Effects of Long Chain Omega-3 Polyunsaturated Fatty Acids on Brain Function in Mildly Hypertensive Older Adults
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
- Inclusion criteria
- Aged 40–85 years
- Clinic SBP between 130–160 mmHg or DBP between 85–100 mmHg (determined at screening)
- Consuming <2 fish/seafood meals per week
- Consuming ≤300 mg/day of LCn-3PUFA from fish oil supplements or enriched foods
- Unlikely to change medication/supplements during the intervention
- Competent in use of computer mouse and keyboard
- Exclusion criteria
- Suspected dementia (Modified-Mini Mental (3MS) examination score of <78/100)
- Smokers or currently on nicotine therapy
- Neurological conditions
- Kidney/liver disease/diabetes
- Major depression as diagnosed by a health care professional
- Impaired vision
- Unsatisfactory TCD signal in the middle cerebral artery (MCA)
- Unwilling to eat or having intolerance to fish or vegetable oil
- Unwilling to provide a blood sample
- Unwilling to maintain pre-enrolment physical activity levels and dietary habits during the trial
2.3. Clinic Visits
2.4. Supplements
2.5. Outcome Assessments
2.5.1. Clinic BP and Arterial Compliance (AC)
2.5.2. Indices of Cerebrovascular Function
2.5.3. Neuropsychological Tests
Oral Trail Making Task (TMT, a Measure of Executive Function)
Oral Serial Subtractions 3 s (SS3) (Measure of Working Memory)
N-Back Test
Computerised Multi-Tasking Test Battery (Purple Framework, UK)
- Test 1—High number tap (attention): A 4 × 4 grid containing digits between 0 and 9 appears. Participants must highlight the highest digits in each grid by clicking on them (e.g., if the highest digit in the grid is “8”, the participant must click all of the 8 s. Once all of the highest digits are successfully highlighted, the grid refreshes and a new (random) grid appears. Participants complete as many grids as they can within the allocated time.
- Test 2—Letter search (verbal memory): A string of random letters appears in a box for a few seconds. Participants try to remember these letters before they disappear. A single (target) letter then appears in the centre of the screen. If the target letter comes from the original list of letters, they click ‘yes’ on the screen with the mouse, otherwise they click ‘no’. Participants have to select a response within 10 s.
- Test 3—Visual Warning (reaction time): The task starts with six red bars beginning to rise upwards at different speeds. When the highest bar reaches the top, digits appear on each bar and a warning sign flashes on the screen, trying to capture the participant’s attention. Participants respond by clicking on the bars in numerical order (from 1 to 6). Once they click on the bars in correct order, the task resets and a new trial begins automatically.
- Test 4—Stroop colour-word test (executive function): Four coloured blocks (blue, yellow, red and green) appear on the right-hand side of the screen. At a set time interval, a colour name appears to the left of the blocks. Participants click on the coloured block on the right that corresponds with the word, regardless of the colour in which the word is written. For example, if the name “blue” appears in red ink, participants should select the blue coloured block.
- Multi-tasking tests (1 + 2 + 3 + 4): All four tasks appear and run at the same time. Participants attempt the tasks simultaneously and equally to assess their divided attention. To determine accuracy in the multi-tasking test battery, their performance of each component task is compared with their performance when they undertook the task individually. Overall performance on the multi-tasking battery is defined as the average accuracy of performance on the High Number Tap, Letter Search and the Stroop colour-word task. Speed of performance on the multi-tasking test battery is the average response time (msec) for all four-tasks during the 5-min test period. The ratio of accuracy to response time serves as a performance index. A higher index indicates better performance.
Dual Tasks with Tapping
Go-no-Go Test
Paper TMT
2.6. Analysis of the Cognitive Outcomes
2.7. CVR to Neuropsychological Tests
2.8. Mood States
2.9. Erythrocyte Fatty Acid Profiles (Omega-3 Index)
2.10. Statistical Analysis
3. Results
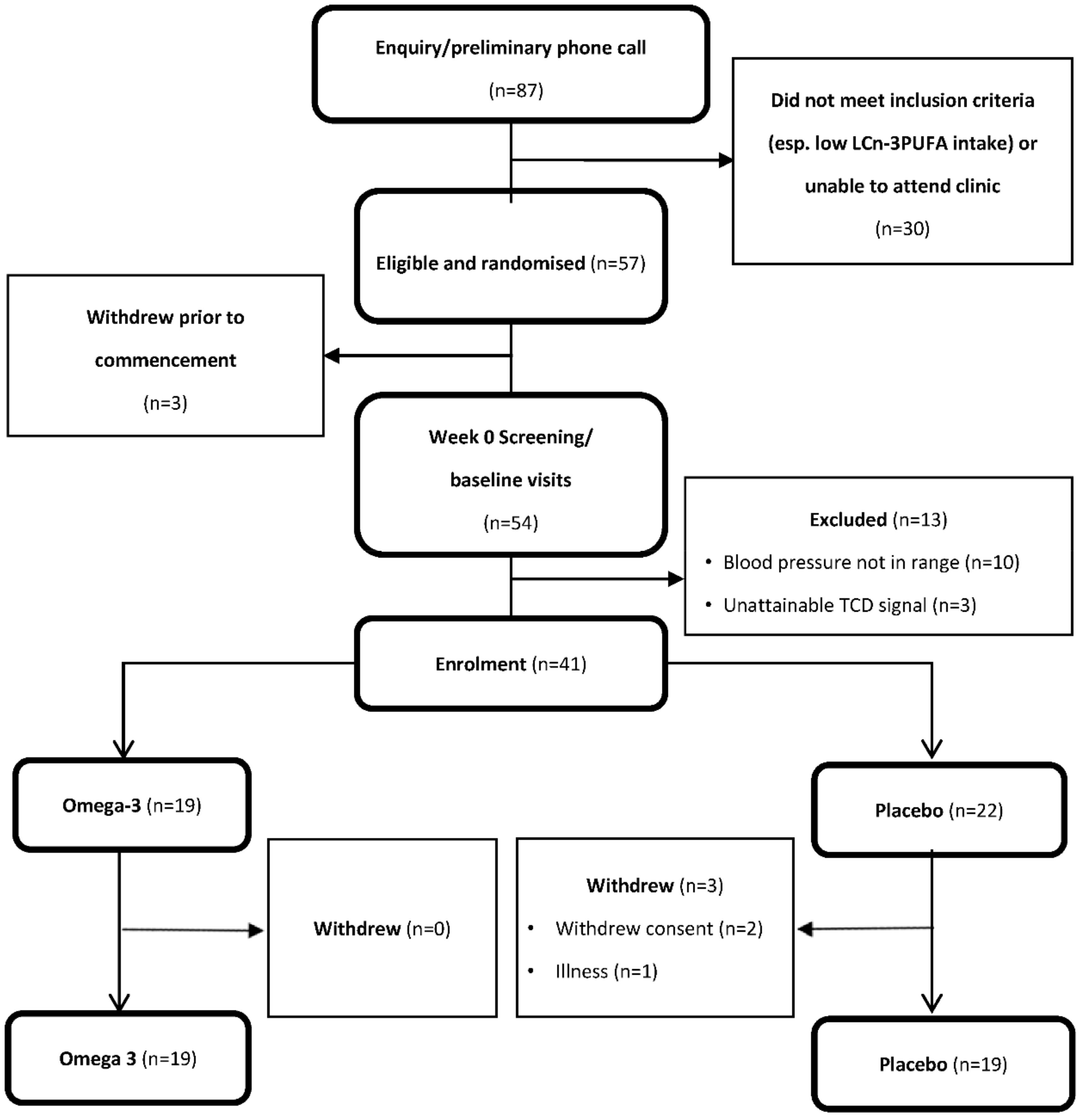
3.1. Participants
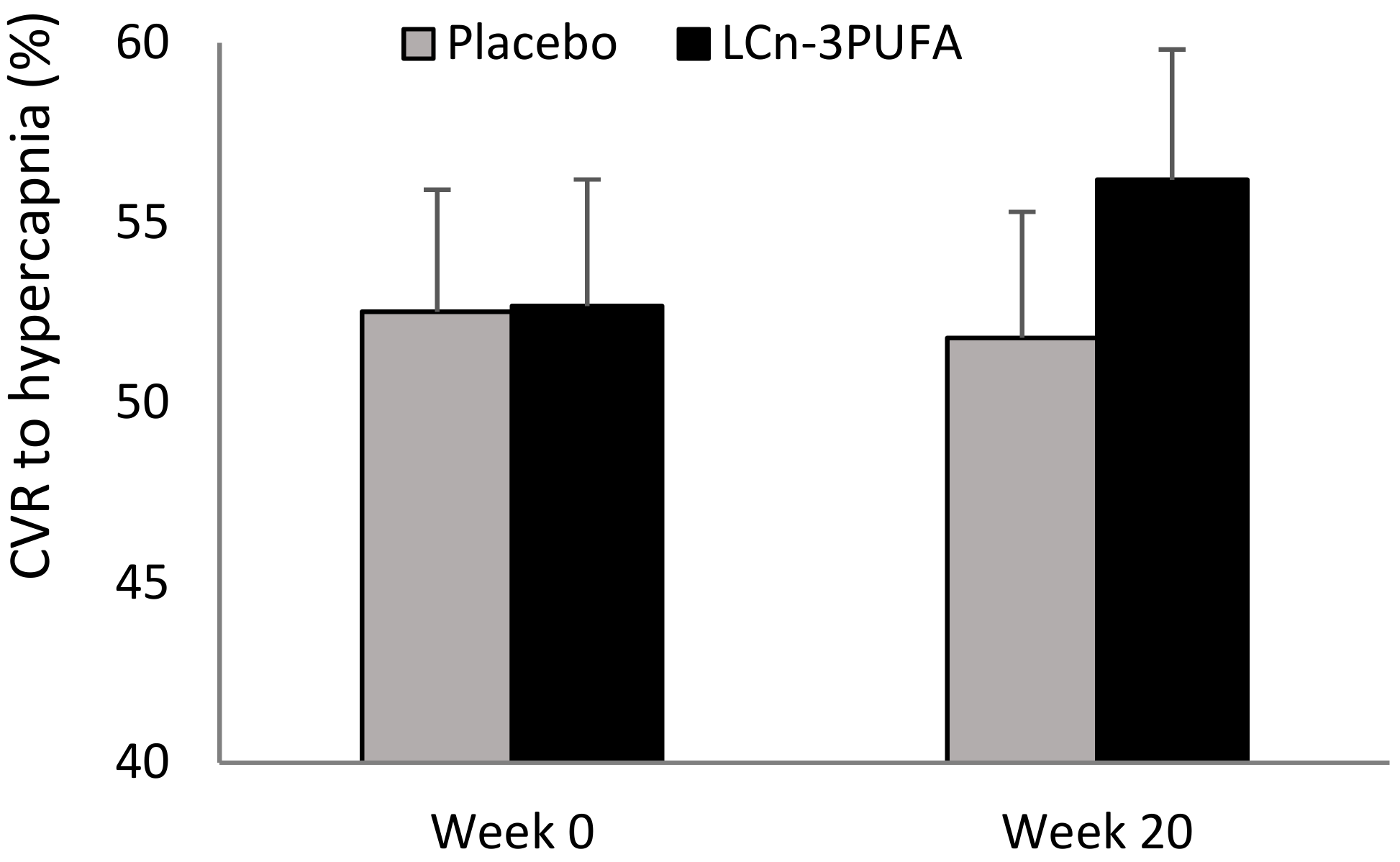
3.2. Primary Outcome—CVR to Hypercapnia
3.3. Secondary Outcomes
3.3.1. Indices of Systemic and Cerebral Vascular Function
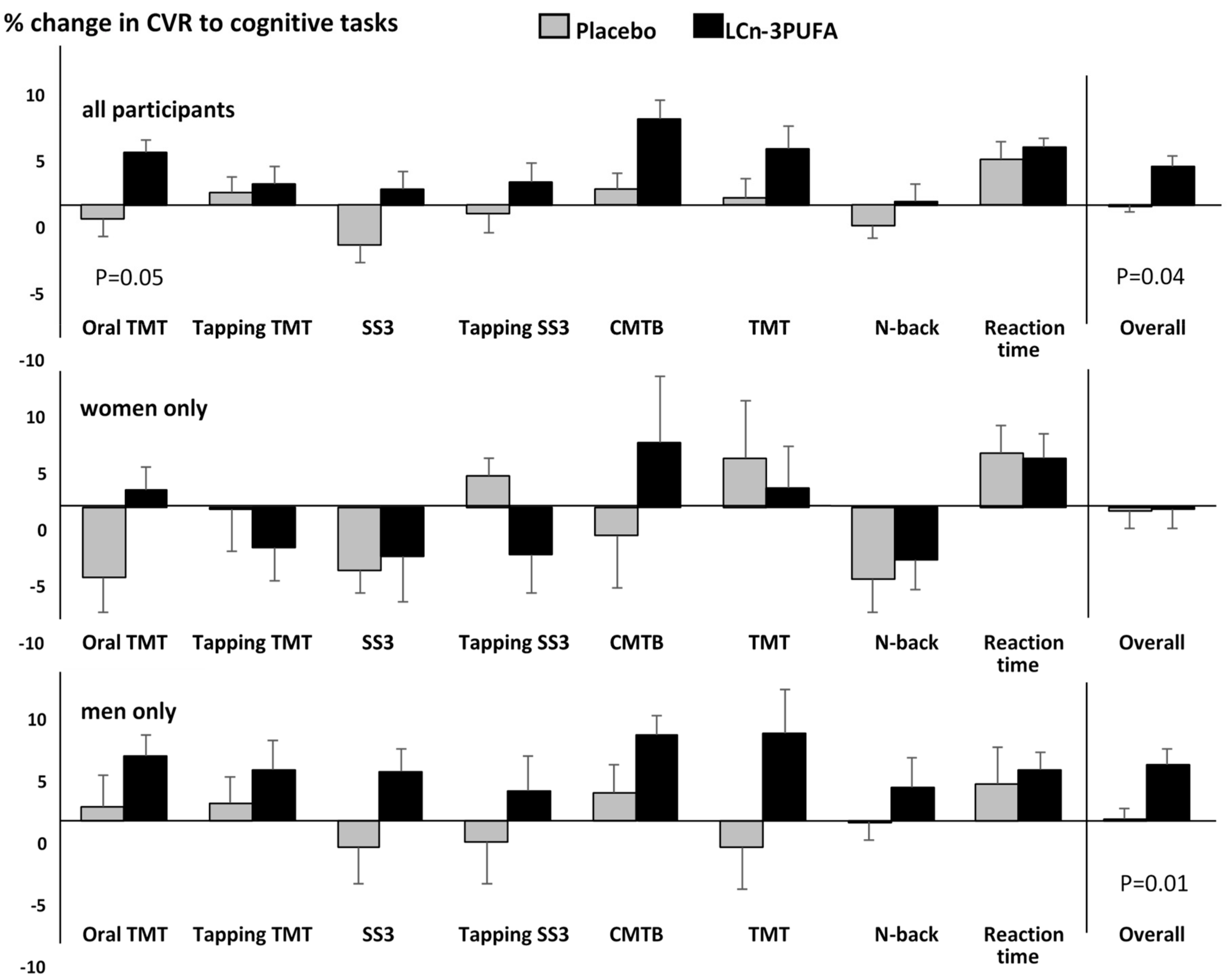
3.3.2. CVR to Cognitive Stimuli (Neurovascular Coupling)
3.3.3. Cognitive Performance
3.3.4. Profile of Mood States
3.3.5. Erythrocyte Fatty Acid Analysis
3.3.6. Correlations between Changes in Erythrocyte Fatty Acids and Changes in Outcome Measures
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sinn, N.; Milte, C.M.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Petkov, J.; Howe, P.R.C. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Nutr. 2012, 107, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Sinn, N.; Howe, P.R.C. Mental health benefits of omega-3 fatty acids may be mediated by improvements in cerebral vascular function. Biosci. Hypotheses 2008, 1, 103–108. [Google Scholar] [CrossRef]
- Kuszewski, J.; Wong, R.; Howe, P. Effects of long-chain omega-3 polyunsaturated fatty acids on endothelial vasodilator function and cognition—Are they interrelated? Nutrients 2017, 9, 487. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.X.; Coates, A.M.; Buckley, J.D.; Howe, P.R.C. Evidence for circulatory benefits of resveratrol in humans. Ann. N. Y. Acad. Sci. 2013, 1290, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Scholey, A.; Howe, P.R. Assessing premorbid cognitive ability in adults with type 2 diabetes mellitus-a review with implications for future intervention studies. Curr. Diabetes Rep. 2014, 14, 547. [Google Scholar] [CrossRef] [PubMed]
- Barbour, J.A.; Howe, P.R.; Buckley, J.D.; Bryan, J.; Coates, A.M. Nut consumption for vascular health and cognitive function. Nutr. Res. Rev. 2014, 27, 131–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nealon, R.S.; Howe, P.R.C.; Jansen, L.; Garg, M.; Wong, R.H.X. Impaired cerebrovascular responsiveness and cognitive performance in adults with type 2 diabetes. J. Diabetes Its Complicat. 2017, 31, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.X.; Evans, H.M.; Howe, P.R.C. Poor cerebrovascular function is an early marker of cognitive decline in healthy postmenopausal women. Alzheimers Dement. Transl. Res. Clin. Interv. 2016, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M.; Howe, P.R.C.; Wong, R.H.X. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Howe, P.R.; Coates, A.M.; Buckley, J.D.; Berry, N.M. Chronic consumption of a wild green oat extract (neuravena) improves brachial flow-mediated dilatation and cerebrovascular responsiveness in older adults. J. Hypertens. 2013, 31, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Barbour, J.A.; Howe, P.R.C.; Buckley, J.D.; Bryan, J.; Coates, A.M. Cerebrovascular and cognitive benefits of high-oleic peanut consumption in healthy overweight middle-aged adults. Nutr. Neurosci. 2017, 20, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Treatment allocation by minimisation. Br. Med. J. 2005, 330, 843–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollock, V.; Cho, D.W.; Reker, D.; Volavka, J. Profile of mood states: The factors and their physiological correlates. J. Nerv. Ment. Dis. 1979, 167, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Eriksdotter, M.; Vedin, I.; Falahati, F.; Freund-Levi, Y.; Hjorth, E.; Faxen-Irving, G.; Wahlund, L.O.; Schultzberg, M.; Basun, H.; Cederholm, T.; et al. Plasma fatty acid profiles in relation to cognition and gender in Alzheimer’s disease patients during oral omega-3 fatty acid supplementation: The omegad study. J. Alzheimers Dis. 2015, 48, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Decsi, T.; Kennedy, K. Sex-specific differences in essential fatty acid metabolism. Am. J. Clin. Nutr. 2011, 94, 1914S–1919S. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Reay, J.L.; Scholey, A.B.; Kennedy, D.O. DHA-rich oil modulates the cerebral haemodynamic response to cognitive tasks in healthy young adults: A near ir spectroscopy pilot study. Br. J. Nutr. 2012, 107, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Reay, J.L.; Scholey, A.B.; Kennedy, D.O. Docosahexaenoic acid-rich fish oil modulates the cerebral hemodynamic response to cognitive tasks in healthy young adults. Biol. Psychol. 2012, 89, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Forster, J.S.; Bell, J.G.; Dick, J.R.; Younger, I.; Kennedy, D.O. DHA supplementation alone or in combination with other nutrients does not modulate cerebral hemodynamics or cognitive function in healthy older adults. Nutrients 2016, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Konagai, C.; Yanagimoto, K.; Hayamizu, K.; Han, L.; Tsuji, T.; Koga, Y. Effects of krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: A randomized controlled trial in healthy elderly volunteers. Clin. Interv. Aging 2013, 8, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Kennedy, D.O. The application of near infrared spectroscopy in nutritional intervention studies. Front. Hum. Neurosci. 2013, 7, 473. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A. Omega-3s and Cognition: Dosage Matters. Global Organization for Epa and DHA Omega-3s (goed) Website 2015. Available online: http://www.goedomega3.com/index.php/blog/2015/08/omega-3s-and-cognition-dosage-matters (accessed on 27 August 2018).
- Phang, M.; Lincz, L.F.; Garg, M.L. Eicosapentaenoic and docosahexaenoic acid supplementations reduce platelet aggregation and hemostatic markers differentially in men and women. J. Nutr. 2013, 143, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Howe, P.R.C.; Buckley, J.D.; Murphy, K.J.; Pettman, T.; Milte, C.; Coates, A.M. Relationship between erythrocyte omega-3 content and obesity is gender dependent. Nutrients 2014, 6, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Placebo (n = 19) | LCn-3PUFA (n = 19) |
|---|---|---|
| Gender (M/F) | 13/6 | 13/6 |
| Age (years) | 64.1 ± 2.3 | 63.2 ± 1.6 |
| 3MS score (%) | 96.3 ± 0.7 | 96.8 ± 0.7 |
| BMI (kg/m2) | 28.8 ± 0.9 | 26.4 ± 0.8 |
| Waist circumference (cm) | 97.9 ± 2.2 | 93.5 ± 2.9 |
| Clinic systolic BP (mmHg) | 141.2 ± 2.0 | 140.4 ± 1.7 |
| Clinic diastolic BP (mmHg) | 79.7 ± 1.7 | 79.4 ± 1.7 |
| LAC (mL/mmHg × 10) | 11.4 ± 0.6 | 12.7 ± 1.0 |
| SAC (ml/mmHg × 100) | 3.3 ± 0.3 | 3.3 ± 0.4 |
| SVR (dyne·sec·cm−5) | 1758 ± 56 | 1755 ± 48 |
| MBFV | 46.7 ± 2.9 | 46.0 ± 1.9 |
| PI | 0.86 ± 0.04 | 0.92 ± 0.05 |
| All Participants | Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | LCn-3PUFA | p | Placebo (n = 13) | LCn-3PUFA (n = 13) | p | Placebo (n = 6) | LCn-3PUFA (n = 6) | p | |
| Systolic BP (mmHg) | −3.1 ± 0.4 | −5.0 ± 2.2 | 0.43 | −4.1 ± 0.4 | −5.3 ± 2.5 | 0.90 | 0.1 ± 0.7 | −4.2 ± 2.7 | 0.52 |
| Diastolic BP (mmHg) | −1.3 ± 1.2 | −4.5 ± 1.1 | 0.12 | −2.7 ± 1.8 | −8.7 ± 2.2 | 0.42 | 6.9 ± 1.6 | −4.6 ± 1.4 | 0.20 |
| LAC (mL/mmHg ×10) | 2.4 ± 0.9 | 2.3 ± 0.8 | 0.96 | 3.4 ± 1.3 | 2.8 ± 0.9 | 0.73 | 1.0 ± 1.3 | 1.2 ± 0.8 | 0.93 |
| SAC (mL/mmHg ×100) | 0.2 ± 0.3 | 0.4 ± 0.3 | 0.62 | 0.3 ± 0.5 | 0.4 ± 0.4 | 0.90 | −0.1 ± 0.1 | 0.3 ± 0.3 | 0.20 |
| SVR * (dyne·s·cm−5) | −28.3 ± 25.2 | −125.2 ± 22.1 | 0.05 | −83.6 ± 30.2 | −153.2 ± 33.1 | 0.4 | 39.1 ± 35.2 | −100.1 ± 32.1 | 0.08 |
| MBFV | −0.5 ± -2.7 | −4.8 ± 2.1 | 0.27 | 1.7 ± 2.7 | −7.6 ± 4.3 | 0.07 | −4.1 ± 4.4 | −2.3 ± 4.6 | 0.51 |
| PI * | 0.02 ± 0.04 | −0.03 ± 0.05 | 0.02 | −0.04 ± 0.04 | −0.00 ± 0.03 | 0.30 | 0.23 ± 0.02 | −0.09 ± 0.04 | 0.05 |
| Week 0 | Week 20 | ∆ (Week 20—Week 0) | p | ||||
|---|---|---|---|---|---|---|---|
| Placebo | LCn-3PUFA | Placebo | LCn-3PUFA | Placebo | LCn-3PUFA | ||
| Oral TMT | −0.1 ± 0.2 | 0.1 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.9 | 0.3 ± 0.2 | 0.65 |
| TMT + tapping | −0.1 ± 0.2 | 0.1 ± 0.2 | 0.4 ± 0.2 | 0.2 ± 0.2 | 0.5 ± 0.2 | 0.1 ± 0.2 | 0.20 |
| Oral SS3 | −0.1 ± 0.3 | 0.1 ± 0.2 | 0.1 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.59 |
| SS3 + tapping | 0.1 ± 0.3 | −0.1 ± 0.2 | 0.3 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.53 |
| CMTB | 0.2 ± 0.3 | −0.2 ± 0.2 | 0.1 ± 0.2 | 0.1 ± 0.3 | −0.1 ± 0.3 | 0.2 ± 0.3 | 0.43 |
| Paper TMT | 0.5 ± 0.3 | 0.2 ± 0.2 | −0.1 ± 0.4 | 0.1 ± 0.3 | −0.5 ± 0.4 | −0.1 ± 0.4 | 0.5 |
| N-back | −0.1 ± 0.2 | 0.1 ± 0.2 | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.2 ± 0.1 | 0.19 |
| Reaction time | 0.1 ± 0.3 | −0.1 ± 0.2 | 0.1 ± 0.2 | 0.3 ± 0.2 | 0.1 ± 0.2 | 0.4 ± 0.2 | 0.35 |
| Overall performance | 0.1 ± 0.2 | 0.1 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.93 |
| Profile of Mood States | Placebo | LCn-3PUFA | Difference (LCn-3 PUFA—Placebo) | p-Value |
|---|---|---|---|---|
| Tension | −2.0 ± 0.7 | −1.7 ± 0.7 | 0.3 ± 0.9 | 0.78 |
| Depression | −1.4 ± 0.6 | 0.3 ± 1.0 | 1.7 ± 1.0 | 0.15 |
| Anger | −1.3 ± 1.0 | 0.1 ± 1.0 | 1.4 ± 1.3 | 0.33 |
| Fatigue | 0.1 ± 1.1 | −0.3 ± 0.5 | −0.4 ± 0.9 | 0.97 |
| Confusion | −0.9 ± 0.4 | 0.0 ± 0.9 | 0.9 ± 0.9 | 0.37 |
| Vigour | 0.2 ± 1.1 | 0.6 ± 0.6 | 0.4 ± 1.0 | 0.76 |
| Total mood disturbances | −5.9 ± 3.0 | −2.1 ± 3.2 | 3.8 ± 3.9 | 0.39 |
| Placebo | LCn-3PUFA | |||||
|---|---|---|---|---|---|---|
| EPA | DHA | O3I | EPA | DHA | O3I | |
| Baseline | ||||||
| All subjects (n = 37) | 1.38 ± 0.10 | 4.34 ± 0.23 | 5.72 ± 0.30 | 1.24 ± 0.10 | 4.73 ± 0.23 | 5.96 ± 0.30 |
| Female (n = 11) | 1.36 ± 0.23 | 4.23 ± 0.48 | 5.58 ± 0.65 | 1.20 ± 0.23 | 4.75 ± 0.48 | 5.95 ± 0.65 |
| Male (n = 26) | 1.39 ± 0.11 | 4.39 ± 0.27 | 5.78 ± 0.34 | 1.25 ± 0.11 | 4.71 ± 0.27 | 5.97±0.34 |
| Week 20 | ||||||
| All subjects (n = 36) | 1.21 ± 0.10 | 4.24 ± 0.21 | 5.44 ± 0.25 | 2.78 ± 0.10 | 7.96 ± 0.21 | 10.74 ± 0.25 |
| Female (n = 11) | 1.10 ± 0.15 | 4.71 ± 0.34 | 5.82 ± 0.43 | 3.07 ± 0.15 | 8.26 ± 0.34 | 11.34 ± 0.43 |
| Male (n = 25) | 1.25 ± 0.12 | 4.04 ± 0.25 | 5.29 ± 0.30 | 2.66 ± 0.12 | 7.83 ± 0.25 | 10.49 ± 0.30 |
| Difference (Week 20—Baseline) | ||||||
| All subjects (n = 36) | −0.17 ± 0.09 | −0.10 ± 0.24 | −0.28 ± 0.28 | 1.54 ± 0.09 * | 3.23 ± 0.24 * | 4.78 ± 0.28 * |
| Female (n = 11) | −0.26 ± 0.15 | 0.48 ± 0.32 | 0.24 ± 0.34 | 1.87 ± 0.15 * | 3.51 ± 0.31 * | 5.39 ± 0.34 * |
| Male (n = 25) | −0.14 ± 0.11 | −0.35 ± 0.30 | −0.49 ± 0.36 | 1.41 ± 0.11 * | 3.1 ± 0.31 * | 4.52 ± 0.36 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howe, P.R.C.; Evans, H.M.; Kuszewski, J.C.; Wong, R.H.X. Effects of Long Chain Omega-3 Polyunsaturated Fatty Acids on Brain Function in Mildly Hypertensive Older Adults. Nutrients 2018, 10, 1413. https://doi.org/10.3390/nu10101413
Howe PRC, Evans HM, Kuszewski JC, Wong RHX. Effects of Long Chain Omega-3 Polyunsaturated Fatty Acids on Brain Function in Mildly Hypertensive Older Adults. Nutrients. 2018; 10(10):1413. https://doi.org/10.3390/nu10101413
Chicago/Turabian StyleHowe, Peter R. C., Hamish M. Evans, Julia C. Kuszewski, and Rachel H. X. Wong. 2018. "Effects of Long Chain Omega-3 Polyunsaturated Fatty Acids on Brain Function in Mildly Hypertensive Older Adults" Nutrients 10, no. 10: 1413. https://doi.org/10.3390/nu10101413
APA StyleHowe, P. R. C., Evans, H. M., Kuszewski, J. C., & Wong, R. H. X. (2018). Effects of Long Chain Omega-3 Polyunsaturated Fatty Acids on Brain Function in Mildly Hypertensive Older Adults. Nutrients, 10(10), 1413. https://doi.org/10.3390/nu10101413






