Use of Dairy and Plant-Derived Lactobacilli as Starters for Cherry Juice Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bacterial Strains
2.3. Fermentation Process and Storage
2.4. Evolution of Bacterial Growth and Acidification of Cherry Juice
2.5. Sugars and Organic Acid Analysis
2.6. Characterization of the Volatile Profile
2.7. Characterization of Polyphenolic Profile of Fermented and Unfermented Cherry Juices
2.8. Statistical Analysis
3. Results
3.1. Fermentation and Acidification of Cherry Juice
3.2. Principal Component Analyses: Overview on Volatiles, Phenolic Compounds, Sugars and Organic Acids
3.3. Sugars and Organic Acid Metabolism
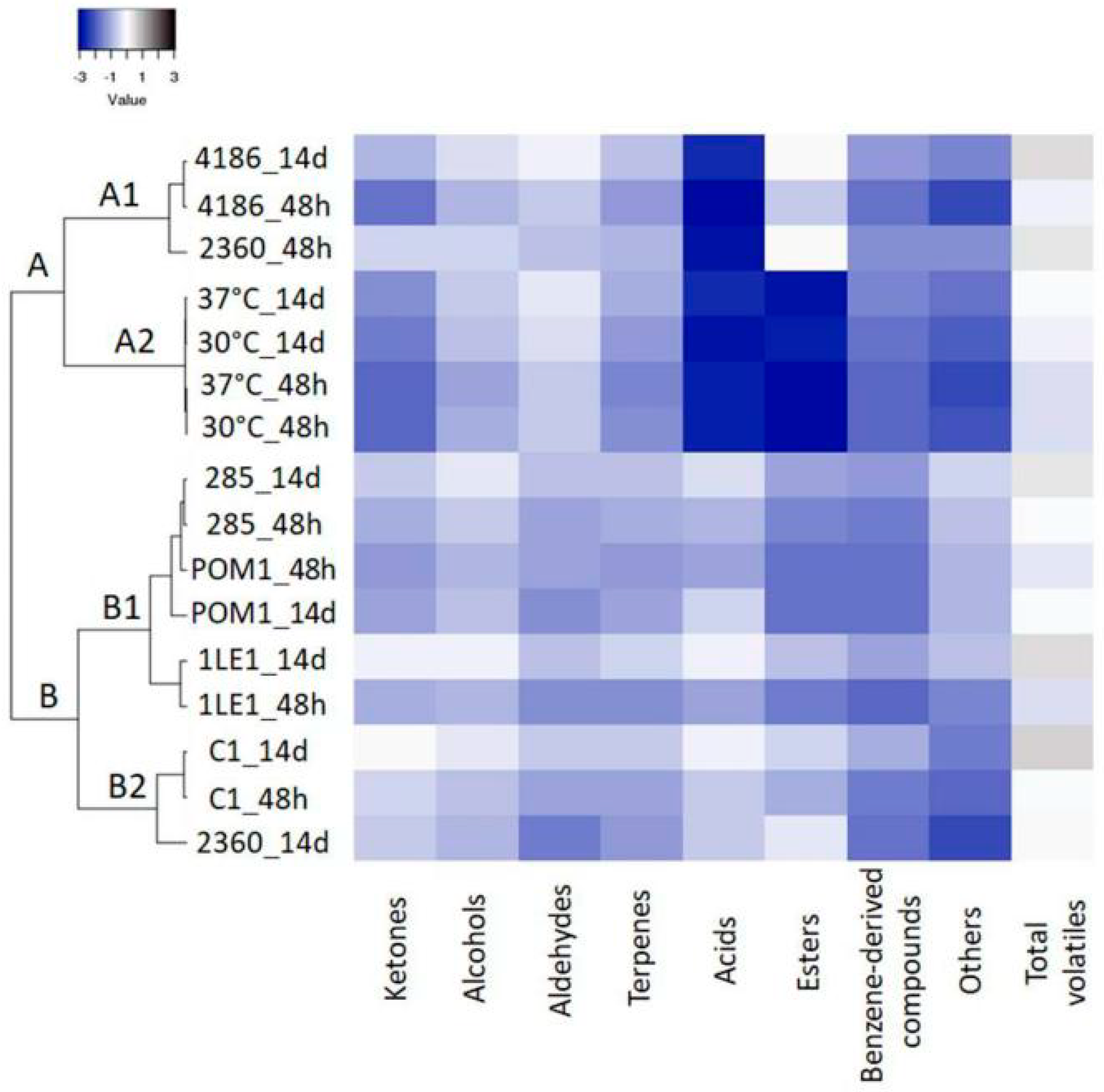
3.4. Characterization of Volatile Profile
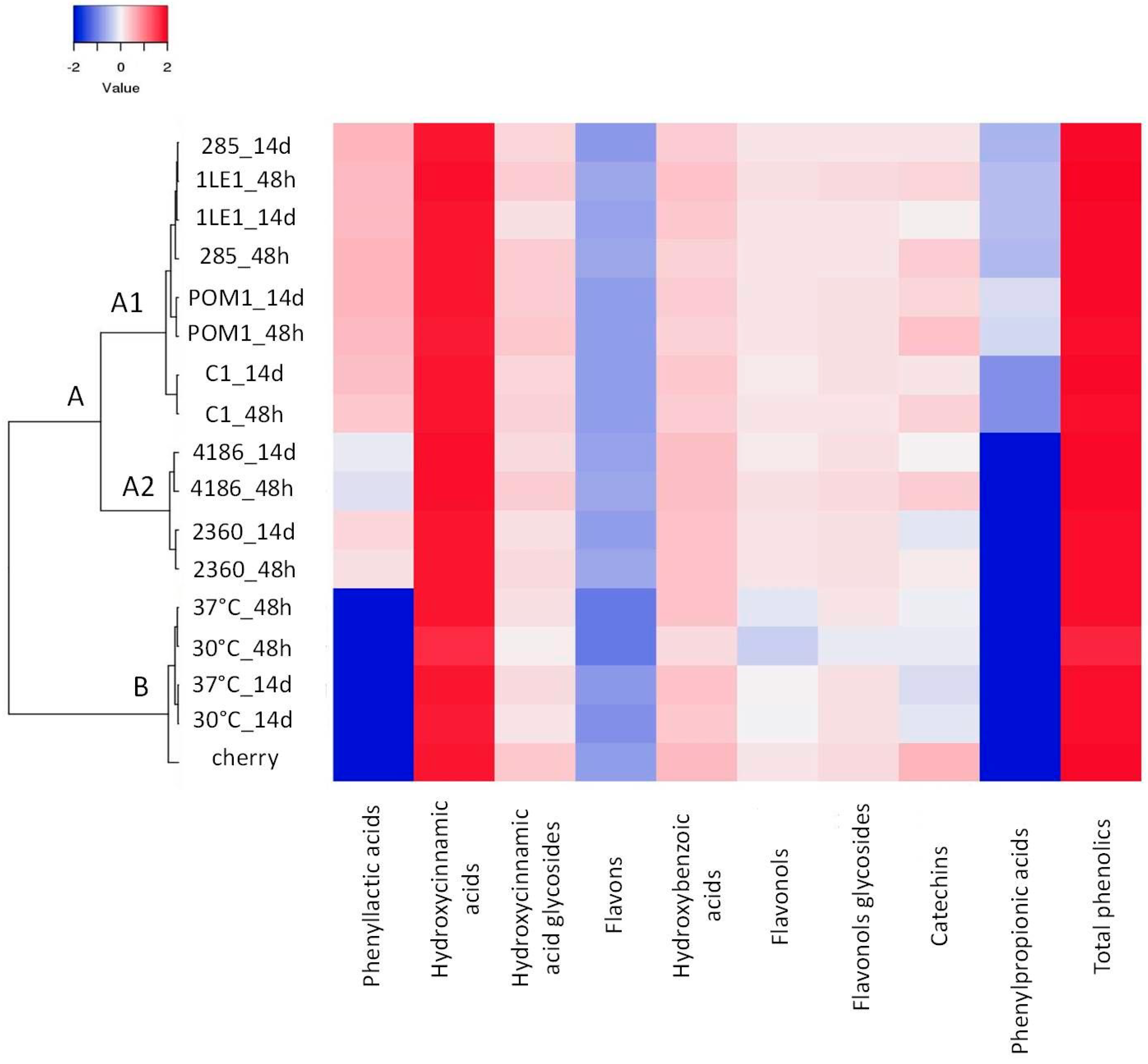
3.5. Characterization of Polyphenolic Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Di Cagno, R.; Surico, R.F.; Minervini, G.; Rizzello, C.G.; Lovino, R.; Servili, M.; Taticchi, A.; Urbani, S.; Gobbetti, M. Exploitation of sweet cherry (Prunus avium L.) puree added of stem infusion through fermentation by selected autochthonous lactic acid bacteria. Food Microbiol. 2011, 28, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Serrano, M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Hernández, A.; López-Corrales, M.; de Guía Córdoba, M.D.G. Physicochemical and sensorial characterisation of four sweet cherry cultivars grown in Jerte Valley (Spain). Food Chem. 2012, 133, 1551–1559. [Google Scholar] [CrossRef]
- Sun, S.Y.; Jiang, W.G.; Zhao, Y.P. Characterization of the aroma-active compounds in five sweet cherry cultivars grown in Yantai (China). Flavour Frag. J. 2010, 25, 206–213. [Google Scholar] [CrossRef]
- Wen, Y.Q.; He, F.; Zhu, B.Q.; Lan, Y.B.; Pan, Q.H.; Li, C.Y.; Reeves, M.J.; Wang, J. Free and glycosidically bound aroma compounds in cherry (Prunus avium L.). Food Chem. 2014, 152, 29–36. [Google Scholar] [CrossRef]
- Ubeda, C.; San-Juan, F.; Concejero, B.; Callejón, R.M.; Troncoso, A.M.; Morales, M.L. Glycosidically bound aroma compounds and impact odourants of four strawberry varieties. J. Agric. Food Chem. 2012, 60, 6095–6102. [Google Scholar] [CrossRef]
- Garcia, C.V.; Quek, S.Y.; Stevenson, R.J.; Winz, R.A. Characterization of the bound volatile extract from baby kiwi (Actinidia arguta). J. Agric. Food Chem. 2011, 59, 8358–8365. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; López de Felipe, F.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Cirlini, M.; Calani, L.; Bernini, V.; Neviani, E.; Del Rio, D.; Galaverna, G.; Lazzi, C. In vitro metabolism of elderberry juice polyphenols by lactic acid bacteria. Food Chem. 2019, 276, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Shah, N.P. Lactic acid bacterial fermentation modified phenolic composition in tea extracts and enhanced their antioxidant activity and cellular uptake of phenolic compounds following in vitro digestion. J. Funct. Foods 2016, 20, 182–194. [Google Scholar] [CrossRef]
- Mantzourani, I.; Nouska, C.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Panayiotidis, M.I.; Galanis, A.; Plessas, S. Production of a Novel Functional Fruit Beverage Consisting of Cornelian Cherry Juice and Probiotic Bacteria. Antioxidants 2018, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional Beverages: The Emerging Side of Functional Foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Dongmo, S.N.; Sacher, B.; Kollmannsberger, H.; Becker, T. Key volatile aroma compounds of lactic acid fermented malt based beverages—Impact of lactic acid bacteria strains. Food Chem. 2017, 229, 565–573. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile profile of elderberry juice: Effect of lactic acid fermentation using L. plantarum, L. rhamnosus and L. casei strains. Food Res. Int. 2018, 105, 412–422. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Ganzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Cirlini, M.; Caligiani, A.; Palla, G. Formation of glucose and fructose acetates during maturation and ageing of balsamic vinegars. Food Chem. 2009, 112, 51–56. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Nualkaekul, S.; Charalampopoulos, D. Survival of Lactobacillus plantarum in model solutions and fruit juices. Int. J. Food Microbiol. 2011, 146, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic responses of Lactobacillus plantarum strains during fermentation and storage of vegetable and fruit juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, R.M. Challenges for the Production of Probiotic Fruit Juices. Beverages 2015, 1, 95–103. [Google Scholar] [CrossRef]
- Fritsch, C.; Heinrich, V.; Vogel, R.F.; Toelstede, S. Phenolic acid degradation potential and growth behavior of lactic acid bacteria in sunflower substrates. Food Microbiol. 2016, 57, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; Ferrer, S.; Monedero, V.; Zúñiga, M. Malic Enzyme and Malolactic Enzyme Pathways Are Functionally Linked but Independently Regulated in Lactobacillus casei BL23. Appl. Environ. Microbiol. 2013, 79, 5509–5518. [Google Scholar] [CrossRef]
- Jyoti, B.D.; Suresha, A.K.; Venkatesha, K.V. Effect of preculturing condition on growth of Lactobacillus rhamnosus on medium containing glucose and citrate. Microbiol. Res. 2004, 159, 35–42. [Google Scholar] [CrossRef]
- Mozzi, F.; Raya, R.R.; Vignolo, G.M. Biotechnology of Lactic Acid Bacteria: Novel Application, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 222–224. [Google Scholar]
- Sinha, N.K.; Sidhu, J.S.; Barta, J.; Wu, J.S.B.; Cano, M.P. Handbook of Fruit and Fruit Processing; Willey-Blackwell: Ames, IA, USA, 2012; pp. 359–367. [Google Scholar]
- Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. The Microbiological Quality of Food. Foodborne Spoilers, 1st ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 237–255. [Google Scholar]
- Todorov, S.D.; De Melo Franco, B.D.G. Lactobacillus Plantarum: Characterization of the Species and Application in Food Production. Food Rev. Int. 2010, 26, 205–229. [Google Scholar] [CrossRef]
- Radler, F.; Bröhl, K. The metabolism of several carboxylic acids by lactic acid bacteria. Eur. Food Res. Technol. 1984, 179, 228–231. [Google Scholar] [CrossRef]
- Du Toit, M.; Pretorius, I.S. Microbial Spoilage and Preservation of Wine: Using Weapons from Nature’s Own Arsenal—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 74–96. [Google Scholar]
- Filannino, P.; Azzi, L.; Cavoski, I.; Vincentini, O.; Rizzello, C.G.; Gobbetti, M.; Di Cagno, R. Exploitation of the health-promoting and sensory properties of organic pomegranate (Punica granatum L.) juices through lactic acid fermentation. Int. J. Food Microbiol. 2013, 163, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Surico, R.F.; Paradiso, A.; De Angelis, M.; Buchin, S.; De Gara, M.; Gobbetti, M. Effect of autochthonous lactic acid bacteria starters on health-promoting and sensory properties of tomato juices. Int. J. Food Microbiol. 2009, 128, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int. J. Food Microbiol. 2017, 248, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sabokbar, N.; Khodaiyan, F. Total phenolic content and antioxidant activities of pomegranate juice and whey based novel beverage fermented by kefir grains. J. Food Sci. Technol. 2016, 53, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Phenolic compounds in plant foods: Chemistry and health benefits. J. Food Sci. Nutr. 2003, 8, 200–218. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of Fermentation with Different Lactic Acid Bacteria and in Vitro Digestion on the Biotransformation of Phenolic Compounds in Fermented Pomegranate Juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef]
- Sanchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Baeza, G.; Bachmair, E.M.; Wood, S.; Mateos, R.; Bravo, L.; Baukje de Roos, B. The colonic metabolites dihydrocaffeic acid and dihydroferulic acid are more effective inhibitors of in vitro platelet activation than their phenolic precursors. Food Funct. 2017, 8, 1333–1342. [Google Scholar] [CrossRef]
- Huang, J.; de Paulisb, T.; May, J.M. Antioxidant effects of dihydrocaffeic acid in human EA.hy926 endothelial cells. J. Nutr. Biochem. 2004, 15, 722–729. [Google Scholar] [CrossRef]
- Silva, I.; Campos, F.M.; Hogg, T.; Couto, J.A. Factors influencing the production of volatile phenols by wine lactic acid bacteria. Int. J. Food Microbiol. 2011, 145, 471–475. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Francesca Valerio, F.; Angelo Visconti, A. Antifungal Activity of Phenyllactic Acid against Molds Isolated from Bakery Products. Appl. Environ. Microbiol. 2003, 69, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; Lavermicocca, P.; Pascale, M.; Visconti, A. Production of phenyllactic acid by lactic acid bacteria: An approach to the selection of strains contributing to food quality and preservation. FEMS Microbiol. Lett. 2004, 233, 289–295. [Google Scholar] [CrossRef] [PubMed]


| Species | Strain | Origin |
|---|---|---|
| L. plantarum | POM1 * | Tomato (plant) |
| C1 * | Carrot (plant) | |
| 1LE1 * | Pineapple (plant) | |
| 285 ** | Minas cheese (dairy) | |
| L. rhamnosus | 2178 ** | Parmigiano Reggiano cheese (dairy) |
| 2140 ** | Parmigiano Reggiano cheese (dairy) | |
| 2360 ** | Parmigiano Reggiano cheese (dairy) | |
| 1473 ** | Parmigiano Reggiano cheese (dairy) | |
| 1019 ** | Parmigiano Reggiano cheese (dairy) | |
| L. casei | 2246 ** | Parmigiano Reggiano cheese (dairy) |
| 2306 ** | Parmigiano Reggiano cheese (dairy) | |
| 2057 ** | Parmigiano Reggiano cheese (dairy) | |
| 2107 ** | Parmigiano Reggiano cheese (dairy) | |
| L. paracasei | 4186 ** | Pecorino cheese (dairy) |
| Species | Strain | Origin | Δ(T48h–T0) | Δ(T14d–T0) |
|---|---|---|---|---|
| L. plantarum | POM1 | Plant | 1.82 ± 0.05 | 1.69 ± 0.03 |
| C1 | Plant | 1.22 ± 0.10 | 1.11 ± 0.04 | |
| 1LE1 | Plant | 1.32 ± 0.07 | 1.39 ± 0.17 | |
| 285 | Dairy | 1.44 ± 0.07 | 1.46 ± 0.03 | |
| L. rhamnosus | 2360 | Dairy | 1.02 ± 0.10 | 0.97 ± 0.03 |
| L. paracasei | 4186 | Dairy | 0.96 ± 0.13 | 0.80 ± 0.14 |
| 48 Hours | ||||||||
| Compound | 37 °C | 2360 | 4186 | 30 °C | 1LE1 | 285 | C1 | POM1 |
| acetoin | 0.001 ± 0.000 | 260.679 ± 34.426 * | 5.909 ± 0.480 | 0.002 ± 0.000 | 71.373 ± 4.486 * | 76.300 ± 18.884 * | 287.902 ± 14.971* | 44.022 ± 1.515 * |
| benzene methanol | 51.130 ± 13.431 | 164.411 ± 4.744 * | 70.560 ± 13.868 | 64.548 ± 0.700 | 70.513 ± 1.502 | 158.829 ± 3.660 * | 95.692 ± 15.202 | 90.078 ± 11.908 * |
| β-linalool | 13.742 ± 3.515 | 39.630 ± 2.624 * | 21.464 ± 2.627 * | 15.670 ± 0.326 | 15.305 ± 0.181 | 28.975 ± 0.424 * | 19.964 ± 2.326 * | 19.593 ± 1.604 * |
| acetic acid | 0.087 ± 0.030 | 0.017 ± 0.017 * | 0.014 ± 0.002 * | 0.013 ± 0.011 | 54.831 ± 18.642 * | 125.405 ± 10.324 * | 184.836 ± 21.933 * | 69.201 ± 9.371 * |
| propyl acetate | 0.009 ± 0.005 | 1186.731 ± 460.827 * | 201.607 ± 26.688 | 0.008 ± 0.005 | 24.680 ± 0.422 * | 27.876 ± 5.804 * | 83.370 ± 6.871 * | 18.539 ± 0.273 * |
| 4-ethylphenol | 0.341 ± 0.016 | 29.025 ± 12.443 * | 1.828 ± 0.232 | 0.503 ± 0.144 | 23.832 ± 4.294 * | 150.460 ± 3.007 * | 8.997 ± 3.734 * | 106.967 ± 6.068 * |
| 14 Days | ||||||||
| Compound | 37 °C | 2360 | 4186 | 30 °C | 1LE1 | 285 | C1 | POM1 |
| acetoin | 0.004 ± 0.005 | 180.078 ± 22.938 * | 65.013 ± 1.293 * | 0.004 ± 0.000 | 580.569 ± 61.646 * | 161.905 ± 50.669 | 1058.883 ± 222.855 * | 63.563 ± 22.691 |
| benzene methanol | 146.075 ± 6.008 | 79.259 ± 21.526 * | 250.467 ± 32.932 * | 99.225 ± 1.022 | 462.867 ± 76.120 * | 311.721 ± 47.740 * | 327.123 ± 54.469 * | 116.204 ± 42.234 |
| β-linalool | 41.464 ± 3.599 | 19.467 ± 2.875 * | 70.918 ± 15.120 * | 27.205 ± 2.210 | 92.675 ± 20.477 * | 57.517 ± 9.385 * | 68.307 ± 4.195 * | 22.431 ± 8.860 |
| acetic acid | 0.024 ± 0.009 | 228.633 ± 71.471 * | 0.354 ± 0.192 | 0.013 ± 0.009 | 535.465 ± 92.920 * | 305.391 ± 5.248 * | 737.615 ± 193.995 * | 257.568 ± 75.381 * |
| propyl acetate | 0.026 ± 0.004 | 509.194 ± 12.345 * | 1230.067 ± 222.171 * | 0.015 ± 0.014 | 160.882 ± 2.895 * | 61.035 ± 14.937 * | 281.546 ± 28.411 * | 16.706 ± 5.548 |
| 4-ethylphenol | 0.848 ± 0.141 | 3.210 ± 0.751 | 21.854 ± 5.943 * | 0.608 ± 0.144 | 129.556 ± 2.648 * | 281.248 ± 21.113 * | 12.287 ± 0.017 * | 136.453 ± 43.612 * |
| 48 Hours | ||||||||
| Compounds | 37 °C | 2360 | 4186 | 30 °C | 1LE1 | 285 | C1 | POM1 |
| p-Hydroxyphenyllactic acid | ND | 0.986 ± 0.121 * | 0.209 ± 0.047 * | ND | 1.229 ± 0.187 * | 1.462 ± 0.089 * | 1.241 ± 0.148 * | 1.134 ± 0.125 * |
| Phenyllactic acid | ND | 0.483 ± 0.062 * | 0.449 ± 0.024 * | ND | 2.098 ± 0.187 * | 1.905 ± 0.150 * | 1.219 ± 0.076 * | 1.930 ± 0.026 * |
| Caffeic acid | 0.633 ± 0.025 | 0.743 ± 0.038 * | 0.722 ± 0.030 * | 0.586 ± 0.033 | 0.406 ± 0.270 | ND | 0.305 ± 0.040 | 0.062 ± 0.014 * |
| p-Coumaric acid | 0.609 ± 0.274 | 0.594 ± 0.061 | 0.486 ± 0.105 | 0.288 ± 0.124 | 0.484 ± 0.134 | ND | 0.354 ± 0.207 | ND |
| Protocatechuic acid | 0.457 ± 0.197 | 0.508 ± 0.023 | 0.382 ± 0.103 | 0.253 ± 0.114 | 0.380 ± 0.131 | ND | 0.308 ± 0.273 | ND |
| Dihydrocaffeic acid | ND | ND | ND | ND | 0.307 ± 0.222 | 0.284 ± 0.043 | 0.131 ± 0.062 | 0.568 ± 0.070 * |
| 14 Days | ||||||||
| Compounds | 37 °C | 2360 | 4186 | 30 °C | 1LE1 | 285 | C1 | POM1 |
| p-Hydroxyphenyllactic acid | ND | 1.272 ± 0.159 * | 0.259 ± 0.030 * | ND | 1.225 ± 0.025 * | 1.473 ± 0.158 * | 1.271 ± 0.115 * | 1.295 ± 0.131 * |
| Phenyllactic acid | ND | 0.559 ± 0.034 * | 0.513 ± 0.006 * | ND | 1.986 ± 0.087 * | 2.150 ± 0.153 * | 1.494 ± 0.141 * | 2.208 ± 0.095 * |
| Caffeic acid | 0.685 ± 0.062 | 0.789 ± 0.050 | 0.668 ± 0.038 | 0.702 ± 0.037 | 0.345 ± 0.241 * | ND | 0.259 ± 0.024 * | 0.092 ± 0.020 * |
| p-Coumaric acid | 0.670 ± 0.294 | 0.472 ± 0.050 | 0.485 ± 0.126 | 0.412 ± 0.103 | 0.337 ± 0.019 | ND | ND | ND |
| Protocatechuic acid | 0.560 ± 0.290 | 0.484 ± 0.170 | 0.497 ± 0.217 | 0.357 ± 0.148 | 0.285 ± 0.052 | ND | 0.459 ± 0.247 | ND |
| Dihydrocaffeic acid | ND | ND | ND | ND | 0.317 ± 0.314 | 0.278 ± 0.022 | 0.124 ± 0.040 | 0.609 ± 0.041 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, A.; Cirlini, M.; Maoloni, A.; Del Rio, D.; Calani, L.; Bernini, V.; Galaverna, G.; Neviani, E.; Lazzi, C. Use of Dairy and Plant-Derived Lactobacilli as Starters for Cherry Juice Fermentation. Nutrients 2019, 11, 213. https://doi.org/10.3390/nu11020213
Ricci A, Cirlini M, Maoloni A, Del Rio D, Calani L, Bernini V, Galaverna G, Neviani E, Lazzi C. Use of Dairy and Plant-Derived Lactobacilli as Starters for Cherry Juice Fermentation. Nutrients. 2019; 11(2):213. https://doi.org/10.3390/nu11020213
Chicago/Turabian StyleRicci, Annalisa, Martina Cirlini, Antonietta Maoloni, Daniele Del Rio, Luca Calani, Valentina Bernini, Gianni Galaverna, Erasmo Neviani, and Camilla Lazzi. 2019. "Use of Dairy and Plant-Derived Lactobacilli as Starters for Cherry Juice Fermentation" Nutrients 11, no. 2: 213. https://doi.org/10.3390/nu11020213
APA StyleRicci, A., Cirlini, M., Maoloni, A., Del Rio, D., Calani, L., Bernini, V., Galaverna, G., Neviani, E., & Lazzi, C. (2019). Use of Dairy and Plant-Derived Lactobacilli as Starters for Cherry Juice Fermentation. Nutrients, 11(2), 213. https://doi.org/10.3390/nu11020213






