Biogeochemical Model Optimization by Using Satellite-Derived Phytoplankton Functional Type Data and BGC-Argo Observations in the Northern South China Sea
Abstract
1. Introduction
2. Methods
2.1. Model Description
2.2. Sensitivity Analysis
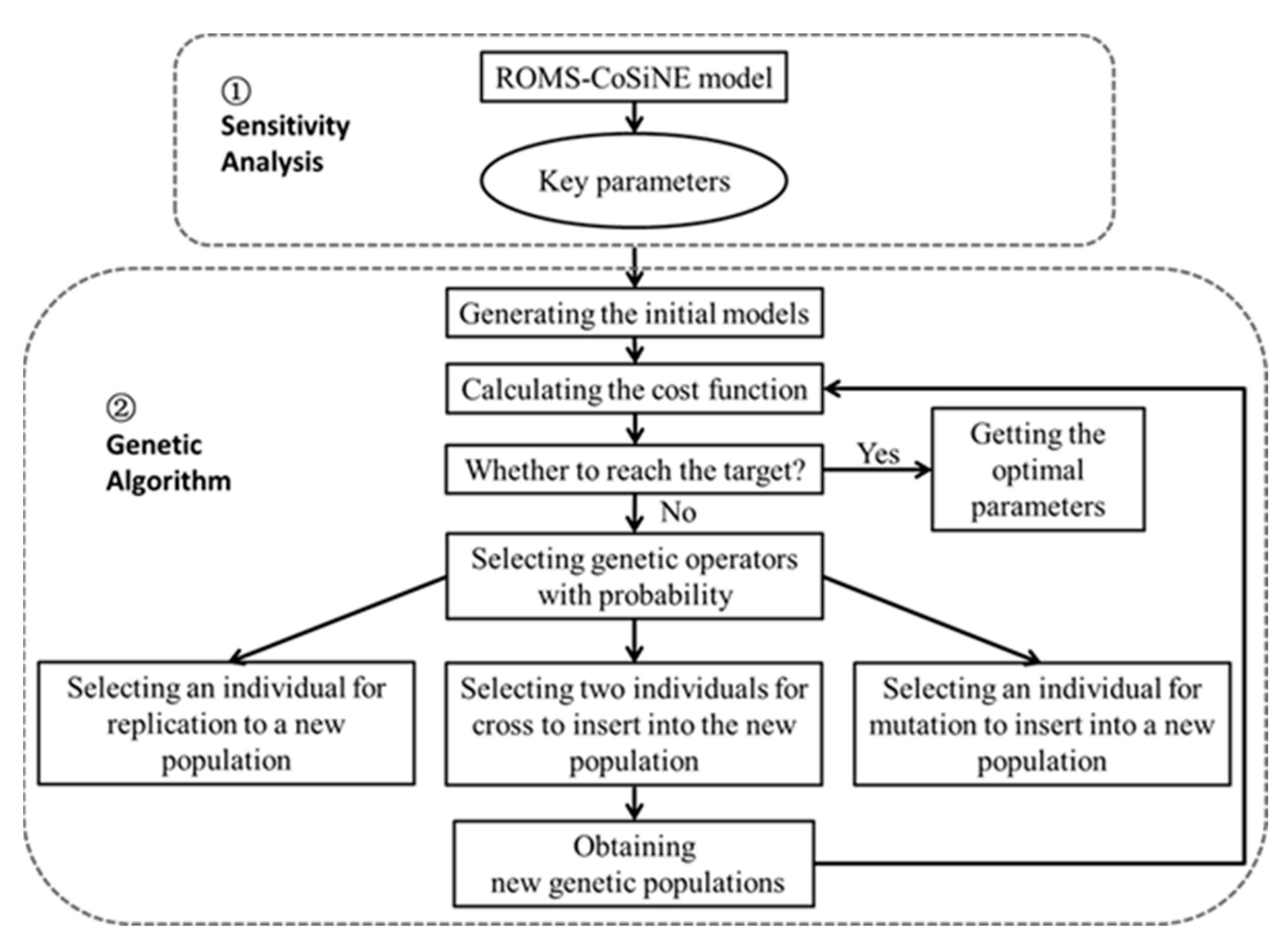
2.3. Genetic Algorithm
2.4. Data and Optimization Experiments
3. Results
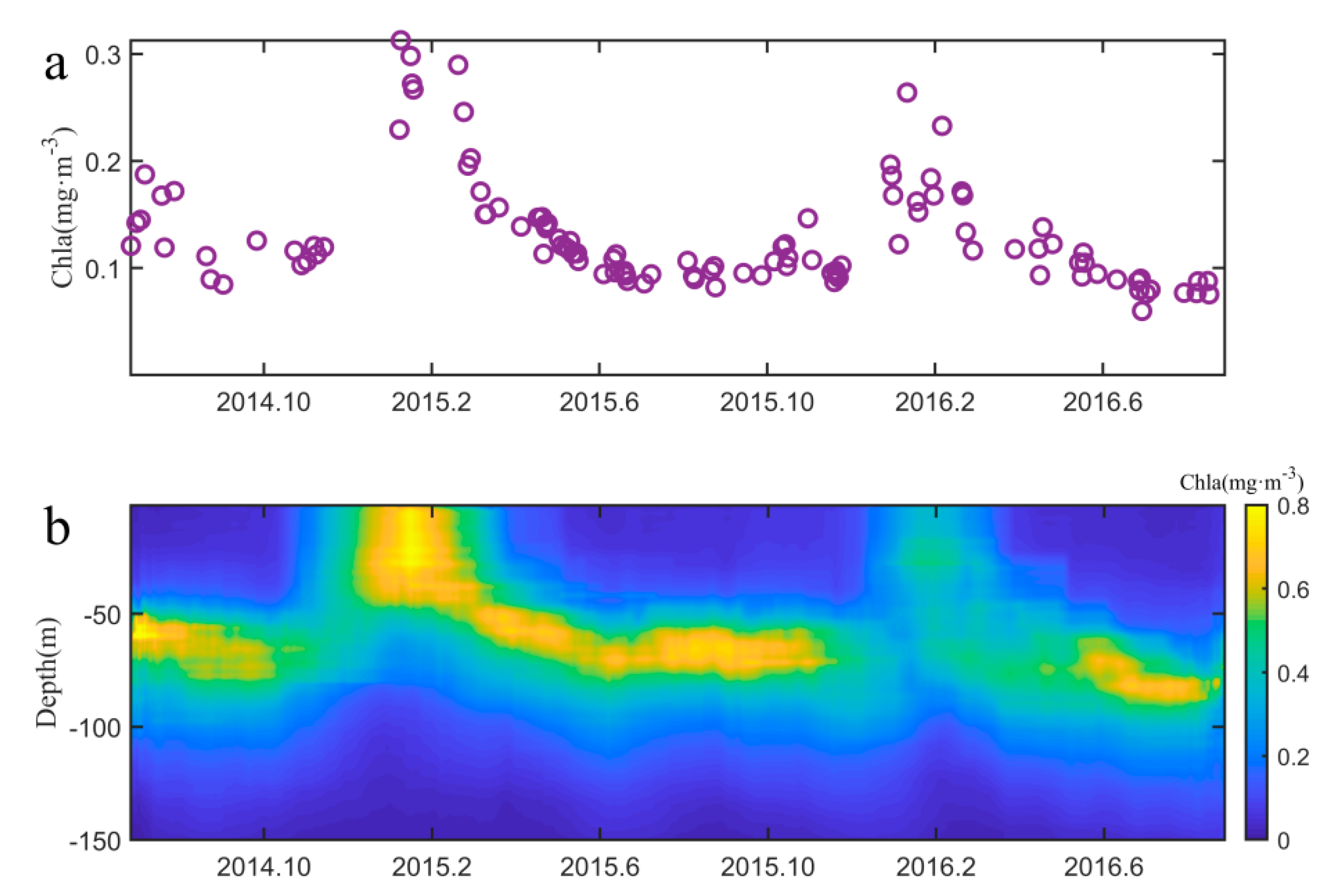
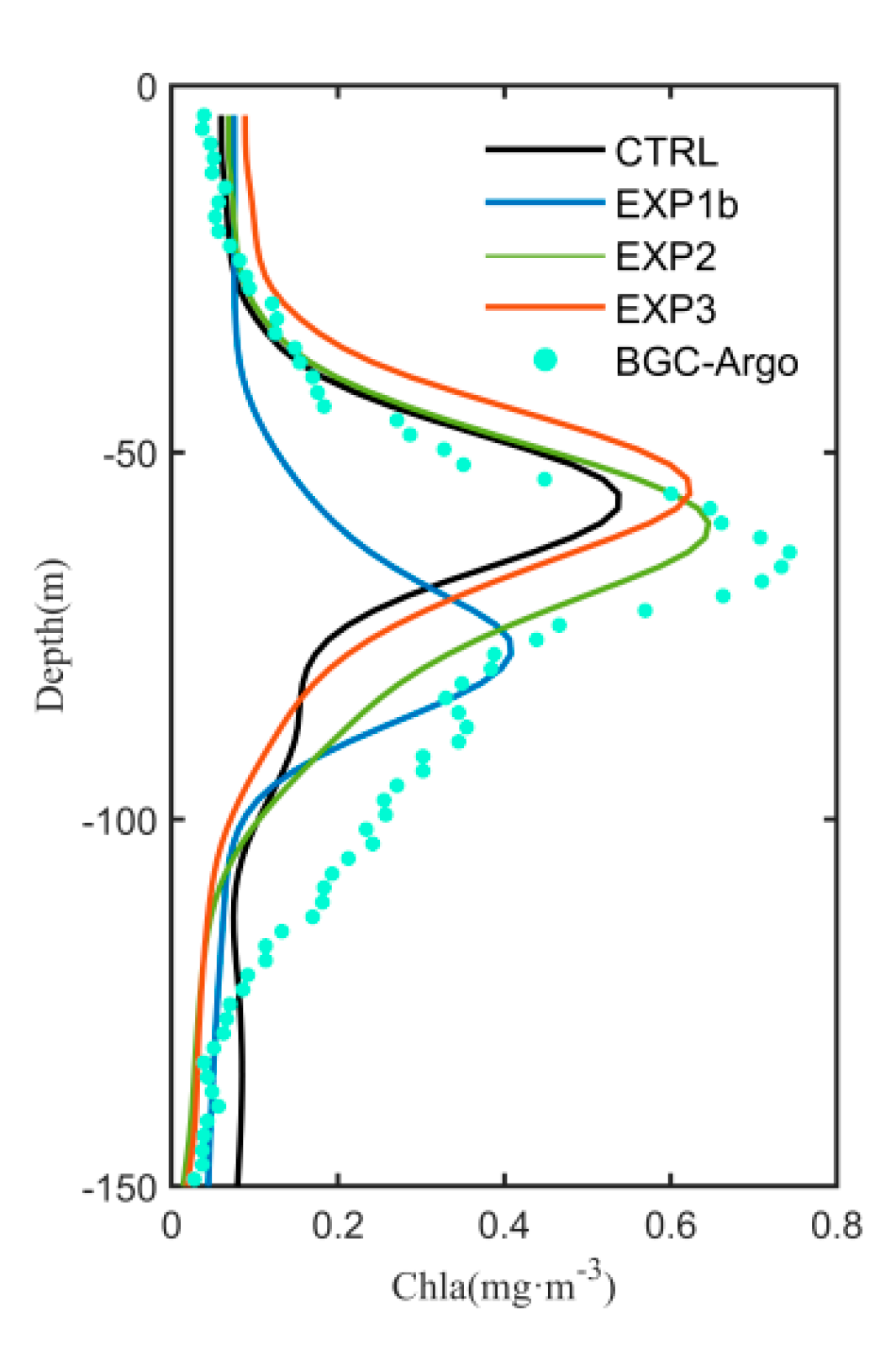
3.1. Seasonal Variation of Chlorophyll-a
3.2. Optimizable Parameter Selection
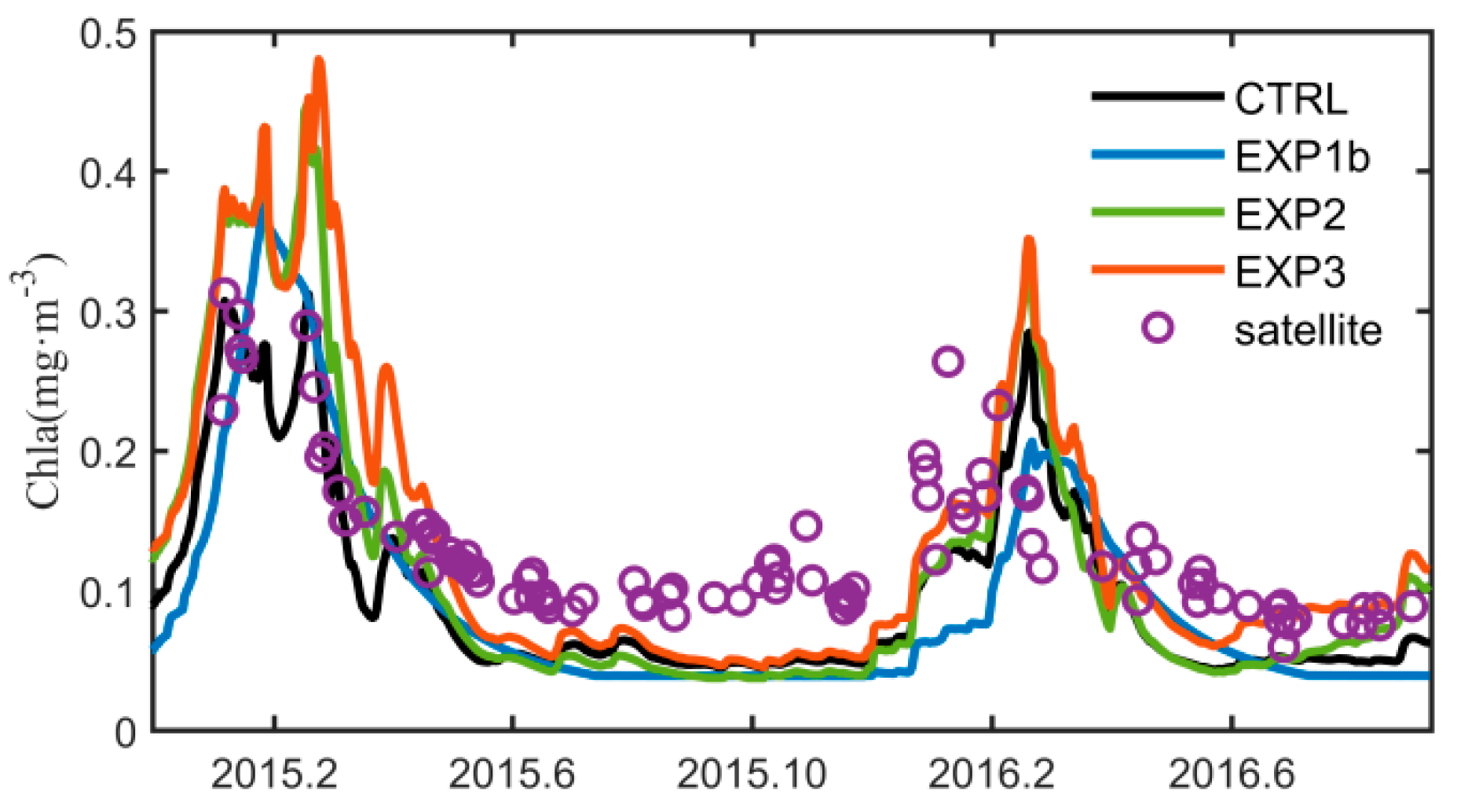
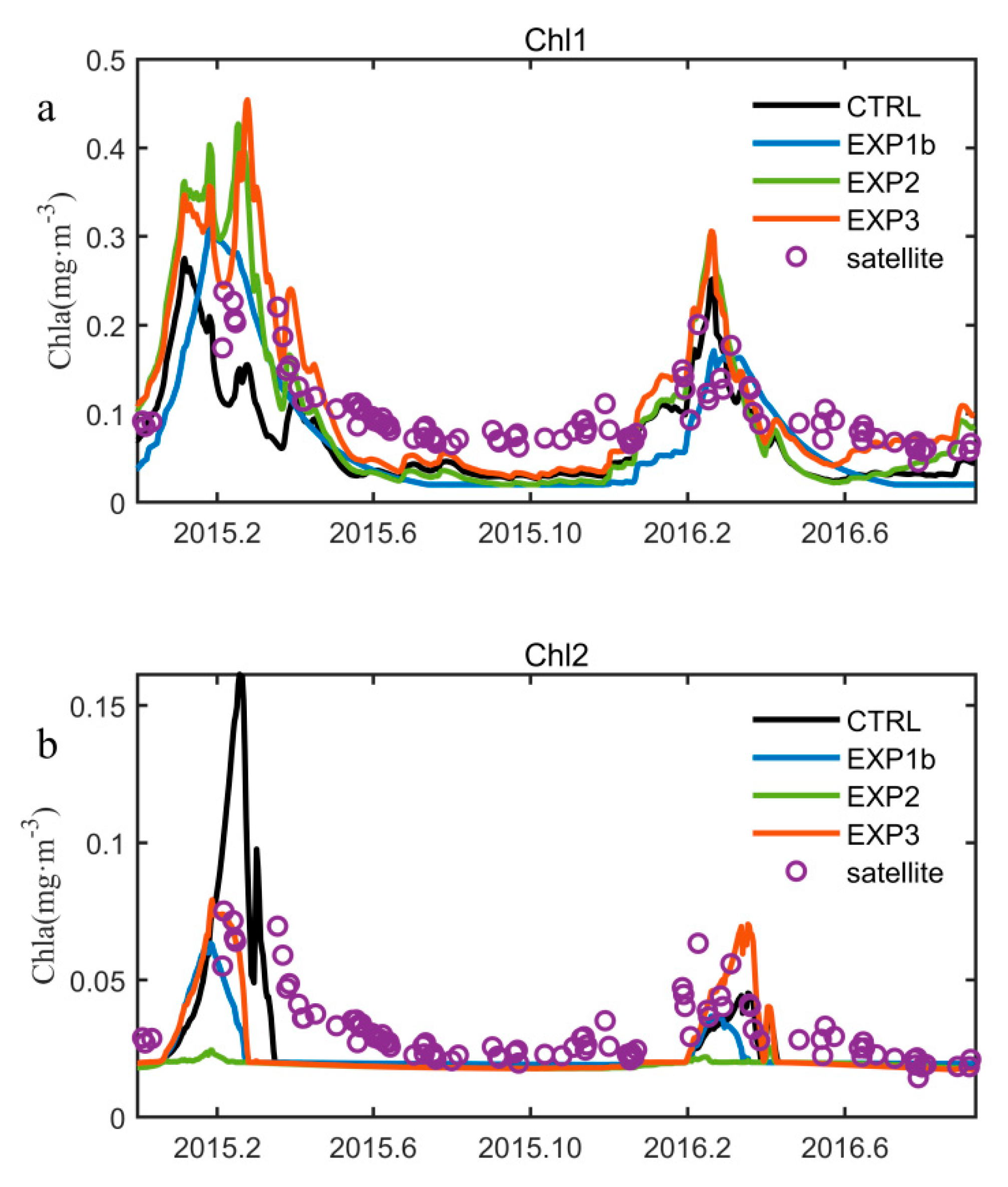
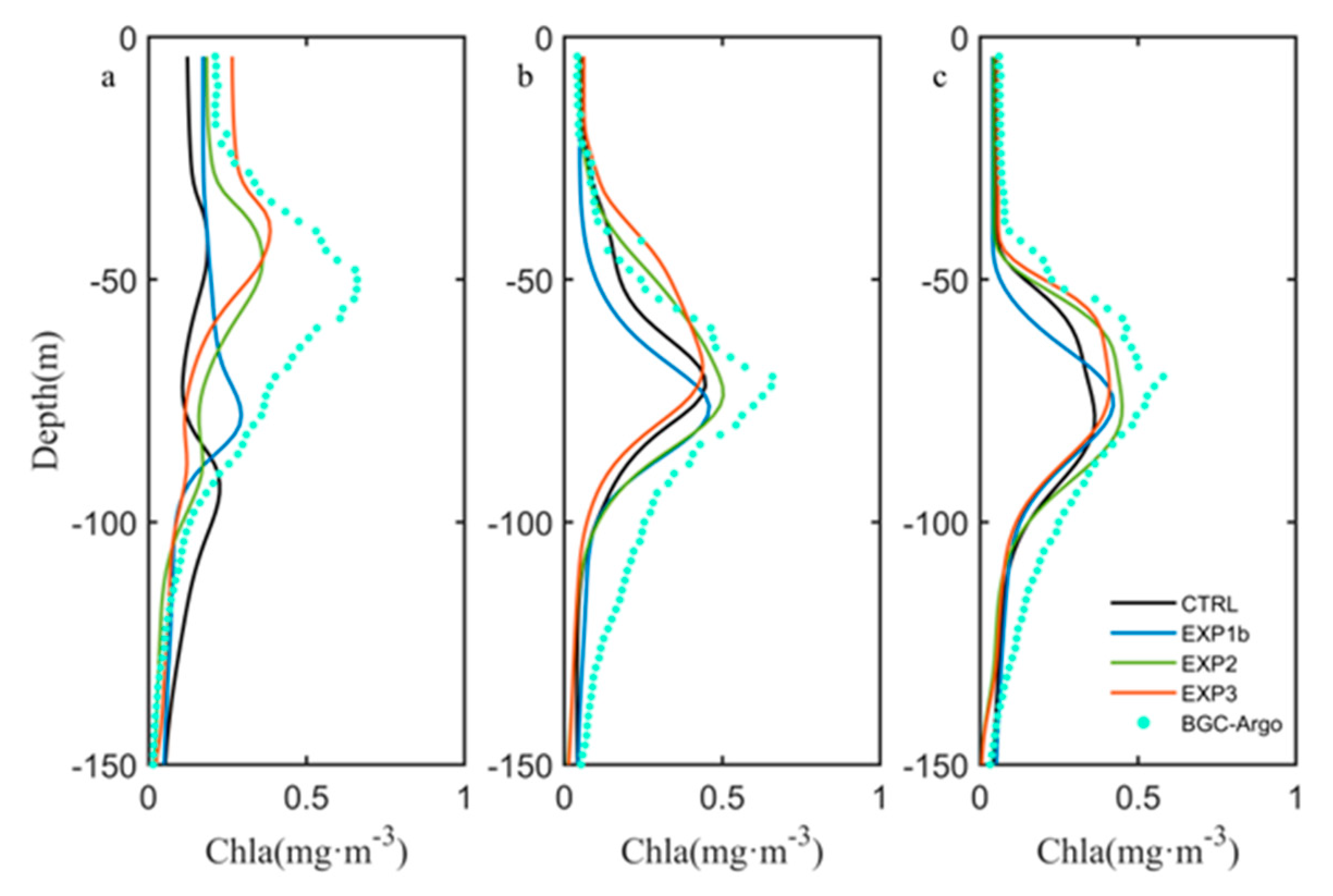
3.3. Optimization Results
4. Discussion
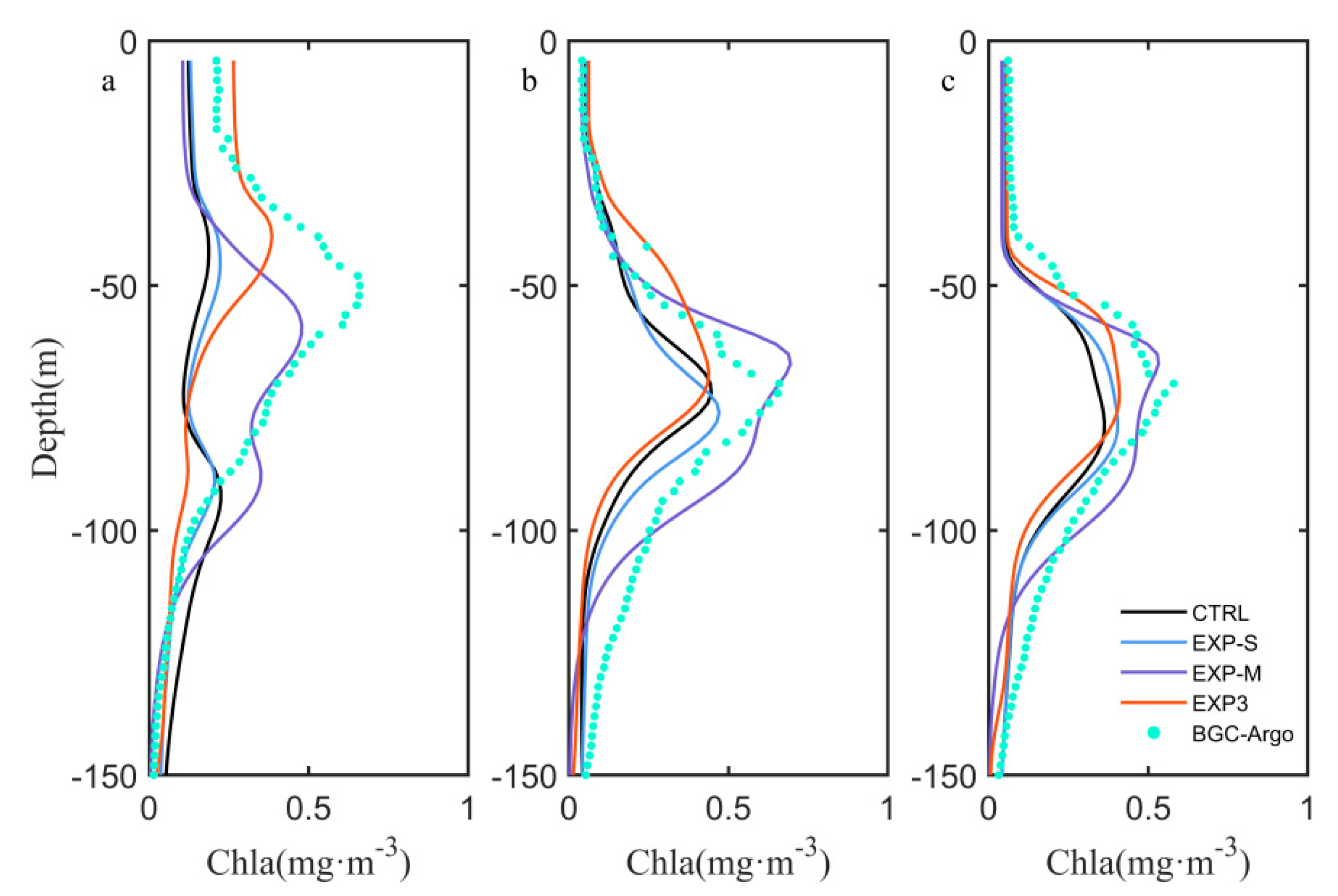
4.1. Influence of Sampling Frequency of Float Data
4.2. Effects of Biological Parameter on Vertical Chlorophyll-a Structure
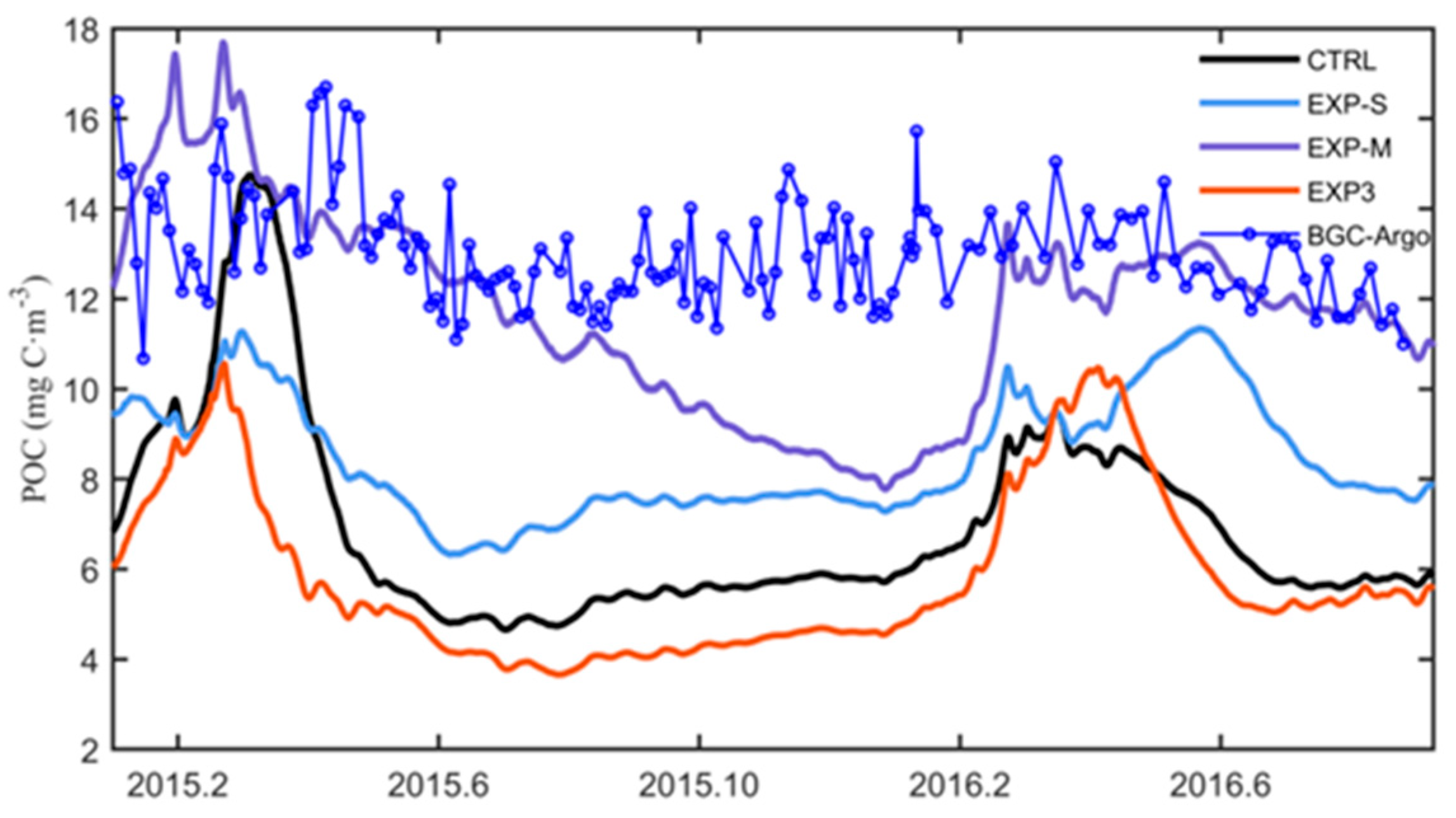
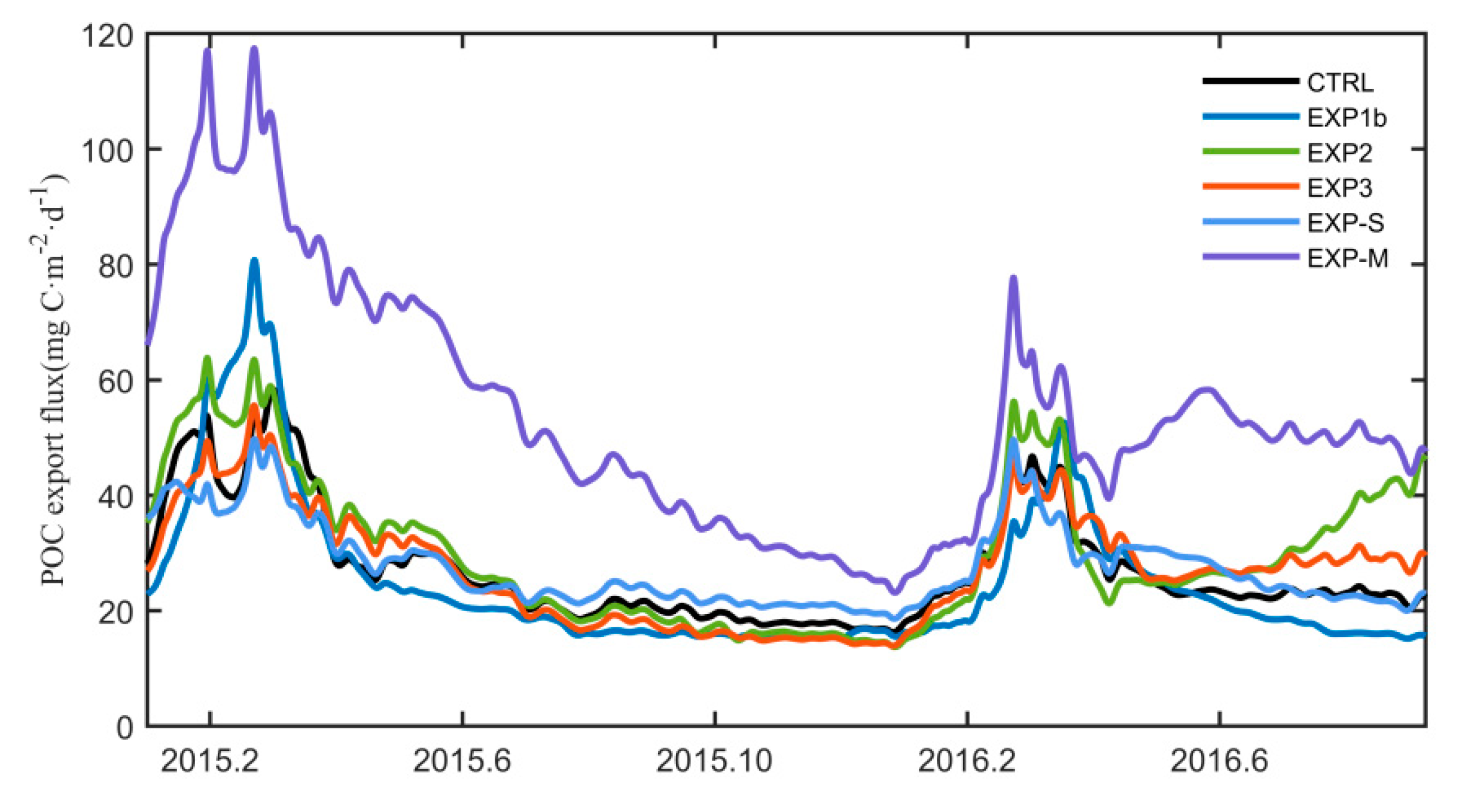
4.3. Impacts on Subsurface POC and Export Flux
5. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
References
- Friedrichs, M.A.M.; Hood, R.R.; Wiggert, J.D. Ecosystem model complexity versus physical forcing: Quantification of their relative impact with assimilated Arabian Sea data. Deep Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 576–600. [Google Scholar] [CrossRef]
- Bisson, K.M.; Siegel, D.A.; Devries, T. How Data Set Characteristics Influence Ocean Carbon Export Models. Glob. Biogeochem. Cycles 2018, 32, 1312–1328. [Google Scholar] [CrossRef]
- Fennel, K.; Losch, M.; Schröter, J.; Wenzel, M. Testing a marine ecosystem model: Sensitivity analysis and parameter optimization. J. Mar. Syst. 2001, 28, 45–63. [Google Scholar] [CrossRef]
- Kuroda, H.; Kishi, M.J. A data assimilation technique applied to estimate parameters for the NEMURO marine ecosystem model. Ecol. Model. 2004, 172, 69–85. [Google Scholar] [CrossRef]
- Dowd, M. Estimating parameters for a stochastic dynamic marine ecological system. Environmetrics 2011, 22, 501–515. [Google Scholar] [CrossRef]
- Mattern, J.P.; Dowd, M.; Fennel, K. Particle filter-based data assimilation for a three-dimensional biological ocean model and satellite observations. J. Geophys. Res. Ocean. 2013, 118, 2746–2760. [Google Scholar] [CrossRef]
- Xiao, Y.; Friedrichs, M.A.M. The assimilation of satellite-derived data into a one-dimensional lower trophic level marine ecosystem model. J. Geophys. Res. Ocean. 2014, 119, 2691–2712. [Google Scholar] [CrossRef]
- Gharamti, M.E.; Samuelsen, A.; Bertino, L.; Simon, E.; Korosov, A.; Daewel, U. Online tuning of ocean biogeochemical model parameters using ensemble estimation techniques: Application to a one-dimensional model in the North Atlantic. J. Mar. Syst. 2017, 168, 1–16. [Google Scholar] [CrossRef]
- Wang, B.; Fennel, K.; Yu, L.; Gordon, C. Assessing the value of biogeochemical argo profiles versus ocean color observations for biogeochemical model optimization in the Gulf of Mexico. Biogeosciences 2020, 17, 4059–4074. [Google Scholar] [CrossRef]
- Xue, H.; Chai, F.; Pettigrew, N.; Xu, D.; Shi, M.; Xu, J. Kuroshio intrusion and the circulation in the South China Sea. J. Geophys. Res. Ocean. 2004, 109, C02017. [Google Scholar] [CrossRef]
- Su, J. Overview of the South China Sea circulation and its influence on the coastal physical oceanography outside the Pearl River Estuary. Cont. Shelf Res. 2004, 24, 1745–1760. [Google Scholar] [CrossRef]
- Liu, K.K.; Chao, S.Y.; Shaw, P.T.; Gong, G.C.; Chen, C.C.; Tang, T.Y. Monsoon-forced chlorophyll distribution and primary production in the South China Sea: Observations and a numerical study. Deep Sea Res. Part I Oceanogr. Res. Pap. 2002, 49, 1387–1412. [Google Scholar] [CrossRef]
- Ning, X.; Chai, F.; Xue, H.; Cai, Y.; Liu, C.; Zhu, G.; Shi, J. Physical-biological oceanographic coupling influencing phytoplankton and primary production in the South China Sea. J. Geophys. Res. Ocean 2004, 109, C10005. [Google Scholar] [CrossRef]
- Shen, S.; Leptoukh, G.G.; Acker, J.G.; Yu, Z.; Kempler, S.J. Seasonal Variations of Chlorophyll a Concentration in the Northern South China Sea. IEEE Geosci. Remote Sens. Lett. 2008, 5, 315–319. [Google Scholar] [CrossRef]
- Tang, S.; Liu, F.; Chen, C. Seasonal and intraseasonal variability of surface chlorophyll a concentration in the South China Sea. Aquat. Ecosyst. Health Manag. 2014, 17, 242–251. [Google Scholar] [CrossRef]
- Geng, B.X.; Xiu, P.; Shu, C.; Zhang, W.Z.; Chai, F.; Li, S.; Wang, D. Evaluating the roles of wind- and buoyancy flux-induced mixing on phytoplankton dynamics in the northern and central South China Sea. J. Geophys. Res. Ocean. 2019, 124, 680–702. [Google Scholar] [CrossRef]
- Gong, X.; Shi, J.; Gao, H. Modeling seasonal variations of subsurface chlorophyll maximum in South China Sea. J. Ocean. Univ. China 2014, 13, 561–571. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Hu, J.; Geng, B. Experiments in optimizing simulations of the subsurface chlorophyll maximum in the South China Sea. J. Mar. Syst. 2016, 156, 1–15. [Google Scholar] [CrossRef]
- Gong, X.; Jiang, W.; Wang, L.; Gao, H.; Boss, E.; Yao, X.; Kao, S.J.; Shi, J. Analytical solution of the nitracline with the evolution of subsurface chlorophyll maximum in stratified water columns. Biogeosciences 2017, 14, 2371–2386. [Google Scholar] [CrossRef]
- Hirata, T.; Hardman-Mountford, N.J.; Brewin, R.J.W. Synoptic relationships between surface Chlorophyll-a and diagnostic pigments specific to phytoplankton functional types. Biogeosciences 2011, 8, 311–327. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Aiken, J.; Alvain, S. Phytoplankton Functional Types from Space; International Ocean-Colour Coordinating Group (IOCCG): Dartmouth, NS, Canada, 2014; p. 15. [Google Scholar]
- Kramer, S.J.; Roesler, C.S.; Sosik, H.M. Bio-optical discrimination of diatoms from other phytoplankton in the surface ocean: Evaluation and refinement of a model for the Northwest Atlantic. Remote Sens. Environ. 2018, 217, 126–143. [Google Scholar] [CrossRef]
- Kramer, S.J.; Siegel, D.A. How Can Phytoplankton Pigments Be Best Used to Characterize Surface Ocean Phytoplankton Groups for Ocean Color Remote Sensing Algorithms? J. Geophys. Res. Ocean. 2019, 124, 7557–7574. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cao, W.; Wang, G.; Hu, S. Satellite-observed variability of phytoplankton size classes associated with a cold eddy in the South China Sea. Mar. Pollut. Bull. 2014, 83, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Brewin, R.J.W.; Stefano, C.; Shubha, S.; Thomas, J.; Gavin, T.; Kieran, C.; Airs, R.L.; Denise, C.; Vanda, B.; Emanuele, O. Uncertainty in ocean-Color estimates of chlorophyll for phytoplankton groups. Front. Mar. Sci. 2017, 4, 104. [Google Scholar] [CrossRef]
- Ciavatta, S.; Brewin, R.J.W.; Skákala, J.; Polimene, L.; De Mora, L.; Artioli, Y.; Allen, J.I. Assimilation of ocean-color plankton functional types to improve marine ecosystem simulations. J. Geophys. Res. Ocean. 2018, 123, 834–854. [Google Scholar] [CrossRef]
- Skákala, J.; Ford, D.; Brewin, R.J.W.; McEwan, R.; Kay, S.; Taylor, B.; de Mora, L.; Ciavatta, S. The assimilation of phytoplankton functional types for operational forecasting in the northwest European shelf. J. Geophys. Res. Ocean. 2018, 123, 5230–5247. [Google Scholar] [CrossRef]
- Ciavatta, S.; Kay, S.; Brewin, R.J.W.; Cox, R.; Di Cicco, A.; Nencioli, F.; Polimene, L.; Sammartino, M.; Santoleri, R.; Skákala, J. Ecoregions in the Mediterranean Sea through the reanalysis of phytoplankton functional types and carbon fluxes. J. Geophys. Res. Ocean. 2019, 124, 6737–6759. [Google Scholar] [CrossRef]
- Pradhan, H.K.; Völker, C.; Losa, S.N.; Bracher, A.; Nerger, L. Global assimilation of ocean-color data of phytoplankton functional types: Impact of different data sets. J. Geophys. Res. Ocean. 2020, 125, e2019JC015586. [Google Scholar] [CrossRef]
- Hoshiba, Y.; Hirata, T.; Shigemitsu, M.; Nakano, H.; Hashioka, T.; Masuda, Y.; Yamanaka, Y. Biological data assimilation for parameter estimation of a phytoplankton functional type model for the western North Pacific. Ocean. Sci. 2018, 14, 371–386. [Google Scholar] [CrossRef]
- Kaufman, D.E.; Friedrichs, M.A.M.; Hemmings, J.C.P.; Smith, W.O., Jr. Assimilating bio-optical glider data during a phytoplankton bloom in the southern Ross Sea. Biogeosciences 2018, 15, 73–90. [Google Scholar] [CrossRef]
- Shchepetkin, A.F.; Mcwilliams, J.C. The regional oceanic modeling system (roms): A split-explicit, free-surface, topography-following-coordinate oceanic model. Ocean. Model. 2005, 9, 347–404. [Google Scholar] [CrossRef]
- Chai, F.; Dugdale, R.C.; Peng, T.H.; Wilkerson, F.P.; Barber, R.T. One-dimensional ecosystem model of the equatorial Pacific upwelling system. Part I: Model development and silicon and nitrogen cycle. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 2713–2745. [Google Scholar] [CrossRef]
- Ma, W.; Xiu, P.; Chai, F.; Li, H. Seasonal variability of the carbon export in the central South China Sea. Ocean. Dyn. 2019, 69, 955–966. [Google Scholar] [CrossRef]
- Geider, R.J.; Macintyre, H.L.; Kana, T.M. Dynamic model of phytoplankton growth and acclimation: Responses of the balanced growth rate and the chlorophyll a:carbon ratio to light, nutrient-limitation and temperature. Mar. Ecol. Prog. Ser. 1997, 148, 187–200. [Google Scholar] [CrossRef]
- Xiu, P.; Chai, F. Modeled biogeochemical responses to mesoscale eddies in the South China Sea. J. Geophys. Res. Ocean. 2011, 116, C10006. [Google Scholar] [CrossRef]
- Guo, L.; Xiu, P.; Chai, F.; Xue, H.; Wang, D.; Sun, J. Enhanced chlorophyll concentrations induced by Kuroshio intrusion fronts in the northern South China Sea. Geophys. Res. Lett. 2017, 44, 11565–11572. [Google Scholar] [CrossRef]
- Geng, B.X.; Xiu, P.; Liu, N.; He, X.Q.; Chai, F. Biological response to the interaction of a mesoscale eddy and the river plume in the northern South China Sea. J. Geophys. Res. Ocean. 2021, 126, e2021JC017244. [Google Scholar] [CrossRef]
- Shaw, P.T.; Chao, S.Y.; Liu, K.K.; Pai, S.C.; Liu, C.T. Winter upwelling off Luzon in the northeastern South China Sea. J. Geophys. Res. Ocean. 1996, 101, 16435–16448. [Google Scholar] [CrossRef]
- Gan, J.; Li, L.; Wang, D.; Guo, X. Interaction of a river plume with coastal upwelling in the northeastern South China Sea. Cont. Shelf Res. 2009, 29, 728–740. [Google Scholar] [CrossRef]
- Jing, Z.Y.; Qi, Y.Q.; Hua, Z.L.; Zhang, H. Numerical study on the summer upwelling system in the northern continental shelf of the South China Sea. Cont. Shelf Res. 2009, 29, 467–478. [Google Scholar] [CrossRef]
- Wong, G.T.F.; Tseng, C.M.; Wen, L.S.; Chung, S.W. Nutrient dynamics and N-anomaly at the SEATS station. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1528–1545. [Google Scholar] [CrossRef]
- Xiu, P.; Chai, F.; Shi, L.; Xue, H.; Chao, Y. A census of eddy activities in the South China Sea during 1993-2007. J. Geophys. Res. Ocean. 2010, 115, C03012. [Google Scholar] [CrossRef]
- Evans, G.T.; Parslow, J.S. A model of annual plankton cycles. Biol. Oceanogr. 1985, 3, 327–347. [Google Scholar] [CrossRef]
- Fasham, M.J.R. Variations in the seasonal cycle of biological production in subarctic oceans: A model sensitivity analysis. Deep Sea Res. Part I Oceanogr. Res. Pap. 1995, 42, 1111–1149. [Google Scholar] [CrossRef]
- Kishi, M.J.; Kashiwai, M.; Ware, D.M.; Megrey, B.A.; Eslinger, D.L.; Werner, F.E.; Noguchi-Aita, M.; Azumaya, T.; Fujii, M.; Hashimoto, S.; et al. NEMURO—a lower trophic level model for the North Pacific marine ecosystem. Ecol. Model. 2007, 202, 12–25. [Google Scholar] [CrossRef]
- Hemmings, J.C.P.; Challenor, P.G.; Yool, A. Mechanistic site-based emulation of a global ocean biogeochemical model (MEDUSA 1.0) for parametric analysis and calibration: An application of the Marine Model Optimization Testbed (MarMOT 1.1). Geosci. Model. Dev. 2015, 8, 697–731. [Google Scholar] [CrossRef]
- Ji, X.; Liu, G.; Gao, S.; Wang, H. Parameter sensitivity study of the biogeochemical model in the China coastal seas. Acta Oceanol. Sin. 2015, 34, 51–60. [Google Scholar] [CrossRef]
- Sankar, S.; Polimene, L.; Marin, L.; Menon, N.N.; Ciavatta, S. Sensitivity of the simulated Oxygen Minimum Zone to biogeochemical processes at an oligotrophic site in the Arabian Sea. Ecol. Model. 2018, 372, 12–23. [Google Scholar] [CrossRef]
- Saltelli, A.; Ratto, M.; Andres, T.; Campolongo, F.; Tarantola, S. Global Sensitivity Analysis the Primer; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Schmitt, L.M. Fundamental study theory of genetic algorithms. Theor. Comput. Sci. 2001, 259, 1–61. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Brewin, R.; Brockmann, C.; Brotas, V.; Platt, T. An Ocean-Colour Time Series for Use in Climate Studies: The Experience of the Ocean-Colour Climate Change Initiative (OC-CCI). Sensors 2019, 19, 4285. [Google Scholar] [CrossRef]
- Boss, E.; Swift, D.; Taylor, L.; Brickley, P.; Zaneveld, R.; Riser, S.; Perry, M.J.; Strutton, P.G. Observations of pigment and particle distributions in the western North Atlantic from an autonomous float and ocean color satellite. Limnol. Oceanogr. 2008, 53, 2112–2122. [Google Scholar] [CrossRef]
- Cullen, J.J. The deep chlorophyll maximum: Comparing vertical profiles of chlorophyll a. Can. J. Fish. Aquat. Sci. 1982, 39, 791–803. [Google Scholar] [CrossRef]
- Xing, X.; Qiu, G.; Boss, E.; Wang, H. Temporal and vertical variations of particulate and dissolved optical properties in the South China Sea. J. Geophys. Res. Ocean. 2019, 124, 3779–3795. [Google Scholar] [CrossRef]
- Bisson, K.M.; Boss, E.; Westberry, T.K.; Behrenfeld, M.J. Evaluating satellite estimates of particulate backscatter in the global open ocean using autonomous profiling floats. Opt. Express 2019, 27, 30191–30203. [Google Scholar] [CrossRef] [PubMed]
- Haëntjens, N.; Della Penna, A.; Briggs, N.; Karp-Boss, L.; Gaube, P.; Claustre, H.; Boss, E. Detecting mesopelagic organisms using biogeochemical-Argo floats. Geophys. Res. Lett. 2020, 47, e2019GL086088. [Google Scholar] [CrossRef] [PubMed]
- Stramski, D.; Reynolds, R.A.; Babin, M.; Kaczmarek, S.; Lewis, M.R.; Röttgers, R.; Sciandra, A.; Stramska, M.; Twardowski, M.S.; Franz, B.A.; et al. Relationships between the surface concentration of particulate organic carbon and optical properties in the eastern South Pacific and eastern Atlantic Oceans. Biogeosciences 2008, 5, 171–201. [Google Scholar] [CrossRef]
- Qiu, G.; Xing, X.; Boss, E.S.; Yan, X.H.; Wang, H. Relationships between optical backscattering, particulate organic carbon, and phytoplankton carbon in the oligotrophic South China Sea basin. Opt. Express 2021, 29, 15159–15176. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.M.; Wong, G.T.F.; Lin, I.I.; Wu, C.R.; Liu, K.K. A unique seasonal pattern in phytoplankton biomass in low-latitude waters in the South China Sea. Geophys. Res. Lett. 2005, 32, L08608. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Wang, H.; Chai, F.; Qiu, G. Physical drivers of chlorophyll variability in the open South China Sea. J. Geophys. Res. Ocean. 2016, 121, 7123–7140. [Google Scholar] [CrossRef]
- Bisson, K.M.; Boss, E.; Werdell, P.J. Seasonal bias in global ocean color observations. Appl. Opt. 2021, 60, 6978–6988. [Google Scholar] [CrossRef]
- Park, M.-S.; Lee, S.; Ahn, J.-H.; Lee, S.-J.; Choi, J.-K.; Ryu, J.-H. Decadal Measurements of the First Geostationary Ocean Color Satellite (GOCI) Compared with MODIS and VIIRS Data. Remote Sens. 2022, 14, 72. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, H.-Y.; Karl, D.M.; Takahashi, M. Nitrogen modulates phytoplankton growth in spring in the South China Sea. Cont. Shelf Res. 2004, 24, 527–541. [Google Scholar] [CrossRef]
- Chen, C.-C.; Shiah, F.-K.; Chung, S.-W.; Liu, K.-K. Winter phytoplankton blooms in the shallow mixed layer of the South China Sea enhanced by upwelling. J. Mar. Syst. 2006, 59, 97–110. [Google Scholar] [CrossRef]
- Huang, B.; Hu, J.; Xu, H.; Cao, Z.; Wang, D. Phytoplankton community at warm eddies in the northern South China Sea in winter 2003/2004. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 1792–1798. [Google Scholar] [CrossRef]
- Beckmann, A.; Hense, I. Beneath the surface: Characteristics of oceanic ecosystems under weak mixing conditions—A theoretical investigation. Prog. Oceanogr. 2007, 75, 771–796. [Google Scholar] [CrossRef]
- De La Rocha, C.L. The Biological Pump. In Treatise on Geochemistry; Elsevier: Oxford, UK, 2007; pp. 1–29. [Google Scholar]
- Zhou, K.; Dai, M.; Maiti, K.; Chen, W.; Xie, Y. Impact of physical and biogeochemical forcing on particle export in the South China Sea. Prog. Oceanogr. 2020, 187, 102403. [Google Scholar] [CrossRef]
- Cai, P.; Zhao, D.; Wang, L.; Huang, B.; Dai, M. Role of particle stock and phytoplankton community structure in regulating particulate organic carbon export in a large marginal sea. J. Geophys. Res. Ocean. 2015, 120, 2063–2095. [Google Scholar] [CrossRef]
- Mouw, C.B.; Barnett, A.; McKinley, G.A.; Gloege, L.; Pilcher, D. Phytoplankton size impact on export flux in the global ocean. Glob. Biogeochem. Cycles 2016, 30, 1542–1562. [Google Scholar] [CrossRef]
- Siegel, D.A.; Buesseler, K.O.; Doney, S.C.; Sailley, S.F.; Behrenfeld, M.J.; Boyd, P.W. Global assessment of ocean carbon export by combining satellite observations and food-web models. Glob. Biogeochem. Cycles 2014, 28, 181–196. [Google Scholar] [CrossRef]
- Garcia-Gorriz, E.; Hoepffner, N.; Ouberdous, M. Assimilation of SeaWiFS data in a coupled physical–biological model of the Adriatic Sea. J. Mar. Syst. 2003, 40, 233–252. [Google Scholar] [CrossRef]
- Tjiputra, J.F.; Polzin, D.; Winguth, A.M.E. Assimilation of seasonal chlorophyll and nutrient data into an adjoint three-dimensional ocean carbon cycle model: Sensitivity analysis and ecosystem parameter optimization. Glob. Biogeochem. Cycles 2007, 21, 1–13. [Google Scholar] [CrossRef]
- Fan, W.; Lv, X. Data assimilation in a simple marine ecosystem model based on spatial biological parameterizations. Ecol. Model. 2009, 220, 1997–2008. [Google Scholar] [CrossRef]
- Xiao, Y.; Friedrichs, M.A.M. Using biogeochemical data assimilation to assess the relative skill of multiple ecosystem models in the Mid-Atlantic Bight: Effects of increasing the complexity of the planktonic food web. Biogeosciences 2014, 11, 3015–3030. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Sathyendranath, S.; Hirata, T.; Lavender, S.J.; Barciela, R.M.; HardmanMountford, N.J. A three-component model of phytoplankton size class for the Atlantic Ocean. Ecol. Model. 2010, 221, 1472–1483. [Google Scholar] [CrossRef]

| Experiment | Observation Data |
|---|---|
| CTRL | - |
| EXP1a | satellite sea surface chlorophyll-a |
| EXP1b | satellite-derived PFT data |
| EXP2 | BGC-Argo profiles of chlorophyll-a |
| EXP3 | PFT data and BGC-Argo profiles of chlorophyll-a |
| EXP-S | PFT data and seasonal averaged BGC-Argo profiles of chlorophyll-a |
| EXP-M | PFT data and monthly averaged BGC-Argo profiles of chlorophyll-a |
| Parameter | Description | Initial Value | Minimum | Maximum | Unit | r2 |
|---|---|---|---|---|---|---|
| reg1 | Z1 excretion rate to ammonium | 0.1 | 0.05 | 0.2 | day−1 | 0.083 |
| gmaxs1 | maximum specific growth rate of S1 | 2.0 | 1.0 | 4.0 | day−1 | 0.018 |
| beta1 | Z1 maximum grazing rate | 0.8 | 0.4 | 1.0 | day−1 | 0.099 |
| beta2 | Z2 maximum grazing rate | 0.4 | 0.2 | 0.8 | day−1 | 0.047 |
| akz2 | half saturation for Z2 grazing | 0.25 | 0.125 | 0.5 | mmol N m−3 | 0.025 |
| amaxs1 | initial slope of P-I curve of S1 | 0.025 | 0.0125 | 0.05 | (W m−2 day)−1 | 0.075 |
| akno3s2 | half saturation of nitrate uptake by S2 | 2.0 | 1.0 | 4.0 | mmol N m−3 | 0.046 |
| bgamma1 | grazing efficiency of Z1 | 0.75 | 0.375 | 1.0 | day−1 | 0.139 |
| Chl2cs2_m | maximum chlorophyll-a to carbon ratio for S2 | 0.065 | 0.03 | 0.08 | mg Chla (mg C)−1 | 0.056 |
| Item | Fs1 | Fs2 | Fv | Fscm |
|---|---|---|---|---|
| CTRL | 0.1945 | 0.0333 | 3.2583 | 0.0542 |
| EXP1a | 0.1782 | 0.0293 | 3.5846 | 0.0477 |
| EXP1b | 0.1424 | 0.0169 | 3.3784 | 0.0516 |
| EXP2 | 0.2020 | 0.0279 | 2.5939 | 0.0368 |
| EXP3 | 0.1600 | 0.0155 | 2.8763 | 0.0404 |
| Item | Fv | Fscm | Fv-s | Fv-m |
|---|---|---|---|---|
| CTRL | 3.2583 | 0.0542 | 1.0583 | 0.9097 |
| EXP-S | 3.0348 | 0.0419 | 0.9812 | 0.8065 |
| EXP-M | 2.6264 | 0.0292 | 0.7438 | 0.5916 |
| EXP3 | 2.8763 | 0.0404 | 0.5826 | 0.6877 |
| Parameter | reg1 | gmaxs1 | beta1 | beta2 | akz2 | amaxs1 | akno3s2 | bgamma1 | Chl2cs2_m |
|---|---|---|---|---|---|---|---|---|---|
| CTRL | 0.1 | 2.0 | 0.8 | 0.4 | 0.25 | 0.025 | 2.0 | 0.75 | 0.065 |
| EXP1a | 0.096 | 2.412 | 0.573 | 0.605 | 0.436 | 0.024 | 1.536 | 0.539 | 0.041 |
| EXP1b | 0.124 | 3.027 | 0.518 | 0.423 | 0.395 | 0.016 | 1.371 | 0.912 | 0.045 |
| EXP2 | 0.118 | 2.303 | 0.679 | 0.649 | 0.384 | 0.026 | 1.647 | 0.821 | 0.057 |
| EXP3 | 0.12 | 1.914 | 0.702 | 0.672 | 0.372 | 0.021 | 1.372 | 0.874 | 0.056 |
| EXP-S | 0.151 | 1.937 | 0.916 | 0.405 | 0.414 | 0.027 | 2.587 | 0.876 | 0.05 |
| EXP-M | 0.092 | 3.471 | 0.624 | 0.468 | 0.305 | 0.048 | 1.05 | 0.743 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, C.; Xiu, P.; Xing, X.; Qiu, G.; Ma, W.; Brewin, R.J.W.; Ciavatta, S. Biogeochemical Model Optimization by Using Satellite-Derived Phytoplankton Functional Type Data and BGC-Argo Observations in the Northern South China Sea. Remote Sens. 2022, 14, 1297. https://doi.org/10.3390/rs14051297
Shu C, Xiu P, Xing X, Qiu G, Ma W, Brewin RJW, Ciavatta S. Biogeochemical Model Optimization by Using Satellite-Derived Phytoplankton Functional Type Data and BGC-Argo Observations in the Northern South China Sea. Remote Sensing. 2022; 14(5):1297. https://doi.org/10.3390/rs14051297
Chicago/Turabian StyleShu, Chan, Peng Xiu, Xiaogang Xing, Guoqiang Qiu, Wentao Ma, Robert J. W. Brewin, and Stefano Ciavatta. 2022. "Biogeochemical Model Optimization by Using Satellite-Derived Phytoplankton Functional Type Data and BGC-Argo Observations in the Northern South China Sea" Remote Sensing 14, no. 5: 1297. https://doi.org/10.3390/rs14051297
APA StyleShu, C., Xiu, P., Xing, X., Qiu, G., Ma, W., Brewin, R. J. W., & Ciavatta, S. (2022). Biogeochemical Model Optimization by Using Satellite-Derived Phytoplankton Functional Type Data and BGC-Argo Observations in the Northern South China Sea. Remote Sensing, 14(5), 1297. https://doi.org/10.3390/rs14051297







