Effect of Cocoa Products and Its Polyphenolic Constituents on Exercise Performance and Exercise-Induced Muscle Damage and Inflammation: A Review of Clinical Trials
Abstract
1. Introduction
2. Polyphenol Antioxidant Profile in Cocoa and Chocolate
3. Literature Search Strategy
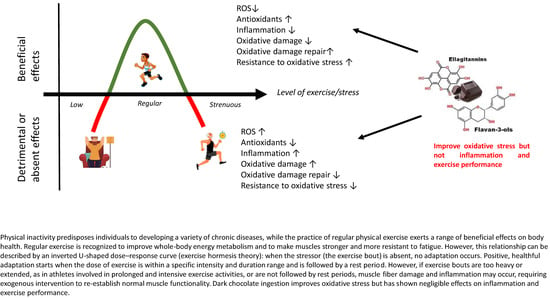
4. Effects of Cocoa Polyphenols on Exercise-Induced Oxidative Stress, Inflammation, and Recovery
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ivanenko, Y.; Gurfinkel, V.S. Human Postural Control. Front. Neurosci. 2018, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [PubMed]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardi, M.; Munoz-Canoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef] [PubMed]
- González, K.; Fuentes, J.; Márquez, J.L. Physical Inactivity, Sedentary Behavior and Chronic Diseases. Korean J. Fam. Med. 2017, 38, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.C.; Liu, S.; Song, Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Mujika, I.; Ronnestad, B.R.; Martin, D.T. Effects of Increased Muscle Strength and Muscle Mass on Endurance-Cycling Performance. Int. J. Sports Physiol. Perform. 2016, 11, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Kang, C.; Zhang, Y. Exercise-induced hormesis and skeletal muscle health. Free Radic. Biol. Med. 2016, 98, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Marathon training and immune function. Sports Med. 2007, 37, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R. Exercise, nutrition and the brain. Sports Med. 2014, 44 (Suppl. 1), S47–S56. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Morgan, D.L. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 2001, 537, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [PubMed]
- Stupka, N.; Tarnopolsky, M.A.; Yardley, N.J.; Phillips, S.M. Cellular adaptation to repeated eccentric exercise-induced muscle damage. J. Appl. Physiol. (1985) 2001, 91, 1669–1678. [Google Scholar] [CrossRef]
- Peake, J.M.; Suzuki, K.; Coombes, J.S. The influence of antioxidant supplementation on markers of inflammation and the relationship to oxidative stress after exercise. J. Nutr. Biochem. 2007, 18, 357–371. [Google Scholar] [CrossRef]
- Peternelj, T.T.; Coombes, J.S. Antioxidant Supplementation during Exercise Training Beneficial or Detrimental? Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef]
- Peterson, J.M.; Bakkar, N.; Guttridge, D.C. NF-kappaB signaling in skeletal muscle health and disease. Curr. Top. Dev. Biol. 2011, 96, 85–119. [Google Scholar]
- Kramer, H.F.; Goodyear, L.J. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J. Appl. Physiol. (1985) 2007, 103, 388–395. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Ichikawa, H.; Yoshida, N.; Yoshikawa, T. Oxidative stress and delayed-onset muscle damage after exercise. Free Radic. Biol. Med. 2004, 37, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Vina, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallen, J.; Ronnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Ostgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Vina, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Pyne, D.B.; Smith, J.A.; Baker, M.S.; Telford, R.D.; Weidemann, M.J. Neutrophil oxidative activity is differentially affected by exercise intensity and type. J. Sci. Med. Sport 2000, 3, 44–54. [Google Scholar] [CrossRef]
- Ascensao, A.; Rebelo, A.; Oliveira, E.; Marques, F.; Pereira, L.; Magalhaes, J. Biochemical impact of a soccer match - analysis of oxidative stress and muscle damage markers throughout recovery. Clin. Biochem. 2008, 41, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Hagglund, M.; Walden, M.; Magnusson, H.; Kristenson, K.; Bengtsson, H.; Ekstrand, J. Injuries affect team performance negatively in professional football: An 11-year follow-up of the UEFA Champions League injury study. Br. J. Sports Med. 2013, 47, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Carlstedt, C.J.; Chen, S.; Carmichael, M.D.; Murphy, E.A. The dietary flavonoid quercetin increases VO(2max) and endurance capacity. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 56–62. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, L.S.; Nieman, D.C.; Dumke, C.L.; Shooter, L.A.; Henson, D.A.; Utter, A.C.; Milne, G.; McAnulty, S.R. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl. Physiol. Nutr. Metab. 2011, 36, 976–984. [Google Scholar] [CrossRef]
- Nieman, D.C.; Williams, A.S.; Shanely, R.A.; Jin, F.; McAnulty, S.R.; Triplett, N.T.; Austin, M.D.; Henson, D.A. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010, 42, 338–345. [Google Scholar] [CrossRef]
- Childs, A.; Jacobs, C.; Kaminski, T.; Halliwell, B.; Leeuwenburgh, C. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic. Biol. Med. 2001, 31, 745–753. [Google Scholar] [CrossRef]
- Bailey, D.M.; Williams, C.; Betts, J.A.; Thompson, D.; Hurst, T.L. Oxidative stress, inflammation and recovery of muscle function after damaging exercise: Effect of 6-week mixed antioxidant supplementation. Eur. J. Appl. Physiol. 2011, 111, 925–936. [Google Scholar] [CrossRef]
- Morrison, D.; Hughes, J.; Della Gatta, P.A.; Mason, S.; Lamon, S.; Russell, A.P.; Wadley, G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015, 89, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef]
- McShea, A.; Leissle, K.; Smith, M.A. The essence of chocolate: A rich, dark, and well-kept secret. Nutrition 2009, 25, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and chocolate in human health and disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Schewe, T.; Heiss, C.; Kelm, M. Cocoa polyphenols and inflammatory mediators. Am. J. Clin. Nutr. 2005, 81, 304S–312S. [Google Scholar] [CrossRef]
- Gammone, M.A.; Efthymakis, K.; Pluchinotta, F.R.; Bergante, S.; Tettamanti, G.; Riccioni, G.; D’Orazio, N. Impact of chocolate on the cardiovascular health. Front. Biosci. (Landmark Ed.) 2018, 23, 852–864. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products—An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Andres-Lacueva, C.; Monagas, M.; Khan, N.; Izquierdo-Pulido, M.; Urpi-Sarda, M.; Permanyer, J.; Lamuela-Raventós, R.M. Flavanol and Flavonol Contents of Cocoa Powder Products: Influence of the Manufacturing Process. J. Agric. Food Chem. 2008, 56, 3111–3117. [Google Scholar] [CrossRef]
- Holt, R.R.; Lazarus, S.A.; Sullards, M.C.; Zhu, Q.Y.; Schramm, D.D.; Hammerstone, J.F.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. Procyanidin dimer B2 [epicatechin-(4beta-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr. 2002, 76, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Sudano, I.; Wolfrum, M.; Thomas, R.; Enseleit, F.; Périat, D.; Kaiser, P.; Hirt, A.; Hermann, M.; Serafini, M.; et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur. Heart J. 2011, 33, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, M.; Conti, A. Theobroma cacao L., the Food of the Gods: A scientific approach beyond myths and claims. Pharmacol. Res. 2010, 61, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. Bioavailability and health effects of cocoa polyphenols. Inflammopharmacology 2009, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, L.M.; Philo, M.; Colquhoun, I.J.; Tapp, H.S.; Saha, S.; Duthie, G.G.; Kemsley, E.K.; de Roos, B.; Kroon, P.A.; Le Gall, G. Acute Consumption of Flavan-3-ol-Enriched Dark Chocolate Affects Human Endogenous Metabolism. J. Proteome Res. 2017, 16, 2516–2526. [Google Scholar] [CrossRef] [PubMed]
- Cavarretta, E.; Peruzzi, M.; Del Vescovo, R.; Di Pilla, F.; Gobbi, G.; Serdoz, A.; Ferrara, R.; Schirone, L.; Sciarretta, S.; Nocella, C.; et al. Dark Chocolate Intake Positively Modulates Redox Status and Markers of Muscular Damage in Elite Football Athletes: A Randomized Controlled Study. Oxid. Med. Cell Longev. 2018, 2018, 4061901. [Google Scholar] [CrossRef] [PubMed]
- Allgrove, J.; Farrell, E.; Gleeson, M.; Williamson, G.; Cooper, K. Regular dark chocolate consumption’s reduction of oxidative stress and increase of free-fatty-acid mobilization in response to prolonged cycling. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 113–123. [Google Scholar] [CrossRef]
- Fraga, C.G.; Actis-Goretta, L.; Ottaviani, J.I.; Carrasquedo, F.; Lotito, S.B.; Lazarus, S.; Schmitz, H.H.; Keen, C.L. Regular consumption of a flavanol-rich chocolate can improve oxidant stress in young soccer players. Clin. Dev. Immunol. 2005, 12, 11–17. [Google Scholar] [CrossRef]
- Patel, R.K.; Brouner, J.; Spendiff, O. Dark chocolate supplementation reduces the oxygen cost of moderate intensity cycling. J. Int. Soc. Sports Nutr. 2015, 12, 47. [Google Scholar] [CrossRef]
- Singh, I.; Quinn, H.; Mok, M.; Southgate, R.J.; Turner, A.H.; Li, D.; Sinclair, A.J.; Hawley, J.A. The effect of exercise and training status on platelet activation: Do cocoa polyphenols play a role? Platelets 2006, 17, 361–367. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Godin, J.P.; Chou, C.J.; Grathwohl, D.; Ross, A.B.; Cooper, K.A.; Williamson, G.; Actis-Goretta, L. The effect of acute dark chocolate consumption on carbohydrate metabolism and performance during rest and exercise. Appl. Physiol. Nutr. Metab. 2014, 39, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Taub, P.R.; Ramirez-Sanchez, I.; Patel, M.; Higginbotham, E.; Moreno-Ulloa, A.; Roman-Pintos, L.M.; Phillips, P.; Perkins, G.; Ceballos, G.; Villarreal, F. Beneficial effects of dark chocolate on exercise capacity in sedentary subjects: Underlying mechanisms. A double blind, randomized, placebo controlled trial. Food Funct. 2016, 7, 3686–3693. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, F.G.; Fisher, M.G.; Thornley, T.T.; Roemer, K.; Pritchett, R.; Freitas, E.C.; Pritchett, K. Cocoa flavanol effects on markers of oxidative stress and recovery after muscle damage protocol in elite rugby players. Nutrition 2019, 62, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Descat, A.; Drittij-Reijnders, M.J.; Weseler, A.R.; Bast, A.; Stahl, W.; Heyman, E.; Meeusen, R. Acute cocoa Flavanols intake has minimal effects on exercise-induced oxidative stress and nitric oxide production in healthy cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2017, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garrido, J.A.; Garcia-Sanchez, J.R.; Garrido-Llanos, S.; Olivares-Corichi, I.M. An association of cocoa consumption with improved physical fitness and decreased muscle damage and oxidative stress in athletes. J. Sports Med. Phys. Fitness 2017, 57, 441–447. [Google Scholar] [PubMed]
- Peschek, K.; Pritchett, R.; Bergman, E.; Pritchett, K. The effects of acute post exercise consumption of two cocoa-based beverages with varying flavanol content on indices of muscle recovery following downhill treadmill running. Nutrients 2013, 6, 50–62. [Google Scholar] [CrossRef]
- Wiswedel, I.; Hirsch, D.; Kropf, S.; Gruening, M.; Pfister, E.; Schewe, T.; Sies, H. Flavanol-rich cocoa drink lowers plasma F(2)-isoprostane concentrations in humans. Free Radic. Biol. Med. 2004, 37, 411–421. [Google Scholar] [CrossRef]
- Davison, G.; Callister, R.; Williamson, G.; Cooper, K.A.; Gleeson, M. The effect of acute pre-exercise dark chocolate consumption on plasma antioxidant status, oxidative stress and immunoendocrine responses to prolonged exercise. Eur. J. Nutr. 2012, 51, 69–79. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Sjodin, B.; Hellsten Westing, Y.; Apple, F.S. Biochemical mechanisms for oxygen free radical formation during exercise. Sports Med. 1990, 10, 236–254. [Google Scholar] [CrossRef]
- Becatti, M.; Mannucci, A.; Barygina, V.; Mascherini, G.; Emmi, G.; Silvestri, E.; Wright, D.; Taddei, N.; Galanti, G.; Fiorillo, C. Redox status alterations during the competitive season in elite soccer players: Focus on peripheral leukocyte-derived ROS. Intern. Emerg. Med. 2017, 12, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, I.; Barroso, O.; Rabin, O. The list of prohibited substances and methods in sport: Structure and review process by the world anti-doping agency. J. Anal. Toxicol. 2011, 35, 608–612. [Google Scholar] [CrossRef] [PubMed]
- World anti-doping Agency. The World Anti-Doping Code International Standard: Substances & Methods Prohibited at All Times. Available online: https://www.wada-ama.org/sites/default/files/wada_2019_english_prohibited_list.pdf (accessed on 1 January 2019).
- Karp, J.R.; Johnston, J.D.; Tecklenburg, S.; Mickleborough, T.D.; Fly, A.D.; Stager, J.M. Chocolate milk as a post-exercise recovery aid. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 78–91. [Google Scholar] [CrossRef]
- Pritchett, K.; Pritchett, R. Chocolate milk: A post-exercise recovery beverage for endurance sports. Med. Sport Sci. 2012, 59, 127–134. [Google Scholar] [PubMed]
- Hatchett, A.; Berry, C.; Oliva, C.; Wiley, D.; St Hilaire, J.; LaRochelle, A. A Comparison between Chocolate Milk and a Raw Milk Honey Solution’s Influence on Delayed Onset of Muscle Soreness. Sports (Basel, Switz.) 2016, 4, 18. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Decroix, L.; Soares, D.D.; Meeusen, R.; Heyman, E.; Tonoli, C. Cocoa Flavanol Supplementation and Exercise: A Systematic Review. Sports Med. 2018, 48, 867–892. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, M.; Angeloni, C.; Hrelia, S. Polyphenols in exercise performance and prevention of exercise-induced muscle damage. Oxid. Med. Cell Longev. 2013, 2013, 825928. [Google Scholar] [CrossRef]
- Pingitore, A.; Lima, G.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef]
- Sansone, R.; Ottaviani, J.I.; Rodriguez-Mateos, A.; Heinen, Y.; Noske, D.; Spencer, J.P.; Crozier, A.; Merx, M.W.; Kelm, M.; Schroeter, H.; et al. Methylxanthines enhance the effects of cocoa flavanols on cardiovascular function: Randomized, double-masked controlled studies. Am. J. Clin. Nutr. 2017, 105, 352–360. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Zhang, P.Y. Polyphenols in Health and Disease. Cell Biochem. Biophys. 2015, 73, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, S. Sport nutrition: A review of the latest guidelines for exercise and sport nutrition from the American College of Sport Nutrition, the International Olympic Committee and the International Society for Sports Nutrition. S. Afr. J. Clin. Nutr. 2013, 26, 6–16. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Davis, J.M.; Angela Murphy, E.; Jenkins, D.P.; Gross, S.J.; Carmichael, M.D.; Quindry, J.C.; Dumke, C.L.; Utter, A.C.; et al. Quercetin’s influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J. Appl. Physiol. (1985) 2007, 103, 1728–1735. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Bluher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year, (Reference) | Study Design | Number of Participants, Sex, Age, Weight | Exercise Protocol | Interventions (mg Polyphenols Daily) | Measurements | Circulating Leucocyte | IL-6 | CK; LDH | Oxidative Stress Markers | EP and R Improvement (Yes/No) |
|---|---|---|---|---|---|---|---|---|---|---|
| Davidson et al. [58] | RCT, crossover design | 14, male, 22 ± 1 years, 71.6 ± 1.6 kg | 2.5 h cycling | 100 g DCHO (39.1 mg catechin, 96.8 mg epicatechin); cocoa-liquor-free control bar (0 mg); 2 h before exercise | Rest, pre-exercise, post-exercise, after 1 h recovery | ↔ by DCHO | ↔ in DCHO | NE | TAS: ↑ in DCHO; F2-isoprostane: ↓ by DCHO | No |
| Decroix et al. [54] | RCT, crossover design | 12, male, 30.5 ± 3 years, 72.8 ± 7.8 kg | 2 cycling sessions of 30 min; | HFCD (900 mg) or LFCD (15 mg) before first exercise session | Baseline, after 100 and 230 min from supplementation; after training sessions | NE | ↔ by CF | NE | TEAC: ↑ by HFCD; MDA: ↔ by HFCD | No |
| Wiswedel et al. [57] | RCT, double-blind, cross-over | 10, male, 20–40 years | Cycling, 29 min | HFCD (187 mg flavanols) or LFCD (14 mg flavanols) 2 h pre-exercise | At baseline and after 2, 4 and 6 h from ingestion | NE | NE | NE | F2-isoprostanes: ↔ by HFCD; ↑ by LFCD | NE |
| Peschek et al. [56] | RCT, single-blind, cross-over | 8, male, 24.6 ± 5.6 years, 73.4 ± 7.0 kg | 30 min downhill running; after 48 h, 5 km running | HFCD (2 × 350 mg) or placebo (0 mg) after downhill running | At baseline, after 24 h, after 48 h, after 5 km running | NE | NE | CK and LDH: ↔ by HFCD | NE | No |
| Stellingwerff et al. [51] | Randomized, single blind, crossover | 9, male, 30.0 ± 6.1 years, 72.8 ± 6.0 kg | 2.5 h cycling, plus a time trial of 15 min | HFCHO (262 mg) or LFCHO (<0.05 mg) acutely (2 h pre-exercise) | After time trial | NE | NE | NE | NE | No |
| First Author, Year, (Reference) | Study Design | Number of Participants, Sex, Age, Weight | Exercise Protocol | Interventions, (Polyphenols Daily mg) | Measurements | Circulating Leucocyte | IL-6 | CK; LDH | Oxidative Stress Markers | EP and R Improvement (Yes/No) |
|---|---|---|---|---|---|---|---|---|---|---|
| Allgrove et al. [47] | RCT, crossover design | 20, male, 22 ± 4 years, 74.6 ± 8 kg | Cycling for 90 min followed by 25 min time trial | 80 g of DCHO (197.4 mg) or 56.8 g of cocoa liquor-free chocolate control (0 mg) bar for 2 weeks before the trial and on the trial day | Before exercise, post-exercise bout, post- exhaustion, and after 1 h of resting recovery | ↔ by DCHO | ↔ by DCHO | NE | TAS: ↔ by DCHO; F2-isoprostanes: ↔ in DCHO; ↑ in control | No |
| Singh et al. [50] | RCT, double-blind, cross-over | 16, male, 23 ± 5 years, 79 ± 11 kg | Cycling, 60 min | Cocoa polyphenol supplement (240 mg) or placebo (0 mg) for 7 days | At baseline and after 8 days | NE | NE | NE | TAS: ↔ by cocoa polyphenol supplement | NE |
| Patel et al. [49] | Randomized, single blind, cross-over | 9, male, 21 ± 1 years, 76 ± 9.3 kg | Cycling, 20 min plus a 2 min time trial | 40 g DCHO (259 mg) or 40 g white chocolate, 2 weeks | At baseline, after 14 days | NE | NE | NE | NE | Yes |
| Fraga et al. [48] | RCT, crossover design | 28, male, 18 ± 1 years, 74.0 ± 0.2 kg | Football, 2 times a week training | FCMCHO (168 mg) or cocoa butter white chocolate for 2 weeks | At baseline and after 2 weeks | NE | NE | CK:↔ by FCMCHO; LDH: ↓ FCMCHO | MDA: ↓ by FCMCHO; OXOd and TRAP: ↔ by FCMCHO | Yes |
| Gonzalez-Garrido et al. [55] | Intervention study with pre/post-design | 15, male, 17.0 ± 1.11 years, 66.98 ± 6.52 kg | Football training five days/week, and 90 min match/week | HFCD (1050 mg) for 5 days | At baseline and after 6 days | NE | NE | CK and LDH: ↓ by HFCD | TBARS: ↔ by HFCD; MDA: ↓ by HFCD; 4-HNE: ↓ by HFCD; Carbonyl groups: ↓ by HFCD; TAS: ↑ by HFCD | Yes |
| de Carvalho et al. [53] | RCT, double-blind | 13, male, 20.69 ± 1.49 years, 87.02 ± 8.03 kg | Daily rugby match for 5 days | HFCD (2 × 308 mg) or LFCD for 5 days | At baseline and after 6 days | NE | NE | CK: ↔ by HFCD | F2-isoprostanes: ↔ by HFCD | No |
| Cavarretta et al. [46] | RCT, double-blind | 20, male, 17.8 ± 0.9 years (control); 17.4 ± 0.5 years (intervention) | 120 min football training, 6 times/week, and 90 min match/week | Normal diet plus 40 g DCHO in tablet (36 mg) or normal diet for 30 days | At baseline and after 60 days | NE | NE | CK and LDH: ↓ by DCHO | HBA: ↓ by DCHO | No |
| Taub et al. [52] | RCT, double-blind, cross-over | 17, male, 49.5 ± 1.6 years, 79 ± 11 kg | Cycling | HFCHO (175 mg) or LFCHO (1.2 mg) for 3 months | At baseline and after 3 months | NE | NE | NE | GSH/GSSG: ↑ by HFCHO; Protein carbonyl: ↓ by HFCHO | Yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaro, M.; Scoditti, E.; Carluccio, M.A.; Kaltsatou, A.; Cicchella, A. Effect of Cocoa Products and Its Polyphenolic Constituents on Exercise Performance and Exercise-Induced Muscle Damage and Inflammation: A Review of Clinical Trials. Nutrients 2019, 11, 1471. https://doi.org/10.3390/nu11071471
Massaro M, Scoditti E, Carluccio MA, Kaltsatou A, Cicchella A. Effect of Cocoa Products and Its Polyphenolic Constituents on Exercise Performance and Exercise-Induced Muscle Damage and Inflammation: A Review of Clinical Trials. Nutrients. 2019; 11(7):1471. https://doi.org/10.3390/nu11071471
Chicago/Turabian StyleMassaro, Marika, Egeria Scoditti, Maria Annunziata Carluccio, Antonia Kaltsatou, and Antonio Cicchella. 2019. "Effect of Cocoa Products and Its Polyphenolic Constituents on Exercise Performance and Exercise-Induced Muscle Damage and Inflammation: A Review of Clinical Trials" Nutrients 11, no. 7: 1471. https://doi.org/10.3390/nu11071471
APA StyleMassaro, M., Scoditti, E., Carluccio, M. A., Kaltsatou, A., & Cicchella, A. (2019). Effect of Cocoa Products and Its Polyphenolic Constituents on Exercise Performance and Exercise-Induced Muscle Damage and Inflammation: A Review of Clinical Trials. Nutrients, 11(7), 1471. https://doi.org/10.3390/nu11071471