Changes in Antioxidants and Sensory Properties of Italian Chocolates and Related Ingredients Under Controlled Conditions During an Eighteen-Month Storage Period
Abstract
:1. Introduction
2. Materials and Methods
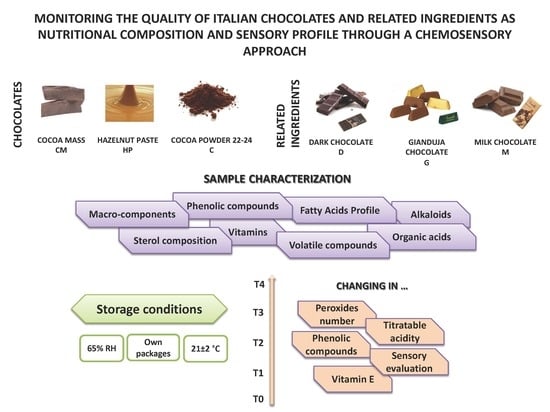
2.1. Samples
- D: cocoa 56% min., cocoa mass, sugar, cocoa butter, emulsifier: soy lecithin, and natural vanilla flavor;
- M: cocoa 31.8% min, milk: 23.5% min., sugar, whole milk powder, cocoa butter, cocoa mass, anhydrous milk fat, emulsifier: soy lecithin, and natural vanilla flavor;
- G: cocoa 21.2% min., Piedmont hazelnut pasta I.G.P. (33%), sugar, whole milk powder, cocoa butter, cocoa mass, anhydrous milk fat, emulsifier: soy lecithin, and natural vanilla flavor.
2.2. Microbiological Analysis
2.3. Chemical Analysis
2.3.1. Humidity, Ash, pH, Acidity, and Fiber
2.3.2. Carotenoids, Retinol, Tocopherols, and Sugars
2.3.3. Proteins
2.3.4. Theobromine, Caffeine, and Polyphenols
2.3.5. Fat Content
2.3.6. Peroxide Value
2.3.7. HPLC Analysis of Organic Acids
2.3.8. GC Analysis of the Volatile Compounds
2.4. Sensory Analysis
2.4.1. Determination of the Sensory Profile of Chocolates and Related Ingredients
2.4.2. Lexicon Generation Process
2.4.3. Sensory Tests of Chocolates and Ingredients to Rate Sensory Attributes
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Analysis
3.2. Nutritional Composition and Related Characterization
3.2.1. Chocolates
3.2.2. Ingredients
3.3. Eighteen-Month Evolution of Chocolates and Ingredients: Nutritional and Sensory Changes
3.3.1. Chemo-Sensory Evolution of Chocolates
3.3.2. Chemo-Sensory Evolution of Ingredients
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Zhang, I.; Li, A.; Manson, J.E.; Sesso, H.D.; Wang, L.; Liu, S. Cocoa flavanol intake and biomarkers for cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J. Nutr. 2016, 146, 2325–2333. [Google Scholar] [CrossRef]
- Ludovici, V.; Barthelmes, J.; Nägele, M.P.; Enseleit, F.; Ferri, C.; Flammer, A.J.; Ruschitzka, F.; Sudano, I. Cocoa, blood pressure, and vascular function. Front. Nutr. 2017, 4, 36. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Shim, J.; Lee, C.Y.; Lee, K.W.; Lee, H.J. Cocoa phytochemicals: Recent advances in molecular mechanisms on health. Crit. Rev. Food Sci. Nutr. 2014, 54, 1458–1472. [Google Scholar] [CrossRef]
- Fung, T. Healthy Eating: A Guide to the New Nutrition; Harvard School of Public Health, Nutrition Department: Boston, MA, USA, 2011; p. 48. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Balestieri, F.; Marini, D. Metodi di Analisi Chimica dei Prodotti Alimentari; Monolite: Roma, Italy, 1996; ISBN 8873310052. [Google Scholar]
- Badrie, N.; Bekele, F.; Sikora, E.; Sikora, M. Cocoa agronomy, quality, nutritional, and health aspects. Crit. Rev. Food Sci. Nutr. 2015, 55, 620–659. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2809. [Google Scholar]
- Afoakwa, E.O. Chocolate Science and Technology; Wiley-Blackwell: Singapore, 2010. [Google Scholar]
- Januszewska, R.; Viaene, J. Acceptance of chocolate by preference cluster mapping across Belgium and Poland. J. Euromark. 2002, 11, 61–86. [Google Scholar] [CrossRef]
- Popov-Raljić, J.V.; Laličić-Petronijević, J.G. Sensory properties and color measurements of dietary chocolates with different compositions during storage for up to 360 days. Sensors 2009, 9, 1996–2016. [Google Scholar] [CrossRef]
- Kumara, B.; Jinap, S.; Che Man, Y.B.; Yusoff, M.S.A. Comparison of colour techniques to measure chocolate fat bloom. Food Sci. Technol. Int. 2003, 9, 295–299. [Google Scholar] [CrossRef]
- Pastor, C.; Santamaria, J.; Chiralt, A.; Aguilera, J.M. Gloss and colour of dark chocolate during storage. Food Sci. Technol. Int. 2007, 13, 27–30. [Google Scholar] [CrossRef]
- Simic, M.G.; Jovanovic, S.V.; Niki, E. Mechanisms of lipid oxidative processes and their inhibition. In Lipid Oxidation in Food; St. Angelo, A.J., Ed.; American Chemical Society: New York, NY, USA, 1992; pp. 14–32. [Google Scholar]
- Jolic, S.M.; Redovnikovic, I.R.; Markovic, K.; Sipusic, D.I.; Delonga, K. Changes of phenolic compounds and antioxidant capacity in cocoa beans processing. Int. J. Food Sci. Technol. 2011, 46, 1793–1800. [Google Scholar] [CrossRef]
- McShea, A.; Ramiro-Puig, E.; Munro, S.B.; Casadesus, G.; Castell, M.; Smith, M.A. Clinical benefit and preservation of flavonols in dark chocolate manufacturing. Nutr. Rev. 2008, 66, 630–641. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, A. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, OJL 338, 1–26.
- ISO. ISO 4833-1:2013. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Microorganisms—Colony-Count Technique at 30 °C; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- ISO. ISO 21528-1:2017. Microbiology of Food and Animal Feeding Stuffs—Horizontal Methods for the Detection and Enumeration of Enterobacteriaceae—Part 1: Detection and Enumeration by MPN Technique with Pre-Enrichment; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- ISO. ISO 4832:2006. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- AOAC International. AOAC International. AOAC 931.04, Moisture in Cocoa Products. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2013. [Google Scholar]
- AOAC International. Total dietary fiber in foods, enzymatic-gravimetric method. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Office International du Cacao et du Chocolat et de la Confiserie (OICCC). Analytical Method; OICCC: Brussels, Belgium, 1972. [Google Scholar]
- AOAC Authors. Official Methods of Analysis Lipids, Fats and Oils Analysis Total Carotenoids—Item 22, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Calvo, P.; Castaño, Á.L.; Hernández, M.T.; González-Gómez, D. Effects of microcapsule constitution on the quality of microencapsulated walnut oil. Eur. J. Lipid Sci. Technol. 2011, 113, 1273–1280. [Google Scholar] [CrossRef]
- Belšcak, A.; Komes, D.; Horzic, D.; Ganic, K.K.; Karlovic, D. Comparative study of commercially available cocoa products in terms of their bioactive composition. Food Res. Int. 2009, 42, 707–716. [Google Scholar] [CrossRef]
- AOAC International. AOAC 939.02-1939, Protein (milk) in milk chocolate. Kjeldahl method. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Adamson, G.E.; Lazarus, A.; Mitchell, A.E.; Prior, R.L.; Cao, G.; Jacobs, P.H.; Kremers, B.G.; Hammerstone, J.F.; Rucker, R.B.; Ritter, K.A.; et al. HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. J. Agric. Food Chem. 1999, 47, 4184–4188. [Google Scholar] [CrossRef]
- López-Martìnez, L.; López-de-Alba, P.L.; Garcìa-Campos, R.; De León-Rodrìguez, L.M. Simultaneous determination of methylxanthines in coffees and teas by UV-Vis spectrophotometry and partial least squares. Anal. Chim. Acta 2003, 493, 83–94. [Google Scholar] [CrossRef]
- Hečimović, I.; Belščak-Cvitanović, A.; Horžić, D.; Komes, D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011, 129, 991–1000. [Google Scholar] [CrossRef]
- EC Commission Regulation no. 2568/91, annex III, on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union 1991, OJL 248, 1.
- Bonvehì, J.S. Investigation of aromatic compounds in roasted cocoa powder. Eur. Food Res. Technol. 2005, 221, 19–29. [Google Scholar] [CrossRef]
- Murray, J.M.; Delahunty, C.M.; Baxter, I.A. Descriptive Sensory Analysis: Past, Present, and Future. Food Res. Int. 2001, 34, 461–471. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J. Sensory Evaluation Practices. In Food and Science Technology Series, 2nd ed.; Taylor, S., Ed.; Academic Press: Cambridge, MA, USA, 1992; ISBN 9780323139762. [Google Scholar]
- ISO. ISO 8589:2007. Sensory Analysis—General Guidance for the Design of Test Rooms; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- ISO. ISO 5492:2008. Sensory Analysis—Vocabulary; ISO: Geneva, Switzerland, 2008. [Google Scholar]
- ISO. ISO 8586:2012. Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors And Expert Sensory Assessors; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Donadini, G.; Fumi, M.D.; Lambri, M. The hedonic response to chocolate and beverage pairing: A preliminary study. Food Res. Int. 2012, 48, 703–711. [Google Scholar] [CrossRef]
- MacFie, H.J.; Bratchell, N.; Greenhoff, K.; Vallis, L.V. Designs to balance the effect of order of presentation and first-order carry-over effect in halls tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- Kemp, S.E.; Hollowood, T.; Hort, J. Sensory Evaluation: A Practical Handbook; Wiley-Blackwell: Singapore, 2009. [Google Scholar] [CrossRef]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Civille, G.V.; Lyon, B.G. Aroma and Flavor Lexicon for Sensory Evaluation: Terms, Definitions, References, and Examples; ASTM Data Series Publication DS 66; American Society for Testing and Materials (ASTM) International: West Conshohocken, PA, USA, 1996. [Google Scholar]
- Thompson, J.L.; Drake, M.A.; Lopetcharat, K.; Yates, M.D. Preference mapping of commercial chocolate milks. J. Food Sci. 2004, 69, S406–S413. [Google Scholar] [CrossRef]
- Ferreira, J.M.M.; Marcacini Azevedo, B.; Luccas, V.; Bolini, H.M.A. Sensory profile and consumer acceptability of prebiotic white chocolate with sucrose substitutes and the addition of goji berry (Lycium barbarum). J. Food Sci. 2017, 82, 818–824. [Google Scholar] [CrossRef]
- Leite, P.B.; Da Silva Bispo, E.; Randomille De Santana, L.R. Sensory profiles of chocolates produced from cocoa cultivars resistant to Moniliophtora Perniciosa. Rev. Bras. Frutic. 2013, 35, 594–602. [Google Scholar] [CrossRef]
- Gills, L.A.; Resurreccion, A.V.A. Sensory and physical properties of peanut butter treated with palm oil and hydrogenated vegetable oil to prevent oil separation. J. Food Sci. 2000, 65, 173–180. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; He, C.; Song, H.; Guo, J.; Wang, Y.; Yang, H.; Su, X. A comparative study of aroma-active compounds between dark and milk chocolate: Relationship to sensory perception. J. Sci. Food Agric. 2015, 95, 1362–1372. [Google Scholar] [CrossRef]
- Marvig, C.L.; Kristiansen, R.M.; Madsen, M.G.; Nielsen, D.S. Identification and characterization of organisms associated with chocolate pralines and sugar syrups used for their production. Int. J. Food Microbiol. 2014, 185, 167–176. [Google Scholar] [CrossRef]
- CREA (Il Centro di Ricerca per gli Alimenti e la Nutrizione). Available online: https://www.crea.gov.it/web/alimenti-e-nutrizione/banche-dati / (accessed on 31 October 2019).
- Elkhori, S.; Parè, J.R.J.; Bélanger, J.M.R.; Pérez, E. The microwave-assisted process (MAPTM1): Extraction and determination of fat from cocoa powder and cocoa nibs. J. Food Eng. 2007, 79, 1110–1114. [Google Scholar] [CrossRef]
- Çakmak, Y.S.; Güler, G.O.; Aktümsek, A. Trans fatty acid contents in chocolates and chocolate wafers in Turkey. Czech J. Food Sci. 2010, 28, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Belščak-Cvitanović, A.; Durgo, K.; Gačina, T.; Horžić, D.; Franekić, J.; Komes, D. Comparative study of cytotoxic and cytoprotective activities of cocoa products affected by their cocoa solids content and bioactive composition. Eur. Food Res. Technol. 2012, 234, 173–186. [Google Scholar] [CrossRef]
- Meng, C.C.; Jalil, A.M.; Ismail, A. Phenolic and theobromine contents of commercial dark, milk and white chocolates on the Malaysian market. Molecules 2009, 14, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Copetti, M.V.; Iamanaka, B.T.; Mororó, R.C.; Pereira, J.L.; Frisvad, J.C.; Taniwaki, M.H. The effect of cocoa fermentation and weak organic acids on growth and ochratoxin A production by Aspergillus species. Int. J. Food Microbiol. 2012, 155, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor formation and character in cocoa and chocolate: A critical Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor chemistry of cocoa and cocoa products—An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Comparison of phenol content and antioxidant capacity of nuts. Ciênc. Tecnol. Aliment. 2010, 30, 254–259. [Google Scholar] [CrossRef] [Green Version]
- Parcerisa, J.; Codony, R.; Boatella, J.; Rafecas, M. Triacylglycerol and phospholipid composition of hazelnut (Corylus avellana L.) lipid fraction during fruit development. J. Agric. Food Chem. 1999, 47, 1410–1415. [Google Scholar] [CrossRef]
- Jalil, A.M.; Ismail, A. Polyphenols in cocoa and cocoa products: Is there a link between antioxidant properties and health? Molecules 2008, 13, 2190–2219. [Google Scholar] [CrossRef]
- Miller, K.B.; Hurst, W.J.; Payne, M.J.; Stuart, D.A.; Apgar, J.; Sweigart, D.S.; Ou, B. Impact of alkalization on the antioxidant and flavanol content of commercial cocoa powders. J. Agric. Food Chem. 2008, 56, 8527–8533. [Google Scholar] [CrossRef] [PubMed]
- Lipp, M.; Simoneau, C.; Ulberth, F.; Anklam, E.; Crews, C.; Brereton, P.; de Greyt, W.; Schwack, W.; Wiedmaier, C. Composition of genuine cocoa butter and cocoa butter equivalents. J. Food Compos. Anal. 2001, 14, 399–408. [Google Scholar] [CrossRef]
- Bignami, C.; Cristofori, V.; Troso, D.; Bertazza, G. Kernel quality and composition of hazelnut (Corylus avellana L.) cultivars. Acta Hortic. 2005, 686, 477–484. [Google Scholar] [CrossRef]
- Ebrahem, K.S.; Richardson, D.G.; Tetley, R.M.; Mehlenbacher, S.A. Oil content, fatty acid composition and vitamin E concentration of hazelnut varieties, compared to other types of nuts and oilseeds. Acta Hortic. 1994, 351, 685–692. [Google Scholar] [CrossRef]
- Botta, R.; Gianotti, C.; Richardson, D.; Suwanagul, A.; Carlos, L.S. Hazelnut variety organic acids, sugars, and total lipid fatty acids. Acta Hortic. 1994, 351, 693–699. [Google Scholar] [CrossRef]
- Machálková, L.; Hřivna, L.; Nedomová, S.; Jůzl, M. The effect of storage temperature on the quality and formation of blooming defects in chocolate confectionery. Potravinarstvo 2015, 9, 39–47. [Google Scholar] [CrossRef]
- Thamke, I.; Dürrschmid, K.; Rohm, H. Sensory description of dark chocolate by consumers. LWT Food Sci. Technol. 2009, 42, 534–539. [Google Scholar] [CrossRef]
- Yadav, P.; Pandey, J.P.; Garc, S.K. Biochemical changes during storage of chocolates. Int. Res. J. Biochem. Bioinform. 2011, 1, 242–247. [Google Scholar]
- Gordon, M.H. Chapter 2—The development of oxidative randicity in foods. In Antioxidants in Food-Practical Applications; Pokorni, J., Yanishlieva, N., Gordon, M., Eds.; Woodhead Publishing Ltd.: Shaston, UK, 2001; pp. 7–21. [Google Scholar]
- Fardelli, A. Effect of Storage Conditions on Hazelnuts (Corylus avellana L.) Quality. Ph.D. Thesis, Università degli Studi della Tuscia, Via Santa Maria in Gradi, Italy, 2008. [Google Scholar]
- Lopez-Uriarte, P.; Bulló, M.; Casas-Agustench, P.; Babio, N.; Salas-Salvadó, J. Nuts and oxidation: A systematic review. Nutr. Rev. 2009, 67, 497–508. [Google Scholar] [CrossRef]
- Subramaniam, P.J. Confectionery products. In The Stability and Shelf-Life of Food; Llcast, D., Subramaniam, P.J., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 221–248. [Google Scholar]
- Lamuela-Raventòs, R.M.; Romero-Pérez, A.I.; Andrés-Lacueva, C.; Tornero, A. Review: Health Effects of Cocoa Flavonoids. Food Sci. Technol. Int. 2005, 11, 159–176. [Google Scholar] [CrossRef]
- Othman, A.; Ismail, A.; Ghani, A.N.; Adenan, I. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007, 100, 1523–1530. [Google Scholar] [CrossRef]
- Kornsteiner, M.; Wagner, K.H.; Elmadfa, I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006, 98, 381–387. [Google Scholar] [CrossRef]
- Bomba, P.C. Shelf life of chocolate confectionery products. In Shelf Life Studies of Foods and Beverages; Charalambous, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 341–351. [Google Scholar]
- Stauffer, M. The flavor of milk chocolate—Changes caused by processing. Manuf. Confect. 2000, 80, 113–118. [Google Scholar]
- Seppanen, C.M.; Song, Q.; Csallany, A.S. The antioxidant functions of tocopherol and tocotrienol homologues in oils, fats, and food systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Baccelloni, S. Recupero di Antiossidanti Naturali dai Sottoprodotti di Trasformazione Dell’industria Agroalimentare: Noccioli di Oliva (Olea europaea L.) Gusci e Perisperma di Nocciole (Corylus avellana L.). Ph.D. Thesis, Università degli Studi della Tuscia, Via Santa Maria in Gradi, Italy, 2006. [Google Scholar]



| Attribute | Description | Range and References | Sample(*) |
|---|---|---|---|
| Brightness | Ability to reflect light; luminescence of color, with descriptions ranging from dull to shiny [42] | Low: Dull; dark chocolate 90% cocoa with fat blooming High: Shiny; dark chocolate 90% cocoa | D – M – G |
| Snap | The noise and force with which the sample breaks or fractures [39] | Low: Gianduja chocolate High: Dark chocolate 90% cocoa | D – M |
| Firmness | Force required for compressing the sample between molar teeth [45] | Low: Milk chocolate High: Dark chocolate 90% cocoa | MC – D – M |
| Crunchiness | Easily broken or ruptured Degree to which the sample fractures into pieces on the first bite with the molars [42] | Low: Dark chocolate 90% cocoa High: Milk chocolate | MC |
| Melting | Chocolate property of melting in mouth while chewing [45] till liquefaction [46] | Low: Dark chocolate 70% cocoa warmed in a microwave oven during 20 s. High: Dark chocolate 70% cocoa warmed in a microwave oven during 40 s. | MC – C – HP – D – M – G |
| Stickiness | The degree a sample sticks to the palate [42,47] | MC – HP – D – M – G | |
| Chewiness | Length of time required to masticate the sample, at a constant rate of force application, to reduce it to a consistency suitable for swallowing [42] | G | |
| Grittiness | Presence of perceptible particles in the oral cavity. The number of solid particles during mastication [42] | MC – C – HP – D – M – G | |
| Astringency | Mouth drying and/or puckering effect which boosts the production of saliva; perceived between tongue and palate or at the back of the front teeth [42,47] | None: Milk chocolate High: Dark chocolate 90% cocoa | MC – C – HP – D |
| Oily | The amount of oil left on mouth surfaces [43] | HP – G | |
| Fatness | Surface textural attributes relating to the perception of the quantity or quality of fat in a product [43] | HP – G | |
| Creaminess | The mouth-feel related to the smoothness of the chocolate as related to fat [44] | D – M – G | |
| Acidity | Citric acid (fruit), acetic acid (vinegar), lactic acid (sour milk), and mineral acid (metallic tasting) [42,46] | MC – C – HP – D – M | |
| Bitterness | The taste on the tongue associated with substances such as caffeine and quinine [42] | None: Distilled water High: Caffeine solution at 0.5% | MC – C – HP – D |
| Sweetness | The taste on the tongue associated with sucrose and other sugars or sweeteners [42] | Low: Sugar solution at 1% High: Sugar solution at 10% | C – HP – D – M – G |
| Cocoa | The flavor associated with cocoa powder or cocoa beans [43] | Low: Powder cocoa solution at 0.5% High: Powder cocoa solution at 5.0% | MC – C – D – M – G |
| Toasted/Roasted | Flavor related to cocoa that is very toasted [46] The aroma associated with popcorn or roasted peanut [48] | Low: Dry cocoa seed without toasting. High: Cocoa seed toasted for 3 h | MC – C – HP – D – G |
| Coffee | The aroma associated with medium-high toasted coffee [39] | MC – C | |
| Nutty | Delicate aroma of indistinguishable nuts without roast. Mixed raw nuts powder (hazelnut, walnut, peanut, and sunflower seeds) [48] | MC – HP – M – G | |
| Caramel | The aroma associated with caramelized sugar [48] | Low: Dry sugar High: Sugar warmed at 120 °C until a brown color | HP – M – G |
| Vanillin | The aroma associated with vanillin [39] | D – M – G |
| Parameter | CM | C | HP | D | M | G |
|---|---|---|---|---|---|---|
| Total microbial count | <5000 | <5000 | <5000 | <5000 | <5000 | <5000 |
| Enterobacteriaceae | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Coliforms | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Parameter | CM | C | HP | D | M | G |
|---|---|---|---|---|---|---|
| Humidity (%) | 1.06 ± 0.03 d | 2.11 ± 0.01 a | 0.75 ± 0.01 e | 0.70 ± 0.03 f | 1.17 ± 0.09 c | 1.37 ± 0.00 b |
| pH | 5.51 ± 0.02 e | 8.31 ± 0.10 a | 5.71 ± 0.01 d | 5.70 ± 0.05 d | 6.29 ± 0.04 b | 6.17 ± 0.03 c |
| Acidity (mg eq stearic acid/100 g) | 361.00 ± 0.01 a | n.d. | 159.00 ± 0.01 c | 184.91 ± 2.84 b | 110.95 ± 5.69 e | 128.02 ± 2.84 d |
| Ash (%) | 4.37 ± 0.97 b | 11.70 ± 0.04 a | 2.05 ± 0.01 c | 1.67 ± 0.03 e | 1.71 ± 0.01 d | 1.65 ± 0.01 f |
| Protein (%) | 14.11 ± 0.63 b | 23.33 ± 0.08 a | 3.90 ± 0.18 f | 7.20 ± 0.01 d | 6.40 ± 0.08 e | 10.10 ± 0.41 c |
| Fat matter (%) | 35.70 ± 0.04 c | 17.90 ± 0.12 f | 66.80 ± 1.75 a | 23.20 ± 0.30 e | 34.20 ± 1.61 d | 37.50 ± 2.30 b |
| Total sugar (%) | 0.80 ± 0.00 e | 1.20 ± 0.03 d | 1.60 ± 0.57 d | 45.60 ± 0.31 b | 54.70 ± 0.25 a | 40.80 ± 1.39 c |
| Fiber (%) * | 12.50 ± 0.06 b | 28.03 ± 0.14 a | 8.83 ± 0.04 c | 7.56 ± 0.04 d | 1.60 ± 0.01 f | 5.45 ± 0.03 e |
| * raw fiber (%) | 11.10 | 13.63 | n.d. | n.d. | n.d. | n.d. |
| Phenols (mg Gallic Acid Equivalents /g) | 2.06 ± 0.25 b | 7.29 ± 0.98 a | 0.31 ± 0.07 e | 2.12 ± 0.18 b | 0.64 ± 0.17 d | 0.99 ± 0.09 c |
| Caffeine (mg/100 g) | 65.67 ± 4.97 b | 97.69 ± 5.46 a | 3.40 ± 0.16 e | 21.51 ± 1.04 c | 2.06 ± 0.93 f | 7.84 ± 2.27 d |
| Theobromine (mg/g) | 6.77 ± 0.68 b | 10.07 ± 0.10 a | 2.04 ± 0.37 d | 7.28 ± 0.59 b | 3.97 ± 0.12 c | 3.63 ± 0.25 c,d |
| 13-Cis-β-Carotene (ppm) | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% |
| 9-Cis-β-Carotene (ppm) | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% |
| All-Trans-α-Carotene (ppm) | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% |
| All-Trans-β-Carotene (ppm) | 0.34 ± 15% | <0.30 ± 15% | <0.10 ± 15% | <0.30 ± 15% | 0.47 ± 15% | 0.30 ± 15% |
| β-Cryptoxanthin (ppm) | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% |
| Retinol (ppm) | <0.10 ± 15% | <0.10 ± 15% | <0.10 ± 15% | <5.00 ± 15% | 82.00 ± 15% | 5.00 ± 15% |
| Vitamin E (ppm) | 6.77 ± 10% d | 9.40 ± 10% c | 304.00 ± 10% a | 8.20 ± 10% c | 6.63 ± 10% d | 114.00 ± 10% b |
| Fatty acid / Sterol | CM | C | HP | D | M | G |
|---|---|---|---|---|---|---|
| Saturated fatty acids | 63.90 | 61.29 | 9.49 | 63.87 | 65.23 | 37.67 |
| Monounsaturated fatty acids | 32.95 | 35.34 | 83.17 | 32.69 | 31.60 | 57.19 |
| Polyunsaturated fatty acids | 3.05 | 3.30 | 7.24 | 3.34 | 3.12 | 5.10 |
| Trans-oleic fatty acids | <0.01 | <0.01 | <0.01 | <0.01 | 0.64 | 0.21 |
| Trans-linoleic fatty acids | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Trans-linolenic fatty acids | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Trans-palmitoleic fatty acids | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| C4:0 Butyric | <0.01 | <0.01 | <0.01 | <0.01 | 0.69 | 0.23 |
| C6:0 Capronic | <0.01 | <0.01 | <0.01 | <0.01 | 0.43 | 0.13 |
| C7:0 Enantiic | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| C8:0 Caprylic | <0.01 | <0.01 | <0.01 | <0.01 | 0.28 | 0.10 |
| C10:0 Capric | <0.01 | <0.01 | <0.01 | <0.01 | 0.58 | 0.22 |
| C10:1 Caproleic | <0.01 | <0.01 | <0.01 | <0.01 | 0.07 | <0.05 |
| C12:0 Lauric | <0.01 | <0.01 | <0.01 | <0.01 | 0.71 | 0.32 |
| C12:1 Lauroleic | <0.01 | <0.01 | <0.01 | <0.01 | <0.05 | <0.05 |
| C13:0 Tridecanoic | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| C13:1 Tridecenoic | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| C14:0 Myristic | 0.10 | 0.11 | <0.05 | 0.21 | 2.34 | 0.98 |
| C14:1 Miristoleic | 0.01 | <0.01 | <0.01 | <0.01 | 0.20 | 0.08 |
| C15:0 Pentadecanoic | <0.05 | <0.01 | <0.01 | 0.05 | 0.26 | 0.12 |
| C15:1 Pentadecenoic | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| C16:0 Palmitic | 26.66 | 27.83 | 6.61 | 25.99 | 26.96 | 16.57 |
| C16:1 Palmitoleic | 0.27 | 0.28 | 0.27 | 0.24 | 0.51 | 0.35 |
| C17.0 Eptadecanoic | 0.22 | 0.23 | <0.05 | 0.23 | 0.38 | 0.16 |
| C17:1 Eptadecenoic | <0.05 | <0.05 | 0.08 | <0.05 | 0.09 | 0.08 |
| C18:0 Stearic | 35.67 | 32.08 | 2.68 | 36.15 | 31.53 | 18.10 |
| C18:1 Oleic | 32.68 | 35.06 | 82.68 | 32.40 | 30.73 | 56.60 |
| C18:2 Linoleic | 2.87 | 3.10 | 7.16 | 3.11 | 2.83 | 4.94 |
| C18:3 Linolenic | 0.18 | 0.20 | 0.08 | 0.23 | 0.29 | 0,16 |
| C20:0 Arachic | 1.01 | 0.90 | 0.15 | 1.03 | 0.90 | 0,57 |
| C:20:1 Eicosenoic | <0.05 | <0.05 | 0.14 | 0.05 | <0.05 | 0.08 |
| C22:0 Behenic | 0.17 | 0.14 | 0.05 | 0.17 | 0.16 | 0.11 |
| C22:1 Erucic | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| C22:0 Lignoceric | 0.07 | <0.01 | <0.05 | 0.09 | 0.08 | 0.06 |
| Cholesterol | 1.00 | 1.00 | 0.30 | 1.30 | 26.80 | 14.70 |
| Brassicasterol | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| 2,4-methylene cholesterol | 0.30 | 0.50 | 0.10 | 0.20 | 0.20 | 0.20 |
| Campesterol | 9.00 | 9.40 | 4.10 | 9.60 | 7.20 | 6.60 |
| Campestanol | 0.20 | 0.30 | 0.40 | 0.20 | 0.10 | 0.20 |
| Stigmasterol | 25.80 | 26.10 | 1.20 | 24.70 | 17.80 | 13.90 |
| Delta-7-campesterol | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Delta-5,23-stigmastadienol | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Clerosterol | 0.80 | 0.70 | 0.70 | 0.70 | 0.50 | 0.90 |
| Beta-sitosterol | 58.70 | 56.30 | 83.40 | 58.40 | 44.20 | 56.70 |
| Sitostanol | 0.70 | 0.70 | 1.40 | 0.70 | 0.40 | 1.00 |
| Delta-5-avenasterol | 2.50 | 2.50 | 6.20 | 2.40 | 2.00 | 3.00 |
| Delta-7,9(11)-stigmastadienol | 0.20 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Delta-5,24-stigmastanediol | 0.40 | 0.20 | 0.80 | 0.80 | 0.20 | 0.80 |
| Delta-7-stigmastenol | 0.40 | 0.90 | 0.70 | 0.60 | 0.20 | 1.00 |
| Delta-7-avenasterol | 0.20 | 0.70 | 0.80 | 0.30 | 0.10 | 0.50 |
| Organic Acids | CM | C | HP | D | M | G |
|---|---|---|---|---|---|---|
| Ossalic acid | 889 ± 27.3 b | 1824 ± 33.2 a | 195 ± 4.6 d | 421 ± 9.7 c | 37 ± 0.4 f | 79 ± 12.7 e |
| Citric acid | 1765 ± 69.7 b | 3640 ± 51.2 a | 547 ± 36.1 d | 1163 ± 98.5 c | 1681 ± 78.5 b | 1253 ± 34.7 c |
| Acetic acid | 902 ± 13.4 b | 2750 ± 5.8 a | 610 ± 7.9 c | 109 ± 0.3 e | 547 ± 34.6 d | 454 ± 78.2 d,e |
| L-malic acid | 444 ± 15.9 b | n.d. | 1524 ± 167.4 a | 399 ± 2.5 c | 194 ± 12.8 d | 1388 ± 54.9 a |
| Lactic acid | 570 ± 3.3 b | n.d. | 17 ± 0.7 e | 194 ± 0.8 d | 874 ± 56.8 a | 434 ± 23.8 c |
| Formic acid | n.d. | n.d. | 208 ± 16.1 b | 843 ± 6.4 a | 229 ± 34.1 b | 202 ± 5.8 b |
| Tartaric acid | 235 ± 15.7 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Succinic acid | 48 ± 0.7 b | n.d. | n.d. | n.d. | 54 ± 4.7 a | n.d. |
| Volatile Compounds | CM | C | HP | D | M | G | References | Descriptors |
|---|---|---|---|---|---|---|---|---|
| 2,5-Dimethylpyrazine | n.d. | 22.69 ± 2.20 a | 5.21 ± 1.54 b | n.d. | 0.46 ± 0.07d | 0.95 ± 0.02 c | 0.23–1.69 | cocoa, roast nuts |
| 2,6-Dimethylpyrazine | 3.32 ± 0.03 b | 2.36 ± 0.47 c | 12.67 ± 2.58 a | 15.94 ± 3.19 a | n.d. | 14.10 ± 1.19 a | 0.11–0.39 | nutty, coffee, green |
| 2,3,5-Trimethylpyrazine | 41.75 ± 7.39 a | 19.18 ± 3.57 b | 3.63 ± 0.45 d | 7.38 ± 2.69 c | 0.12 ± 0.02 f | 0.33 ± 0.17 e | 0.21–1.71 | cocoa, roast nuts, peanut |
| 2,3,5,6-Tetramethylpyrazine | 6.50 ± 1.51 a | 4.50 ± 2.11a b | 2.03 ± 1.26 c | 8.15 ± 1.89 a | 1.58 ± 0.11 c | 4.53 ± 0.57 a,b | 0.52–8.28 | chocolate, cocoa, coffee |
| Benzaldehyde | 0.59 ± 0.31 d | 3.86 ± 0.12b | 0.48 ± 0.04 e | 7.52 ± 3.50 a | 1.80 ± 0.60 c | 0.73 ± 0.29 d | 0.5–1.89 | bitter |
| 2-Acetyl-5-methylfuran | n.d. | 5.06 ± 0.92 a | 1.43 ± 0.16 b | 1.76 ± 0.45 b | 0.50 ± 0.14 c | 0.43 ± 0.01 c | ||
| 2-Phenylacetaldehyde | 0.55 ± 0.07 c | 2.15 ± 0.75 b | 4.02 ± 0.35 a | 1.47 ± 1.12b c | 2.59 ± 0.54 b | 1.55 ± 0.07b c | 2–8.90 | berry, nutty |
| α-Terpenilformato | 0.16 ± 0.01 c | n.d. | 0.72 ± 0.11 b | n.d. | 0.71 ± 0.22 b | 2.89 ± 0.34 a | 0–0.38 | herbaceous, citrus |
| Benzyl acetate | 0.37 ± 0.29 d | n.d. | 1.92 ± 0.01 a | 0.59 ± 0.12 c | 0.45 ± 0.04 d | 1.32 ± 0.13 b | 0–0.033 | floral, jasmine |
| Octanoic acid | 0.40 ± 0.29 b | 1.93 ± 0.96 a | 1.12 ± 0.09 a | 1.62 ± 0.02 a | 0.61 ± 0.23 b | 0.85 ± 0.01 a | 0.021–0.37 | unpleasant, oily, fatty |
| 2-Acetyl pyrrole | 0.18 ± 0.03 c | 2.74 ± 1.71 a | 1.52 ± 0.31 a | n.d. | 0.36 ± 0.01 b | 1.58 ± 1.11 a | 0.021–0.38 | bread, walnut, licorice |
| 3-Hydroxy-2-methylpyridine | n.d. | 1.63 ± 0.06 b | 1.84 ± 0.32 b | 4.90 ± 1.94 a | 0.59 ± 0.04 d | 0.94 ± 0.06 c | 0.14–0.38 | wizened |
| 2,3-Dihydro-3,5-dihydro-6-methyl-4-pyrone | n.d. | n.d. | n.d. | n.d. | 0.50 ± 0.14 | n.d. | 0.28–1.87 | roasted |
| 3,5-Hydroxy-6-methyl-4-pyrone | n.d. | n.d. | n.d. | n.d. | 2.59 ± 0.54 | n.d. | 0.02–0.37 | roasted |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roda, A.; Lambri, M. Changes in Antioxidants and Sensory Properties of Italian Chocolates and Related Ingredients Under Controlled Conditions During an Eighteen-Month Storage Period. Nutrients 2019, 11, 2719. https://doi.org/10.3390/nu11112719
Roda A, Lambri M. Changes in Antioxidants and Sensory Properties of Italian Chocolates and Related Ingredients Under Controlled Conditions During an Eighteen-Month Storage Period. Nutrients. 2019; 11(11):2719. https://doi.org/10.3390/nu11112719
Chicago/Turabian StyleRoda, Arianna, and Milena Lambri. 2019. "Changes in Antioxidants and Sensory Properties of Italian Chocolates and Related Ingredients Under Controlled Conditions During an Eighteen-Month Storage Period" Nutrients 11, no. 11: 2719. https://doi.org/10.3390/nu11112719