Waterborne Polymer Coating Material Modified with Nano-SiO2 and Siloxane for Fabricating Environmentally Friendly Coated Urea
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation and Preparation of Hydrophobic Waterborne Coating Films
2.2.1. Formulation and Preparation of Waterborne Coating Films
2.2.2. Formulation and Preparation of Hydrophobic Waterborne Polymer Films
2.3. Fabrication of Hydrophobic-Waterborne-Polymer-Coated Urea (SWCU)
2.4. Determination of Water Absorbency and Porosity
2.4.1. Determination of Water Absorption
2.4.2. Determination of Porosity
2.5. Characterization
2.6. N-Release Characteristics of Hydrophobic WCU
3. Results and Discussion
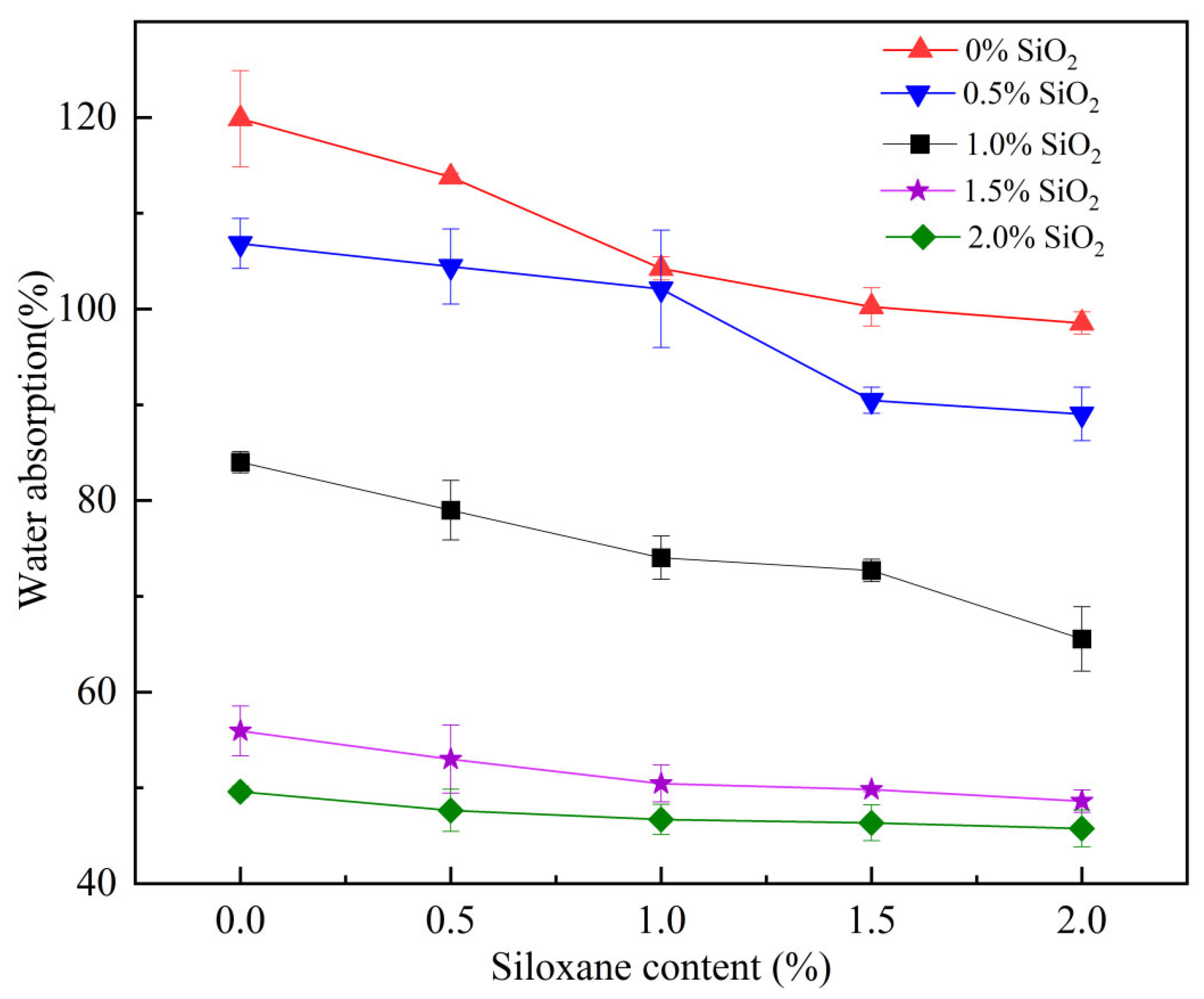
3.1. Water Absorption
3.2. Porosity
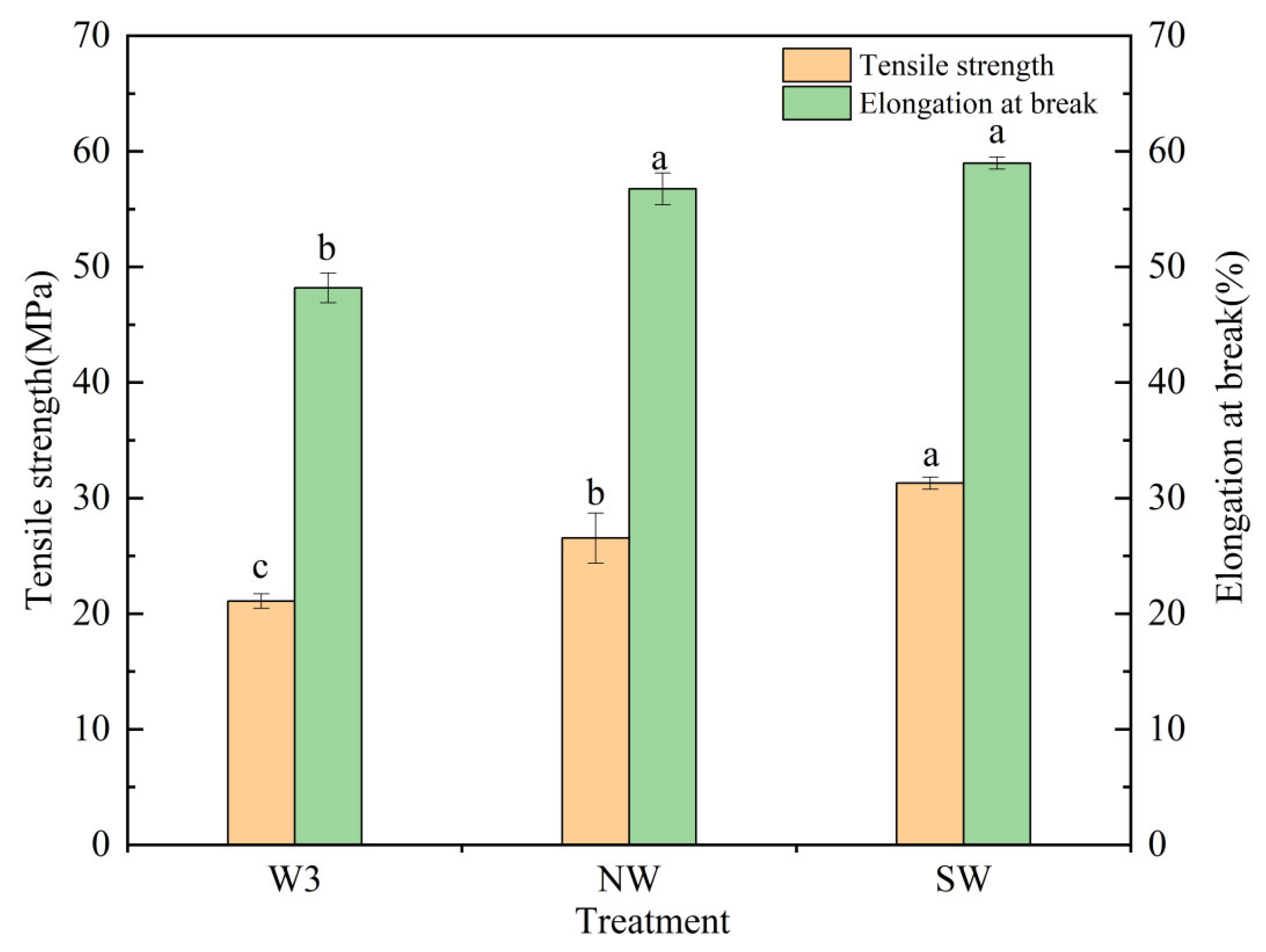
3.3. Mechanical Strength
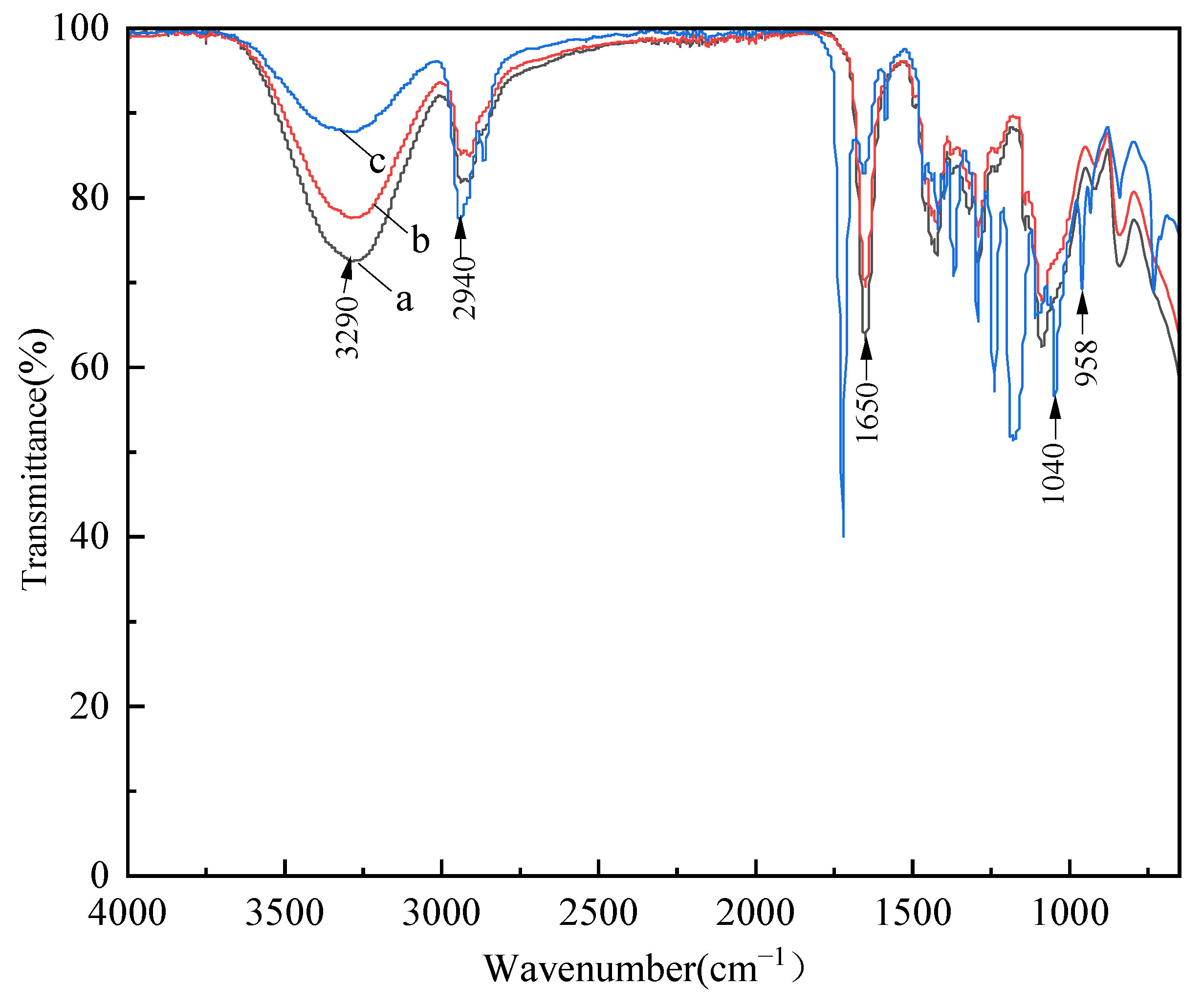
3.4. FT-IR Analysis
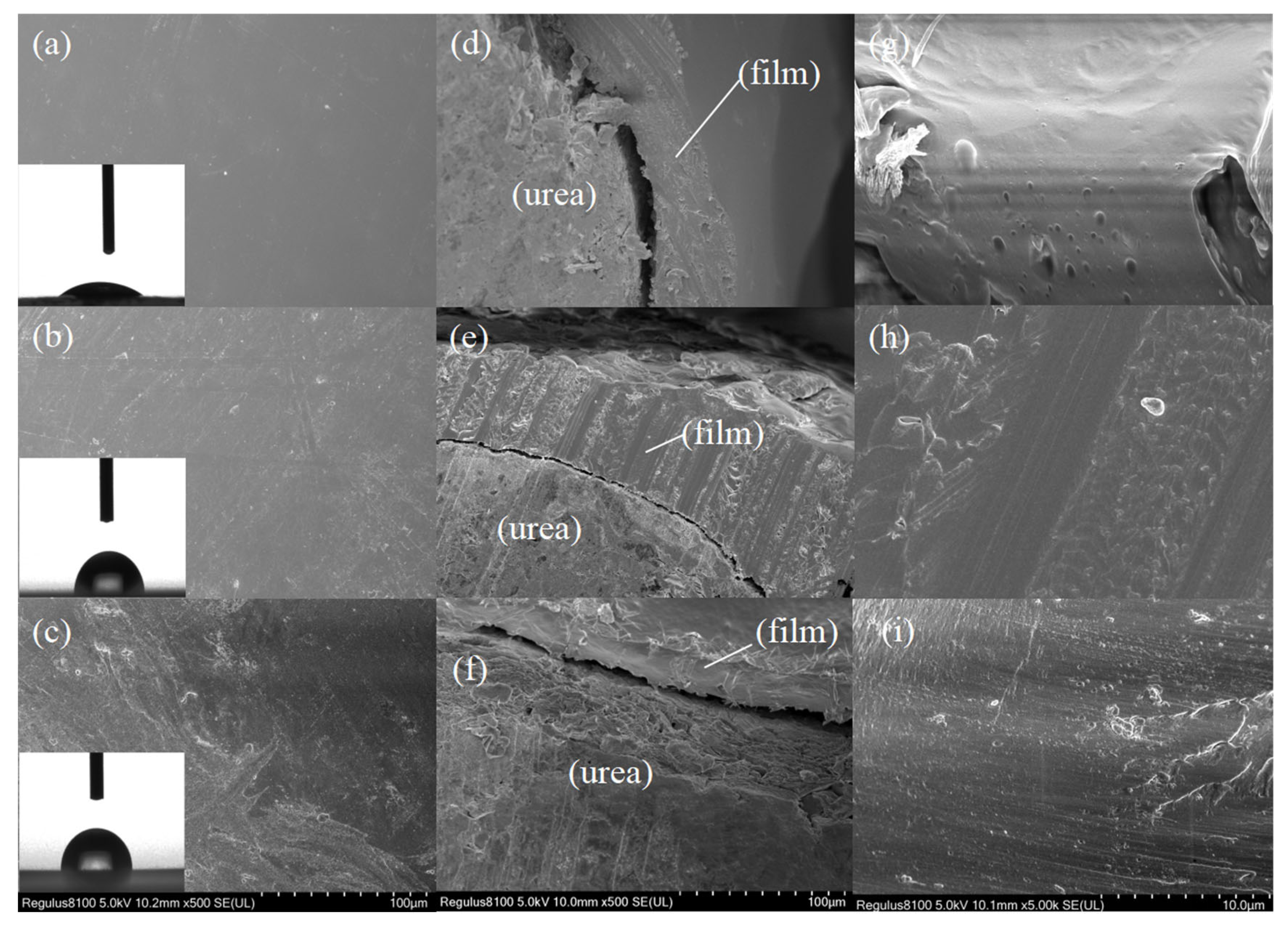
3.5. Morphological Characterization
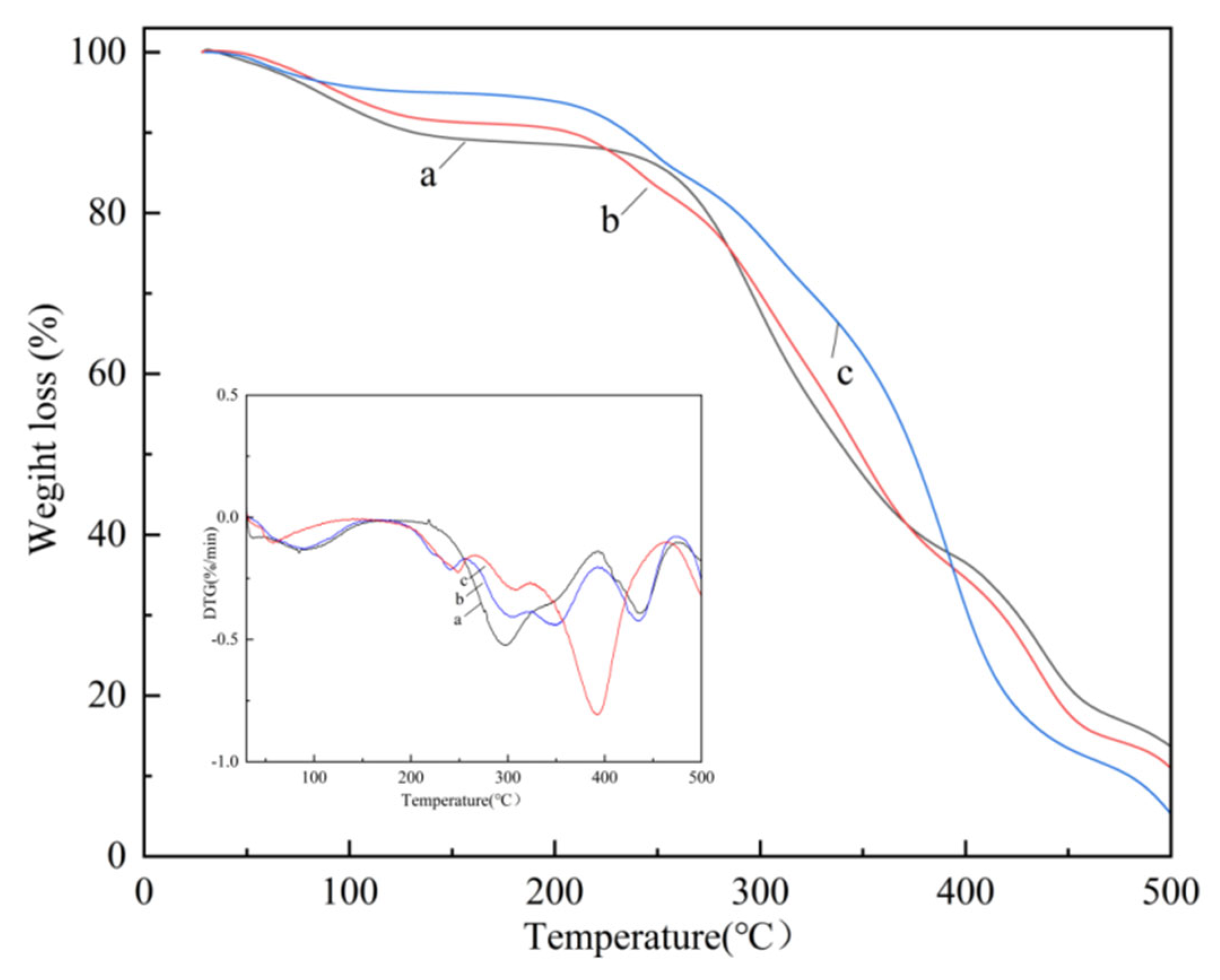
3.6. Thermal Behaviors
3.7. XPS Spectra
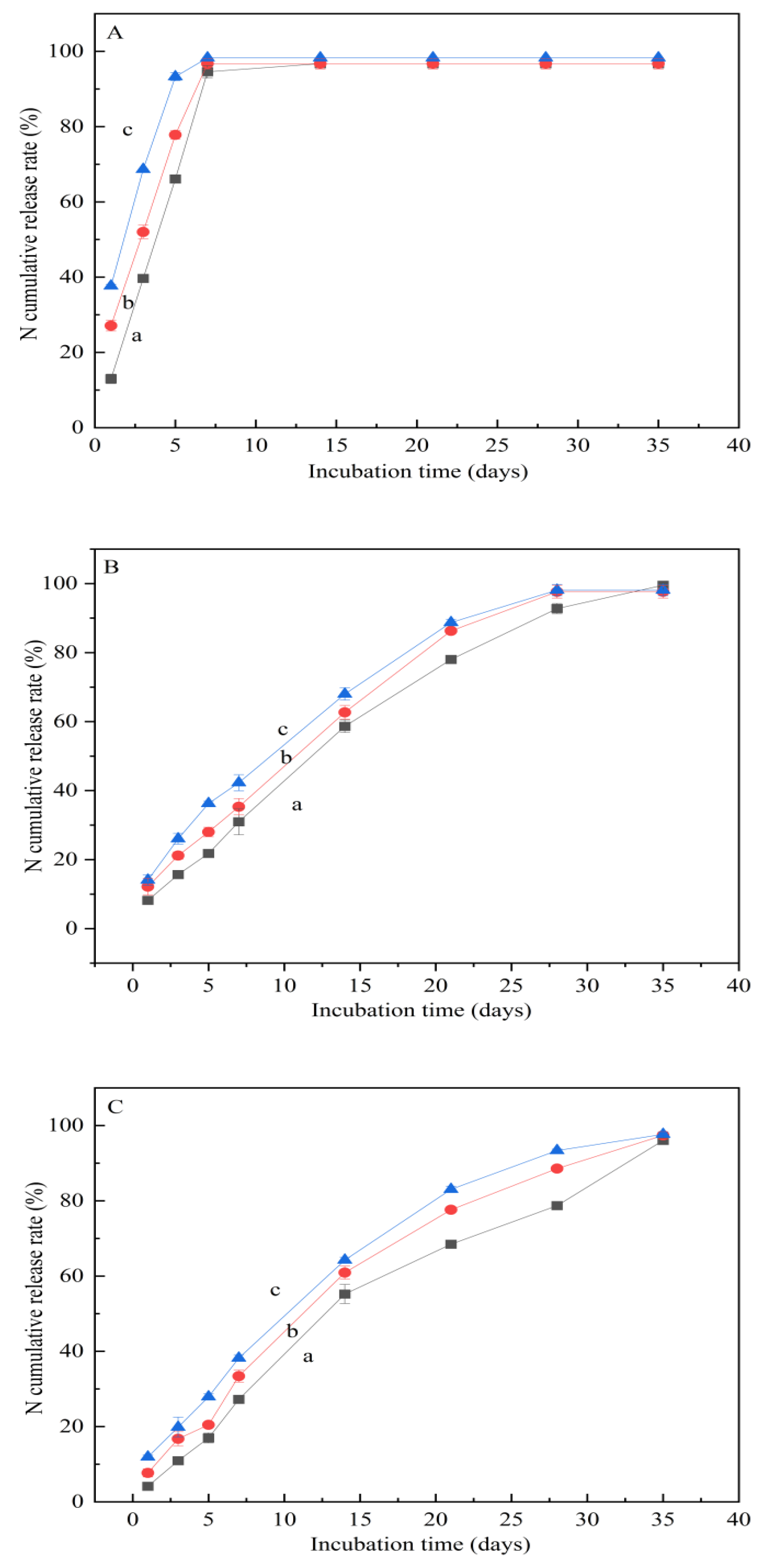
3.8. N-Release Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, S.Q.; Pang, M.H.; Li, H.T.; Zou, G.Y.; Liang, L.N. Introduction of bio-based hard segment as an alternative strategy to environmentally friendly polyurethane coated urea. Ind. Crops Prod. 2023, 203, 117164. [Google Scholar] [CrossRef]
- Zhao, C.H.; Tian, H.Y.; Zhang, Q.; Liu, Z.G.; Wang, J. Preparation of urea-containing starch-castor oil superabsorbent polyurethane coated urea and investigation of controlled nitrogen release. Carbohydr. Polym. 2021, 253, 117240. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.I.; Adewale, O.F.; Arun, G.; Olalekan, O.B.; Omolola, A.; Stacey, F.; Adam, F.B.; Vuyo, M.; Marshall, K.; Ashwil, K. Biofertilizer: The future of food security and food safety. Microorganisms 2022, 10, 1220. [Google Scholar] [CrossRef]
- Tang, J.; Xu, J.; Fan, H.; Li, R.; Shi, M.; Liu, Y.; Wang, B.; Liu, P. Preparation of hydroxy-terminated polydimethylsiloxane and nano-SiO2 hydrophobic polyurethane coated urea and investigation of controlled nitrogen release. Chem. Eng. Sci. 2025, 302, 120728. [Google Scholar] [CrossRef]
- Pang, M.H.; Liu, Z.R.; Li, H.Y.; Liang, L.N.; Li, X.; Li, L.X. Effect of fatty acids on vegetable-oil-derived sustainable polyurethane coatings for controlled-release fertilizer. Coatings 2024, 14, 1183. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, J.N.; Li, B.Y.; Ren, D.; Chen, X.; Yu, J.; Qi, Z. Multiscale construction of bifunctional electrocatalysts for long-lifespan rechargeable zinc–air batteries. Adv. Funct. Mater. 2020, 36, 2003619. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, F.P.; Dai, J.; Zhang, Q.P.; Jiang, M.; Xiong, Y.Z. Recycled-oil-based polyurethane modified with organic silicone for controllable release of coated fertilizer. Polymers 2019, 11, 454. [Google Scholar] [CrossRef]
- Lu, P.F.; Zhang, M.; Li, Q.; Xu, Y. Structure and properties of controlled release fertilizers coated with thermosetting Resin. Polym.-Plast. Technol. Eng. 2013, 52, 381–386. [Google Scholar] [CrossRef]
- Yang, X.D.; Jiang, R.F.; Lin, Y.Z.; Li, Y.T.; Li, J.; Zhao, B.Q. Nitrogen release characteristics of polyethylene-coated controlled-release fertilizers and their dependence on membrane pore structure. Particuology 2018, 36, 158–164. [Google Scholar] [CrossRef]
- Ma, X.X.; Chen, J.Q.; Yang, Y.C.; Su, X.R.; Zhang, S.G. Siloxane and polyether dual modification improves hydrophobicity and interpenetrating polymer network of bio-polymer for coated fertilizers with enhanced slow release characteristics. Chem. Eng. J. 2018, 350, 1125–1134. [Google Scholar] [CrossRef]
- Kassem, I.; Ablouh, E.H.; Bouchtaoui, F.Z.E.; Mohamed, J.; Mounir, E.A. Polymer coated slow/controlled release granular fertilizers: Fundamentals and research trends. Prog. Mater. Sci. 2024, 144, 101269. [Google Scholar] [CrossRef]
- Elhassani, C.E.; Abdelouahed, E.G.; Younes, E.; Salma, E.; Karim, D.; Soumia, A.; Mohamed, Z. Lignin nanoparticles filled chitosan/polyvinyl alcohol polymer blend as a coating material of urea with a slow-release property. J. Appl. Polym. Sci. 2023, 140, e53755. [Google Scholar] [CrossRef]
- Ibrahim, K.A.; Naz, M.Y.; Shukrullah, S.; Sulaiman, S.; Abdul, G.; Abdel-Salam, N.M. Nitrogen pollution impact and remediation through low cost starch based biodegradable polymers. Sci. Rep. 2020, 10, 5927. [Google Scholar] [CrossRef]
- Ranjith, R.; Balraj, S.; Ganesh, J.; Milton, M.C.J. Therapeutic agents loaded chitosan-based nanofibrous mats as potential wound dressings: A review. Mater. Today Chem. 2019, 12, 386–395. [Google Scholar] [CrossRef]
- Bakshia, P.; Selvakumara, D.; Kadirvelub, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial-a review on recent modifications and applications. Int. J. Biol. Macromol. 2019, 150, 1072–1083. [Google Scholar] [CrossRef]
- Kabiri, S.; Andelkovic, R.D.; Silva, D.; Fien, D.; Roslyn, B.; Tavakkoli, E.; Losic, D.; Mclaughlin, M. Engineered phosphate fertilizers with dual-release properties. Ind. Eng. Chem. Res. 2020, 59, 5512–5524. [Google Scholar] [CrossRef]
- Aslam, M.; Mazhar, A.K.; Zulfiqar, A.R. Investigation of structural and thermal properties of distinct nanofillers-doped PVA composite films. J. Polym. Bull. 2018, 76, 73–86. [Google Scholar] [CrossRef]
- Das, P.; Ray, S.K.; Kuila, S.B.; Samant, H.S.; Singha, N.R. Systematic choice of crosslinker and filler for pervaporation membrane: A case study with dehydration of isopropyl alcohol–water mixtures by polyvinyl alcohol membranes. Sep. Purif. Technol. 2011, 81, 159–173. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, L.; Wang, L.; Qi, Y.; Zhao, Y.; Lu, H.; Lu, L.; Zhang, D.; Wang, Z.; Zhang, H. Preparation and application of degradable lignin/poly (Vinyl Alcohol) polymers as urea slow-release coating materials. Molecules 2024, 29, 1699. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Alexandre, M.E.; Alexander, D.M.; Alexandr, G.D.; Kirill, A.E. Synergistic effect of superhydrophobicity and oxidized layers on corrosion resistance of aluminum alloy surface textured by nanosecond laser treatment. ACS Appl. Mater. Emelyanenko Interfaces 2015, 7, 19500–19508. [Google Scholar] [CrossRef]
- Wang, R.; Jie, Z.; Meng, K.X.; Wang, H.; Deng, T.; Gao, X.F.; Jiang, L. Bio-inspired superhydrophobic closely packed aligned nanoneedle architectures for enhancing condensation heat transfer. Adv. Funct. Mater. 2018, 28, 1800634. [Google Scholar] [CrossRef]
- Xie, J.Z.; Yang, Y.C.; Gao, B.; Wan, Y.S.; Li, Y.C.; Xu, J.; Zhao, Q.H. Biomimetic superhydrophobic biobased polyurethane-coated fertilizer with atmosphere “Outerwear”. ACS Appl. Mater. Interfaces 2017, 9, 15868–15879. [Google Scholar] [CrossRef]
- Chen, S.L.; Han, Y.Y.; Yang, M.; Zhu, X.Q.; Liu, C.T.; Liu, H.D.; Zou, H.T. Hydrophobically modified water-based polymer for slow-release urea formulation. Prog. Org. Coat. 2020, 149, 105964. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Du, C.W.; Zhou, J.M.; Ma, F. The facile modification of polyacrylate emulsion via hexadecane to enhance controlled-release profiles of coated urea. Sci. Rep. 2018, 8, 12279. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.D.; Li, X.W.; Xu, J.B.; Zhang, F.A. Organosilicon-modified double crosslinked waterborne polyurethane coatings. Prog. Org. Coat. 2024, 190, 108372. [Google Scholar] [CrossRef]
- Xiong, M.N.; Bo, Y.; Zhou, S.X.; Wu, L.M. Study on acrylic resin/titania organic–inorganic hybrid materials prepared by the sol–gel process. Polymer 2004, 45, 2967–2976. [Google Scholar] [CrossRef]
- Dural, S.; Şebnem, S.C.; Nilhan, K.A. Improving the mechanical, thermal and surface properties of polyaspartic ester bio-based polyurea coatings by incorporating silica and titania. Mater. Today Commun. 2024, 38, 107654. [Google Scholar] [CrossRef]
- Feng, Y.H.; Wei, X.; Huang, J.; Zhang, P. Thermo-optic response and mechanical properties of hybrid polyurethane elastomers via silicon group-induced microphase separation evolution. React. Funct. Polym. 2023, 193, 105739. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Suwanakood, P.; Saengsuwan, S. Biodegradable hydrogels of cassava starch-g-polyacrylic acid/natural rubber/polyvinyl alcohol as environmentally friendly and highly efficient coating material for slow-release urea fertilizers. J. Ind. Eng. Chem. 2021, 101, 237–252. [Google Scholar] [CrossRef]
- Santos, A.M.P.; Alexandre, C.B.; Ana, C.C.P.B.; Raphael, A.B.G.; Jerusa, S.G.; Marcello, G.T. New organomineral complex from humic substances extracted from poultry wastes: Synthesis, characterization and controlled Release Study. J. Braz. Chem. Soc. 2018, 29, 140–150. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, F.; Yan, L.; Liu, J.; Zhang, T.H.; Su, X.H.; Wang, W.T.; Yang, Y.C.; Xu, J.; Xie, J.Z. Double-modified biopolymer-coatings based on recyclable poplar-catkin: Efficient performance, controlled-release mechanism and rice application. Prog. Org. Coat. 2024, 186, 107980. [Google Scholar] [CrossRef]
- Yang, M.; Gao, H.; Zou, H.T.; Sun, D. Siloxane and lauric acid copper dual modification improves hydrophobicity and elasticity of all biopolymer coated fertilizers with enhanced nitrogen release abilities. Ind. Crops Prod. 2025, 226, 120620. [Google Scholar] [CrossRef]
- Chen, S.L.; Yang, M.; Han, Y.Y.; Liu, H.D.; Zou, H.T. Hydrophobically modified sustainable bio-based polyurethane for controllable release of coated urea. Eur. Polym. J. 2020, 142, 110114. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, Y.; Jin, J.; Sun, J.; Tian, X. Polyvinyl alcohol composite hydrogels/epoxidized natural rubber composites (CMCS/PVA/CS-ENR) with core-shell structure as biomass coating material for slow-release nitrogen fertilizer. Prog. Org. Coat. 2023, 183, 107744. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.T.; Zhang, H.; Huang, Y.; Yang, J.Z.; Sun, D.P. Facile approach to the fabrication of 3D cellulose nanofibrils (CNFs) reinforced poly(vinyl alcohol) hydrogel with ideal biocompatibility. Carbohydr. Polym. 2017, 173, 547–555. [Google Scholar] [CrossRef]
- Zhao, J.R.; Li, X.L.; Liu, F.X.; Zhao, W.Y.; Chen, S.L. Smart fertilizer with temperature-responsive behavior coated using a naturally derived material. J. Appl. Polym. Sci. 2025, 142, 56721. [Google Scholar] [CrossRef]
- Jungsinyatam, P.; Pitchayaporn, S.; Sayant, S. Multicomponent biodegradable hydrogels based on natural biopolymers as environmentally coating membrane for slow-release fertilizers: Effect of crosslinker type. Sci. Total Environ. 2022, 843, 157050. [Google Scholar] [CrossRef]
- Salimi, M.; Channab, B.E.; Ayoub, E.I.; Mohamed, Z.; Elaheh, M. A comprehensive review on starch: Structure, modification, and applications in slow/controlled-release fertilizers in agriculture. Carbohydr. Polym. 2023, 322, 121326. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, J.W.; Jiang, S.M.; Fu, C.; Li, Y.F.; Xiang, H.; Lu, R.H.; Zhu, J.; Yu, B. Enhancing slow-release performance of biochar-based fertilizers with kaolinite-infused polyvinyl alcohol/starch coating: From fertilizer development to field application. Int. J. Biol. Macromol. 2025, 302, 140665. [Google Scholar] [CrossRef]
- Assimi, T.E.; Omar, L.; Abdellatif, E.M.; Mehdi, K.; Abdelmalek, D.; Redouane, B.; Rachid, B.; Mustapha, R.; Mohammed, L. Sustainable coating material based on chitosan-clay composite and Paraffin wax for Slow-Release DAP Fertilizer. Int. J. Biol. Macromol. 2020, 161, 492–502. [Google Scholar] [CrossRef]
- Han, Y.Y.; Chen, S.L.; Xie, B.Y.; Wang, Y.Q.; Fan, Y.W.; Meng, Q.Y.; Zou, H.T.; Zhang, Y.L. Waterborne polymer modified with zeolite for environment-friendly slow-release coated urea. J. Appl. Polym. Sci. 2023, 140, e53633. [Google Scholar] [CrossRef]
- Azeem, B.; Kuzilati, K.; Muhammad, N.; Lau, K.K.; Mohammed, K.A.; Zakaria, A.Q.; Salman, R.N.; Noureddine, E. Production and Characterization of Controlled Release Urea Using Biopolymer and Geopolymer as Coating Materials. Polymers 2020, 12, 400. [Google Scholar] [CrossRef] [PubMed]


| Levels | PVA (%) | PVP (%) | Chitosan (%) | Starch (%) |
|---|---|---|---|---|
| 1 | 4 | 1 | 1 | 1 |
| 2 | 6 | 2 | 2 | 2 |
| 3 | 8 | 3 | 3 | 3 |
| Treatment | PVA (%) | PVP (%) | CS (%) | Starch (%) | Water Absorbency (%) |
|---|---|---|---|---|---|
| W1 | 4 | 1 | 1 | 1 | 174.87 b |
| W2 | 4 | 2 | 2 | 2 | 153.03 c |
| W3 | 4 | 3 | 3 | 3 | 119.86 d |
| W4 | 6 | 1 | 2 | 3 | 174.96 b |
| W5 | 6 | 2 | 3 | 1 | 153.72 c |
| W6 | 6 | 3 | 1 | 2 | 149.75 c |
| W7 | 8 | 1 | 3 | 2 | 173.54 b |
| W8 | 8 | 2 | 1 | 3 | 186.35 a |
| W9 | 8 | 3 | 2 | 1 | 170.68 b |
| Field Capacity (%) | Richard Equation | ||||
|---|---|---|---|---|---|
| SWCU | |||||
| a | xc | d | k | R2 | |
| 40% | 148.68 | 0.41 | 0.01 | 0.03 | 0.99 |
| 60% | 103.41 | 7.32 | 0.68 | 0.09 | 0.99 |
| 80% | 103.01 | 6.2 | 0.79 | 0.1 | 0.99 |
| Coating Material | Fertilizer | Culture Method | Coating Methods | Drying Temperature (°C) | Thickness (%) | Release rate (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Polyvinyl alcohol + Chitosan + Starch + polyvinylpyrrolidone + Nano-SiO2 + Siloxane | Urea | Soil | Rotating pan | 70 | 7 | 78.7% after 28 days | This work |
| Polyvinyl alcohol + Chitosan + Starch + Sodium carboxymethyl cellulose + Zeolite | Urea | Soil | Fluidized bed | 65 | 7 | 75.9% after 42 days | [41] |
| Polyvinyl alcohol + Starch + Citric acid + Fly ash + NaOH | Urea | Soil | Fluidized bed | 80 | 7 | 88.6% after 37 days | [42] |
| Natural rubber + Chitosan + Polyvinyl alcohol | Urea | Water | Rotating pan | 50 | - | 62.7% after 10 days | [34] |
| Polyvinyl alcohol + Starch + Kaolinite + Biochar | Urea | Soil | Rotating pan | - | - | 19.1% after 29 days | [39] |
| Starch + Chitosan + Acrylic acid-co-acrylamide | Urea | Soil | Rotating pan | 70 | - | 78.3% in 30 days | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Liu, F.; Zhao, W.; Zhao, J.; Li, X.; Wang, J. Waterborne Polymer Coating Material Modified with Nano-SiO2 and Siloxane for Fabricating Environmentally Friendly Coated Urea. Sustainability 2025, 17, 6987. https://doi.org/10.3390/su17156987
Chen S, Liu F, Zhao W, Zhao J, Li X, Wang J. Waterborne Polymer Coating Material Modified with Nano-SiO2 and Siloxane for Fabricating Environmentally Friendly Coated Urea. Sustainability. 2025; 17(15):6987. https://doi.org/10.3390/su17156987
Chicago/Turabian StyleChen, Songling, Fuxin Liu, Wenying Zhao, Jianrong Zhao, Xinlin Li, and Jianfei Wang. 2025. "Waterborne Polymer Coating Material Modified with Nano-SiO2 and Siloxane for Fabricating Environmentally Friendly Coated Urea" Sustainability 17, no. 15: 6987. https://doi.org/10.3390/su17156987
APA StyleChen, S., Liu, F., Zhao, W., Zhao, J., Li, X., & Wang, J. (2025). Waterborne Polymer Coating Material Modified with Nano-SiO2 and Siloxane for Fabricating Environmentally Friendly Coated Urea. Sustainability, 17(15), 6987. https://doi.org/10.3390/su17156987






