Lutein Intake and Blood Lutein Concentration Are Positively Associated with Physical Activity in Adults: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
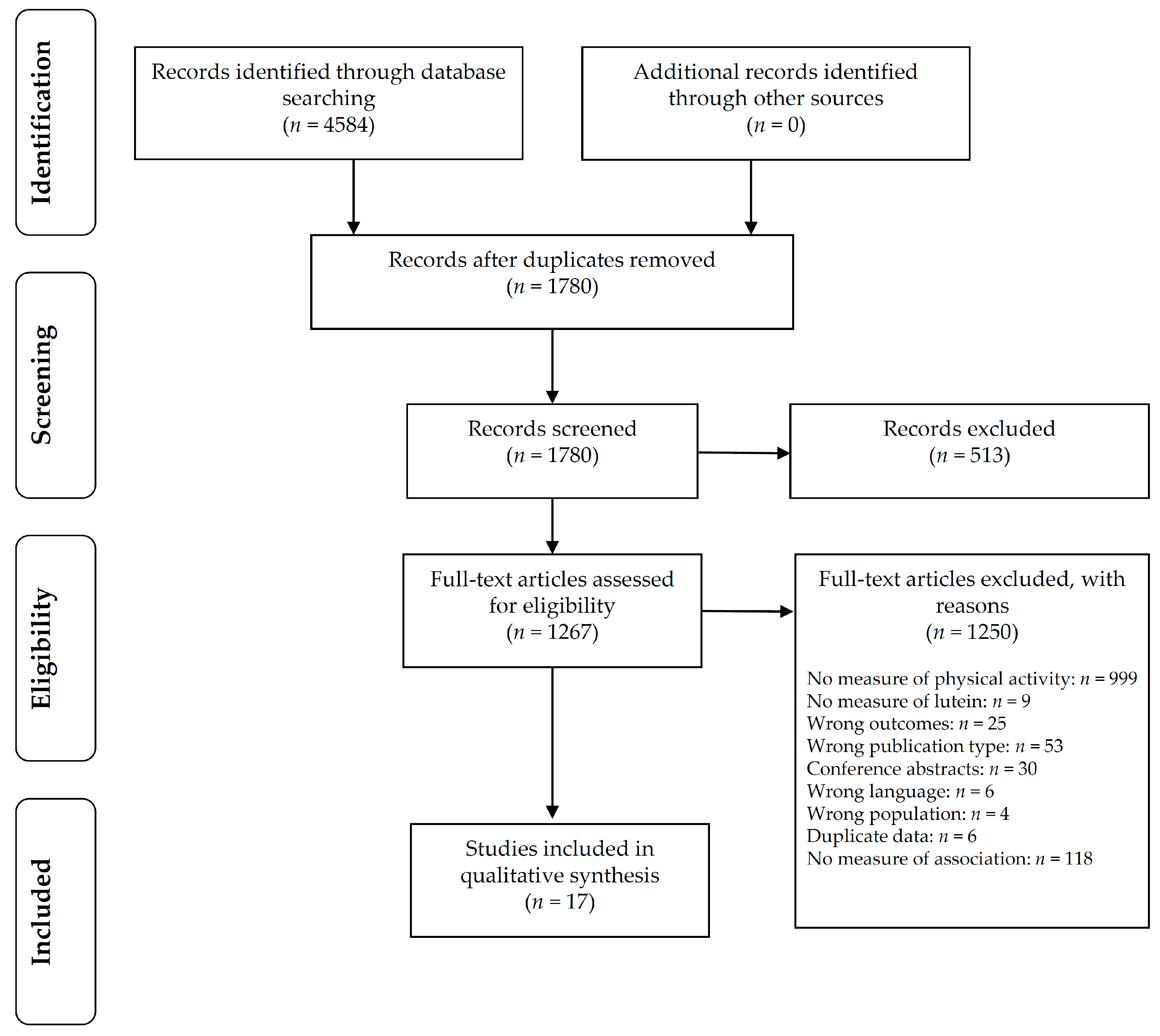
3. Results
3.1. Synthesis of Results
3.2. Risk of Bias within Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Australian Bureau of Statistics. National Health Survey: First Results 2014-15; Australian Bureau of Statistics: Canberra, Australia, 2015.
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Masse, L.C.; Tilert, T.; McDowell, M. Physical activity in the united states measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Impact of Physical Inactivity as a Risk Factor for Chronic Conditions: Australian Burden of Disease Study; Australian Institute of Health and Welfare: Canberra, Australia, 2017. [Google Scholar]
- Australian Department of Health. Australia’s Physical Activity and Sedentary Behaviour Guidelines. Available online: http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-strateg-phys-act-guidelines/$File/FS-Adults-18-64-Years.PDF (accessed on 24 November 2015).
- Slentz, C.; Houmard, J.; Kraus, W. Modest exercise prevents the progressive disease associated with physical inactivity. Exerc. Sport Sci. Rev. 2007, 35, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Sommerburg, O.; Keunen, J.; Bird, A.; van Kuijk, F. Fruits and vegetables that are sources for lutein and zeaxanthin: The macular pigment in human eyes. Br. J. Opthalmol. 1998, 82, 907–910. [Google Scholar] [CrossRef]
- Mares-Perlman, J.; Millen, A.; Ficek, T.; Hankinson, S. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. J. Nutr. 2002, 132, 518S–524S. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hagio, M.; Inoue, R.; Mitani, T.; Yajima, M.; Hara, H.; Yajima, T. Long-term oral feeding of lutein-fortified milk increases voluntary running distance in rats. PLoS ONE 2014, 9, e93529. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.L.; Coates, A.M.; Howe, P.R.; Bryan, J.; Matsumoto, M.; Buckley, J.D. Increases in plasma lutein through supplementation are correlated with increases in physical activity and reductions in sedentary time in older adults. Nutrients 2014, 6, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.; Elias, M.; Alkerwi, A.; Buckley, J. Intake of lutein-rich vegetables is associated with higher levels of physical activity. Nutrients 2015, 7, 8058–8071. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.; Buckley, J.; Coates, A.M.; Buckley, E. Does Dietary Intake of Lutein Affect Physical Activity Behavior in Adults? A Systematic Review and Meta-Analysis. Available online: https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016046749 (accessed on 29 August 2016).
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (axis). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Gross, M.D.; Jacobs, D.R., Jr. Association of serum carotenoids and tocopherols with γ- glutamyltransferase: The cardiovascular risk development in young adults (cardia) study. Clin. Chem. 2004, 50, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Ohira, T.; Hozawa, A.; Iribarren, C.; Daviglus, M.L.; Matthews, K.A.; Gross, M.D.; Jacobs, D.R., Jr. Longitudinal association of serum carotenoids and tocopherols with hostility: The cardia study. Am. J. Epidemiol. 2008, 167, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Tanaka, K.; Kiyohara, C.; Hirohata, T.; Tomita, Y.; Ishibashi, M.; Kido, K. Relationship of alcohol use, physical activity and dietary habits with serum carotenoids, retinol and alpha-tocopherol among male Japanese smokers. Int. J. Epidemiol. 1997, 26, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Hamulka, J.; Wawrzyniak, A.; Gadomska, M.; Gronowska-Senger, A.B.; Bawa, S. The influence of selected demographic and lifestyle factors on lutein intakes by groups of polish women. Int. J. Food Sci. Nutr. 2009, 60, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gaziano, J.M.; Norkus, E.P.; Buring, J.E.; Sesso, H.D. Associations of plasma carotenoids with risk factors and biomarkers related to cardiovascular disease in middle-aged and older women. Am. J. Clin. Nutr. 2008, 88, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Ainsworth, B.E. Associations of food consumption, serum vitamins and metabolic syndrome risk with physical activity level in middle-aged adults: The national health and nutrition examination survey (nhanes) 2005–2006. Public Health Nutr. 2016, 19, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, T.A.; Curran-Celantano, J.; Cooper, D.A.; Hammond, B.R., Jr.; Danis, R.P.; Pratt, L.M.; Riccardi, K.A.; Filloon, T.G. Macular pigment optical density in a midwestern sample. Ophthalmology 2001, 108, 730–737. [Google Scholar] [CrossRef]
- Coyne, T.; Ibiebele, T.I.; McNaughton, S.; Rutishauser, I.H.E.; O’Dea, K.; Hodge, A.M.; McClintock, C.; Findlay, M.G.; Lee, A. Evaluation of brief dietary questions to estimate vegetable and fruit consumption—Using serum carotenoids and red-cell folate. Public Health Nutr. 2005, 8, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thornquist, M.D.; Neuhouser, M.L.; Kristal, A.R.; Neumark-Sztainer, D.; Cooper, D.A.; Patterson, R.E.; Cheskin, L.J. Diet and lifestyle correlates of lutein in the blood and diet. J. Nutr. 2002, 132, 525S–530S. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.; Potter, J.D. Physical activity and colon cancer: Confounding or interaction? Med. Sci. Sports Exerc. 2002, 34, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Stimpson, J.P.; Nash, A.C.; Ju, H.; Eschbach, K. Neighborhood deprivation is associated with lower levels of serum carotenoids among adults participating in the third national health and nutrition examination survey. J. Am. Diet. Assoc. 2007, 107, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Tormo, M.J.; Navarro, C.; Chirlaque, M.D.; Barber, X.; Argilaga, S.; Agudo, A.; Amiano, P.; Barricarte, A.; Beguiristain, J.M.; Dorronsoro, M.; et al. Physical sports activity during leisure time and dietary intake of foods and nutrients in a large spanish cohort. Int. J. Sport Nutr. Exerc. 2003, 13, 47–64. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, S.J.; McCullough, M.L.; Song, W.O.; Fernandez, M.L.; Koo, S.I.; Chun, O.K. Dietary carotenoids are associated with cardiovascular disease risk biomarkers mediated by serum carotenoid concentrations. J. Nutr. 2014, 144, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Gruber, M.; Chappell, R.; Millen, A.; LaRowe, T.; Moeller, S.M.; Iannaccone, A.; Kritchevsky, S.B.; Mares, J. Correlates of serum lutein + zeaxanthin: Findings from the third national health and nutrition examination survey. J. Nutr. 2004, 134, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Sahli, M.W.; Mares, J.A.; Meyers, K.J.; Klein, R.; Brady, W.E.; Klein, B.E.K.; Ochs-Balcom, H.M.; Donahue, R.P.; Millen, A.E. Dietary intake of lutein and diabetic retinopathy in the atherosclerosis risk in communities study (aric). Ophthalmic Epidemiol. 2016, 23, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.M.; Parekh, N.; Tinker, L.; Ritenbaugh, C.; Blodi, B.; Wallace, R.B.; Mares, J.A.; CAREDS Research Study Group. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the carotenoids in age-related eye disease study (careds): Ancillary study of the women’s health initiative. Arch. Ophthalmol. 2006, 124, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council of Australia. Nhmrc Levels of Evidence and Grades for Recommendations for Developers of Clinical Practice Guidelines. Available online: https://www.nhmrc.gov.au/_files_nhmrc/file/guidelines/developers/nhmrc_levels_grades_evidence_120423.pdf (accessed on 5 December 2016).
- Hamulka, J.; Koczara, J.; Gronek, M. Lutein content of selected polish foods and estimation of its intake. Pol. J. Food Nutr. Sci. 2005, 14/15, 201–206. [Google Scholar]
- The atherosclerosis risk in communities (aric) study: Design and objectives. The aric investigators. Am. J. Epidemiol. 1989, 129, 687–702. [Google Scholar]
- Natarajan, L.; Flatt, S.W.; Sun, X.; Gamst, A.C.; Major, J.M.; Rock, C.L.; Al-Delaimy, W.; Thomson, C.A.; Newman, V.A.; Pierce, J.P.; et al. Validity and systematic error in measuring carotenoid consumption with dietary self-report instruments. Am. J. Epidemiol. 2006, 163, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, C.; Betts, N.; Hoos, T.; Glenn, M. Young adult exercisers and nonexercisers differ in food attitudes, perceived dietary changes, and food choices. Int. J. Sport Nutr. 1996, 6, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Charreire, H.; Kesse-Guyot, E.; Bertrais, S.; Simon, C.; Chaix, B.; Weber, C.; Touvier, M.; Galan, P.; Hercberg, S.; Oppert, J.M. Associations between dietary patterns, physical activity (leisure-time and occupational) and television viewing in middle-aged french adults. Br. J. Nutr. 2011, 105, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Olinto, M.T.; Willett, W.C.; Gigante, D.P.; Victora, C.G. Sociodemographic and lifestyle characteristics in relation to dietary patterns among young Brazilian adults. Public Health Nutr. 2011, 14, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Ricci, C.; Baumeister, S.E.; Leitzmann, M.F. Replacing sedentary time with physical activity in relation to mortality. Med. Sci. Sports Exerc. 2016, 48, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Fishman, E.I.; Steeves, J.A.; Zipunnikov, V.; Koster, A.; Berrigan, D.; Harris, T.A.; Murphy, R. Association between objectively measured physical activity and mortality in nhanes. Med. Sci. Sports Exerc. 2016, 48, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. 4364.0.55.004—Australian Health Survey: Physical Activity, 2011–12. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4364.0.55.004Chapter1002011-12 (accessed on 5 June 2017).
- Vishwanathan, R.; Neuringer, M.; Snodderly, D.M.; Schalch, W.; Johnson, E.J. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr. Neurosci. 2013, 16, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.W., Jr.; Smith, J.W.; Kuchan, M.J.; Mohn, E.S.; Johnson, E.J.; Rubakhin, S.S.; Wang, L.; Sweedler, J.V.; Neuringer, M. Lutein and brain function. Foods 2015, 4, 547–564. [Google Scholar] [CrossRef] [PubMed]
| Reference | Study Design | NHMRC Level of Evidence | CA Score | Sample Characteristics | Physical Activity Outcome Measure | Physical Activity Measure | Lutein Outcome Measure | Measure of Lutein Status | Measure of Association (Type, Strength, Direction, and Significance) |
|---|---|---|---|---|---|---|---|---|---|
| Randomised control trials | |||||||||
| Thomson et al. (2014) [10] | RCT | II | 7 | Australia n = 44 Sedentary, but otherwise healthy males and females 68.8 ± 6.4 years BMI 25.3 ± 2.6 kg/m2 | Subjective and objective Amount of sedentary PA, light PA and mod–vig PA by exercise diary Cut-point PA levels using accelerometer (7164 ActiGraph) over 7-day period | Lutein (Mean values) Sed: 235 min/day (SD 61), light PA: 301 min/day (SD 88), mod-vig PA: 22 min/day (SD 14) Accelerometer 235,292 counts per day (SD 82, 693) Placebo Sed: 219 mins/day (SD 46), light PA: 341 min/day (SD 76), mod-vig PA: 24 min/day (SD 18) Accelerometer 273,760 counts per day (SD 85, 018) | Objective HPLC (plasma lutein and zeaxanthin separately) | Lutein: mean 10.3 ug/dL (SD 2.5) Placebo: mean 10.1 ug/dL (SD 3.6) | (1) Correlation SEDENTARY: Plasma lutein and time spent sedentary: r = −0.36 (p = 0.03) PA: Plasma lutein and activity counts: r = 0.29, p = 0.08 (2) % change correlation SEDENTARY: Time sedentary and relative % change in plasma lutein: r = −0.39 (p = 0.02) PA: % difference lutein with % difference activity, r = 0.36, p = 0.03 |
| Cross sectional studies ^ | |||||||||
| Choi et al. (2016) [20] | Cross sectional | IV | 17 | USA n = 1661 NHANES 40–70 years | Objective Accelerometer daily steps (sedentary [s], intermediate [i], and active [a]) | Male: s <6802 steps/day i = 6082–10, 698 steps/day a >10, 698 steps/day Female: s < 5785 steps/day i = 5785–9225 steps/day a ≥ 9226 steps/day | Objective Serum lutein and zeaxanthin | Male: s = 14.0 ug/dL mean (SE 0.7) i = 16.3 ug/dL mean (SE 0.6) a = 18.9 ug/dL mean (SE 0.6) Female: s = 14.8 ug/dL mean (SE 0.6) i = 17.1 ug/dL mean (SE 0.5) a = 18.6 ug/dL mean (SE 0.5) | ANCOVA tertiles of step counts. Covariates: age, BMI, total energy intake M: positive increase serum lutein/zeaxanthin with increasing tertile step counts (p < 0.01) F: positive increase serum lutein/zeaxanthin with increasing tertile step counts (p < 0.01) >26% higher serum lutein + zexanthin in active vs sedentary participants Associated with >57% difference in accelerometer step counts between sedentary and active participants |
| Coyne et al. (2005) [22] | Cross sectional | IV | 17 | Australia n = 1598 Random sample of adults ≥25 years | Subjective Self-report questionnaire previous week. PA time: sum of time walking or moderate intensity activity plus double time vigorous activity | Sufficiently active >150 min/week In-sufficiently active <150 min/week Sedentary 0 min/week | Objective HPLC serum lutein/zeaxanthin | Active: 0.39 umol/L mean (95% CI 0.37–0.41) In-sufficiently active: 0.39 umol/L mean. (95% CI 0.37–0.41) Sedentary: 0.37 umol/L mean (95% CI 0.34–0.39) | ANOVA No significant difference serum lutein/zeaxanthin across PA tertiles, p > 0.01 |
| Gruber et al. (2004) [28] | Cross sectional | IV | 15 | USA n = 7059 NHANES ≥40 years | Subjective Interview | PA ‘yes’ by quintiles of serum lutein/zeaxanthin Q1: 51%, Q3: 62%, Q5: 69%. | Objective HPLC serum lutein/zeaxanthin | Q1: 0.02–0.25 umol/L Q3: 0.33–0.44 umol/L Q5: 0.58–4.45 umol/L | Participants who were physically active had 13% higher serum lutein/zeaxanthin than those who were not active, p < 0.01 |
| Kitamura et al. (1997) [17] | Cross sectional | IV | 15 | Japan n = 194 Healthy male smokers (>15 cigarettes/day) 24–60 years | Subjective Self-reported questionnaire (closed questions) | Average duration walking/day (<30 min, 30 min to 1 h, 1–2 h, >2 h) Frequency participating in sports (none, occasional, frequent) | Objective HPLC (serum lutein) | HPLC: Mean 39.2 ug/dL (95% CI 37.5–41.0) | Spearman rank correlation coefficient (adjusted for age) Between walking time and serum lutein, r = 0.01 (p > 0.05) Between frequency of sport participation and serum lutein, r = 0.12 (p < 0.05) |
| Lee et al. (2004) [15] | Cross sectional | IV | 15 | USA n = 3128 Black and white males and females 17–35 years | Subjective CARDIA PA history, Minnesota Leisure time PA questionnaire (Simplified version) | Data not reported | Objective HPLC serum lutein/zeaxanthin | Data not reported | Linear regression analysis r = 0.08 (p < 0.01) |
| Slattery & Potter (2002) [24] | Cross sectional (case control) | IV | 15 | USA Colon cancer cases n = 1993 Control n = 2410 30–79 years | Subjective CARDIA PA questionnaire (Scoring 1 = no vigorous leisure time PA) 2 = 1–250 cal/week. 3 = 251–1000 cal/week. 4 ≥ 1000 cal/week) | Men: Case: 1: n = 233, 2: n = 312, 3: n = 329, 4: n = 225. Control: 1: n = 216, 2: n = 314, 3: n = 379, 4: n = 380 Women: Case: 1: n = 326, 2: n = 233, 3: n = 189, 4: n = 146. Control: 1: n = 318, 2: n = 314, 3: n = 264, 4: n = 224 | Subjective Nutrient values calculated using Minnesota NCC database | Data not reported | (1) Correlation coefficient: Male: r = 0.08 (p < 0.05) Female: r = 0.05 (p > 0.05) |
| Hamulka et al. (2009) [18] | Cross sectional | IV | 14 | Poland n = 100 Female 48.6 ± 16.2 years BMI 24.6 kg/m2 | Subjective Self-reported questionnaire | Sedentary, moderate, high (values not reported) | Subjective Dietary lutein intake from food records | Sed: mean 2.02 mg/day (SD 0.67) Mod: mean 2.29 mg/day (SD 1.21) High: mean 1.85 mg/day (SD 0.74) | Non-parametric Kruskal–Wallis ANOVA, p = 0.33 Spearman rank correlation: Crude: r = 0.105 (p = 0.30) Adjusted for age, BMI, place of dwelling and level of education: r = −0.062 (p = 0.55) |
| Sahli et al. (2016) [29] | Cross sectional | IV | 14 | USA n = 1430 Black and white male and female 45–65 years Diabetes mellitus | Subjective Modified Baecke PA questionnaire | By quintiles of lutein intake Q1: PA at work 2.2 (SD 0.6) Sports in leisure time 2.3 (SD 0.8) Other leisure time PA 2.3 (SD 0.6) Q4: PA at work 2.1 (SD 1.0) Sports in leisure time 2.4 (SD 0.7) Other leisure time physical activity 2.3 (SD 0.6) | Subjective (outcome measure not reported) | Q1: mean 435.2 ug/1000 kcal (SD 165.1) Q4: mean 4853.1 ug/1000 kcal (SD 2695.3) | ANOVA PA at work index p = 0.29 Sports in leisure time index p = 0.89 Other leisure time PA index p = 0.25 |
| Stimpson et al. (2007) [25] | Cross sectional | IV | 13 | USA n = 17, 002 NHANES ≥17 years | Subjective Self-reported questionnaire | PA score of 0: n = 11,757 PA score >1: n = 5236 Missing: n = 9 | Objective HPLC serum lutein/zeaxanthin | PA score 0: mean 22.62 ug/dL (SD 12.59) PA score ≥1: mean 23.17 ug/dL (SD 13.33) | Multivariate linear regression ≥1, using high PA as a reference B value = −1.10 (SE 0.34) p < 0.01. Indicating that as serum lutein + zeaxanthin increased physical inactivity decreased |
| Tormo et al. (2003) [26] | Cross sectional | IV | 13 | Spain n = 37, 287 Healthy male and female 50.9 ± 7.2 years BMI 28.4 ± 3.4 kg/m2 31% smokers 17% heavy drinkers | Subjective PA questionnaire | 0–0.5 h/week, >0.5–2 h/week, >2–3 h/week, >3 h/week | Subjective Lutein intake, food recalls against Food Composition table | 0–0.5 h/week (ref): mean 784.7 ug/day (SD 826.0) >0.5–2 h/week mean 898.8 ug/day (SD 828.6) >2–3 h/week mean 935.6 (SD 910.7) >3 h/week mean 854.7 ug/day (SD 840.0) | p < 0.05 for ANOVA comparing mean value of each PA category with reference level p < 0.05 for ANCOVA comparing mean PA category with reference level adjusted by age, BMI, current smoking, excessive alcohol drinking, secondary/higher education, sedentary PA at work and interaction of education with PA at work ≥600% difference in hours of PA |
| Ciulla et al. (2001) [21] | Cross sectional | IV | 12 | USA n = 280 Male and female 18–50 years 26% smokers | Subjective Self-reported questionnaire | Number of times exercise per week | Objective Serum lutein/zeaxanthin (umol/L) Subjective FFQ lutein/zeaxanthin (ug/day) | Serum: 0.372 umol/L mean (SD 0.169) Intake: 1102 ug/day mean (SD 839) | Spearman correlation coefficient Serum: r = 0.02, p > 0.05 Intake: r = 0.25, p < 0.05 |
| Moeller et al. (2006) [30] | Cross sectional | IV | 12 | USA n = 1787 Female 50–79 years | Subjective Self-reported questionnaire | Physical activity levels Low: 12 MET/week High PA: 18 MET/week | Objective HPLC serum lutein/zeaxanthin Subjective Dietary intake questionnaire | Dietary lutein intake Low: mean 792 ug/day (SD 169) High: mean 2868 ug/day (SD 919) | No measure of association between serum lutein and physical activity reported 50% difference in physical activity between low and high dietary intake groups (t-test, p ≤ 0.001). |
| Ohira et al. (2008) [16] | Cross sectional | IV | 12 | USA n = 3579 18–30 years | Subjective Self-reported total PA score, habitual PA, and participation in 13 different PA categories (vigorous to moderate) over 12 months | Total CARDIA PA history score (arbitrary units) | Objective HPLC 12-h fasting serum lutein/zeaxanthin | Data not reported | Correlation coefficient, adjusted for age, gender, race, and serum lipid r = 0.06, p < 0.01 |
| Wang et al. (2008) [19] | Cross sectional | IV | 11 | USA n = 2895 Female ≥45 years Self-reported free from cardiovascular disease and cancer (except non-melanoma skin cancer) | Subjective Questionnaire: self-reported vigorous PA | Rarely/never (ref), <1 time/week, 1–3 times/week, >4 times/week | Objective HPLC serum lutein/zeaxanthin | Reported as mean (95% CI) Rarely/never (ref): 0.279 umol/L (0.271–0.286) <1 time/week: 0.284 umol/L (0.274–0.296) 1–3 times/week 0.300 umol/L (0.290–0.309) >4-times/week: 0.310 umol/L (0.293–0.328) | Serum lutein + zeaxanthin significantly higher in 1–3 times/week and >4 times/week compared with rarely or never (t-test) p < 0.001 >400% difference between reference and >4 times per week of physical activity |
| Rock et al. (2002) [23] | Cross sectional | IV | 9 | USA n = 2786 Male and female 44 ± 16 years BMI 27.5 ± 6.1 kg/m2 | Subjective Questionnaire, PA minutes/day | <30 min/day (ref), 30–60 min/day or >60 min/day | Subjective Dietary lutein/zeaxanthin intake | Mean intake 1347 (891) ug/day | % difference in dietary intake of lutein + zeaxanthin from reference physical activity group (<30 min/day) 30–60 min/day: 10.3% higher dietary intake (5.6–15.3%) p = 0.05 >60 min/day: 19.3% higher dietary intake (9.6–29.8%) p = 0.05 |
| Wang et al. (2014) [27] | Cross sectional | IV | 9 | USA n = 2856 NHANES Male and female ≥ 20 years | Subjective Questionnaire | <2.5 MET h/week (ref) 2.5–<4 MET h/week 4–<11.5 MET h/week >11.5 MET h/week | Subjective Dietary lutein/zeaxanthin intake | <2.5 MET h/week: dietary intake of 0.65 mg/day (0.59–0.71) 2.5–<4 MET h/week: dietary intake of 0.84 mg/day (0.75–0.94) 4–<11.5 MET h/week: dietary intake of 0.82 mg/day (0.73-0.92) >11.5 MET h/week: dietary intake of 0.75 mg/day (0.68–0.83) | Multivariate model geometric means (95% CIs) Dietary lutein/zeaxanthin intake for 2.5–<4 MET h/week and 4–<11.5 MET h/week significantly different from <2.5 MET-h/week (ref) (p < 0.017) >400% difference in physical activity between dietary lutein intake of 0.65 mg/day (<2.5 MET h/week group) and dietary intake of 0.82–0.84 mg/day (2.5 to <11.5 MET h/week groups) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooke, M.C.; Coates, A.M.; Buckley, E.S.; Buckley, J.D. Lutein Intake and Blood Lutein Concentration Are Positively Associated with Physical Activity in Adults: A Systematic Review. Nutrients 2018, 10, 1186. https://doi.org/10.3390/nu10091186
Cooke MC, Coates AM, Buckley ES, Buckley JD. Lutein Intake and Blood Lutein Concentration Are Positively Associated with Physical Activity in Adults: A Systematic Review. Nutrients. 2018; 10(9):1186. https://doi.org/10.3390/nu10091186
Chicago/Turabian StyleCooke, Madeline C., Alison M. Coates, Elizabeth S. Buckley, and Jonathan D. Buckley. 2018. "Lutein Intake and Blood Lutein Concentration Are Positively Associated with Physical Activity in Adults: A Systematic Review" Nutrients 10, no. 9: 1186. https://doi.org/10.3390/nu10091186
APA StyleCooke, M. C., Coates, A. M., Buckley, E. S., & Buckley, J. D. (2018). Lutein Intake and Blood Lutein Concentration Are Positively Associated with Physical Activity in Adults: A Systematic Review. Nutrients, 10(9), 1186. https://doi.org/10.3390/nu10091186







