Monobutyrin and Monovalerin Affect Brain Short-Chain Fatty Acid Profiles and Tight-Junction Protein Expression in ApoE-Knockout Rats Fed High-Fat Diets
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Permission
2.2. Animals and Diets
2.3. Experimental Design
2.4. Analyses
2.4.1. Lipids
2.4.2. Intestinal In Vivo Permeability
2.4.3. Mucosal Thickness
2.4.4. SCFAs
2.4.5. Gene Expression in Small Intestine and Brain
2.4.6. Cytokines in Blood and Brain and Liver Enzymes
2.5. Statistical Analyses and Calculations
3. Results
3.1. Effects of Monobutyrin and Monovalerin on Serum and Liver Lipids
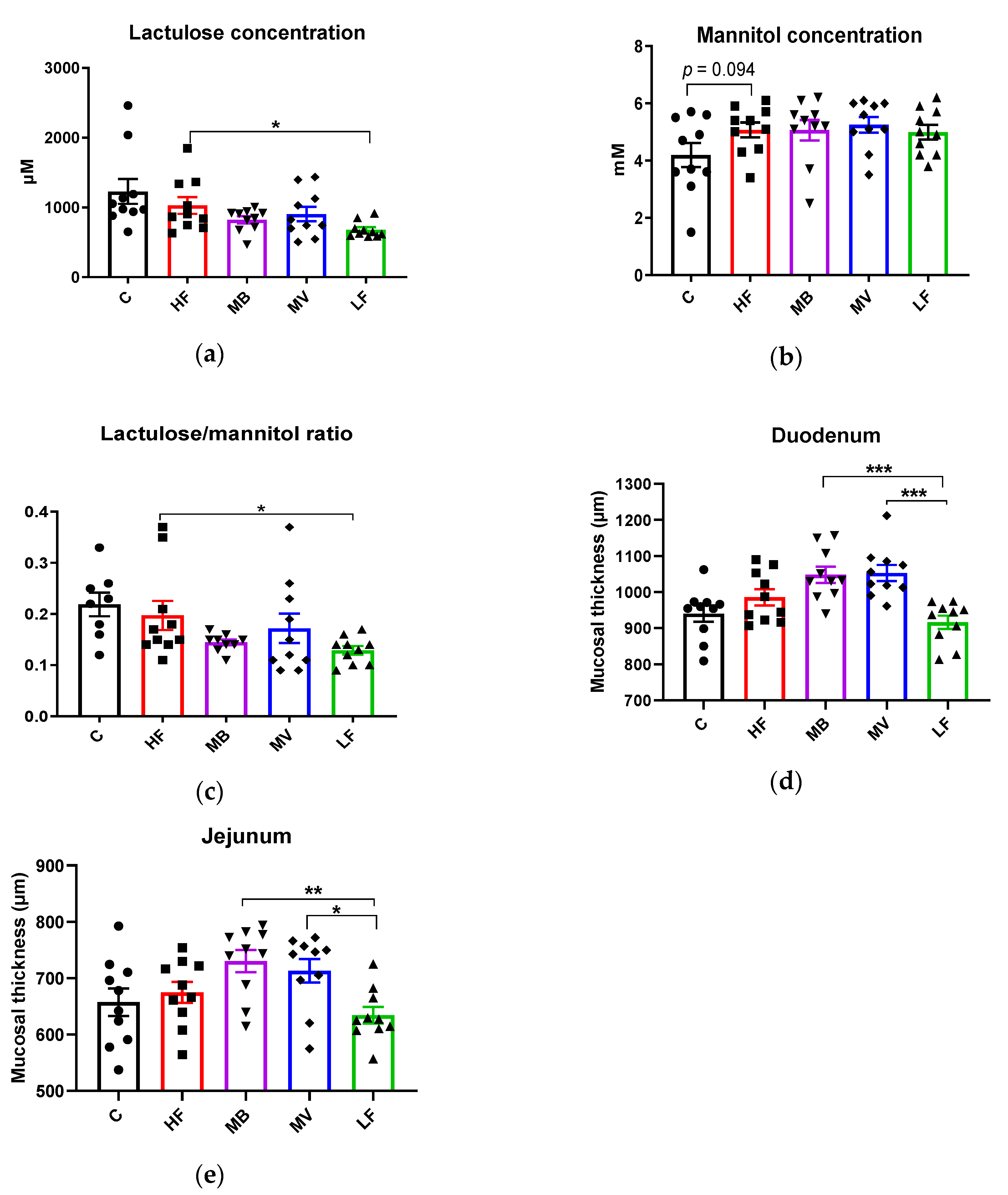
3.2. Effects of Monobutyrin and Monovalerin on Intestinal Permeability and Mucosal Thickness
3.3. Monobutyrin and Monovalerin Influence the SCFA Profile in the Blood and Brain
3.3.1. Portal Serum
3.3.2. Brain
3.4. Monobutyrin and Monovalerin Upregulate the Expression of Tight Junction Proteins and GPR109A Receptor
3.4.1. Small Intestine (Jejunal)
3.4.2. Brain
3.5. Monobutyrin and Monovalerin Affect Inflammation-Related Biomarkers
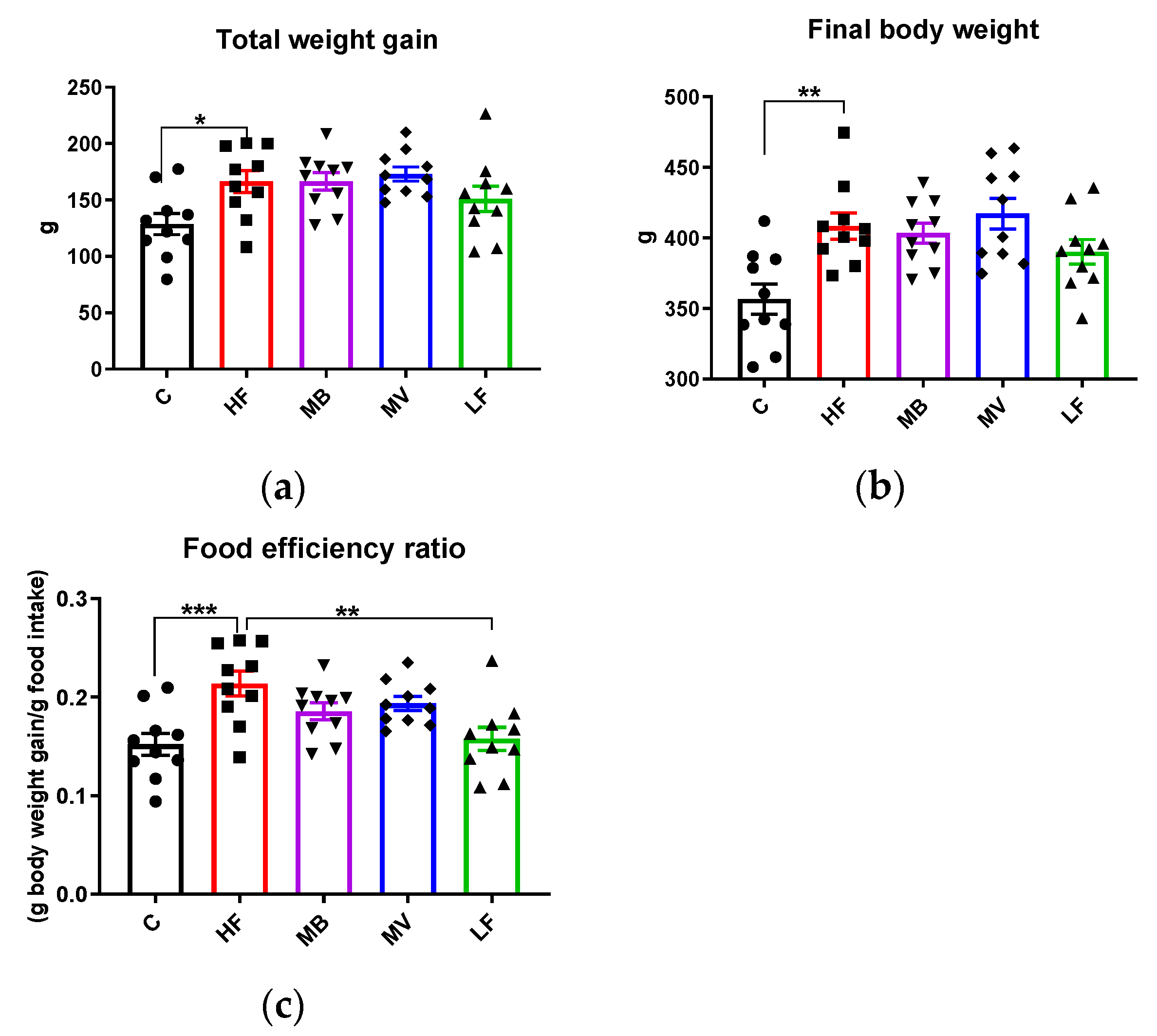
3.6. Food Intake, Body and Organ Weights
3.7. Multivariate Data Analysis and Correlations
3.7.1. Multivariate Data Analysis
3.7.2. Correlations
4. Discussion
4.1. Effects of Monovalerin on Lipid Metabolism
4.2. Impact of Monobutyrin and Monovalerin on the Brain SCFA Profile
4.3. Monobutyrin and Monovalerin Improve the Gut–Brain Barrier Function
4.4. Effects of Monobutyrin on IL-10
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Von Frankenberg, A.D.; Marina, A.; Song, X.; Callahan, H.S.; Kratz, M.; Utzschneider, K.M. A high-fat, high-saturated fat diet decreases insulin sensitivity without changing intra-abdominal fat in weight-stable overweight and obese adults. Eur. J. Nutr. 2017, 56, 431–443. [Google Scholar] [CrossRef]
- Ghanim, H.; Batra, M.; Abuaysheh, S.; Green, K.; Makdissi, A.; Kuhadiya, N.D.; Chaudhuri, A.; Dandona, P. Antiinflammatory and ROS Suppressive Effects of the Addition of Fiber to a High-Fat High-Calorie Meal. J. Clin. Endocrinol. Metab. 2017, 102, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Guillemot-Legris, O.; Muccioli, G.G. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Berge, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012, 61, 543–553. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Lewis, K.; Lutgendorff, F.; Phan, V.; Soderholm, J.D.; Sherman, P.M.; McKay, D.M. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm. Bowel. Dis. 2010, 16, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, C.; Huang, R.; Song, J.; Li, D.; Xia, M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2018, 56, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Hooker, J.M.; Otto, N.; Win, K.; Muench, L.; Shea, C.; Carter, P.; King, P.; Reid, A.E.; Volkow, N.D.; et al. Whole-body pharmacokinetics of HDAC inhibitor drugs, butyric acid, valproic acid and 4-phenylbutyric acid measured with carbon-11 labeled analogs by PET. Nucl. Med. Biol. 2013, 40, 912–918. [Google Scholar] [CrossRef]
- De Chaves, E.P.; Narayanaswami, V. Apolipoprotein E and cholesterol in aging and disease in the brain. Future Lipidol. 2008, 3, 505–530. [Google Scholar] [CrossRef]
- Poirier, J. Apolipoprotein E and cholesterol metabolism in the pathogenesis and treatment of Alzheimer’s disease. Trends. Mol. Med. 2003, 9, 94–101. [Google Scholar] [CrossRef]
- Methia, N.; Andre, P.; Hafezi-Moghadam, A.; Economopoulos, M.; Thomas, K.L.; Wagner, D.D. ApoE deficiency compromises the blood brain barrier especially after injury. Mol. Med. 2001, 7, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Charreau, B.; Tesson, L.; Soulillou, J.P.; Pourcel, C.; Anegon, I. Transgenesis in rats: Technical aspects and models. Transgenic Res. 1996, 5, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Rodrigues, H.G.; Festuccia, W.T.; Crisma, A.R.; Alves, V.S.; Martins, A.R.; Amaral, C.L.; Fiamoncini, J.; Hirabara, S.M.; Sato, F.T.; et al. Tributyrin attenuates obesity-associated inflammation and insulin resistance in high-fat-fed mice. Am. J. Physiol. Endoc. M 2012, 303, E272–E282. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Prykhodko, O.; Fak Hallenius, F.; Nyman, M. Effects of monobutyrin and tributyrin on liver lipid profile, caecal microbiota composition and SCFA in high-fat diet-fed rats. J. Nutr. Sci. 2017, 6, e51. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Prykhodko, O.; Fak Hallenius, F.; Nyman, M. Monovalerin and trivalerin increase brain acetic acid, decrease liver succinic acid, and alter gut microbiota in rats fed high-fat diets. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Prykhodko, O.; Hallenius, F.F.; Nyman, M. Monobutyrin Reduces Liver Cholesterol and Improves Intestinal Barrier Function in Rats Fed High-Fat Diets. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Agusti, A.; Garcia-Pardo, M.P.; Lopez-Almela, I.; Campillo, I.; Maes, M.; Romani-Perez, M.; Sanz, Y. Interplay Between the Gut-Brain Axis, Obesity and Cognitive Function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef]
- Teixeira, T.F.; Souza, N.C.; Chiarello, P.G.; Franceschini, S.C.; Bressan, J.; Ferreira, C.L.; Peluzio Mdo, C. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin. Nutr. 2012, 31, 735–740. [Google Scholar] [CrossRef]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J.; et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef]
- ApoE Knockout Rat Model. Available online: https://www.horizondiscovery.com/apoe-knockout-rat-tgra3710 (accessed on 1 April 2018).
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef]
- Meddings, J.B.; Gibbons, I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology 1998, 114, 83–92. [Google Scholar] [CrossRef]
- Arevalo Sureda, E.; Prykhodko, O.; Westrom, B. Early effects on the intestinal barrier and pancreatic function after enteral stimulation with protease or kidney bean lectin in neonatal rats. Br. J. Nutr. 2018, 119, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Wolinski, J.; Slupecka, M.; Westrom, B.; Prykhodko, O.; Ochniewicz, P.; Arciszewski, M.; Ekblad, E.; Szwiec, K.; Skibo, G.; Kovalenko, T.; et al. Effect of feeding colostrum versus exogenous immunoglobulin G on gastrointestinal structure and enteric nervous system in newborn pigs. J. Anim. Sci. 2012, 90 (Suppl. 4), 327–330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.H.; Liu, J.F.; Nyman, M.; Jonsson, J.A. Determination of short-chain fatty acids in serum by hollow fiber supported liquid membrane extraction coupled with gas chromatography. J. Chromatogr. B 2007, 846, 202–208. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, Y.; Su, L.; He, K.; Wang, Q.; Zhang, Y.; Yang, D.; Yang, Y.; Ma, S. Apolipoprotein E-deficient rats develop atherosclerotic plaques in partially ligated carotid arteries. Atherosclerosis 2015, 243, 589–592. [Google Scholar] [CrossRef]
- Rune, I.; Rolin, B.; Lykkesfeldt, J.; Nielsen, D.S.; Krych, L.; Kanter, J.E.; Bornfeldt, K.E.; Kihl, P.; Buschard, K.; Josefsen, K.; et al. Long-term Western diet fed apolipoprotein E-deficient rats exhibit only modest early atherosclerotic characteristics. Sci. Rep. 2018, 8, 5416. [Google Scholar] [CrossRef]
- Diaz-Zagoya, J.C.; Hurtado, M.E.; Gonzalez, J. Alteration of cholesterol synthesis in rat liver as induced by 4-methyl-5-hydroxy valeric acid. Experientia 1976, 32, 1138–1140. [Google Scholar] [CrossRef]
- Lee, M.H.; Hong, I.; Kim, M.; Lee, B.H.; Kim, J.H.; Kang, K.S.; Kim, H.L.; Yoon, B.I.; Chung, H.; Kong, G.; et al. Gene expression profiles of murine fatty liver induced by the administration of valproic acid. Toxicol. Appl. Pharmacol. 2007, 220, 45–59. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, B.H.; Lee, Y.S.; Kang, K.S. Defective cholesterol traffic and neuronal differentiation in neural stem cells of Niemann-Pick type C disease improved by valproic acid, a histone deacetylase inhibitor. Biochem. Biophys. Res. Commun. 2007, 360, 593–599. [Google Scholar] [CrossRef]
- Szczesniak, O.; Hestad, K.A.; Hanssen, J.F.; Rudi, K. Isovaleric acid in stool correlates with human depression. Nutr. Neurosci. 2016, 19, 279–283. [Google Scholar] [CrossRef]
- Ribeiro, C.A.; Leipnitz, G.; Amaral, A.U.; de Bortoli, G.; Seminotti, B.; Wajner, M. Creatine administration prevents Na+,K+-ATPase inhibition induced by intracerebroventricular administration of isovaleric acid in cerebral cortex of young rats. Brain Res. 2009, 1262, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Lacher, S.K.; Mayer, R.; Sichardt, K.; Nieber, K.; Muller, C.E. Interaction of valerian extracts of different polarity with adenosine receptors: Identification of isovaltrate as an inverse agonist at A1 receptors. Biochem. Pharmacol. 2007, 73, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Zabin, I.; Bloch, K. Studies on the utilization of isovaleric acid in cholesterol synthesis. J. Biol. Chem. 1951, 192, 267–273. [Google Scholar] [PubMed]
- Liu, J.; Sun, J.; Wang, F.; Yu, X.; Ling, Z.; Li, H.; Zhang, H.; Jin, J.; Chen, W.; Pang, M.; et al. Neuroprotective Effects of Clostridium butyricum against Vascular Dementia in Mice via Metabolic Butyrate. Biomed. Res. Int. 2015, 2015, 412946. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ling, Z.; Wang, F.; Chen, W.; Li, H.; Jin, J.; Zhang, H.; Pang, M.; Yu, J.; Liu, J. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci. Lett. 2016, 613, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Leeds, P.; Chuang, D.M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009, 110, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Winkler, E.A.; Sagare, A.P.; Singh, I.; LaRue, B.; Deane, R.; Zlokovic, B.V. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010, 68, 409–427. [Google Scholar] [CrossRef]
- Kirk, J.; Plumb, J.; Mirakhur, M.; McQuaid, S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J. Pathol. 2003, 201, 319–327. [Google Scholar] [CrossRef]
- Zeng, H.; Briske-Anderson, M. Prolonged butyrate treatment inhibits the migration and invasion potential of HT1080 tumor cells. J. Nutr. 2005, 135, 291–295. [Google Scholar] [CrossRef]
- Papi, A.; Ferreri, A.M.; Rocchi, P.; Guerra, F.; Orlandi, M. Epigenetic modifiers as anticancer drugs: Effectiveness of valproic acid in neural crest-derived tumor cells. Anticancer. Res. 2010, 30, 535–540. [Google Scholar]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Toth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Chakrabarty, P.; Li, A.; Ceballos-Diaz, C.; Eddy, J.A.; Funk, C.C.; Moore, B.; DiNunno, N.; Rosario, A.M.; Cruz, P.E.; Verbeeck, C.; et al. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron 2015, 85, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Breitner, J.C.; Baker, L.D.; Montine, T.J.; Meinert, C.L.; Lyketsos, C.G.; Ashe, K.H.; Brandt, J.; Craft, S.; Evans, D.E.; Green, R.C.; et al. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers Dement. 2011, 7, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Leoutsakos, J.M.; Muthen, B.O.; Breitner, J.C.; Lyketsos, C.G.; Team, A.R. Effects of non-steroidal anti-inflammatory drug treatments on cognitive decline vary by phase of pre-clinical Alzheimer disease: Findings from the randomized controlled Alzheimer’s Disease Anti-inflammatory Prevention Trial. Int. J. Geriatr. Psychiatry 2012, 27, 364–374. [Google Scholar] [CrossRef]
- Cheng, F.; Lienlaf, M.; Perez-Villarroel, P.; Wang, H.W.; Lee, C.; Woan, K.; Woods, D.; Knox, T.; Bergman, J.; Pinilla-Ibarz, J.; et al. Divergent roles of histone deacetylase 6 (HDAC6) and histone deacetylase 11 (HDAC11) on the transcriptional regulation of IL10 in antigen presenting cells. Mol. Immunol. 2014, 60, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Terova, G.; Diaz, N.; Rimoldi, S.; Ceccotti, C.; Gliozheni, E.; Piferrer, F. Effects of Sodium Butyrate Treatment on Histone Modifications and the Expression of Genes Related to Epigenetic Regulatory Mechanisms and Immune Response in European Sea Bass (Dicentrarchus Labrax) Fed a Plant-Based Diet. PLoS ONE 2016, 11, e0160332. [Google Scholar] [CrossRef]
- Wu, S.; Li, R.W.; Li, W.; Li, C.J. Transcriptome characterization by RNA-seq unravels the mechanisms of butyrate-induced epigenomic regulation in bovine cells. PLoS ONE 2012, 7, e36940. [Google Scholar] [CrossRef]
- Ajamian, F.; Salminen, A.; Reeben, M. Selective regulation of class I and class II histone deacetylases expression by inhibitors of histone deacetylases in cultured mouse neural cells. Neurosci. Lett. 2004, 365, 64–68. [Google Scholar] [CrossRef]
- Aguilar, E.C.; Leonel, A.J.; Teixeira, L.G.; Silva, A.R.; Silva, J.F.; Pelaez, J.M.; Capettini, L.S.; Lemos, V.S.; Santos, R.A.; Alvarez-Leite, J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFkappaB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef]



| C | HF | MB | MV | LF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Liver Tissue | ||||||||||
| Total CHOL (mg) | 527 ** | 22 | 692 | 32 | 712 | 42 | 697 | 38 | 685 | 39 |
| TG (mg) | 868 ** | 88 | 2503 | 335 | 2900 | 345 | 2577 | 356 | 2490 | 254 |
| Tail Vein–week 2 | ||||||||||
| Total CHOL (mmol/L) | 4 **** | 0.2 | 8 | 0.4 | 8 ††† | 0.4 | 8 †† | 0.4 | 6 ** | 0.3 |
| TG (mmol/L) | 2.2 ** | 0.2 | 3.7 | 0.2 | 3.9 ††† | 0.3 | 4.0 ††† | 0.3 | 2.1 *** | 0.1 |
| LDL-c (mmol/L) | 3 **** | 0.1 | 4 | 0.2 | 4 | 0.2 | 5 | 0.3 | 3 ** | 0.1 |
| HDL-c (mmol/L) | 2 **** | 0.1 | 4 | 0.1 | 4 †††† | 0.2 | 4 †††† | 0.2 | 2 **** | 0.1 |
| LDL-c/HDL-c | 1.7 * | 0.1 | 1.2 | 0.1 | 1.2 ††† | 0.1 | 1.2 ††† | 0.1 | 1.9 ** | 0.1 |
| Tail Vein–week 4 | ||||||||||
| Total CHOL (mmol/L) | 6 **** | 0.2 | 10 | 0.5 | 10 | 0.2 | 9 | 0.3 | 7 **** | 0.3 |
| TG (mmol/L) | 1.7 **** | 0.1 | 3.6 | 0.3 | 3.3 †††† | 0.2 | 3.4 †††† | 0.2 | 2.0 **** | 0.1 |
| LDL-c (mmol/L) | 3 *** | 0.3 | 6 | 0.3 | 6 | 0.3 | 6 | 0.3 | 4 * | 0.1 |
| HDL-c (mmol/L) | 2 **** | 0.1 | 3 | 0.2 | 3 ††† | 0.1 | 3 †††† | 0.2 | 2 **** | 0.1 |
| LDL-c/HDL-c | 2.1 | 0.1 | 1.8 | 0.1 | 2.0 | 0.1 | 2.0 | 0.1 | 2.1 | 0.1 |
| Portal Vein–week 5 | ||||||||||
| Total CHOL (mmol/L) | 3.9 **** | 0.2 | 7.8 | 0.4 | 8.1 ††† | 0.3 | 8.7 ††† | 0.5 | 5.2 **** | 0.1 |
| TG (mmol/L) | 2.1 *** | 0.2 | 4.1 | 0.3 | 4.2 † | 0.2 | 4.6 ††† | 0.2 | 2.7 * | 0.2 |
| LDL-c (mmol/L) | 2.3 *** | 0.1 | 4.7 | 0.1 | 4.8 † | 0.3 | 5.4 †† | 0.3 | 3.2 * | 0.1 |
| HDL-c (mmol/L) | 1.1 **** | 0.1 | 2.5 | 0.1 | 2.5 †††† | 0.1 | 2.9 * †††† | 0.1 | 1.3 **** | 0.0 |
| LDL-c/HDL-c | 2.1 | 0.1 | 2.0 | 0.2 | 1.9 † | 0.1 | 1.9 † | 0.1 | 2.4 * | 0.1 |
| C | HF | MB | MV | LF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Portal vein (µmol/L) | ||||||||||
| Total | 595 | 31 | 539 | 43 | 625 | 45 | 647 | 34 | 592 | 66 |
| Acetic acid | 487 | 26 | 435 | 35 | 509 | 33 | 527 | 27 | 474 | 54 |
| Propionic acid | 39 | 4 | 38 | 6 | 44 | 6 | 41 | 5 | 49 | 6 |
| Butyric acid | 33 | 2 | 34 | 4 | 42 | 5 | 30 | 2 | 36 | 4 |
| Valeric acid | 3 | 0.3 | 4 | 0.4 | 3 | 0.6 | 15 **** | 1.9 | 4 | 0.7 |
| Isovaleric acid | 14 | 2 | 14 | 3 | 14 | 2 | 23 | 3 | 18 | 2 |
| Brain (µmol) | ||||||||||
| Total | 77 | 5 | 73 | 5 | 75 | 5 | 62 | 5 | 74 | 6 |
| Acetic acid | 73 | 5 | 69 | 4 | 71 | 5 | 58 | 5 | 70 | 6 |
| Propionic acid | 1.2 | 0.1 | 1.3 | 0.1 | 1.2 | 0.1 | 1.1 † | 0.2 | 1.4 | 0.1 |
| Butyric acid | 0.20 | 0.10 | 0.06 | 0.06 | 0.32 *† | 0.07 | 0.21 | 0.09 | 0.04 | 0.04 |
| Valeric acid | 1.4 | 0.1 | 1.7 | 0.2 | 1.6 † | 0.2 | 1.6 †† | 0.1 | 1.1 * | 0.1 |
| Isovaleric acid | 0.9 | 0.1 | 0.9 | 0.1 | 0.8 † | 0.1 | 0.5 *†† | 0.1 | 1.1 | 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.D.; Hållenius, F.F.; Lin, X.; Nyman, M.; Prykhodko, O. Monobutyrin and Monovalerin Affect Brain Short-Chain Fatty Acid Profiles and Tight-Junction Protein Expression in ApoE-Knockout Rats Fed High-Fat Diets. Nutrients 2020, 12, 1202. https://doi.org/10.3390/nu12041202
Nguyen TD, Hållenius FF, Lin X, Nyman M, Prykhodko O. Monobutyrin and Monovalerin Affect Brain Short-Chain Fatty Acid Profiles and Tight-Junction Protein Expression in ApoE-Knockout Rats Fed High-Fat Diets. Nutrients. 2020; 12(4):1202. https://doi.org/10.3390/nu12041202
Chicago/Turabian StyleNguyen, Thao Duy, Frida Fåk Hållenius, Xue Lin, Margareta Nyman, and Olena Prykhodko. 2020. "Monobutyrin and Monovalerin Affect Brain Short-Chain Fatty Acid Profiles and Tight-Junction Protein Expression in ApoE-Knockout Rats Fed High-Fat Diets" Nutrients 12, no. 4: 1202. https://doi.org/10.3390/nu12041202
APA StyleNguyen, T. D., Hållenius, F. F., Lin, X., Nyman, M., & Prykhodko, O. (2020). Monobutyrin and Monovalerin Affect Brain Short-Chain Fatty Acid Profiles and Tight-Junction Protein Expression in ApoE-Knockout Rats Fed High-Fat Diets. Nutrients, 12(4), 1202. https://doi.org/10.3390/nu12041202





