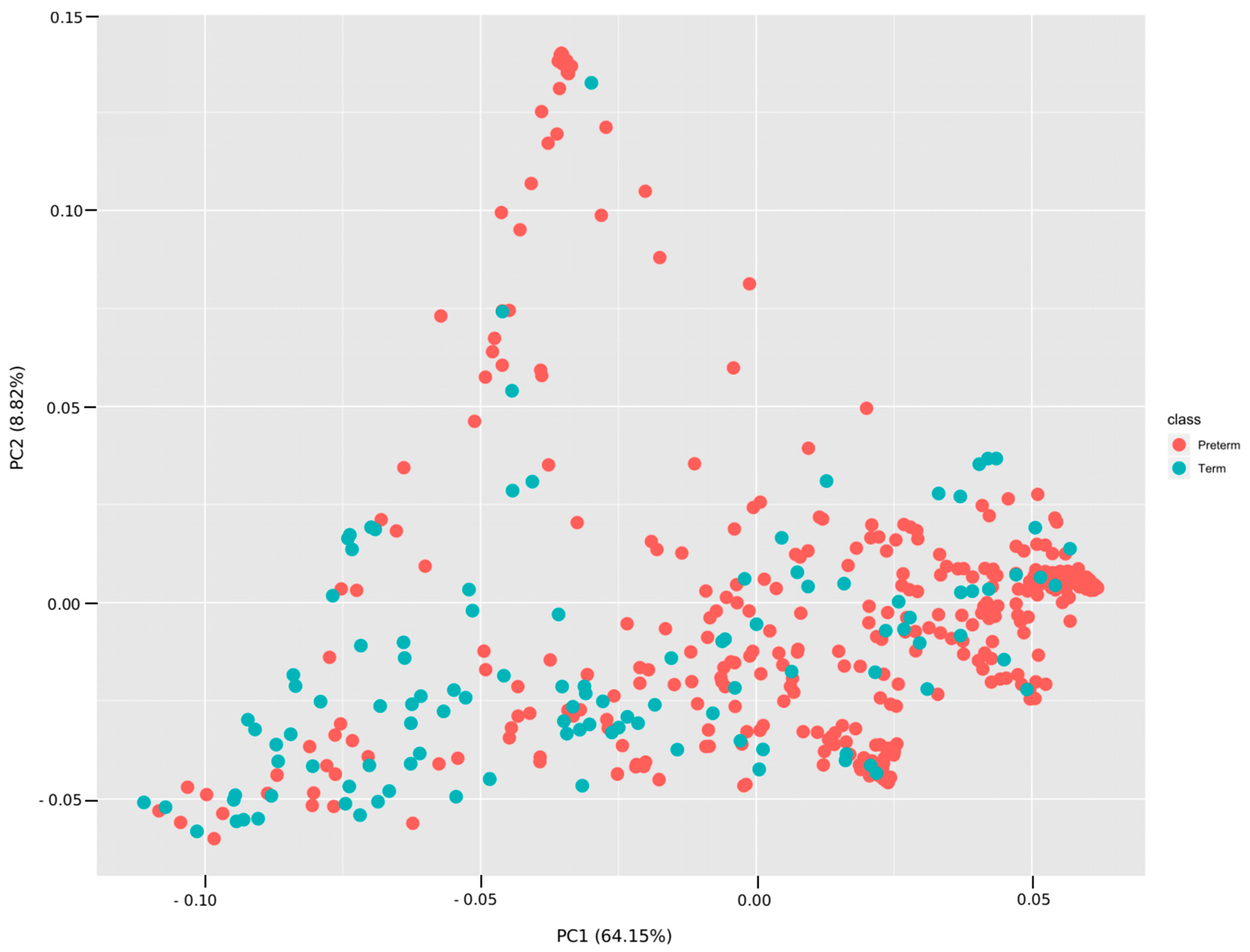
3.3. Analyses Based on Distribution of ASVs
3.4. Analyses Based on Distribution of Metabolic Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef]
- Shah, P.; Nathan, E.; Doherty, D.; Patole, S. Optimising enteral nutrition in growth restricted extremely preterm neonates—A difficult proposition. J. Matern. Fetal Neonatal Med. 2015, 28, 1981–1984. [Google Scholar] [CrossRef]
- Corpeleijn, W.E.; Vermeulen, M.J.; van den Akker, C.H.; van Goudoever, J.B. Feeding very-low-birth-weight infants: Our aspirations versus the reality in practice. Ann. Nutr. Metab. 2011, 58, 20–29. [Google Scholar] [CrossRef]
- McDonald, B.; McCoy, K.D. Maternal microbiota in pregnancy and early life. Science 2019, 365, 984–985. [Google Scholar] [CrossRef]
- Jašarević, E.; Bale, T.L. Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front. Neuroendocrinol. 2019, 55, 10097. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef]
- Diaz Heitz, R. Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. Semin. Fetal Neonatal Med. 2016, 21, 410–417. [Google Scholar] [CrossRef]
- Yee, A.L.; Miller, E.; Dishaw, L.J.; Gordon, J.M.; Ji, M.; Dutra, S.; Ho, T.T.; Gilbert, J.A.; Groer, M. Longitudinal Microbiome Composition and Stability Correlate with Increased Weight and Length of Very-Low-Birth-Weight Infants. MSystems 2019, 4, e00229-18. [Google Scholar] [CrossRef]
- Fenton, T.R.; Chan, H.T.; Madhu, A.; Griffin, I.J.; Hoyos, A.; Ziegler, E.E.; Groh-Wargo, S.; Carlson, S.J.; Senterre, T.; Anderson, D.; et al. Preterm Infant Growth Velocity Calculations: A Systematic Review. Pediatrics 2017, 139, e20162045. [Google Scholar] [CrossRef]
- Johnson, M.J.; Wootton, S.A.; Leaf, A.A.; Jackson, A.A. Preterm birth and body composition at term equivalent age: A systematic review and meta-analysis. Pediatrics 2012, 130, e640–e649. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Groer, M.W.; Miller, E.M.; D’Agata, M.; Ho, T.B.; Dutra, S.; Yoo, J.Y.; Yee, A.L.; Gilbert, J.A.; Dishaw, L.J. Contributors to Dysbiosis in Very Low Birth Weight Infants. J. Obstet. Gynecol. Neonatal Nurs. 2020, in press. [Google Scholar] [CrossRef]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S. Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 2019, 76, 3917–3937. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatri. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Iwai, S.; Weinmaier, T.; Schmidt, B.L.; Albertson, D.G.; Poloso, N.J.; Dabbagh, K.; DeSantis, T.Z. Piphillin: Improved prediction of metagenomic content by direct inference from human microbiomes. PloS one 2016, 11, e0166104. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Dogra, S.; Sakwinska, O.; Soh, S.E.; Ngom-Bru, C.; Bruck, W.M.; Berger, B.; Brussow, H.; Karnani, N.; Lee, Y.S.; Yap, F.; et al. Rate of establishing the gut microbiota in infancy has consequences for future health. Gut Microbes 2015, 6, 321–325. [Google Scholar] [CrossRef]
- Togo, A.; Dufour, J.C.; Lagier, J.C.; Dubourg, G.; Raoult, D.; Million, M. Repertoire of human breast and milk microbiota: A systematic review. Future Microbiol. 2019, 14, 623–641. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.; Firek, B.A.; Miller, C.S.; Sharon, I.; Thomas, B.C.; Baker, R.; Morowitz, M.J.; Banfield, J.F. Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome 2014, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Leroy, F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int. J. Food Microbiol. 2011, 149, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chavez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017, 357, 570–575. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Cebra, J.J. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 1999, 69, 1046s–1051s. [Google Scholar] [CrossRef]
- Arboleya, S.; Sanchez, B.; Solis, G.; Fernandez, N.; Suarez, M.; Hernandez-Barranco, A.M.; Milani, C.; Margolles, A.; de Los Reyes-Gavilan, C.G.; Ventura, M.; et al. Impact of Prematurity and Perinatal Antibiotics on the Developing Intestinal Microbiota: A Functional Inference Study. Int. J. Mol. Sci. 2016, 17, 649. [Google Scholar] [CrossRef]
- Younge, N.E.; Newgard, C.B.; Cotten, C.M.; Goldberg, R.N.; Muehlbauer, M.J.; Bain, J.R.; Stevens, R.D.; O’Connell, T.M.; Rawls, J.F.; Seed, P.C.; et al. Disrupted Maturation of the Microbiota and Metabolome among Extremely Preterm Infants with Postnatal Growth Failure. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Grier, A.; Qiu, X.; Bandyopadhyay, S.; Holden-Wiltse, J.; Kessler, H.A.; Gill, A.L.; Hamilton, B.; Huyck, H.; Misra, S.; Mariani, T.J.; et al. Impact of prematurity and nutrition on the developing gut microbiome and preterm infant growth. Microbiome 2017, 5, 158. [Google Scholar] [CrossRef]
- Chin, A.M.; Hill, D.R.; Aurora, M.; Spence, J.R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Biol. 2017, 66, 81–93. [Google Scholar] [CrossRef]
- Quigley, E.M. Microflora modulation of motility. J. Neurogastroenterol. Motil. 2011, 17, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Arya, S.; Choudhary, S.; Jain, S.K. Amniotic fluid: Source of trophic factors for the developing intestine. World J. Gastrointest. Pathophysiol. 2016, 7, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Jain, S.K. Protective effects of amniotic fluid in the setting of necrotizing enterocolitis. Pediatri. Res. 2017, 82, 584–595. [Google Scholar] [CrossRef] [PubMed]
- McElroy, S.J.; Weitkamp, J.H. Innate Immunity in the Small Intestine of the Preterm Infant. NeoReviews 2011, 12, e517–e526. [Google Scholar] [CrossRef] [PubMed]
- Rogosch, T.; Kerzel, S.; Hoss, K.; Hoersch, G.; Zemlin, C.; Heckmann, M.; Berek, C.; Schroeder, H.W., Jr.; Maier, R.F.; Zemlin, M. IgA response in preterm neonates shows little evidence of antigen-driven selection. J. Immunol. 2012, 189, 5449–5456. [Google Scholar] [CrossRef] [PubMed]
- Hard, A.L.; Nilsson, A.K.; Lund, A.M.; Hansen-Pupp, I.; Smith, L.E.H.; Hellstrom, A. Review shows that donor milk does not promote the growth and development of preterm infants as well as maternal milk. Acta Paediatr. 2019, 108, 998–1007. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Boquien, C.Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of Human Milk for Preterm Infants: Update and Recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front. Pediatri. 2019, 7, 76. [Google Scholar] [CrossRef]
- Steele, C. Best Practices for Handling and Administration of Expressed Human Milk and Donor Human Milk for Hospitalized Preterm Infants. Front. Nutr. 2018, 5, 76. [Google Scholar] [CrossRef]
- Ziegler, E.E. Human milk and human milk fortifiers. World Rev. Nutr. Diet. 2014, 110, 215–227. [Google Scholar]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lonnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatri. 2018, 6, 313. [Google Scholar] [CrossRef]
- Bitman, J.; Wood, L.; Hamosh, M.; Hamosh, P.; Mehta, N.R. Comparison of the lipid composition of breast milk from mothers of term and preterm infants. Am. J. Clin. Nutr. 1983, 38, 300–312. [Google Scholar] [CrossRef]
- Miliku, K.; Duan, Q.L.; Moraes, T.J.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; Subbarao, P.; Field, C.J.; et al. Human milk fatty acid composition is associated with dietary, genetic, sociodemographic, and environmental factors in the CHILD Cohort Study. Am. J. Clin. Nutr. 2019, 110, 1370–1383. [Google Scholar] [CrossRef]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host Microbe 2019, 25, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.M.; Murphy, B.P.; Kiely, M.E. Optimising preterm nutrition: Present and future. Proc. Nutr. Soc. 2016, 75, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Eidelman, A.I.; Schanler, R.J. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar]
- Mosca, F.; Gianni, M.L. Human milk: Composition and health benefits. Pediatr. Med. Chir. 2017, 39, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.G.; Christensen, S.H.; Lind, M.V.; Michaelsen, K.F. Human milk composition and infant growth. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. MMBR 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth—First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef]
- Ho, T.T.B.; Groer, M.W.; Kane, B.; Yee, A.L.; Torres, B.A.; Gilbert, J.A.; Maheshwari, A. Dichotomous development of the gut microbiome in preterm infants. Microbiome 2018, 6, 157. [Google Scholar] [CrossRef]
- Groer, M.W.; Gregory, K.E.; Louis-Jacques, A.; Thibeau, S.; Walker, W.A. The very low birth weight infant microbiome and childhood health. Birth Defects Res. C Embryo Today 2015, 105, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Nunez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate production by probiotic bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef] [PubMed]


| Group | Unique IDs | Total Specimen |
|---|---|---|
| VLBW Infants | 84 | 375 |
| Term infants | 15 | 112 |
| Total | 99 | 561 |
| KO Number | Category | Sub-Category | Q-Value | Co-Efficient |
|---|---|---|---|---|
| ko00511 | Glycan biosynthesis and metabolism | Other glycan degradation | 3.51 × 10−29 | 0.017720 |
| ko00600 | Lipid metabolism | Sphingolipid metabolism | 2.68 × 10−28 | 0.016071 |
| ko04142 | Transport and catabolism | Lysosome | 4.87 × 10−34 | 0.015629 |
| ko00603 | Glycan biosynthesis and metabolism | Glycosphingolipid biosynthesis –globo and isoglobo series | 1.40 × 10−26 | 0.013687 |
| ko00523 | Metabolism of terpenoids and polyketides | Polyketide sugar unit biosynthesis | 1.56 × 10−26 | 0.012467 |
| ko01230 | Metabolism | Biosynthesis of amino acids | 4.04 × 10−13 | 0.011963 |
| ko00521 | Biosynthesis of other secondary metabolites | Streptomycin biosynthesis | 7.87 × 10−33 | 0.011445 |
| ko00531 | Glycan biosynthesis and metabolism | Glycosaminoglycan degradation | 5.90 × 10−30 | 0.010446 |
| ko01130 | Lipid metabolism | Steroid hormone biosynthesis | 4.78 × 10−13 | 0.009016 |
| ko00513 | Glycan biosynthesis and metabolism | Various types of N-glycan biosynthesis | 5.96 × 10-23 | 0.008769 |
| ko00604 | Glycan biosynthesis and metabolism | Glycosphingolipid biosynthesis - ganglio series | 6.84 × 10−23 | 0.008754 |
| ko04974 | Digestive system | Protein digestion and absorption | 2.39 × 10−26 | 0.008621 |
| ko04920 | Endocrine system | Adipocytokine signaling pathway | 1.79 × 10−15 | 0.007461 |
| ko00460 | Metabolism of other amino acids | Cyanoamino acid metabolism | 7.62 × 10−21 | 0.007188 |
| ko00525 | Biosynthesis of other secondary metabolites | Acarbose and validamycin biosynthesis | 1.23 × 10−15 | 0.006739 |
| ko03010 | Translation | Ribosome | 1.13 × 10−4 | 0.006631 |
| ko01210 | Metabolism | 2-Oxocarboxylic acid metabolism | 7.45 × 10−13 | 0.006483 |
| ko00250 | Amino acid metabolism | Alanine, aspartate and glutamate metabolism | 1.12 × 10−18 | 0.006323 |
| ko00340 | Amino acid metabolism | Histidine metabolism | 1.20 × 10−11 | 0.006178 |
| ko00311 | Biosynthesis of other secondary metabolites | N-Glycan biosynthesis | 4.12 × 10−38 | 0.006095 |
| KO Number | Category | Sub-Category | Q-Value | Co-Efficient |
|---|---|---|---|---|
| ko02020 | Signal transduction | Two-component system | 9.9 × 10−20 | −0.02705 |
| ko02060 | Membrane transport | Phosphotransferase system | 1.1 × 10−27 | −0.02545 |
| ko02040 | Cell motility | Flagellar assembly | 2.3 × 10−8 | −0.02141 |
| ko02026 | Cellular community—prokaryotes | Biofilm formation—Escherichia coli | 5.6 × 10−7 | −0.01179 |
| ko00130 | Metabolism of cofactors and vitamins | Ubiquinone and other terpenoid-quinone biosynthesis | 5.8 × 10−18 | −0.01034 |
| ko01503 | Drug resistance: antimicrobial | Cationic antimicrobial peptide (CAMP) resistance | 4.2 × 10−13 | −0.01026 |
| ko00920 | Energy metabolism | Sulfur metabolism | 3.4 × 10−9 | −0.00897 |
| ko05111 | Cellular community—prokaryotes | Biofilm formation—Vibrio cholerae | 5.2 × 10−9 | |
| ko01220 | Metabolism | Degradation of aromatic compounds | 1.1 × 10−11 | −0.00851 |
| ko00362 | Xenobiotics biodegradation and metabolism | Benzoate degradation | 9.2 × 10−17 | −0.00797 |
| ko02010 | Membrane transport | ABC transporters | 6.4 × 10−14 | −0.00791 |
| ko00053 | Carbohydrate metabolism | Ascorbate and aldarate metabolism | 4.6 × 10−9 | −0.00783 |
| ko05132 | Infectious disease: bacterial | Salmonella infection | 1.7 × 10−13 | −0.00763 |
| ko05133 | Infectious disease: bacterial | Pertussis | 2.6 × 10−5 | −0.00762 |
| ko01053 | Metabolism of terpenoids and polyketides | Biosynthesis of siderophore group non-ribosomal peptides | 9.8 × 10−13 | −0.00729 |
| ko00040 | Carbohydrate metabolism | Pentose and glucuronate interconversions | 6.7 × 10−8 | −0.00728 |
| ko02030 | Cell motility | Bacterial chemotaxis | 9.0 × 10−3 | −0.00727 |
| ko00633 | Xenobiotics biodegradation and metabolism | Nitrotoluene degradation | 4.6 × 10−10 | −0.00718 |
| ko01120 | Metabolism | Microbial metabolism in diverse environments | 6.5 × 10−10 | −0.00713 |
| ko00310 | Amino acid metabolism | Lysine degradation | 5.8 × 10−11 | −0.00699 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groer, M.; Miller, E.M.; Sarkar, A.; Dishaw, L.J.; Dutra, S.V.; Youn Yoo, J.; Morgan, K.; Ji, M.; Ho, T. Predicted Metabolic Pathway Distributions in Stool Bacteria in Very-Low-Birth-Weight Infants: Potential Relationships with NICU Faltered Growth. Nutrients 2020, 12, 1345. https://doi.org/10.3390/nu12051345
Groer M, Miller EM, Sarkar A, Dishaw LJ, Dutra SV, Youn Yoo J, Morgan K, Ji M, Ho T. Predicted Metabolic Pathway Distributions in Stool Bacteria in Very-Low-Birth-Weight Infants: Potential Relationships with NICU Faltered Growth. Nutrients. 2020; 12(5):1345. https://doi.org/10.3390/nu12051345
Chicago/Turabian StyleGroer, Maureen, Elizabeth M. Miller, Anujit Sarkar, Larry J. Dishaw, Samia V. Dutra, Ji Youn Yoo, Katherine Morgan, Ming Ji, and Thao Ho. 2020. "Predicted Metabolic Pathway Distributions in Stool Bacteria in Very-Low-Birth-Weight Infants: Potential Relationships with NICU Faltered Growth" Nutrients 12, no. 5: 1345. https://doi.org/10.3390/nu12051345
APA StyleGroer, M., Miller, E. M., Sarkar, A., Dishaw, L. J., Dutra, S. V., Youn Yoo, J., Morgan, K., Ji, M., & Ho, T. (2020). Predicted Metabolic Pathway Distributions in Stool Bacteria in Very-Low-Birth-Weight Infants: Potential Relationships with NICU Faltered Growth. Nutrients, 12(5), 1345. https://doi.org/10.3390/nu12051345





