Validation of a Dietary Questionnaire to Screen Omega-3 Fatty Acids Levels in Healthy Adults
Abstract
1. Introduction
2. Methods
2.1. Recruitment
2.2. Determination of the Dietary Criteria
2.3. Dietary Assessment Questionnaire
2.4. Calculation of Dietary Intake of EPA and DHA
2.5. Omega-3 Index and Blood Fatty Acids Levels
2.6. Sample Size
2.7. Statistical Analysis
3. Results
3.1. Volunteer Characteristics and Blood Levels of Omega-3 PUFAs
3.2. Dietary Consumption of EPA and DHA
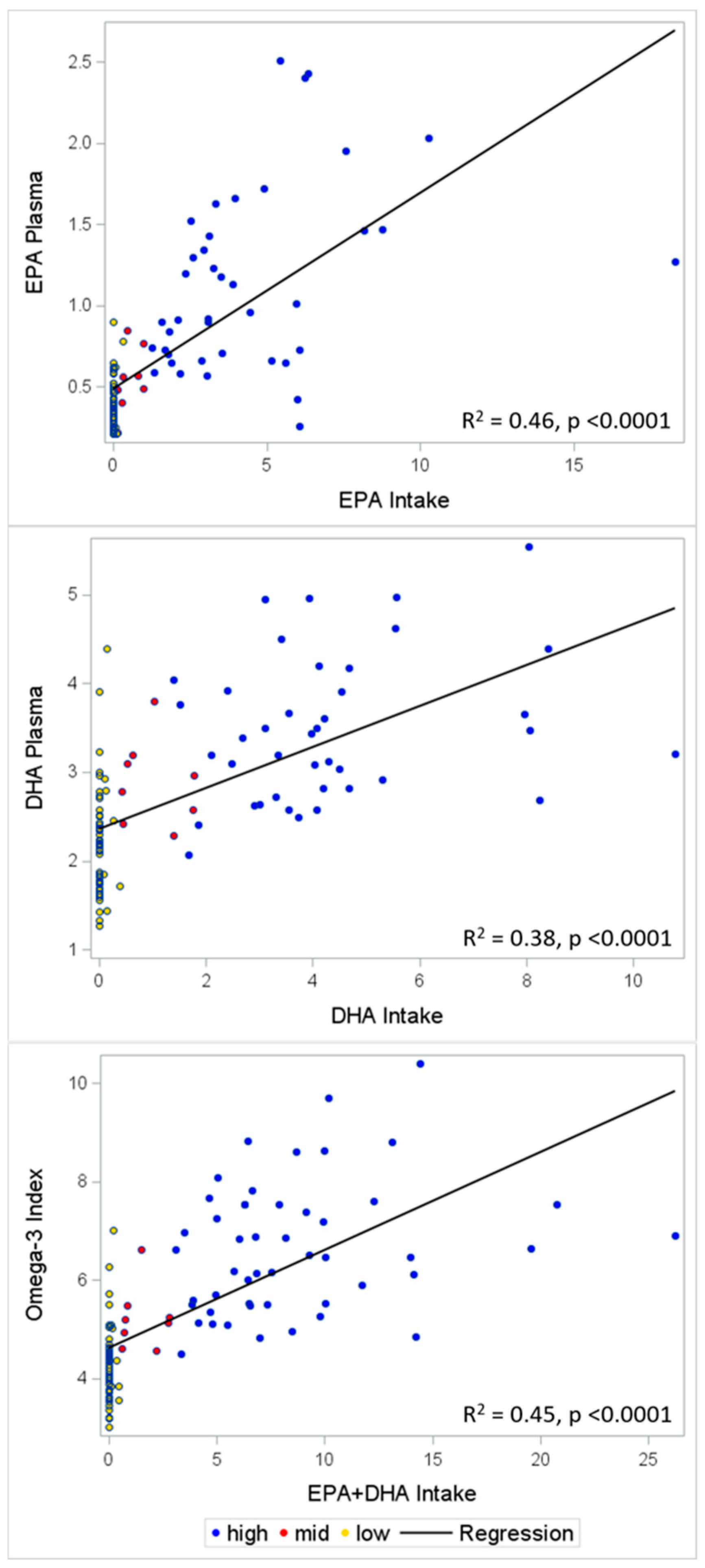
3.3. Validation
3.4. Sensitivity and Specificity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
Abbreviations
| ALA | alpha-linolenic acid |
| BMI | body mass index |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| FFQ | food frequency questionnaire |
| PUFAs | polyunsaturated fatty acids |
| USDA | United States Department of Agriculture |
References
- Penberthy, L.T.; Dahman, B.A.; Petkov, V.I.; DeShazo, J.P. Effort required in eligibility screening for clinical trials. J. Oncol. Pract. 2012, 8, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.R.; Fujimoto, W.Y.; Marrero, D.G.; Brenneman, T.; Charleston, J.B.; Edelstein, S.L.; Fisher, E.B.; Jordan, R.; Knowler, W.C.; Lichterman, L.C.; et al. The Diabetes Prevention Program: recruitment methods and results. Control. Clin. Trials 2002, 23, 157–171. [Google Scholar] [PubMed]
- Clark, M.A.; Neighbors, C.J.; Wasserman, M.R.; Armstrong, G.F.; Drnach, M.L.; Howie, S.L.; Hawthorne, T.L. Strategies and cost of recruitment of middle-aged and older unmarried women in a cancer screening study. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Gismondi, P.M.; Hamer, D.H.; Leka, L.S.; Dallal, G.; Fiatarone Singh, M.A.; Meydani, S.N. Strategies, time, and costs associated with the recruitment and enrollment of nursing home residents for a micronutrient supplementation clinical trial. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- 2015–2020 Dietary Guidelines—Health.gov. Available online: https://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 7 May 2019).
- Qin, Y.; Zhou, Y.; Chen, S.-H.; Zhao, X.-L.; Ran, L.; Zeng, X.-L.; Wu, Y.; Chen, J.-L.; Kang, C.; Shu, F.-R.; et al. Fish Oil Supplements Lower Serum Lipids and Glucose in Correlation with a Reduction in Plasma Fibroblast Growth Factor 21 and Prostaglandin E2 in Nonalcoholic Fatty Liver Disease Associated with Hyperlipidemia: A Randomized Clinical Trial. PLoS ONE 2015, 10, e0133496. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Maki, K.C.; Palacios, O.M.; Bell, M.; Toth, P.P. Use of supplemental long-chain omega-3 fatty acids and risk for cardiac death: An updated meta-analysis and review of research gaps. J. Clin. Lipidol. 2017, 11, 1152–1160. [Google Scholar] [CrossRef]
- Alexander, D.D.; Miller, P.E.; Van Elswyk, M.E.; Kuratko, C.N.; Bylsma, L.C. A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies of Eicosapentaenoic and Docosahexaenoic Long-Chain Omega-3 Fatty Acids and Coronary Heart Disease Risk. Mayo Clin. Proc. 2017, 92, 15–29. [Google Scholar] [CrossRef]
- Nielsen, A.A.; Jørgensen, L.G.M.; Nielsen, J.N.; Eivindson, M.; Grønbaek, H.; Vind, I.; Hougaard, D.M.; Skogstrand, K.; Jensen, S.; Munkholm, P.; et al. Omega-3 fatty acids inhibit an increase of proinflammatory cytokines in patients with active Crohn’s disease compared with omega-6 fatty acids. Aliment. Pharmacol. Ther. 2005, 22, 1121–1128. [Google Scholar] [CrossRef]
- Shen, W.; Gaskins, H.R.; McIntosh, M.K. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J. Nutr. Biochem. 2014, 25, 270–280. [Google Scholar] [CrossRef]
- Kaliannan, K.; Wang, B.; Li, X.-Y.; Kim, K.-J.; Kang, J.X. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci. Rep. 2015, 5, 11276. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Hermannstädter, H.M.; Fiebach, J.B.; Schreiber, S.J.; Schuchardt, J.P.; Hahn, A.; Flöel, A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb. Cortex 2014, 24, 3059–3068. [Google Scholar] [CrossRef]
- Shinto, L.; Quinn, J.; Montine, T.; Dodge, H.H.; Woodward, W.; Baldauf-Wagner, S.; Waichunas, D.; Bumgarner, L.; Bourdette, D.; Silbert, L.; et al. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J. Alzheimers Dis. 2014, 38, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Hughes, M.; Rowsell, R.; Cockerell, R.; Pipingas, A.; Crewther, S.; Crewther, D. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Hum. Psychopharmacol. 2014, 29, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T.; Ohta, H.; Onoe, Y.; Tsugawa, N.; Shiraki, M. Intake of omega-3 fatty acids contributes to bone mineral density at the hip in a younger Japanese female population. Osteoporos. Int. 2017, 28, 2887–2891. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.M.; Kerstetter, J.E.; Kenny, A.M.; Insogna, K.L.; Walsh, S.J. An investigation of the association between omega 3 FA and bone mineral density among older adults: Results from the National Health and Nutrition Examination Survey years 2005–2008. Osteoporos. Int. 2014, 25, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Koren, N.; Simsa-Maziel, S.; Shahar, R.; Schwartz, B.; Monsonego-Ornan, E. Exposure to omega-3 fatty acids at early age accelerate bone growth and improve bone quality. J. Nutr. Biochem. 2014, 25, 623–633. [Google Scholar] [CrossRef]
- Rodriguez-Santana, Y.; Ochoa, J.J.; Lara-Villoslada, F.; Kajarabille, N.; Saavedra-Santana, P.; Hurtado, J.A.; Peña, M.; Diaz-Castro, J.; Sebastian-Garcia, I.; Machin-Martin, E.; et al. Cytokine distribution in mothers and breastfed children after omega-3 LCPUFAs supplementation during the last trimester of pregnancy and the lactation period: A randomized, controlled trial. Prostaglandins Leukot. Essent. Fatty Acids 2017, 126, 32–38. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Englund-Ögge, L.; Haugen, M.; Birgisdottir, B.E.; Knutsen, H.K.; Sengpiel, V.; Myhre, R.; Alexander, J.; Nilsen, R.M.; Jacobsson, B.; et al. Maternal intake of seafood and supplementary long chain n-3 poly-unsaturated fatty acids and preterm delivery. BMC Pregnancy Childbirth 2017, 17, 41. [Google Scholar]
- Haghiac, M.; Yang, X.; Presley, L.; Smith, S.; Dettelback, S.; Minium, J.; Belury, M.A.; Catalano, P.M.; Hauguel-de Mouzon, S. Dietary Omega-3 Fatty Acid Supplementation Reduces Inflammation in Obese Pregnant Women: A Randomized Double-Blind Controlled Clinical Trial. PLoS ONE 2015, 10, e0137309. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, W.; Perera, F.; Maugeri, U.; Mrozek-Budzyn, D.; Miller, R.L.; Flak, E.; Mroz, E.; Jacek, R.; Spengler, J.D. Effects of prenatal and perinatal exposure to fine air pollutants and maternal fish consumption on the occurrence of infantile eczema. Int. Arch. Allergy Immunol. 2011, 155, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Calder, P.C. Dietary alpha-linolenic acid and health-related outcomes: A metabolic perspective. Nutr. Res. Rev. 2006, 19, 26–52. [Google Scholar] [CrossRef] [PubMed]
- Pawlosky, R.J.; Hibbeln, J.R.; Novotny, J.A.; Salem, N. Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J. Lipid Res. 2001, 42, 1257–1265. [Google Scholar] [PubMed]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Del Gobbo, L.; Tintle, N.L. The Omega-3 Index and relative risk for coronary heart disease mortality: Estimation from 10 cohort studies. Atherosclerosis 2017, 262, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Bigornia, S.J.; Harris, W.S.; Falcón, L.M.; Ordovás, J.M.; Lai, C.-Q.; Tucker, K.L. The Omega-3 Index Is Inversely Associated with Depressive Symptoms among Individuals with Elevated Oxidative Stress Biomarkers. J. Nutr. 2016, 146, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Van der Wurff, I.S.M.; von Schacky, C.; Berge, K.; Zeegers, M.P.; Kirschner, P.A.; de Groot, R.H.M. Association between Blood Omega-3 Index and Cognition in Typically Developing Dutch Adolescents. Nutrients 2016, 8, 13. [Google Scholar] [CrossRef]
- Ferguson, J.J.A.; Veysey, M.; Lucock, M.; Niblett, S.; King, K.; MacDonald-Wicks, L.; Garg, M.L. Association between omega-3 index and blood lipids in older Australians. J. Nutr. Biochem. 2016, 27, 233–240. [Google Scholar] [CrossRef]
- Meyer, B.J.; Byrne, M.K.; Collier, C.; Parletta, N.; Crawford, D.; Winberg, P.C.; Webster, D.; Chapman, K.; Thomas, G.; Dally, J.; et al. Baseline omega-3 index correlates with aggressive and attention deficit disorder behaviours in adult prisoners. PLoS ONE 2015, 10, e0120220. [Google Scholar]
- Parker, H.M.; O’Connor, H.T.; Keating, S.E.; Cohn, J.S.; Garg, M.L.; Caterson, I.D.; George, J.; Johnson, N.A. Efficacy of the Omega-3 Index in predicting non-alcoholic fatty liver disease in overweight and obese adults: A pilot study. Br. J. Nutr. 2015, 114, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Sands, S.A.; Reid, K.J.; Windsor, S.L.; Harris, W.S. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids 2005, 40, 343–347. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.I.; Bird, J.K. Impact of Biological Feedback and Incentives on Blood Fatty Acid Concentrations, Including Omega-3 Index, in an Employer-Based Wellness Program. Nutrients 2017, 9, 842. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.; Marshall, K.; Hartunian-Sowa, S.; McBurney, M. Omega-3 Status among Family Physicians: A Catalyst for Increased Patient Recommendations. FASEB J. 2017, 31, 971.6. [Google Scholar]
- Harris, W.S.; Pottala, J.V.; Lacey, S.M.; Vasan, R.S.; Larson, M.G.; Robins, S.J. Clinical correlates and heritability of erythrocyte eicosapentaenoic and docosahexaenoic acid content in the Framingham Heart Study. Atherosclerosis 2012, 225, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Parks, C.A.; Brett, N.R.; Agellon, S.; Lavery, P.; Vanstone, C.A.; Maguire, J.L.; Rauch, F.; Weiler, H.A. DHA and EPA in red blood cell membranes are associated with dietary intakes of omega-3-rich fish in healthy children. Prostaglandins Leukot. Essent. Fatty Acids 2017, 124, 11–16. [Google Scholar] [CrossRef]
- Jackson, K.H.; Polreis, J.M.; Tintle, N.L.; Kris-Etherton, P.M.; Harris, W.S. Association of reported fish intake and supplementation status with the omega-3 index. Prostaglandins Leukot. Essent. Fatty Acids 2019, 142, 4–10. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Mazzucco, S.; Biolo, G.; Farcomeni, A.; Ramaccini, C.; Antonaroli, S.; Monti, M.; Muscaritoli, M. Effect of Oral Docosahexaenoic Acid (DHA) Supplementation on DHA Levels and Omega-3 Index in Red Blood Cell Membranes of Breast Cancer Patients. Front. Physiol. 2017, 8, 549. [Google Scholar] [CrossRef]
- Bailey, R.L.; Miller, P.E.; Mitchell, D.C.; Hartman, T.J.; Lawrence, F.R.; Sempos, C.T.; Smiciklas-Wright, H. Dietary screening tool identifies nutritional risk in older adults. Am. J. Clin. Nutr. 2009, 90, 177–183. [Google Scholar] [CrossRef]
- Rifas-Shiman, S.L.; Willett, W.C.; Lobb, R.; Kotch, J.; Dart, C.; Gillman, M.W. PrimeScreen, a brief dietary screening tool: Reproducibility and comparability with both a longer food frequency questionnaire and biomarkers. Public Health Nutr. 2001, 4, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Lanier, S.A.; Hutchins, H.L.; Pescatello, L.S.; Johnson, M.K.; Bartoshuk, L.M. Food preference questionnaire as a screening tool for assessing dietary risk of cardiovascular disease within health risk appraisals. J. Am. Diet Assoc. 2007, 107, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the “malnutrition universal screening tool” (‘MUST’) for adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef]
- Ritenbaugh, C.; Aickin, M.; Taren, D.; Teufel, N.; Graver, E.; Woolf, K.; Alberts, D.S. Use of a food frequency questionnaire to screen for dietary eligibility in a randomized cancer prevention phase III trial. Cancer Epidemiol. Biomarkers Prev. 1997, 6, 347–354. [Google Scholar] [PubMed]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z.; Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Subar, A.F. Developing dietary assessment tools. J. Am. Diet Assoc. 2004, 104, 769–770. [Google Scholar] [CrossRef] [PubMed]
- Kretser, A.; Murphy, D.; Starke-Reed, P. A partnership for public health: USDA branded food products database. J. Food Compost. Anal. 2017, 64, 10–12. [Google Scholar] [CrossRef]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef]
- Office of Dietary Supplements - Omega-3 Fatty Acids. Available online: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/ (accessed on 7 May 2019).
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; American Heart Association. Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Papanikolaou, Y.; Brooks, J.; Reider, C.; Fulgoni, V.L. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: Results of an analysis using observational data from NHANES 2003–2008. Nutr. J. 2014, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Polreis, J. Measurement of the Omega-3 Index in Dried Blood Spots. Ann. Clin. Res. 2016, 4. [Google Scholar] [CrossRef]
- Shiraishi, M.; Haruna, M.; Matsuzaki, M.; Murayama, R.; Sasaki, S. The biomarker-based validity of a brief-type diet history questionnaire for estimating eicosapentaenoic acid and docosahexaenoic acid intakes in pregnant Japanese women. Asia. Pac. J. Clin. Nutr. 2015, 24, 316–322. [Google Scholar] [PubMed]
- Brenna, J.T.; Salem, N.; Sinclair, A.J.; Cunnane, S.C. International Society for the Study of Fatty Acids and Lipids, ISSFAL alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.V.; Davis, B.C.; Garg, M.L. Omega-3 polyunsaturated fatty acids and vegetarian diets. Med. J. Aust. 2013, 199, S22–S26. [Google Scholar] [CrossRef]
- Rosell, M.S.; Lloyd-Wright, Z.; Appleby, P.N.; Sanders, T.A.B.; Allen, N.E.; Key, T.J. Long-chain n-3 polyunsaturated fatty acids in plasma in British meat-eating, vegetarian, and vegan men. Am. J. Clin. Nutr. 2005, 82, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; Shakya-Shrestha, S.; Lentjes, M.A.H.; Wareham, N.J.; Khaw, K.-T. Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the product-precursor ratio [corrected] of α-linolenic acid to long-chain n-3 polyunsaturated fatty acids: Results from the EPIC-Norfolk cohort. Am. J. Clin. Nutr. 2010, 92, 1040–1051. [Google Scholar]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef]
- Köhler, A.; Sarkkinen, E.; Tapola, N.; Niskanen, T.; Bruheim, I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects—A randomized, single-dose, cross-over trial. Lipids Health Dis. 2015, 14, 19. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; Kralovec, J.; Bailey-Hall, E.; Smeberg, V.; Stark, J.G.; Salem, N. Similar eicosapentaenoic acid and docosahexaenoic acid plasma levels achieved with fish oil or krill oil in a randomized double-blind four-week bioavailability study. Lipids Health Dis. 2015, 14, 99. [Google Scholar] [CrossRef]
- Ulven, S.M.; Kirkhus, B.; Lamglait, A.; Basu, S.; Elind, E.; Haider, T.; Berge, K.; Vik, H.; Pedersen, J.I. Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA, in healthy volunteers. Lipids 2011, 46, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Jones, P.J.H. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipids Health Dis. 2013, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.H.; Sinclair, A.J.; Lewandowski, P.A.; Su, X.Q. Postprandial long-chain n-3 polyunsaturated fatty acid response to krill oil and fish oil consumption in healthy women: A randomised controlled, single-dose, crossover study. Asia. Pac. J. Clin. Nutr. 2018, 27, 148–157. [Google Scholar] [PubMed]
- Cicero, A.F.; Colletti, A. Krill oil: Evidence of a new source of polyunsaturated fatty acids with high bioavailability. Clin. Lipidol. 2015, 10, 1–4. [Google Scholar] [CrossRef]
- Swierk, M.; Williams, P.G.; Wilcox, J.; Russell, K.G.; Meyer, B.J. Validation of an Australian electronic food frequency questionnaire to measure polyunsaturated fatty acid intake. Nutrition 2011, 27, 641–646. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ingram, M.A.; Stonehouse, W.; Russell, K.G.; Meyer, B.J.; Kruger, R. The New Zealand PUFA semiquantitative food frequency questionnaire is a valid and reliable tool to assess PUFA intakes in healthy New Zealand adults. J. Nutr. 2012, 142, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
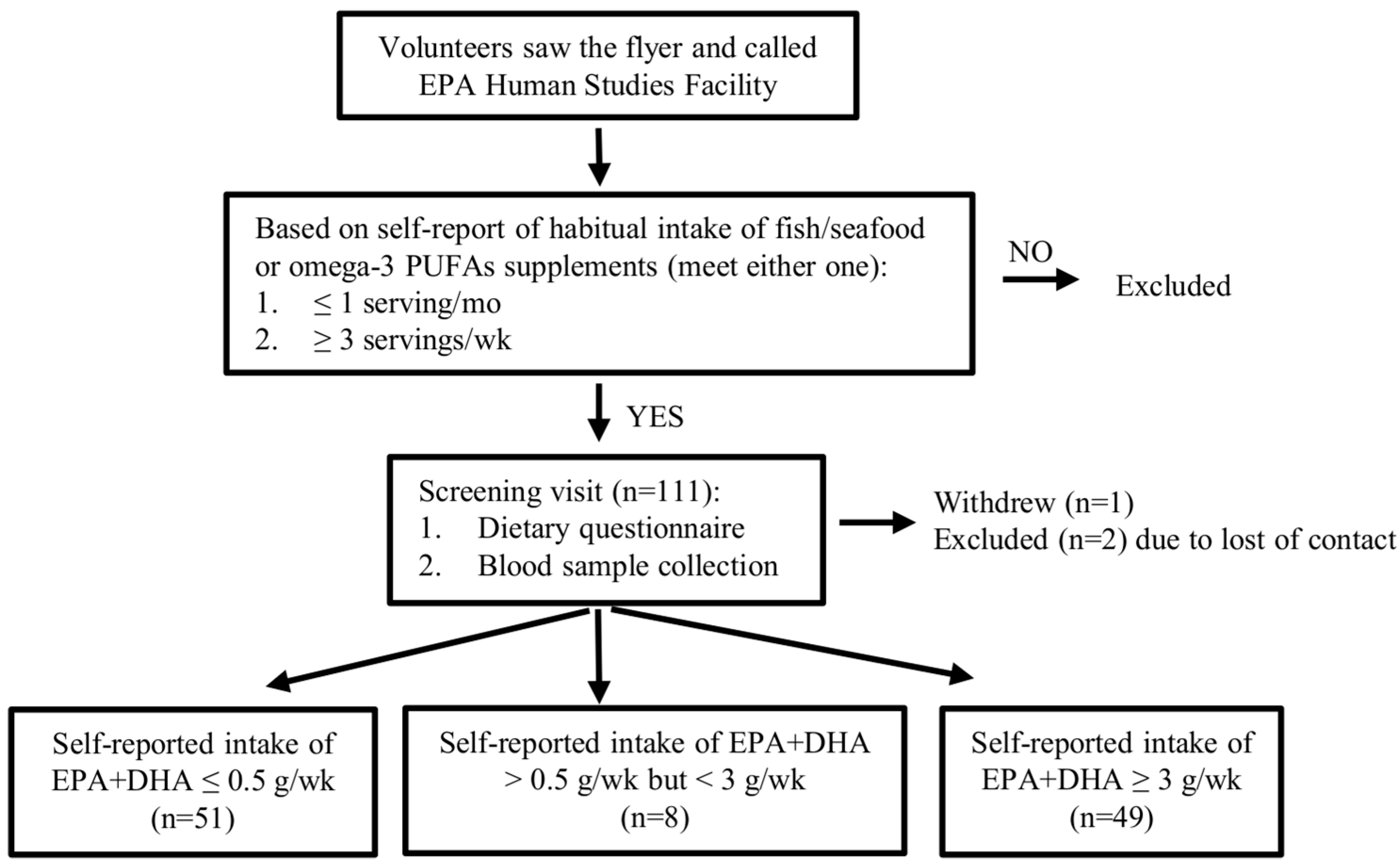

| Low Intake (n = 51) 2 | Medium Intake (n = 8) 3 | High Intake (n = 49) 4 | |
|---|---|---|---|
| Age (years) | 37.2 (8.8) | 40.5 (8.3) | 39.8 (9.9) |
| Gender | |||
| Male | 16 (31%) | 1 (13%) | 21 (43%) |
| Female | 35 (69%) | 7 (88%) | 28 (57%) |
| Race/Ethnicity | |||
| Non-Hispanic white | 33 (65%) | 3 (38%) | 28 (57%) |
| African American | 15 (29%) | 4 (50%) | 13 (26%) |
| Asian American | 1 (2%) | 0 (0%) | 4 (8%) |
| Other 5 | 2 (4%) | 1 (13%) | 4 (8%) |
| Marital status | |||
| Single | 27 (53%) | 5 (63%) | 19 (39%) |
| Married | 19 (37%) | 3 (38%) | 25 (51%) |
| Separated or divorced | 5 (10%) | 0 (0%) | 5 (10%) |
| Education | |||
| High school or trade school | 6 (12%) | 1 (13%) | 6 (12%) |
| College | 25 (49%) | 1 (13%) | 22 (45%) |
| Graduate school | 20 (39%) | 6 (75%) | 21 (43%) |
| BMI (kg/m2) 6 | |||
| Self-report (n = 108) | 24.7 (3.8) | 23.7 (3.0) | 25.3 (3.2) |
| Actual (n = 75) | 25.8 (4.2) | 22.6 (3.8) | 25.6 (3.9) |
| Self-reported intake (g/week) | |||
| EPA † | 0.013 (0.049) | 0.52 (0.37) | 4.5 (3.1) *** |
| DHA † | 0.025 (0.074) | 1.0 (0.58) | 4.3 (2.1) *** |
| EPA + DHA † | 0.038 (0.11) | 1.5 (0.93) | 8.6 (4.7) *** |
| Blood level (weight% from the whole fatty acids) | |||
| EPA † | 0.39 (0.15) | 0.54 (0.20) | 1.2 (0.52) *** |
| DHA † | 2.2 (0.61) | 2.9 (0.49) | 3.5 (0.8) *** |
| Omega-3 index | |||
| ≤4% † | 21 (41%) | 0 (0%) | 0 *** |
| 4–5.5% † | 26 (51%) | 7 (88%) | 10 (20%) *** |
| ≥5.5% † | 4 (8%) | 1 (13%) | 39 (80%) *** |
| Omega-6:Omega-3 † | 9.6 (1.8) | 7.5 (1.2) | 5.9 (1.3) *** |
| Food Item | Number of People Reported any Intake | Averaged Servings Consumed per Week (Servings) 1 | Averaged EPA Content per Serving 2 | Averaged DHA Content per Serving 2 | |
|---|---|---|---|---|---|
| Mean | Range | (g/Serving) | (g/Serving) | ||
| Seafood | |||||
| Bass, seabass | 4 | 1.69 | 0.75–3 | 0.175 | 0.473 |
| Catfish, farmed | 1 | 1 | na 3 | 0.042 | 0.109 |
| Clams | 2 | 0.32 | 0.3–0.33 | 0.117 | 0.124 |
| Cod | 7 | 1.3 | 0.33–3 | 0.003 | 0.131 |
| Crab | 7 | 2.88 | 0.33–16 | 0.251 | 0.1 |
| Flounder | 5 | 1.22 | 0.5–3 | 0.207 | 0.219 |
| Halibut | 1 | 0.25 | na | 0.077 | 0.318 |
| Herring, canned | 2 | 0.75 | 0.5–1 | 0.825 | 1.002 |
| Grouper | 1 | 0.5 | na | 0.03 | 0.181 |
| Lobster | 2 | 0.63 | 0.25–1 | 0.29 | 0.118 |
| Mackerel, canned | 4 | 3.08 | 1.33–6 | 0.369 | 0.677 |
| Mahi Mahi | 1 | 1 | na | 0.022 | 0.096 |
| Oyster, farmed | 2 | 0.5 | 0.5–0.5 | 0.195 | 0.179 |
| Porgy | 1 | 0.25 | na | 0.088 | 0.451 |
| Salmon 4 | |||||
| Canned | 3 | 1.21 | 0.67–1.67 | 0.402 | 0.597 |
| Steak | 43 | 1.92 | 0.25–6.67 | 0.341–0.587 | 0.595–1.238 |
| Sardine, canned | 5 | 1.02 | 0.67–2 | 0.402 | 0.433 |
| Scallop | 1 | 1.33 | na | 0.141 | 0.169 |
| Shrimp | 17 | 1.73 | 0.25–5 | 0.145 | 0.122 |
| Snapper | 1 | 1 | na | 0.041 | 0.232 |
| Tilapia | 11 | 1.62 | 0.5–4 | 0.004 | 0.111 |
| Trout, rainbow, farmed | 6 | 0.7 | 0.25–2 | 0.284 | 0.697 |
| Tuna 4 | |||||
| Canned, albacore or white | 35 | 1.54 | 0.25–8.33 | 0.198 | 0.535 |
| Steak | 6 | 0.74 | 0.33–1.33 | 0.04–0.309 | 0.19–0.97 |
| Fortified food | |||||
| DHA fortified eggs 5 | 5 | 6.5 | 1.5–12 | 0 | 0.075 |
| DHA fortified milk | 4 | 2.1 | 1–3.5 | 0 | 0.032 |
| Self-Reported EPA Intake | Self-Reported DHA Intake | Self-Reported EPA + DHA Intake | EPA Level in the Blood | DHA Level in the Blood | Omega-3 Index | |
|---|---|---|---|---|---|---|
| Self-reported EPA intake | 1 | |||||
| Self-reported DHA intake | 0.82 *** | 1 | ||||
| Self-reported EPA + DHA intake | 0.96 *** | 0.95 *** | 1 | |||
| EPA level in the blood | 0.67 *** | 0.67 *** | 0.68 *** | 1 | ||
| DHA level in the blood | 0.51 *** | 0.62 *** | 0.59 *** | 0.71 *** | 1 | |
| Omega-3 index | 0.62 *** | 0.69 *** | 0.67 *** | 0.87 *** | 0.96 *** | 1 |
| EPA | DHA | Omega-3 Index | |
|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Self-reported intake | |||
| Crude | 0.12 (0.09, 0.15) | 0.23 (0.17, 0.29) | 0.20 (0.16, 0.24) |
| Adjusted 1 | 0.12 (0.10, 0.15) | 0.25 (0.19, 0.30) | 0.20 (0.16, 0.24) |
| Reported Intake | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Agreement | Kappa |
|---|---|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Low | 100% (100%, 100%) | 66% (53%, 78%) | 41% (32%, 50%) | 100% (100%, 100%) | 72% (64%, 81%) | 0.42 (0.28, 0.57) |
| High | 89% (79%, 99%) | 84% (75%, 94%) | 80% (72%, 87%) | 92% (86%, 97%) | 86% (80%, 93%) | 0.72 (0.59, 0.85) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, W.; Weaver, A.M.; Salazar, C.; Samet, J.M.; Diaz-Sanchez, D.; Tong, H. Validation of a Dietary Questionnaire to Screen Omega-3 Fatty Acids Levels in Healthy Adults. Nutrients 2019, 11, 1470. https://doi.org/10.3390/nu11071470
Shen W, Weaver AM, Salazar C, Samet JM, Diaz-Sanchez D, Tong H. Validation of a Dietary Questionnaire to Screen Omega-3 Fatty Acids Levels in Healthy Adults. Nutrients. 2019; 11(7):1470. https://doi.org/10.3390/nu11071470
Chicago/Turabian StyleShen, Wan, Anne M. Weaver, Claudia Salazar, James M. Samet, David Diaz-Sanchez, and Haiyan Tong. 2019. "Validation of a Dietary Questionnaire to Screen Omega-3 Fatty Acids Levels in Healthy Adults" Nutrients 11, no. 7: 1470. https://doi.org/10.3390/nu11071470
APA StyleShen, W., Weaver, A. M., Salazar, C., Samet, J. M., Diaz-Sanchez, D., & Tong, H. (2019). Validation of a Dietary Questionnaire to Screen Omega-3 Fatty Acids Levels in Healthy Adults. Nutrients, 11(7), 1470. https://doi.org/10.3390/nu11071470





