Do Dietary Supplements and Nutraceuticals Have Effects on Dental Implant Osseointegration? A Scoping Review
Abstract
1. Introduction
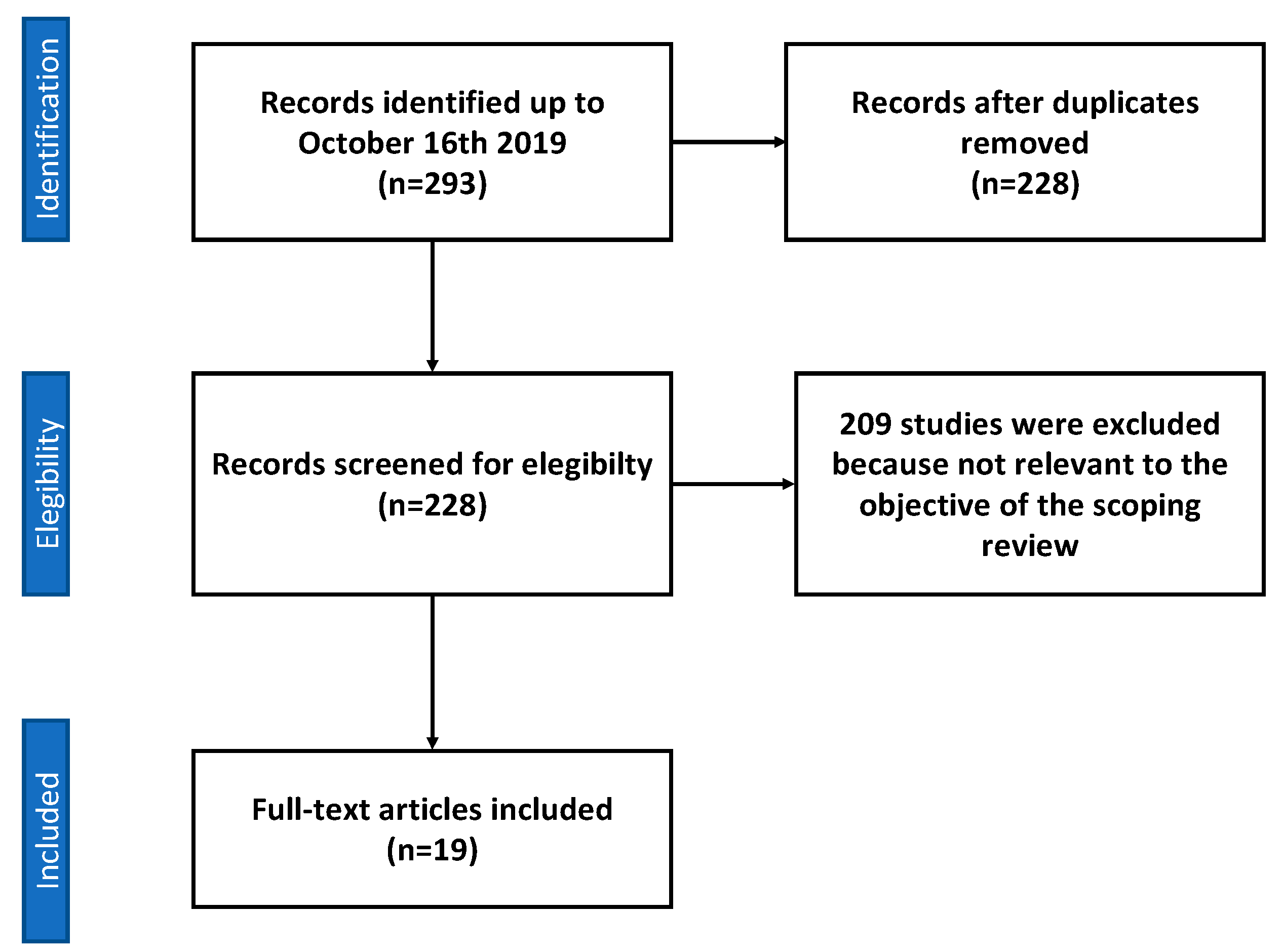
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
3. Results
3.1. Vitamin D
3.1.1. Animal Studies
3.1.2. Clinical Studies
3.1.3. Magnesium
3.1.4. Resveratrol
3.1.5. Mixtures of Micronutrients
3.2. Vitamin C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zarb, G.A.; Albrektsson, T. Osseointegration—A requiem for the periodontal ligament? Int. J. Periodontol. Restor. Dent. 1991, 11, 88–91. [Google Scholar]
- Goiato, M.C.; Dos Santos, D.M.; Santiago, J.F., Jr.; Moreno, A.; Pellizzer, E.P. Longevity of dental implants in type IV bone: A systematic review. Int. J. Oral Maxillofac. Surg. 2014, 43, 1108–1116. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef]
- Esposito, M.; Thomsen, P.; Ericson, L.E.; Lekholm, U. Histopathologic observations on early oral implant failures. Int. J. Oral Maxillofac. Implants 1999, 14, 798–810. [Google Scholar]
- Huynh-Ba, G.; Friedberg, J.R.; Vogiatzi, D.; Ioannidou, E. Implant failure predictors in the posterior maxilla: A retrospective study of 273 consecutive implants. J. Periodontol. 2008, 79, 2256–2261. [Google Scholar] [CrossRef] [PubMed]
- Sverzut, A.T.; Stabile, G.A.; de Moraes, M.; Mazzonetto, R.; Moreira, R.W. The influence of tobacco on early dental implant failure. J. Oral Maxillofac. Surg. 2008, 66, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Urban, T.; Kostopoulos, L.; Wenzel, A. Immediate implant placement in molar regions: Risk factors for early failure. Clin. Oral Implants Res. 2012, 23, 220–227. [Google Scholar] [CrossRef] [PubMed]
- WHO Micronutrients. Available online: https://www.who.int/nutrition/topics/micronutrients/en/ (accessed on 27 December 2019).
- Iolascon, G.; Gimigliano, R.; Bianco, M.; De Sire, A.; Moretti, A.; Giusti, A.; Malavolta, N.; Migliaccio, S.; Migliore, A.; Napoli, N.; et al. Are Dietary Supplements and Nutraceuticals Effective for Musculoskeletal Health and Cognitive Function? A Scoping Review. J. Nutr. Health Aging 2017, 21, 527–538. [Google Scholar] [CrossRef]
- Montalvany-Antonucci, C.C.; Zicker, M.C.; Oliveira, M.C.; Macari, S.; Madeira, M.F.M.; Andrade IJr Ferreira, A.V.; Silva, T.A. Diet versus jaw bones: Lessons from experimental models and potential clinical implications. Nutrition 2018, 45, 59–67. [Google Scholar] [CrossRef]
- Aguirre, J.I.; Akhter, M.P.; Kimmel, D.B.; Pingel, J.; Xia, X.; Williams, A.; Jorgensen, M.; Edmonds, K.; Lee, J.Y.; Reinhard, M.K.; et al. Enhanced alveolar bone loss in a model of non-invasive periodontitis in rice rats. Oral Dis. 2012, 18, 459–468. [Google Scholar] [CrossRef]
- Kametaka, S.; Miyazaki, T.; Inoue, Y.; Hayashi, S.I.; Takamori, A.; Miyake, Y.; Suginaka, H. The effect of ofloxacin on experimental periodontitis in hamsters infected with Actinomyces viscosus. J. Periodontol. 1989, 60, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Maki, K. High-fat diet induced periodontitis in mice through lipopolysaccharides (LPS) receptor signaling: Protective action of estrogens. BMC Obes. 2016, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque Taddei, S.R.; Madeira, M.F.M.; de Abreu Lima, I.L.; Queiroz-Junior, C.M.; Moura, A.P.; Oliveira, D.D.; Andrade, I., Jr.; da Glória Souza, D.; Teixeira, M.M.; da Silva, T.A. Effect of Lithothamnium sp and calcium supplements in strain- and infection-induced bone resorption. Angle Orthod. 2014, 84, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Barò, M.A.; Rocamundi, M.R.; Viotto, O.J.; Ferreyra, R.S. Alveolar wound healing in rats fed on high sucrose diet. Acta Odontol. Latinoam. 2013, 26, 97–103. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Dermience, M.; Lognay, G.; Mathieu, F.; Goyens, P. Effects of thirty elements on bone metabolism. J. Trace Elem. Med. Biol. 2015, 32, 86–106. [Google Scholar] [CrossRef]
- Farley, J.R.; Wergedal, J.E.; Baylink, D.J. Fluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cells. Science 1983, 222, 330–332. [Google Scholar] [CrossRef]
- Dommisch, H.; Kuzmanova, D.; Jönsson, D.; Grant, M.; Chapple, I. Effect of micronutrient malnutrition on periodontal disease and periodontal therapy. Periodontology 2000 2018, 78, 129–153. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Riordon, D.R.; Chan, J.S.; Krieger, N.S. Decreased potassium stimulates bone resorption. Am. J. Physiol. Ren. Physiol. 1997, 272, F774–F780. [Google Scholar] [CrossRef]
- Ornstrup, M.J.; Harsløf, T.; Sørensen, L.; Stenkjær, L.; Langdahl, B.L.; Pedersen, S.B. Resveratrol Increases Osteoblast Differentiation in Vitro Independently of Inflammation. Calcif. Tissue Int. 2016, 99, 155–163. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, G.J.; Yoo, H.S.; Song, D.H.; Chung, K.H.; Lee, K.J.; Koo, Y.T.; An, J.H. Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/β-Catenin/ATF4 Signaling Pathways. Nutrients 2019, 11, 506. [Google Scholar] [CrossRef]
- Kim, H.-N.; Lee, J.-H.; Jin, W.J.; Lee, Z.H. α-Tocopheryl Succinate Inhibits Osteoclast Formation by Suppressing Receptor Activator of Nuclear Factor-kappaB Ligand (RANKL) Expression and Bone Resorption. J. Bone Metab. 2012, 19, 111–120. [Google Scholar] [CrossRef]
- Myneni, V.D.; Mezey, E. Regulation of bone remodeling by vitamin K2. Oral Dis. 2017, 23, 1021–1028. [Google Scholar] [CrossRef]
- Yamaguchi, M. Nutritional factors and bone homeostasis: Synergistic effect with zinc and genistein in osteogenesis. Mol. Cell. Biochem. 2012, 366, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.C.C.; Cardozo, F.T.G.S.; Magini, R.S.; Simões, C.M.O. Retinoic acid increases the effect of bone morphogenetic protein type 2 on osteogenic differentiation of human adipose-derived stem cells. J. Appl. Oral Sci. 2019, 27, e20180317. [Google Scholar] [CrossRef]
- Fratoni, V.; Brandi, M.L. B vitamins, homocysteine and bone health. Nutrients 2015, 7, 2176–2192. [Google Scholar] [CrossRef]
- Mangano, F.; Ghertasi Oskouei, S.; Paz, A.; Mangano, N.; Mangano, C. Low serum vitamin D and early dental implant failure: Is there a connection? A retrospective clinical study on 1740 implants placed in 885 patients. J. Dent. Res. Dent. Clin. Dent. Prospects 2018, 12, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.; Schuder, K.; Hof, M.; Heuberer, S.; Seemann, R.; Dvorak, G. Does osteoporosis influence the marginal peri-implant bone level in female patients? A cross-sectional study in a matched collective. Clin. Implant Dent. Relat. Res. 2017, 19, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Mortellaro, C.; Mangano, N.; Mangano, C. Is Low Serum Vitamin D Associated with Early Dental Implant Failure? A Retrospective Evaluation on 1625 Implants Placed in 822 Patients. Mediat. Inflamm. 2016, 2016, 5319718. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Grunert, S.; Woelber, J.P.; Nelson, K.; Semper-Hogg, W. Vitamin D deficiency in early implant failure: Two case reports. Int. J. Implant Dent. 2016, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Bryce, G.; MacBeth, N. Vitamin D deficiency as a suspected causative factor in the failure of an immediately placed dental implant: A case report. J. R. Naval Med. Serv. 2014, 100, 328–332. [Google Scholar]
- Liu, W.; Zhang, S.; Zhao, D.; Zou, H.; Sun, N.; Liang, X.; Dard, M.; Lanske, B.; Yuan, Q. Vitamin D supplementation enhances the fixation of titanium implants in chronic kidney disease mice. PLoS ONE 2014, 9, e95689. [Google Scholar] [CrossRef]
- Zhou, C.; Li, Y.; Wang, X.; Shui, X.; Hu, J. 1,25Dihydroxy vitamin D3 improves titanium implant osseointegration in osteoporotic rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, S174–S178. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Yu, T.; Yang, X.Y.; Li, F.; Ma, L.; Yang, Y.; Liu, X.G.; Wang, Y.Y.; Gong, P. Vitamin D3 and insulin combined treatment promotes titanium implant osseointegration in diabetes mellitus rats. Bone 2013, 52, 1–8. [Google Scholar] [CrossRef]
- Akhavan, A.; Noroozi, Z.; Shafiei, A.A.; Haghighat, A.; Jahanshahi, G.R.; Mousavi, S.B. The effect of vitamin D supplementation on bone formation around titanium implants in diabetic rats. Dent. Res. J. 2012, 9, 582–587. [Google Scholar] [CrossRef]
- Dvorak, G.; Fügl, A.; Watzek, G.; Tangl, S.; Pokorny, P.; Gruber, R. Impact of dietary vitamin D on osseointegration in the ovariectomized rat. Clin. Oral Implants Res. 2012, 23, 1308–1313. [Google Scholar] [CrossRef]
- Kelly, J.; Lin, A.; Wang, C.J.; Park, S.; Nishimura, I. Vitamin D and bone physiology: Demonstration of vitamin D deficiency in an implant osseointegration rat model. J. Prosthodont. Implant Esthet. Reconstr. Dent. 2009, 18, 473–478. [Google Scholar] [CrossRef]
- Belluci, M.M.; Giro, G.; Del Barrio, R.A.L.; Pereira, R.M.R.; Marcantonio, E., Jr.; Orrico, S.R.P. Effects of magnesium intake deficiency on bone metabolism and bone tissue around osseointegrated implants. Clin. Oral Implants Res. 2011, 22, 716–721. [Google Scholar] [CrossRef]
- Del Barrio, R.A.; Giro, G.; Belluci, M.M.; Pereira, R.M.; Orrico, S.R. Effect of severe dietary magnesium deficiency on systemic bone density and removal torque of osseointegrated implants. Int. J. Oral Maxillofac. Implants 2010, 25, 1125–1130. [Google Scholar]
- Ribeiro, F.V.; Pimentel, S.P.; Corrêa, M.G.; Bortoli, J.P.; Messora, M.R.; Casati, M.Z. Resveratrol reverses the negative effect of smoking on peri-implant repair in the tibia of rats. Clin. Oral Implants Res. 2019, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Casarin, R.C.; Casati, M.Z.; Pimentel, S.P.; Cirano, F.R.; Algayer, M.; Pires, P.R.; Ghiraldini, B.; Duarte, P.M.; Ribeiro, F.V. Resveratrol improves bone repair by modulation of bone morphogenetic proteins and osteopontin gene expression in rats. Int. J. Oral Maxillofac. Surg. 2014, 43, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, S.P.; Casarin, R.C.; Ribeiro, F.V.; Cirano, F.R.; Rovaris, K.; Haiter Neto, F.; Casati, M.Z. Impact of micronutrients supplementation on bone repair around implants: microCT and counter-torque analysis in rats. J. Appl. Oral Sci. 2016, 24, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Watanabe, T.; Nakada, H.; Sato, H.; Tanimoto, Y.; Sakae, T.; Kimoto, S.; Mijares, D.; Zhang, Y.; Kawai, Y. Improved Bone Micro Architecture Healing Time after Implant Surgery in an Ovariectomized Rat. J. Hard Tissue Biol. 2016, 25, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nakada, H.; Takahashi, T.; Fujita, K.; Tanimoto, Y.; Sakae, T.; Kimoto, S.; Kawai, Y. Potential for acceleration of bone formation after implant surgery by using a dietary supplement: An animal study. J. Oral Rehabil. 2015, 42, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, L.; Lin, Y.F.; Xie, G.F. Role of vitamin C in wound healing after dental implant surgery in patients treated with bone grafts and patients with chronic periodontitis. Clin. Implant Dent. Relat. Res. 2018, 20, 793–798. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Smoking and dental implants, a systematic review and meta-analysis. J. Dent. 2015, 43, 487–498. [Google Scholar] [CrossRef]
- Arora, M.; Schwarz, E.; Sivaneswaran, S.; Banks, E. Cigarette smoking and tooth loss in a cohort of older Australians, the 45 and Up Study. J. Am. Dent. Assoc. 2010, 141, 1242–1249. [Google Scholar] [CrossRef]
- Dietary Supplement Products & Ingredients. FDA. Available online: https://www.fda.gov/food/dietary-supplements/dietary-supplement-products-ingredients (accessed on 27 December 2019).
- DeFelice, S. The NutraCeutical Revolution: Fueling a Powerful, New International Market. Presented at the Harvard University Advanced Program in Biomedical Research Management and Development, Como, Italy; 1989. Available online: https://fimdefelice.org/library/the-nutraceutical-revolution-fueling-a-powerful-new-international-market/ (accessed on 27 December 2019).
- Zeisel, S.H. Regulation of “nutraceuticals”. Science 1999, 285, 1853–1855. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Fulgoni, V.L., III; Keast, D.R.; Auestad, N.; Quann, E.E. Nutrients from dairy foods are difficult to replace in diets of Americans: Food pattern modeling and an analyses of the National Health and Nutrition Examination Survey 2003–2006. Nutr. Res. 2011, 31, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Nastri, L.; Guida, L.; Annunziata, M.; Ruggiero, N.; Rizzo, A. Vitamin D modulatory effect on cytokines expression by human gingival fibroblasts and periodontal ligament cells. Minerva Stomatol. 2018, 67, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Bashutski, J.D.; Eber, R.M.; Kinney, J.S.; Benavides, E.; Maitra, S.; Braun, T.M.; Giannobile, W.V.; McCauley, L.K. The impact of vitamin D status on periodontal surgery outcomes. J. Dent. Res. 2011, 90, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Salomó-Coll, O.; Maté-Sánchez de Val, J.E.; Ramírez-Fernandez, M.P.; Hernández-Alfaro, F.; Gargallo-Albiol, J.; Calvo-Guirado, J.L. Topical applications of vitamin D on implant surface for bone-to-implant contact enhance: A pilot study in dogs part II. Clin. Oral Implants Res. 2016, 27, 896–903. [Google Scholar] [CrossRef]
- Boyera, N.; Galey, I.; Bernard, B.A. Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. Int. J. Cosmet. Sci. 1998, 20, 151–158. [Google Scholar] [CrossRef] [PubMed]
- DePhillipo, N.N.; Aman, Z.S.; Kennedy, M.I.; Begley, J.P.; Moatshe, G.; LaPrade, R.F. Efficacy of Vitamin C Supplementation on Collagen Synthesis and Oxidative Stress After Musculoskeletal Injuries: A Systematic Review. Orthop. J. Sports Med. 2018, 6, 2325967118804544. [Google Scholar] [CrossRef]
- DiPietro, S.G.L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar]
| Nutrient or Non-Nutrient Compound | Effect |
|---|---|
| Calcium | 99% of calcium in the body is in the form of hydroxyapatite, which is bone and tooth mineral [17]. Relationship between calcium and maintenance of normal bone and tooth assessed with a favorable outcome (European Food Safety Authority (EFSA) opinion). |
| Fluorides | Stimulates osteoblast growth and bone formation, increasing bone mineral density (BMD) [18], and supports tooth mineralization (EFSA opinion). |
| Magnesium | Essential for the conversion of vitamin D into its active form and necessary for calcium absorption and metabolism [19], and maintenance of normal bone and teeth (EFSA opinion). |
| Potassium | Potassium citrate helps maintain acid–base balance and support bone health, counteracting bone resorption [20]. However, a cause-and-effect relationship is not established between the dietary intake of potassium salts of citric acid and maintenance of normal bone (EFSA opinion). |
| Resveratrol | Active substance found in food, such as red grapes, peanuts, and berries, with anti-inflammatory and antioxidant effects; it additionally provides an inhibitory effect on osteoclast differentiation and potentially induces bone formation [21] (EFSA opinion about bone and tooth health not available). |
| Vitamin C (ascorbic acid) | Enhances osteoblastogenesis and inhibits osteoclastogenesis via Wnt/β-catenin signaling [22]. Vitamin C contributes to normal function of bones and teeth (EFSA opinion). |
| Vitamin D | Modulates calcium and phosphate metabolism; it promotes growth, bone mineralization of the skeleton and teeth [17], and maintenance of normal bone and teeth (EFSA opinion). |
| Vitamin E (alpha-tocopherol) | Reduces the expression of receptor activator of nuclear factor kappa B (NF-κB) ligand (RANKL) in osteoblasts and inhibits osteoclastogenesis [23]. However, a cause-and-effect relationship is not established between the dietary intake of vitamin E and maintenance of normal bone and teeth (EFSA opinion). |
| Vitamin K2 (MK7) | Stimulates osteoblasts differentiation, protects these cells from apoptosis [24], and maintains normal bone (EFSA opinion). |
| Zinc | Stimulates osteoblast proliferation, differentiation, and mineralization, which may facilitate bone formation [25,26] and maintain normal bone (EFSA opinion). |
| Vitamin A | Increases the effect of bone morphogenetic proteins (BMPs) on osteogenic differentiation [27]. A cause-and-effect relationship is not established between the dietary intake of vitamin A and maintenance of normal bone and teeth (EFSA opinion). |
| B Vitamins | Deficiency in folic acid and vitamins B6 and B12 can result in increased serum homocysteine that leads to endothelial dysfunction (decreased bone blood flow) and enhanced osteoclast activity (bone resorption). Moreover, hyperhomocysteinemia interferes with cross-linking of collagen (altered bone matrix) [28]. However, a cause-and-effect relationship is not established between the dietary intake of B vitamins and maintenance of normal bone and teeth (EFSA opinion). |
| Author, Year | Nutraceutical Compound | Study Design/Experimental Model | Main Aim | Results |
|---|---|---|---|---|
| Mangano et al., 2018 [29] | Vitamin D | Retrospective study | To investigate the correlation between serum levels of vitamin D and early dental implant failure | In patients with serum levels of vitamin D < 10 ng/mL, there were 11.1% early dental implant failures (EDIF; failures that occurred before prosthesis positioning), 4.4% EDIFs in patients with vitamin D levels between 10 and 30 ng/mL, and 2.9% EDIFs in patients with levels > 30 ng/mL. No statistically significant correlation was found between EDIF and vitamin D serum levels, but a clear trend toward an increased incidence of EDIF with lowering of serum vitamin D levels was reported. |
| Wagner et al., 2017 [30] | Vitamin D | Retrospective parallel group | To evaluate the influence of osteoporosis on the marginal peri-implant bone level | Osteoporosis was shown to have a significant negative influence on the marginal bone loss (MBL) at the mesial and the distal implant aspect. Vitamin D positively and significantly affected the MBL, showing beneficial effects on the peri-implant bone formation. |
| Mangano et al., 2016 [31] | Vitamin D | Retrospective study | To investigate the correlation between early dental implant failure and low serum levels of vitamin D | There were 9% EDIFs in patients with serum levels of vitamin D < 10 ng/mL, 3.9% EDIFs in patients with vitamin D levels between 10 and 30 ng/mL, and 2.2% EDIFs in patients with vitamin D levels > 30 ng/mL. Although there was an increasing trend in the incidence of early implant failures with the worsening of vitamin D deficiency, the difference between these 3 groups was not statistically significant. |
| Fretwurst et al., 2016 [32] | Vitamin D | Case series | To evaluate the correlation between vitamin D deficiency and early implant failure | After vitamin D supplementation, implant placement was successful in 2 patients with previous early implant failures. |
| Bryce & Macbeth, 2014 [33] | Vitamin D | Case report | To investigate the influence of vitamin D deficiency in the osseointegration process of a dental implant | Authors reported a case of a patient that received dental extraction and the insertion of an immediate implant that failed to osseointegrate. Medical investigations revealed that he was severely vitamin D-deficient and that this may have contributed to the implant failure. |
| Liu et al., 2014 [34] | Vitamin D | Animal study | To investigate the effect of Vitamin D supplementation on implant osseointegration in CKD mice. | In rats with chronic kidney disease (CKD), vitamin D supplementation led to bone-to-implant contact rate (BIC) and bone volume/total volume levels higher than the CKD group without supplementation and comparable to rats without CKD. Also, at the push-in test, the CKD + vitamin D group had better results than the CKD group, which were comparable to the control group. |
| Zhou et al., 2012 [35] | Vitamin D | Animal study | Investigate the effects of 1,25(OH)2D3 on implant osseointegration in osteoporotic rats | Vitamin D supplementation in osteoporotic rats led to formation of more cancellous bone around implants, an increase of bone volume by 96.0% in terms of osseointegration, by 94.4% in terms of mean trabecular number, by 112.5% in terms of mean trabecular thickness, by 51.8% in terms of trabecular connective density, and by 38.0% in terms of connective density, as well as a decrease in terms of trabecular separation by 39.3%. Vitamin D increased bone area density by 1.2-fold and bone-to-implant contact by 1.5-fold and increased the maximal push-out force by 2.0-fold. |
| Wu et al., 2012 [36] | Vitamin D | Animal study | Effect of insulin and vitamin D3 on implant osseointegration in diabetic mellitus rats | Vitamin D and insulin combined treatment of diabetic rats led to an improvement of bone volume per total volume, percentage of osseointegration, mean trabecular thickness, mean trabecular number, connective density, maximal push-out force, and ultimate shear strength, BIC, and bone area ratio (BA), while the mean trabecular separation decreased. These indexes showed values comparable to those of healthy control rats. |
| Akhavan et al., 2012 [37] | Vitamin D | Animal study | Compare the effect of vitamin D administration on bone to implant contact in diabetic rats | At the histological analysis 3 weeks after implant insertion, diabetic rats reported a BIC level of 44 ± 19, while diabetic rats receiving vitamin D had a level of 57 ± 20. At 6 weeks, the control group reported BIC level of 70 ± 29 and the vitamin D group had a level of 65 ± 22. Considering these results, vitamin D seems not to have an effect on osseointegration of implants in diabetic rats. |
| Dvorak et al., 2012 [38] | Vitamin D (deficiency) | Animal study | Impact of vitamin D supplementation on the process of osseointegration | Vitamin D depletion in ovariectomized rats led to a significant decrease in bone-to-implant contact in the cortical area compared to rats fed with a standard vitamin D diet, while no significant reduction in BIC was observed in the medullar and the periosteal compartment. |
| Kelly et al., 2008 [39] | Vitamin D (deficiency) | Animal study | To evaluate the effect of a common deficiency of vitamin D on implant osseointegration in the rat model | Vitamin D deficiency in rats, 14 days after implant insertion, led to a lower push-in test and a lower BIC, compared to rats without deficiency. SEM analyses showed that the calcified tissues after push-in test, in the vitamin D deficiency groups, fractured between the implant and the surrounding tissue, resulting in exposed implant surface. |
| Belluci et al., 2011 [40] | Magnesium | Animal study | To evaluate the effect of magnesium dietary deficiency on bone metabolism and bone tissue around implants with established osseointegration | Rats fed with a diet with 90% magnesium reduction presented loss of systemic bone mass, decreased cortical bone thickness, and lower values of removal torque of the implants. |
| Del Barrio et al., 2010 [41] | Magnesium | Animal study | To evaluate the effect of severe magnesium dietary deficiency on systemic bone density and biomechanical resistance of bone tissue to the removal torque of osseointegrated implants | Magnesium intake reduction of 90% in diet of rats led to a statistically lower removal torque of the implants compared to rats fed with the recommended magnesium content, while no difference was demonstrated between the group with a 75% magnesium reduction and the control group. |
| Ribeiro et al., 2018 [42] | Resveratrol | Animal study | To investigate the effect of resveratrol on peri-implant repair, and its influence on bone-related markers in rats | Systemic assumption of resveratrol positively affected biomechanical retention of titanium implants, measured as torque removal values, and determined a higher BIC in smoking rats, when compared to smoking + placebo rat group. |
| Casarin et al., 2014 [43] | Resveratrol | Animal study | To investigate the effect of resveratrol on bone healing and its influence on the gene expression of osteogenic markers | Resveratrol increased the counter-torque values of implant removal when compared to placebo therapy and increased bone healing of critical size defects in rats. |
| Pimentel et al., 2016 [44] | Calcium, magnesium, zinc, and vitamin D3 | Animal study | To investigate the effect of micronutrients supplementation on the bone repair around implants | Rats receiving calcium, magnesium, zinc, and vitamin D intake for 30 days after implant insertion showed counter-torque values with no statistical difference compared to rats that received a placebo solution. Neither bone volume per total volume nor BIC showed a statistically significant difference between the 2 groups. |
| Takahashi et al., 2016 [45] | Synthetic bone mineral (dicalcium phosphate dihydrate + magnesium and zinc chlorides) | Animal study | To investigate whether oral intake of synthetic bone mineral improves peri-implant bone formation and bone micro architecture | Synthetic bone mineral (SBM; a mixture of dicalcium phosphate dihydrate and magnesium and zinc chlorides) intake led to a significantly higher bone volume per total volume, trabecular thickness, trabecular star volume compared to rats fed without SBM. The bone surface ratio of the rats that were fed with SBM was significantly lower than that of the rats fed without SBM. The trabecular number of the rats fed with SBM was not significantly increased compared to rats fed without SBM. Rats fed without SBM had no bone formation at 2 weeks, while bone formation was clearly observed in rats fed with SBM at 2 and 4 weeks after implantation. In rats fed without SBM at 4 weeks after implantation, irregular bone bands around the implants were observed. |
| Watanabe et al., 2015 [46] | Synthetic bone mineral (dicalcium phosphate dihydrate + magnesium and zinc chlorides) | Animal study | To investigate the effect of synthetic bone mineral in accelerating peri-implant bone formation | Pull-out strength was greatly higher in the SBM group compared to control group at 2 and 4 weeks. Bone mineral density was approximately double in the SBM group compared to control group both at 2 and 4 weeks, and this result was confirmed also by bone mineral density (BMD) color imaging. Microscopy observation showed green fluorescence in the SBM group at 2 and 4 weeks and only at 4 weeks in the control group. |
| Li et al., 2018 [47] | Vitamin C | Parallel group | To explore the effects of vitamin C supplementation in wound healing, following the placement of dental implants with or without bone grafts and patients with chronic periodontitis | Patients that received implants with guided bone regeneration (GBR) or with Bio-Oss collagen grafts and received vitamin C supplements, 14 days post-surgery, showed significantly improved wound healing compared with patients receiving the same surgical therapy but without vitamin C supplements. Patients suffering from chronic periodontitis that received implants showed significantly better wound healing at 7 and 14 days when they had vitamin C supplements compared to patients without supplements. Vitamin C showed no postoperative pain relief proprieties in any group. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastri, L.; Moretti, A.; Migliaccio, S.; Paoletta, M.; Annunziata, M.; Liguori, S.; Toro, G.; Bianco, M.; Cecoro, G.; Guida, L.; et al. Do Dietary Supplements and Nutraceuticals Have Effects on Dental Implant Osseointegration? A Scoping Review. Nutrients 2020, 12, 268. https://doi.org/10.3390/nu12010268
Nastri L, Moretti A, Migliaccio S, Paoletta M, Annunziata M, Liguori S, Toro G, Bianco M, Cecoro G, Guida L, et al. Do Dietary Supplements and Nutraceuticals Have Effects on Dental Implant Osseointegration? A Scoping Review. Nutrients. 2020; 12(1):268. https://doi.org/10.3390/nu12010268
Chicago/Turabian StyleNastri, Livia, Antimo Moretti, Silvia Migliaccio, Marco Paoletta, Marco Annunziata, Sara Liguori, Giuseppe Toro, Massimiliano Bianco, Gennaro Cecoro, Luigi Guida, and et al. 2020. "Do Dietary Supplements and Nutraceuticals Have Effects on Dental Implant Osseointegration? A Scoping Review" Nutrients 12, no. 1: 268. https://doi.org/10.3390/nu12010268
APA StyleNastri, L., Moretti, A., Migliaccio, S., Paoletta, M., Annunziata, M., Liguori, S., Toro, G., Bianco, M., Cecoro, G., Guida, L., & Iolascon, G. (2020). Do Dietary Supplements and Nutraceuticals Have Effects on Dental Implant Osseointegration? A Scoping Review. Nutrients, 12(1), 268. https://doi.org/10.3390/nu12010268







