Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals
Abstract
1. Introduction
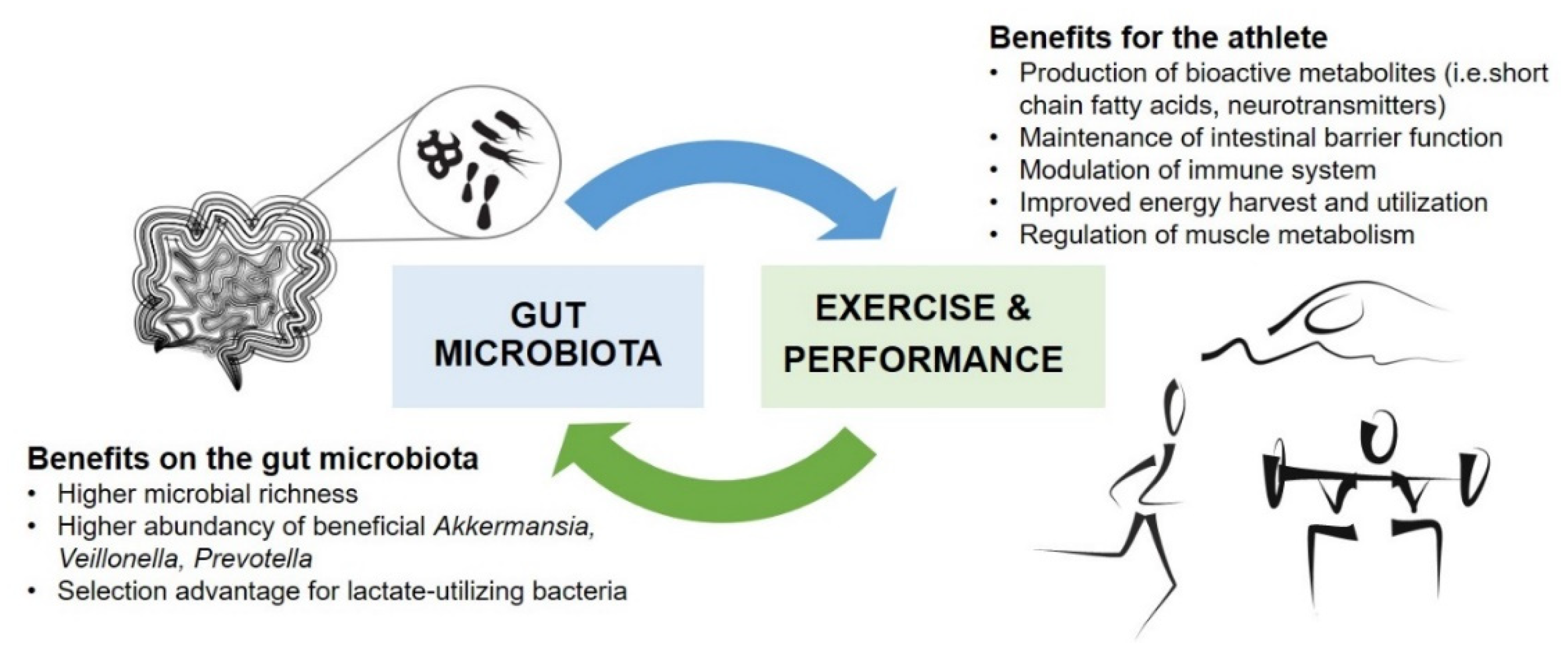
2. Gut Microbiota and Physical Performance
2.1. Gut Microbiota in Athletes
| Subjects | Training Regimen, Exercise Protocol | Dietary Intake | Main Results | Reference |
|---|---|---|---|---|
| Athletes: | ||||
| Rugby players vs. BMI-matched sedentary controls n = 86, males Age 29 ± 4 y | Habitual training and exercise | Self-reported intake by FFQ In athletes, higher total energy, macronutrient and fiber intake. Protein intake 22 E% in athletes, 16 E% in low-BMI and 15 E% in high-BMI controls | In athletes, higher α-diversity and Akkermansia spp. abundance vs. sedentary controls. Protein intake was positively correlated with microbial diversity. | [12] |
| Rugby players vs. BMI-matched sedentary controls n = 86, males Age 29 ± 4 y | Habitual training and exercise | Self-reported intake by FFQ In athletes, higher total energy, macronutrient and fiber intake. Protein 22 E% in athletes vs. 16 E% in low-BMI and 15 E% in high-BMI controls | In athletes, fecal SCFAs, microbial pathways for antibiotic biosynthesis, and amino acids and carbohydrate metabolism were increased. | [30] |
| Professional cyclists vs. amateur cyclists n = 33 (22/M, 11/F) Age 19–49 y | Habitual training | Dietary intake data collected by questionnaire, reported and analyzed as overall dietary patterns. | Prevotella spp. abundance was positively correlated with the amount of exercise and branched chain amino acid and carbohydrate metabolism pathways. Professional cyclists had increased Methanobrevibacter smithii transcripts and upregulated genes involved in the production of methane compared with amateur cyclists. No correlations between overall diet and gut microbiota clusters. | [13] |
| Cross-country runners n = 18, males Age: Control group 35.4 ± 9.0 y Protein group 34.9 ± 9.5 y | Habitual endurance training | Habitual diet by FFQ No differences in habitual dietary intake within or between groups, at baseline or after the intervention. Dietary intervention: habitual diet and whey isolate (10 g) + beef hydrolysate (10 g) or maltodextrin (control) for 10 weeks | After the intervention, higher Bacteroidetes and lower Firmicutes abundance in the protein group. Bifidobacterium longum was reduced after intervention in the protein group. No changes in microbiota composition in the control group, from pre- to post-intervention. No differences within or between groups in fecal SCFA, before or after the intervention. | [32] |
| Bodybuilders, long-distance runners vs. sedentary subjects n = 45, males Age: Bodybuilders 25 ± 3 y, distance runners 20 ± 1 y, sedentary 26 ± 2 y | Habitual training and exercise | Self-recorded 3-day food diary Bodybuilders had a high-protein and distance runners had a low-dietary-fiber dietary pattern. Dietary fiber intake was below recommendation in all groups. | Compositional differences in bodybuilders and runners associated with exercise type and diet. No difference in microbial diversity between groups. In distance runners, protein intake was negatively correlated with microbial diversity. | [33] |
| Highly trained ultra-endurance rowers n = 4, males Age 26.5 ± 1.3 y | ca. 5000 km rowing race over 34 days | Self-reported intake (FFQ), detailed daily record pre-race and during the race No fresh produce consumed during race. Pre-race fiber intake: 21.45 g/day, intra-race 23.1 g/day. Only small changes in intra-race macronutrient intake compared with pre-race | After the race, increased diversity and butyrate-producing species including Roseburia hominis and changes in microbial composition were observed. | [34] |
| Elite race walkers n = 21, males Age 20–35 y | 3-week structured program of intensified training | Dietary intervention for 3 weeks with planned and individualized menus. Subjects allocated into High-carbohydrate diet (HCHO) Periodized-carbohydrate diet (PCHO), or Low-carbohydrate, high-fat diet (LCHF) (ketogenic) group | At baseline, microbiota profiles could be separated into Prevotella- or Bacteroides-dominating enterotypes. HCHO and PCHO resulted in minor changes, whereas LCHF resulted in stronger changes in microbial composition. LCHF was associated with reduced Faecalibacterium, Bifidobacterium, and Veillonella spp. Increased Bacteroides and Dorea spp. in the LCHF group was associated with decreased performance. | [35] |
| Marathon runners: n = 15 (4/M, 11/F) Mean age 27.1 y; Non-runners: n = 11 (5/M, 6/F) Mean age 29.2 y; Ultramarathon and rower athletes: n = 11 (5/M, 6/F) Age not reported | Habitual training and a marathon Type of exercise not reported for the cohort of ultra-marathon and rower athletes | Dietary intake data collected by questionnaire | In marathon runners, the relative abundance of Veillonella spp. increased post-marathon. In ultramarathon and rower athletes, the relative abundance of the methylmalonyl-CoA pathway (degrading lactate into propionate) in the gut microbiome increased post-exercise. No correlations between dairy, protein, grains, fruits, or vegetables and Veillonella spp. abundance was observed among marathon runners. | [14] |
| Non-athletes and sedentary subjects: | ||||
| Healthy subjects n = 39 (22/M, 17/F) Age 18–35 y | VO2Peak test to assess CRF and to allocate subjects into groups (low, average, and high CRF) | 24-h dietary recall interview No significant differences in dietary intake between groups. | CRF correlated with microbial diversity and butyrate production. | [36] |
| Active vs. sedentary women n = 40 Active: 30.7 ± 5.9 y, BMI 24.4 ± 4.5 kg/m2; Sedentary: 32.2 ± 8.7 y, BMI 22.9 ± 3.0 kg/m2 | Habitual physical activity measured by accelerometer. | Self-reported food intake (FFQ) Fiber, fruit, and vegetable intake significantly higher in the active group. | Higher abundance of Faecalibacterium prausnitzii, Roseburia hominis and Akkermansia muciniphila in active women. Physical activity was not associated with differences in microbiota richness. | [37] |
| Lean and obese sedentary subjects n = 32 Lean: n = 18 (9/M, 9/F), mean age 25.10 y; Obese: n = 14 (3/M, 11/F), mean age 31.14 y | Exercise intervention study: 6 weeks of moderate-to-vigorous intensity aerobic exercise and 6 weeks without exercise | Maintenance of habitual diet during the intervention. A designed 3-day food menu, based on previous reported habitual diet, before fecal sample collection. | At baseline, the composition of gut microbiota differed between lean and obese subjects, but after exercise training, no difference was observed between lean and obese subjects. Exercise increased fecal SCFA and SCFA producing bacteria in lean subjects. | [15] |
| Children and teenagers n = 267 (178/M, 89/F) Age 7–18 y | Self-reported physical activity | Type of diet reported as omnivore or vegetarian. | Gut microbiota composition was affected by BMI, exercise frequency, and diet type. Firmicutes were significantly enriched in subjects with more frequent exercise. | [38] |
| Overweight sedentary women n = 17 Age 36.8 ± 3.9 y BMI 31.8 ± 4.4 kg/m2 | Habitual physical activity. Exercise intervention study: 6-week control period without exercise, 6-week programmed endurance exercise, on a bicycle ergometer | Habitual diet Self-reported 3-day food record No changes in intake of total energy, macronutrients or fiber from baseline, after control or exercise period. A modest increase in energy from starch | Exercise did not affect α-diversity. Exercise increased Akkermansia spp. and reduced Proteobacteria abundance. No significant changes in BMI or total fat mass after exercise. Significant reduction in android fat mass. | [16] |
| Healthy subjects n = 37 (20/M, 17/F) Age 25.7 ± 2.2 y | VO2max test to assess CRF | Habitual diet recorded for 7 days | CRF correlated with Firmicutes/Bacteroidetes ratio. No correlation between dietary factors or BMI and Firmicutes/Bacteroidetes ratio. | [39] |
| Elderly community-dwelling men n = 373 Age 78–98 y | Habitual physical activity, measured by activity sensor, for 5 days. Step count as primary physical activity variable | Self-reported food intake (FFQ) Step count was not associated with food or alcohol intake. | Physical activity was not associated with α-diversity but was positively associated with β-diversity. Increased physical activity was associated with greater Faecalibacterium and Lachnospira spp. prevalence. | [40] |
| Elderly sedentary women n = 29 Age 65–77 y | Exercise intervention study: resistance training (trunk muscles) or aerobic exercise (brisk walking) for 12 weeks | Self-reported food intake (FFQ) No changes in energy or nutrient intake after interventions. | Brisk walking increased the relative abundance of Bacteroides spp. Bacteroides spp. abundance was positively associated with improved CRF after aerobic training but not with improved CRF after resistance training. | [17] |
2.2. Impacts of Exercise Interventions on Gut Microbiota
2.3. Effects of Targeted Gut Microbiota Modulation on Physical Performance
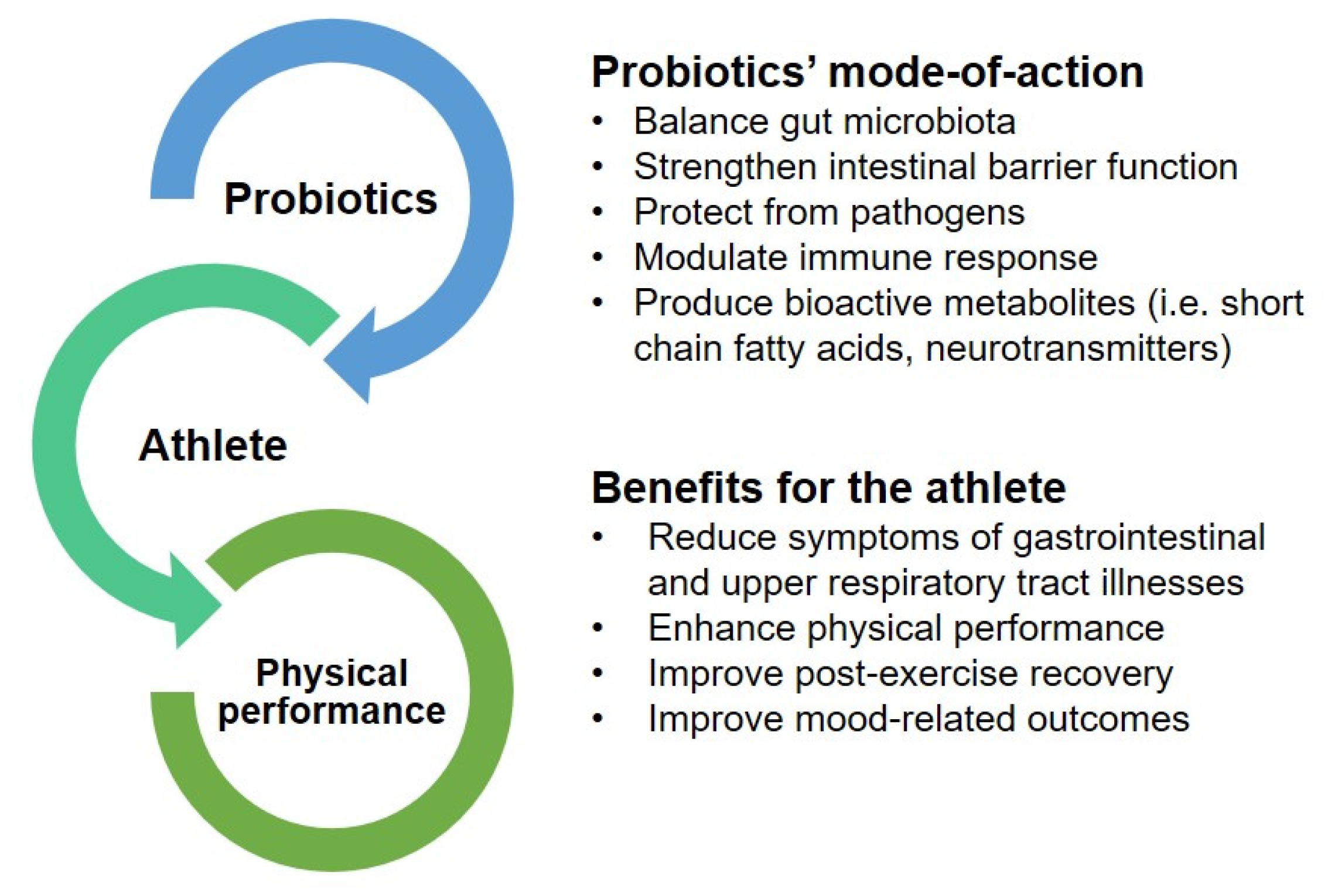
3. Probiotics as a Potential Ergogenic Aid to Enhance Physical Performance
3.1. Reduction of Gastrointestinal and Upper Respiratory Tract Symptoms
3.2. Enhancement of Physical Performance
| Subjects | Design | Exercise Protocol and/or Intervention | Probiotic Supplementation | Main Results | Reference |
|---|---|---|---|---|---|
| Animal studies: | |||||
| 6-week-old male ICR mice 3 groups n = 8/group | Animal study | Forelimb grip strength Forced swim-to-exhaustion test, with loads 15-min swim test to determine recovery and fatigue-related biomarkers | L. plantarum TWK10 (LP10) Dosing per group: 0, 2.05 × 108; or 1.03 × 109 CFU/kg/day for 6 weeks | PRO improved forelimb grip strength and exhaustive swimming time. Blood lactate, ammonia, and CK levels were lower in PRO mice after a 15-min swim compared with those in control mice. Type I muscle fiber type increased, and relative muscle weight increased in PRO mice vs. control mice. | [88] |
| 6-week-old male ICR mice 4 groups n = 8/group | Animal study | Forelimb grip strength Forced swim-to-exhaustion test with loads 10-min and 90-min swim tests, to determine recovery and fatigue-related biomarkers | A kefir drink with L. fermentum DSM 32,784 (LF26), L. helveticus DSM 32,787 (LH43), L. paracasei DSM 32,785 (LPC12), L. rhamnosus DSM 32,786 (LRH10), and S. thermophilus DSM 32,788 (ST30) Kefir dosing per group: 0, 2.15, 4.31, or 10.76 g/kg/day for 4 weeks | Kefir supplementation increased time-to exhaustion, and improved forelimb grip strength. Blood lactate, ammonia, blood urea nitrogen, and CK levels were lower after exercise in kefir-fed mice compared with control mice, in a dose-dependent manner. Glycogen contents in the liver and muscle were higher in kefir-supplemented mice compared with control mice. | [89] |
| 11-week-old male Wistar rats 2 groups n = 13/group | Animal study | Incremental speed exercise on a treadmill, until exhaustion Treadmill chamber, coupled with gas-analyzer, to assess VO2max | Saccharomyces boulardii (strain not reported) 3 × 108 CFU/kg/day for 10 days | PRO supplementation moderately improved aerobic performance. PRO mice ran approx. 8 min longer than control mice (until exhaustion) and had higher maximal speed. | [90] |
| 7-week-old male ICR mice 4 groups n = 10/group | Animal study | Forelimb grip strength Forced swim-to-exhaustion test, with loads 10-min and 90-min swim tests, to determine recovery and fatigue-related biomarkers | B. longum subsp. longum OLP-01 isolated from a female weightlifter Dosing per group: 0, 2.05 × 109, 4.10 × 109, or 1.03 × 1010 CFU/kg/day for 4 weeks | PRO improved forelimb grip strength and swim-to-exhaustion time, in a dose-dependent manner. Blood lactate and ammonia levels were lower after the acute swim test in PRO vs. control mice. After a 90-min swim test, blood urea nitrogen and CK levels were lower in PRO mice compared with those in control mice. PRO increased hepatic and muscular glycogen contents, observed at autopsy. | [98] |
| 6-week-old male ICR mice 4 groups n = 10/group | Animal study | Forelimb grip strength Forced swim-to-exhaustion test, with loads 10-min and 90-min swim tests, to determine recovery and fatigue-related biomarkers | L. salivarius subsp. salicinius SA-03, isolated from a female weightlifter’s gut microbiota Dosing per group: 0, 2.05 × 109, 4.10 × 109, or 1.03 × 1010 CFU/kg/day for 4 weeks | PRO improved forelimb grip strength and swim-to-exhaustion time, in a dose-dependent manner. Blood lactate and ammonia levels were lower and blood glucose levels were higher after acute tests in the PRO groups vs. control group. After a 90-min swim, blood CK levels were lower in PRO groups compared to the control group. PRO increased hepatic and muscular glycogen contents, observed at autopsy. | [99] |
| Clinical studies: | |||||
| Swimmers | |||||
| Highly trained competitive swimmers n = 17, females Age not reported | Randomized, double-blind, placebo-controlled | 6 weeks of intensified off-season training, including swimming and resistance exercise. Performance assessment: Vertical jump force plate test, aerobic and anaerobic swim performance test Cognitive assessment: stress and recovery during the intensified exercise training load (the Recovery-Stress Questionnaire for Athletes) | B. longum 35,624; 1 × 109 CFU bacteria/day for 6 weeks | No significant differences in exercise performance or systemic inflammation markers (at rest) between PRO and PLA. Differences in cognitive outcomes were detected showing more favorable sport recovery related scores in the PRO group. | [93] |
| Swimmers n = 46, females Age 13.8 ± 1.8 y | Randomized, placebo-controlled | Normal exercise regimen Performance assessment: 400-m free- swimming record, Harvard step test to, measure VO2max | L. acidophilus SPP, L. delbrueckii subsp. bulgaricus, B. bifidum, and S. salivarus subsp. thermophilus, strains not reported 400 mL of probiotic yogurt/day with 4 × 1010 CFU/mL for 8 weeks | Significant improvement in VO2max in the PRO group. No differences in 400-m swimming times between PRO and PLA groups. | [82] |
| Endurance runners | |||||
| Elite distance runners n = 20, males Age 27.3 ± 6.4 y | Randomized, double-blind, placebo-controlled, crossover | Habitual winter-season training Performance assessment: A treadmill running test until exhaustion, at the start of the study period and the end of each study month | L. fermentum VRI-003; 1.2 × 1010 CFU bacteria/day for 4 weeks Cross-over study, with 1-month wash-out | No difference in performance outcomes with PRO compared to PLA. The number of illness days during PRO supplementation was significantly lower than with PLA (30 vs. 72 days). IFN-γ response was moderately higher with the PRO than with PLA. | [81] |
| Endurance-trained runners n = 8, males Age 26 ± 6 y | Randomized, blinded, placebo-controlled, cross-over | Habitual training Bout of exercise: 2-h running exercise at 60% VO2max in hot ambient conditions | L. casei (strain not reported) 1 × 1011 CFU/day for 7 days Cross-over study, with 1-month wash-out | No differences in hydration status between PRO and PLA. Inflammatory cytokine levels were not different between PRO and PLA, either pre-exercise or post-exercise (1, 2, 4, and 24 h after running). | [100] |
| Endurance-trained runners n = 8, males Age 26 ± 6 y | Randomized, blinded, placebo-controlled, cross-over | Habitual training Bout of exercise: 2-h running exercise, at 60% VO2max, in hot, ambient conditions | L. casei (strain not reported) 1 × 1011 CFU/day for 7 days Cross-over study with 1-month wash-out | PRO and PLA did not differ in salivary anti-microbial protein or serum cortisol responses during the post-exercise period (1, 2, 4, and 24 h after running). | [101] |
| Runners n = 10, males Age 27 ± 2 y | Randomized, double-blind, placebo-controlled, cross-over | Normal training Performance assessment: Running to fatigue, at 80% of ventilatory threshold, at 35 °C and 40% humidity | Multispecies probiotic, strains not specified; L. acidophilus, L. rhamnosus, L. casei, L. plantarum, L. fermentum, B. lactis, B. breve, B. bifidum, and S. thermophilus 45 × 109 CFU/day for 4 weeks, cross-over study with a 3-week wash-out | PRO increased run time to fatigue (PRO 37:44 vs. PLA 33:00 min:sec). A moderate, non-significant reduction in pre-exercise and post-exercise serum lipopolysaccharide (LPS) levels for PRO compared to PLA. No difference between PRO and PLA in plasma IL-6, IL-10, and IL-1Ra or GI permeability after exercise in the heat. | [77] |
| Marathon runners n = 42, males Age 39.5 ± 9.4 y | Randomized, double-blind, placebo-controlled | Usual training Bout of exercise: marathon run | L. casei Shirota 40 × 109 CFU/day for 30 days | PRO maintained salivary immune protection and increased anti-inflammatory response on the upper airways, immediately after the marathon. Serum TNF-α level was significantly lower immediately post-marathon in the PRO group compared to that in the PLA group | [102] |
| Marathon runners n = 119 (105/M, 14/F) Average age 40 y | Randomized, double-blind, placebo-controlled | 3-month training period, 6-day preparation period Bout of exercise: marathon run | L. rhamnosus GG 4.0 × 1010 bacteria in drink/day (or 1 × 1010 in tablet/day) for 3 months | PRO did not differ from PLA in ox-LDL or antioxidant activity, pre- or post-marathon. | [103] |
| Marathon runners n = 24 (20/M, 4/F) Age 22–50 y | Randomized, double-blind, placebo- controlled, matched-pairs | Habitual training routine Performance assessment/Bout of exercise: Marathon race (no baseline assessment) | L. acidophilus CUL60, L. acidophilus CUL21, B. bifidum CUL20, and B. animalis subsp. lactis CUL34 2.5 × 1010 CFU/day for 28 days | No difference in marathon times between PRO and PLA. During the final third of the race, the reduction in average relative speed was greater in PLA compared to PRO. GI symptoms were lower in PRO compared to PLA during the final third. No difference in post-race serum IL-6, IL-8, IL-10, and cortisol levels between groups. | [104] |
| Ultramarathon runners n = 32 (26/M, 6/F) Age 23–53 y | Randomized, controlled (single-blind for glutamine supplementation) | Training for a marathon, ultra-marathon race of 294 km Performance assessment: A graded exercise test, to maximal exhaustion, on a motorized treadmill, VO2max test, pre-marathon, time-to-completion in ultra- marathon race | PRO: Multi-strain probiotic, daily dose 30 × 109 CFU comprising of 10 × 109 CFU L. acidophilus CUL-60 (NCIMB 30,157), 10 × 109 CFU L. acidophillus CUL-21 (NCIMB 30,156), 9.5 × 109 CFU B. bifidum CUL-20 (NCIMB 30,172), and 0.5 × 109 CFU B. animalis subsp. lactis CUL-34 (NCIMB 30,153 + 55.8 g fructooligosaccharides PRO + glutamine: Daily dose 2 × 109 CFU L. acidophilus CUL-60 (NCIMB 30,157), 2 × 109 CFU L. acidophilus CUL-21 (NCIMB 30156), 5 × 107 CFU B. bifidum CUL-20 (NCIMB 30,172), 9.5 × 108 CFU B. animalis subsp. lactis CUL-34 (NCIMB 30,153), and 5x 109 CFU L. salivarius CUL61 (NCIMB 30,211) + 0.9 g glutamine 12 weeks before the marathon | No difference in pre-race VO2max or in time-to-completion for ultra-marathon between PRO, PRO + glutamine, and control groups. PRO and PRO + glutamine had no effects on immune activation via extracellular heat-shock protein eHsp72 signaling at post-race. | [94] |
| Cyclists, triathletes | |||||
| Competitive cyclists n = 99 (64/M, 35/F) Age 35 ± 9 y/M and 36 ± 9 y/F | Randomized, double-blind, placebo-controlled | Habitual training (physical activity recorded) Performance assessment: an incremental cycle ergometer performance test (peak power output, VO2max) | L. fermentum VRI-003 PC 1 × 109 CFU/day for 11 weeks | PRO did not affect training patterns or performance in VO2 max testing. Acute exercise-induced changes in anti- and pro-inflammatory cytokines were attenuated with PRO. | [71] |
| Triathletes Study I: n = 18, Study II: n = 16 Sex not reported Age 19–26 y | Randomized, double-blind, placebo-controlled | 8 weeks of programmed training before a sprint triathlon (Study I) or full triathlon competition (Study II) Performance assessment: Wingate and 85% VO2max test (after full triathlon) | L. plantarum PS128 3 × 1010 CFU/day Study I: last 4 weeks of training Study II: last 3 weeks of training | In Study II, performance during recovery from a full triathlon was decreased in the PLA group and maintained at the pre-triathlon level in the PRO group. PRO group had lower blood TNF-α, IFN-γ, IL-6, and IL-8 levels compared to PLA, immediately after exercise (Study I/II), with levels significantly lower in PRO group 3 h after full triathlon (Study II). Anti-inflammatory IL-10 was higher in the PRO group, immediately after exercise (Study II) compared with that in the PLA group. No differences in muscle damage or fatigue markers detected between groups (Study I/II) except, lower CK in PRO vs. PLA, 3 h after full triathlon (Study II). Oxidative stress marker (MPO) was lower in PRO after exercise, with no differences 3 h post-exercise. | [105] |
| Elite athletes (badminton, triathlon, cycling, alpinism, karate, savate, kayak, judo, tennis, and swimming) n = 50 (36/M, 14/F) Age 18–28 y | Randomized, double-blind, placebo-controlled | Habitual training >11 h/week, self-reported training loads Performance assessment: VO2max, by a graded cardiopulmonary test, on a treadmill Cognitive assessment: Profile of mood and state (POMS) questionnaire | L. helveticus Lafti L10 2 × 1010 CFU/day for 14 weeks | No difference in VO2max and treadmill performance between PRO and PLA. Increase in the subjective feeling of vigor in the PRO group, but no difference in other cognitive scores between groups. | [84] |
| Recreational triathletes n = 30 (25/M, 5/F) Age 35 ± 1 y | Randomized, double-blind, placebo-controlled | Standardized training program for the previous 6 months Performance assessment/Bout of exercise: a long-distance triathlon (no baseline assessment) | Multistrain probiotic, daily dose 30 × 109 CFU (10 × 109 CFU L. acidophilus CUL-60 (NCIMB 30,157), 10 × 109 CFU L. acidophillus CUL-21 (NCIMB 30,156), 9.5 × 109 CFU B. bifidum CUL-20 (NCIMB 30,172), 0.5 × 109 CFU B. animalis subsp. lactis CUL-34 (NCIMB 30,153)) + 55.8 g fructo-oligosaccharides, alone or in combination with 600 mg N-acetyl carnitine + 400 mg α-lipoic acid for 12 weeks before and 6 days after triathlon | Non-significantly faster times were reported for PRO during swim and cycle stages, and a trend towards an overall faster time was reported compared to PLA (~86 min faster). No baseline measurements on performance were assessed. PRO reduced post-race plasma endotoxin levels, whereas PLA had no effect. | [73] |
| Team sports | |||||
| Division I volleyball and soccer athletes n = 23, females Age 19.6 ± 1.0 y | Randomized, double-blind, placebo-controlled | Offseason resistance training protocol Performance assessment: 1RM testing (bench press, squat, deadlift), isometric midthigh pull, vertical jump height, pro-agility test | Bacillus subtilis DE111 5 × 109 CFU/day for 10 weeks | PRO had no effect on strength or athletic performance but significantly reduced percentage of body fat percentage. | [96] |
| Division I baseball athletes n = 25, males Age 20.1 ± 1.5 y | Randomized, double-blind, placebo-controlled | Resistance training program Performance assessment: 1RM testing (squat, deadlift), pro-agility test, 10-yard sprint, standing long jump | Bacillus subtilis DE111 1 × 109 CFU/day for 12 weeks | No differences between PRO and PLA in strength, performance, or body composition. PRO reduced TNF-α levels, but no differences in IL-10, cortisol, zonulin, or testosterone levels observed between PRO and PLA. | [95] |
| Highly trained athletes n = 29 (13/M, 16/F) Age 20–35 years | Randomized, double-blind, placebo-controlled | Normal training Performance assessment: Cycle ergometer exercise test until exhaustion | B. bifidum W23, B. lactis W51, Enterococcus faecium W54, L. acidophilus W22, L. brevis W63, and L. lactis W58 1 × 1010 CFU/day for 12 weeks | No difference in performance between groups. Weekly training loads were significantly higher in PRO compared to PLA (8.0 ± 2.3 vs. 6.6. ± 4.3 h/week). Exercise-induced reduction in tryptophan levels in PLA but not in the PRO group. PRO reduced the incidence of URT infections. | [85] |
| Active non-athletes | |||||
| Resistance trained subjects n = 15, males Age 25 ± 4 y | Randomized, double-blind, placebo-controlled, crossover | Muscle-damaging eccentric exercise bout Performance assessment: isometric peak torque, after muscle damaging-exercise | S. thermophilus FP4, and B. breve BR03 5 × 109 CFU of each/day for 21 days | PRO attenuated performance decrements caused by muscle-damaging exercise during the recovery period. No effects of PRO on muscle soreness, range of motion, or plasma creatine kinase. PRO lowered resting IL-6 concentrations that were sustained until 48 h post-exercise. | [106] |
| Recreational exercisers n = 29, males Age 21.5 ± 2.8 y | Single-blind, crossover (casein first, after washout, PRO+casein) | Single-leg exercise bout Performance assessment: Anaerobic power by modified Wingate test, single-leg vertical jump, strength, by 1RM testing in the one-legged leg press, after muscle damaging-exercise | Bacillus coagulans BC30 1 × 109 CFU/day + 20 g casein for 14 days | PRO + casein increased perceived recovery status and reduced muscle soreness after exercise compared with casein alone. PRO + casein maintained post-exercise Wingate peak power at the pre-exercise level, whereas casein alone demonstrated reduced post-exercise performance. For 1RM leg-press and vertical jump power, no differences between groups in post-exercise performance. | [107] |
| Physically active subjects n = 27, females Age 18–25 y | Controlled, randomized | Habitual moderate exercise Performance assessment: treadmill running until exhaustion, VO2max test (Bruce test) | Probiotic not specified 450 g of probiotic yogurt/day for 2 weeks | No difference in VO2max between PRO and PLA. PRO yogurt increased antioxidant enzyme activities and reduced MMP2 and MMP9 levels before and after exhaustive exercise. No significant differences between PRO and PLA in high-sensitivity CRP, IL-6, and TNF-α after intense exercise. | [108] |
| Physically active students n = 11, sex not reported Age 22 ± 1 y | Non-controlled | Habitual training including endurance exercise Bout of exercise: 2-h cycling at 60% of VO2max | L. acidophilus, L. delbrueckii subsp. bulgaricus, Lactococcus lactis subsp. lactis, L. casei, L. helveticus, L. plantarum, L. rhamnosus, L. salivarius subsp. salivarius, B. breve, B. bifidum, B. infantis, B. longum, Bacillus subtilis, S. thermophilus minimum 2 × 109 CFU/capsule, 3 capsules/day for 30 days | Rating of perceived exertion during exercise was not different between PRO and PLA. PRO did not affect salivary antimicrobial proteins at rest or in response to an acute bout of prolonged exercise. | [109] |
| Students n = 67, males and females (n not specified by sex) Age 18–24 y | Controlled | The exercise groups completed structured, long-distance, endurance run training, whereas the active group maintained their usual exercise routine. Performance assessment: 1.5-mile (2.41 km) walk or run | Probiotic kefir, probiotic strain and dose not specified 15 weeks | No effect of PRO on 1.5-mile completion time. PRO attenuated exercise-induced inflammation, measured as serum CRP levels. | [110] |
| Students of physical education n = 30, males Average age: PRO 21.56 y, PLA 21.28 y | Randomized, matched pairs | Habitual training and training program by the study Performance assessment: Cooper test, maximum aerobic power, using Bulk test on a laboratory treadmill | Probiotic strains unspecified, included S. thermophilus and/or L. delbrueckii subsp. bulgaricus 1 × 105 CFU/g in 200 mL yogurt/day for 10 weeks | PRO improved VO2max and aerobic performance. PRO decreased serum high-sensitivity CRP and increased HDL levels. | [111] |
| Healthy participants n = 16, males Age 20–40 y | Randomized, double-blind, placebo-controlled | Habitual exercise Performance assessment: Treadmill running at 85% VO2max workload, until exhaustion. | L. plantarum TWK10 1 × 1011 CFU/day for 6 weeks | PRO improved time-to-exhaustion (PLA vs. PRO: 817 ± 79 s vs. 1292 ± 204 s). Blood glucose was higher in PRO vs. PLA after exhaustive exercise. No differences in post-exercise blood lactate, free fatty acid, CK levels between PRO and PLA. | [91] |
| Healthy participants n = 54, (27/M, 27/F) Age 20–30 y | Double-blind, placebo-controlled | Habitual exercise Performance assessment: treadmill running, at 60% VO2max and 85% VO2max workload, until exhaustion | L. plantarum TWK10 3 × 1010 CFU/day or 9 × 1010 CFU/day for 6 weeks | Exhaustion time was increased in both PRO groups and were longer compared to PLA. Improvement in exercise capacity was dose-dependent. PRO reduced serum lactate during and after exercise compared to PLA. Muscle mass increased in the high-dose PRO group. | [92] |
| Healthy sedentary individuals n = 41, males Age 19–26 y | Randomized, parallel, placebo-controlled | Circuit training protocol, including resistance exercises, 3 times a week Performance assessment: muscular strength (peak torque) and power via an isokinetic dynamometer | L. acidophilus BCMC 12,130, L. casei BCMC 12,313, L. lactis BCMC 12,451, B. bifidum BCMC 02,290, B. infantis BCMC 02,129 and B. longum BCMC 02,120 6 × 1010 CFU/day for 12 weeks | PRO did not show superior effects to PLA on muscular strength (peak torque) and power. PRO alone and exercise alone increased post-intervention serum IL-10 concentrations from pre-intervention levels. PRO and PLA with or without exercise, had no effects on serum IL-6 concentration. | [97] |
| Healthy elderly individuals with stretching experience n = 29 (14/M, 25/F) Age > 65 y | Randomized, double-blind, placebo-controlled | Moderate resistance exercise training, in instructed classes and at home Cognitive assessment: General cognitive performance (incl. tests for accuracy, reaction time), mental state (scoring for depression, anxiety, and overall mental state) | B. longum BB536, B. infantis M-63, B. breve M-16V and B. breve B-3 5 × 1010 CFU/day (1.25 × 1010 CFU each probiotic/day) for 12 weeks | An increase in the general cognitive function scores was observed in PRO and PLA groups, at 12 weeks. PRO group showed a decrease in anxiety-depression scores, body weight, BMI and body fat. | [112] |
3.3. Improvement in Post-Exercise Recovery
3.4. Improvements in Mood-Related Outcomes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Ning, M.X.; Chen, D.K.; Ma, W.T. Interactions Between the Gut Microbiota and the Host Innate Immune Response Against Pathogens. Front. Immunol. 2019, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology 2012, 37, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef]
- Larsen, O.F.; Claassen, E. The mechanistic link between health and gut microbiota diversity. Sci. Rep. 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Neish, A.S.J.G. Microbes in gastrointestinal health and disease. Gastroenterology 2009, 136, 65–80. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R.J.N. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for a gut-muscle axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef]
- McKinney, J.; Lithwick, D.J.; Morrison, B.N.; Nazzari, H.; Isserow, S.H.; Heilbron, B.; Krahn, A.D. The health benefits of physical activity and cardiorespiratory fitness. Br. Columbia Med. J. 2016, 58, 131–137. [Google Scholar]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Munukka, E.; Ahtiainen, J.P.; Puigbo, P.; Jalkanen, S.; Pahkala, K.; Keskitalo, A.; Kujala, U.M.; Pietila, S.; Hollmen, M.; Elo, L.; et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front. Microbiol. 2018, 9, 2323. [Google Scholar] [CrossRef]
- Morita, E.; Yokoyama, H.; Imai, D.; Takeda, R.; Ota, A.; Kawai, E.; Hisada, T.; Emoto, M.; Suzuki, Y.; Okazaki, K. Aerobic exercise training with Brisk walking increases intestinal Bacteroides in healthy elderly women. Nutrients 2019, 11, 868. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Vepsäläinen, O.; Suomalainen, M.; Kolmeder, C.; Varjosalo, M.; Miettinen, S.; Kukkonen, K.; Savilahti, E.; Kuitunen, M. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Hibberd, A.; Yde, C.; Ziegler, M.; Honoré, A.H.; Saarinen, M.T.; Lahtinen, S.; Stahl, B.; Jensen, H.; Stenman, L. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Eutamene, H.; Bueno, L. Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut 2007, 56, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Yun, M.; Oh, Y.J.; Choi, H.-J. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. J. Microbiol. 2018, 56, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Charreire, H.; Kesse-Guyot, E.; Bertrais, S.; Simon, C.; Chaix, B.; Weber, C.; Touvier, M.; Galan, P.; Hercberg, S.; Oppert, J.-M.J.B.J.o.N. Associations between dietary patterns, physical activity (leisure-time and occupational) and television viewing in middle-aged French adults. Brit. J. Nutr. 2011, 105, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Sheflin, A.M.; Melby, C.L.; Carbonero, F.; Weir, T.L. Linking dietary patterns with gut microbial composition and function. Gut Microbes 2017, 8, 113–129. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiu, C.C.; Li, Y.P.; Huang, W.C.; Te Huang, Y.; Huang, C.C.; Chuang, H.L. Effect of intestinal microbiota on exercise performance in mice. J. Strength. Cond. Res. 2015, 29, 552–558. [Google Scholar] [CrossRef]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rué, O.; Mariadassou, M. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E158–E171. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell. Fact. 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, D.; Bressa, C.; Bailén, M.; Hamed-Bousdar, S.; Naclerio, F.; Carmona, M.; Pérez, M.; González-Soltero, R.; Montalvo-Lominchar, M.G.; Carabaña, C. Effect of a protein supplement on the gut microbiota of endurance athletes: A randomized, controlled, double-blind pilot study. Nutrients 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Jang, L.G.; Choi, G.; Kim, S.W.; Kim, B.Y.; Lee, S.; Park, H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: An observational study. J. Int. Soc. Sports Nutr. 2019, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Keohane, D.M.; Woods, T.; O’Connor, P.; Underwood, S.; Cronin, O.; Whiston, R.; O’Sullivan, O.; Cotter, P.; Shanahan, F.; Molloy, M.G. Four men in a boat: Ultra-endurance exercise alters the gut microbiome. J. Sci. Med. Sport 2019, 22, 1059–1064. [Google Scholar] [CrossRef]
- Murtaza, N.; Burke, L.M.; Vlahovich, N.; Charlesson, B.; O’Neill, H.; Ross, M.L.; Campbell, K.L.; Krause, L.; Morrison, M. The effects of dietary pattern during intensified training on stool microbiota of elite race walkers. Nutrients 2019, 11, 261. [Google Scholar] [CrossRef]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef]
- Bai, J.; Hu, Y.; Bruner, D. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7–18 years old children from the American Gut Project. Pediatr. Obes. 2019, 14, e12480. [Google Scholar] [CrossRef]
- Durk, R.P.; Castillo, E.; Márquez-Magaña, L.; Grosicki, G.J.; Bolter, N.D.; Lee, C.M.; Bagley, J.R. Gut microbiota composition is related to cardiorespiratory fitness in healthy young adults. Int. J. Sport. Nutr. Exerc. Metab. 2019, 29, 249–253. [Google Scholar] [CrossRef]
- Langsetmo, L.; Johnson, A.; Demmer, R.; Fino, N.; Orwoll, E.; Ensrud, K.; Hoffman, A.R.; Cauley, J.A.; Shmagel, A.; Meyer, K. The Association between Objectively Measured Physical Activity and the Gut Microbiome among Older Community Dwelling Men. J. Nutr. Health Aging 2019, 23, 538–546. [Google Scholar] [CrossRef]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Knight, R. Dietary effects on human gut microbiome diversity. Br. J. Nutr. 2015, 113, S1–S5. [Google Scholar] [CrossRef]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global recommendations on physical activity for health. Geneva World Health Organ. 2010, 60. [Google Scholar]
- Cintineo, H.P.; Arent, M.A.; Antonio, J.; Arent, S.M. Effects of Protein Supplementation on Performance and Recovery in Resistance and Endurance Training. Front. Nutr. 2018, 5, 83. [Google Scholar] [CrossRef]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Agostini, D.; Gervasi, M.; Annibalini, G.; Amatori, S.; Ferrini, F.; Sisti, D.; Piccoli, G.; Barbieri, E.; Sestili, P. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients 2020, 12, 17. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef]
- Choi, J.J.; Eum, S.Y.; Rampersaud, E.; Daunert, S.; Abreu, M.T.; Toborek, M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ. Health Perspect. 2013, 121, 725–730. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE 2013, 8, e65465. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, N.; Li, Z.; Wang, X.; Shi, H.; Xue, C.; Li, R.W.; Tang, Q. Chondroitin sulfate disaccharides modified the structure and function of the murine gut microbiome under healthy and stressed conditions. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Pescador, S.; Porras, D.; García-Mediavilla, M.V.; Martínez-Flórez, S.; Juarez-Fernández, M.; Cuevas, M.J.; Mauriz, J.L.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis. Model Mech. 2019, 12, dmm039206. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xu, S.; Huang, H.; Liang, J.; Wu, Y.; Li, C.; Yuan, H.; Zhao, X.; Lai, X.; Hou, S. Influence of excessive exercise on immunity, metabolism, and gut microbial diversity in an overtraining mice model. Scand. J. Med. Sci. Sports 2018, 28, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Chen, Y.-H.; Chuang, H.-L.; Chiu, C.-C.; Huang, C.-C. Investigation of the Effects of Microbiota on Exercise Physiological Adaption, Performance, and Energy Utilization Using a Gnotobiotic Animal Model. Front. Microbiol. 2019, 10, 1906. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H. IOC consensus statement: Dietary supplements and the high-performance athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef]
- Ducray, H.; Globa, L.; Pustovyy, O.; Roberts, M.; Rudisill, M.; Vodyanoy, V.; Sorokulova, I. Prevention of excessive exercise-induced adverse effects in rats with Bacillus subtilis BSB3. J. Appl. Microbiol. 2020, 128, 1163. [Google Scholar] [CrossRef] [PubMed]
- Ünsal, C.; Ünsal, H.; Ekici, M.; Koc Yildirim, E.; Üner, A.; Yildiz, M.; Güleş, Ö.; Ekren Aşici, G.; Boyacioğlu, M.; Balkaya, M. The effects of exhaustive swimming and probiotic administration in trained rats: Oxidative balance of selected organs, colon morphology, and contractility. Physiol. Int. 2018, 105, 309–324. [Google Scholar] [CrossRef]
- Lollo, P.; Cruz, A.; Morato, P.; Moura, C.; Carvalho-Silva, L.; Oliveira, C.A.F.d.; Faria, J.; Amaya-Farfan, J. Probiotic cheese attenuates exercise-induced immune suppression in Wistar rats. J. Dairy Sci. 2012, 95, 3549–3558. [Google Scholar] [CrossRef]
- Lollo, P.C.B.; de Moura, C.S.; Morato, P.N.; Cruz, A.G.; de Freitas Castro, W.; Betim, C.B.; Nisishima, L.; José de Assis, F.F.; Junior, M.M.; Fernandes, C.O. Probiotic yogurt offers higher immune-protection than probiotic whey beverage. Food Res. Int. 2013, 54, 118–124. [Google Scholar] [CrossRef]
- Appukutty, M.; Ramasamy, K.; Rajan, S.; Vellasamy, S.; Ramasamy, R.; Radhakrishnan, A. Effect of orally administered soy milk fermented with Lactobacillus plantarum LAB12 and physical exercise on murine immune responses. Benef. Microbes 2015, 6, 491–496. [Google Scholar] [CrossRef]
- Coffey, V.G.; Hawley, J.A. Concurrent exercise training: Do opposites distract? J. Physiol. 2017, 595, 2883–2896. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Perna, S.; Giacosa, A.; Peroni, G.; Castellazzi, A.M. Using probiotics in clinical practice: Where are we now? A review of existing meta-analyses. Gut Microbes 2017, 8, 521–543. [Google Scholar] [CrossRef]
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015, CD006895. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; Tauler, P. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 55–64. [Google Scholar] [CrossRef]
- Kekkonen, R.A.; Vasankari, T.J.; Vuorimaa, T.; Haahtela, T.; Julkunen, I.; Korpela, R. The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 352–363. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Cripps, A.W.; Hopkins, W.G.; Eskesen, D.C.; Jairath, A.; Christophersen, C.T.; Conlon, M.A.; Fricker, P.A. Lactobacillus fermentum (PCC(R)) supplementation and gastrointestinal and respiratory-tract illness symptoms: A randomised control trial in athletes. Nutr. J. 2011, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Suckling, C.A.; Peedle, G.Y.; Murphy, J.A.; Dawkins, T.G.; Roberts, M.G. An Exploratory Investigation of Endotoxin Levels in Novice Long Distance Triathletes, and the Effects of a Multi-Strain Probiotic/Prebiotic, Antioxidant Intervention. Nutrients 2016, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Haywood, B.A.; Black, K.E.; Baker, D.; McGarvey, J.; Healey, P.; Brown, R.C. Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players. J. Sci. Med. Sport 2014, 17, 356–360. [Google Scholar] [CrossRef]
- De Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal complaints during exercise: Prevalence, etiology, and nutritional recommendations. Sports Med. 2014, 44 (Suppl. 1), S79–S85. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Cripps, A.; Christophersen, C.T.; Conlon, M.A.; Fricker, P.A. Gut Balance, a synbiotic supplement, increases fecal Lactobacillus paracasei but has little effect on immunity in healthy physically active individuals. Gut Microbes 2012, 3, 221–227. [Google Scholar] [CrossRef]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.D.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2014, 114, 93–103. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Nieman, D.C. Exercise, upper respiratory tract infection, and the immune system. Med. Sci. Sports Exerc. 1994, 26, 128–139. [Google Scholar] [CrossRef]
- Colbey, C.; Cox, A.J.; Pyne, D.B.; Zhang, P.; Cripps, A.W.; West, N.P. Upper Respiratory Symptoms, Gut Health and Mucosal Immunity in Athletes. Sports Med. 2018, 48, 65–77. [Google Scholar] [CrossRef]
- Cox, A.J.; Pyne, D.B.; Saunders, P.U.; Fricker, P.A. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br. J. Sports Med. 2010, 44, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Salarkia, N.; Ghadamli, L.; Zaeri, F.; Sabaghian Rad, L. Effects of probiotic yogurt on performance, respiratory and digestive systems of young adult female endurance swimmers: A randomized controlled trial. Med. J. Islam. Repub. Iran 2013, 27, 141–146. [Google Scholar]
- West, N.P.; Horn, P.L.; Pyne, D.B.; Gebski, V.J.; Lahtinen, S.J.; Fricker, P.A.; Cripps, A.W. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin. Nutr. 2014, 33, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Michalickova, D.; Minic, R.; Dikic, N.; Andjelkovic, M.; Kostic-Vucicevic, M.; Stojmenovic, T.; Nikolic, I.; Djordjevic, B. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: A randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 2016, 41, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Geiger, D.; Schauer, M.; Gostner, J.M.; Gatterer, H.; Burtscher, M.; Fuchs, D. Probiotic Supplements Beneficially Affect Tryptophan-Kynurenine Metabolism and Reduce the Incidence of Upper Respiratory Tract Infections in Trained Athletes: A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients 2016, 8, 752. [Google Scholar] [CrossRef]
- Kellmann, M.; Bertollo, M.; Bosquet, L.; Brink, M.; Coutts, A.J.; Duffield, R.; Erlacher, D.; Halson, S.L.; Hecksteden, A.; Heidari, J.; et al. Recovery and Performance in Sport: Consensus Statement. Int. J. Sports Physiol. Perform. 2018, 13, 240–245. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition; Novel Foods and Food Allergens (EFSA NDA Panel); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.; Kearney, J.; Knutsen, H.; Maciuk, A.; Mangelsdorf, I.; et al. Guidance on the scientific requirements for health claims related to muscle function and physical performance. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Huang, W.C.; Lin, J.S.; Chen, Y.M.; Ho, S.T.; Huang, C.C.; Tung, Y.T. Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef]
- Soares, A.D.N.; Wanner, S.P.; Morais, E.S.S.; Hudson, A.S.R.; Martins, F.S.; Cardoso, V.N. Supplementation with Saccharomyces boulardii Increases the Maximal Oxygen Consumption and Maximal Aerobic Speed Attained by Rats Subjected to an Incremental-Speed Exercise. Nutrients 2019, 11, 2352. [Google Scholar] [CrossRef]
- Huang, W.C.; Hsu, Y.J.; Li, H.; Kan, N.W.; Chen, Y.M.; Lin, J.S.; Hsu, T.K.; Tsai, T.Y.; Chiu, Y.S.; Huang, C.C. Effect of Lactobacillus Plantarum TWK10 on Improving Endurance Performance in Humans. Chin. J. Physiol. 2018, 61, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lee, M.C.; Lee, C.C.; Ng, K.S.; Hsu, Y.J.; Tsai, T.Y.; Young, S.L.; Lin, J.S.; Huang, C.C. Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients 2019, 11, 2836. [Google Scholar] [CrossRef] [PubMed]
- Carbuhn, A.F.; Reynolds, S.M.; Campbell, C.W.; Bradford, L.A.; Deckert, J.A.; Kreutzer, A.; Fry, A.C. Effects of Probiotic (Bifidobacterium longum 35624) Supplementation on Exercise Performance, Immune Modulation, and Cognitive Outlook in Division I Female Swimmers. Sports 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.; Chrismas, B.C.R.; Suckling, C.A.; Roberts, J.D.; Foster, J.; Taylor, L. Chronic probiotic supplementation with or without glutamine does not influence the eHsp72 response to a multi-day ultra-endurance exercise event. Appl. Physiol. Nutr. Metab. 2017, 42, 876–883. [Google Scholar] [CrossRef]
- Townsend, J.R.; Bender, D.; Vantrease, W.C.; Sapp, P.A.; Toy, A.M.; Woods, C.A.; Johnson, K.D. Effects of Probiotic (Bacillus subtilis DE111) Supplementation on Immune Function, Hormonal Status, and Physical Performance in Division I Baseball Players. Sports 2018, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.C.; Townsend, J.R.; Johnson, S.B.; Toy, A.M.; Vantrease, W.C.; Bender, D.; Crimi, C.C.; Stowers, K.L.; Ruiz, M.D.; VanDusseldorp, T.A.; et al. Effects of Probiotic (Bacillus subtilis) Supplementation During Offseason Resistance Training in Female Division I Athletes. J. Strength. Cond. Res. 2018, 10. [Google Scholar] [CrossRef]
- Ibrahim, N.S.; Muhamad, A.S.; Ooi, F.K.; Meor-Osman, J.; Chen, C.K. The effects of combined probiotic ingestion and circuit training on muscular strength and power and cytokine responses in young males. Appl. Physiol. Nutr. Metab. 2018, 43, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Hsu, Y.J.; Chuang, H.L.; Hsieh, P.S.; Ho, H.H.; Chen, W.L.; Chiu, Y.S.; Huang, C.C. In Vivo Ergogenic Properties of the Bifidobacterium longum OLP-01 Isolated from a Weightlifting Gold Medalist. Nutrients 2019, 11, 2003. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Hsieh, S.H.; Kuo, Y.W.; Sung, H.C.; Huang, C.C. Lactobacillus salivarius Subspecies salicinius SA-03 is a New Probiotic Capable of Enhancing Exercise Performance and Decreasing Fatigue. Microorganisms 2020, 8, 545. [Google Scholar] [CrossRef]
- Gill, S.K.; Allerton, D.M.; Ansley-Robson, P.; Hemmings, K.; Cox, M.; Costa, R.J. Does Short-Term High Dose Probiotic Supplementation Containing Lactobacillus casei Attenuate Exertional-Heat Stress Induced Endotoxaemia and Cytokinaemia? Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 268–275. [Google Scholar] [CrossRef]
- Gill, S.K.; Teixeira, A.M.; Rosado, F.; Cox, M.; Costa, R.J. High-Dose Probiotic Supplementation Containing Lactobacillus casei for 7 Days Does Not Enhance Salivary Antimicrobial Protein Responses to Exertional Heat Stress Compared With Placebo. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 150–160. [Google Scholar] [CrossRef]
- Vaisberg, M.; Paixao, V.; Almeida, E.B.; Santos, J.M.B.; Foster, R.; Rossi, M.; Pithon-Curi, T.C.; Gorjao, R.; Momesso, C.M.; Andrade, M.S.; et al. Daily Intake of Fermented Milk Containing Lactobacillus casei Shirota (Lcs) Modulates Systemic and Upper Airways Immune/Inflammatory Responses in Marathon Runners. Nutrients 2019, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Valimaki, I.A.; Vuorimaa, T.; Ahotupa, M.; Kekkonen, R.; Korpela, R.; Vasankari, T. Decreased training volume and increased carbohydrate intake increases oxidized LDL levels. Int. J. Sports Med. 2012, 33, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.N.; Sparks, A.S.; Doran, D.A.; Fleming, S.C.; Langan-Evans, C.; Kirk, B.; Fearn, R.; Morton, J.P.; Close, G.L. Four weeks of probiotic supplementation reduces GI symptoms during a marathon race. Eur. J. Appl. Physiol. 2019, 119, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Wei, C.C.; Huang, C.C.; Chen, W.L.; Huang, H.Y. The Beneficial Effects of Lactobacillus plantarum PS128 on High-Intensity, Exercise-Induced Oxidative Stress, Inflammation, and Performance in Triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Purpura, M.; Stone, J.D.; Turner, S.M.; Anzalone, A.J.; Eimerbrink, M.J.; Pane, M.; Amoruso, A.; Rowlands, D.S.; Oliver, J.M. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 Supplementation Attenuates Performance and Range-of-Motion Decrements Following Muscle Damaging Exercise. Nutrients 2016, 8, 642. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Shields, K.A.; Lowery, R.P.; De Souza, E.O.; Partl, J.M.; Hollmer, C.; Purpura, M.; Wilson, J.M. Probiotic Bacillus coagulans GBI-30, 6086 reduces exercise-induced muscle damage and increases recovery. PeerJ 2016, 4, e2276. [Google Scholar] [CrossRef]
- Mazani, M.; Nemati, A.; Baghi, A.N.; Amani, M.; Haedari, K.; Alipanah-Mogadam, R. The effect of probiotic yoghurt consumption on oxidative stress and inflammatory factors in young females after exhaustive exercise. J. Pak. Med. Assoc. 2018, 68, 1748–1754. [Google Scholar]
- Muhamad, A.; Gleeson, M. Effects of a 14-strain probiotics supplement on salivary antimicrobial proteins at rest and in response to an acute bout of prolonged exercise. Int. J. Sports Sci. 2014, 4, 7. [Google Scholar] [CrossRef]
- O’Brien, K.V.; Stewart, L.K.; Forney, L.A.; Aryana, K.J.; Prinyawiwatkul, W.; Boeneke, C.A. The effects of postexercise consumption of a kefir beverage on performance and recovery during intensive endurance training. J. Dairy Sci. 2015, 98, 7446–7449. [Google Scholar] [CrossRef]
- Salehzadeh, K. The effects of probiotic yogurt drink on lipid profile, CRP and record changes in aerobic athletes. Int. J. Life Sci. 2015, 9, 32–37. [Google Scholar] [CrossRef]
- Inoue, T.; Kobayashi, Y.; Mori, N.; Sakagawa, M.; Xiao, J.Z.; Moritani, T.; Sakane, N.; Nagai, N. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef. Microbes 2018, 9, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, O.; Douzi, W.; Theurot, D.; Bosquet, L.; Dugue, B. An Evidence-Based Approach for Choosing Post-exercise Recovery Techniques to Reduce Markers of Muscle Damage, Soreness, Fatigue, and Inflammation: A Systematic Review with Meta-Analysis. Front. Physiol. 2018, 9, 403. [Google Scholar] [CrossRef]
- Gepner, Y.; Hoffman, J.R.; Shemesh, E.; Stout, J.R.; Church, D.D.; Varanoske, A.N.; Zelicha, H.; Shelef, I.; Chen, Y.; Frankel, H.; et al. Combined effect of Bacillus coagulans GBI-30, 6086 and HMB supplementation on muscle integrity and cytokine response during intense military training. J. Appl. Physiol. 2017, 123, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Zaragoza, J.; Purpura, M.; Iametti, S.; Marengo, M.; Tinsley, G.M.; Anzalone, A.J.; Oliver, J.M.; Fiore, W.; Biffi, A.; et al. Probiotic Administration Increases Amino Acid Absorption from Plant Protein: A Placebo-Controlled, Randomized, Double-Blind, Multicenter, Crossover Study. Probiotics Antimicrob. Proteins 2020. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Vitellio, P.; Chira, A.; De Angelis, M.; Dumitrascu, D.L.; Portincasa, P. Probiotics in Psychosocial Stress and Anxiety. A Systematic Review. J. Gastrointestin. Liver Dis. 2020, 29, 77–83. [Google Scholar] [CrossRef]
- Sashihara, T.; Nagata, M.; Mori, T.; Ikegami, S.; Gotoh, M.; Okubo, K.; Uchida, M.; Itoh, H. Effects of Lactobacillus gasseri OLL2809 and α-lactalbumin on university-student athletes: A randomized, double-blind, placebo-controlled clinical trial. Appl. Physiol. Nutr. Metab. 2013, 38, 1228–1235. [Google Scholar] [CrossRef]
- Sawada, D.; Kuwano, Y.; Tanaka, H.; Hara, S.; Uchiyama, Y.; Sugawara, T.; Fujiwara, S.; Rokutan, K.; Nishida, K. Daily intake of Lactobacillus gasseri CP2305 relieves fatigue and stress-related symptoms in male university Ekiden runners: A double-blind, randomized, and placebo-controlled clinical trial. J. Funct. Foods 2019, 57, 465–476. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals. Nutrients 2020, 12, 2936. https://doi.org/10.3390/nu12102936
Marttinen M, Ala-Jaakkola R, Laitila A, Lehtinen MJ. Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals. Nutrients. 2020; 12(10):2936. https://doi.org/10.3390/nu12102936
Chicago/Turabian StyleMarttinen, Maija, Reeta Ala-Jaakkola, Arja Laitila, and Markus J. Lehtinen. 2020. "Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals" Nutrients 12, no. 10: 2936. https://doi.org/10.3390/nu12102936
APA StyleMarttinen, M., Ala-Jaakkola, R., Laitila, A., & Lehtinen, M. J. (2020). Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals. Nutrients, 12(10), 2936. https://doi.org/10.3390/nu12102936





