Abstract
Urea has served as the primary nitrogenous fertilizer globally since the early 1950s. It is widely recognized as the most concentrated nitrogen source, containing approximately 46% nitrogen. Presently, around 220 million t/year of urea compounds are manufactured globally to fit the requirements of the agricultural sector. However, a significant drawback of this is that approximately 30–35% of the urea used in soil can be lost to the environment because of its limited effectiveness. Enhancing the efficiency of urea utilization can be achieved by regulating the release of urea-nitrogen in the soil. Numerous researchers have reported that the use of slow or controlled fertilizers can regulate the release and accumulation of nitrogen in the soil. Moreover, the augmentation of soil nitrogen levels can be accomplished by using the slow or controlled release of urea fertilizers. The regulation of the release process can play a vital role in the peril of N loss. This can be effectively alleviated by delaying the release of nitrogen in ammonium form configuration for several days. This delay functions to diminish nitrogen losses, which are caused by the rapid hydrolysis of urea, and loss by leaching or volatilization. Therefore, this review aims to comprehensively explore the use of conventional urea and various materials employed for modifying urea. It will explain the distinctions among modification processes and their respective mechanisms. This review will also discuss the pros and cons of applying slow- and controlled-release nitrogen, the impact of modified urea compounds on crop productivity, and nitrogen use efficiency (NUE).
1. Introduction
Presently, several challenges have emerged with the growing world population. The most important challenge is food security. Crop production requires nitrogenous fertilizers in relatively large amounts, it’s making the nutrient most often deficient, especially with the loss of soil fertility.
Urea, as one of the most important nitrogenous fertilizers, is a major player in plant nutrition and soil fertility. Urea is the cheapest and richest fertilizer in nitrogen (46%). Despite urea being the root of nitrogenous fertilization, it has less effectiveness in comparison with other nitrogen fertilizers. The urea’s inadequate efficiency arises from its rapid release in the soil, leading to nitrogen loss. Upon application of urea fertilizer to the soil, it can move away from the plant’s root or soil system and become unattainable for the plants due to many processes such as denitrification, leaching, immobilization, and fixation [1,2,3,4]. Moreover, crops often recover only a small portion of nitrogen from soluble N fertilizers like urea, typically ranging between 30% and 40%. This inefficiency comes with a significant environmental cost [3,5,6,7,8].
Therefore, nitrogen loss through NH3 volatilization, caused by surface-applicable urea, is a critical environmental issue since it is influenced by soil properties and meteorological conditions. All of the aforementioned factors resulted in reduced urea use efficiency and an increased demand for nitrogen fertilizers. The agricultural sector will need to use even greater quantities of fertilizer to live up to the rising food demand [9]. The use of conventional urea impairs agronomic nitrogen use efficiency (ANUE), limiting yields [4,10].
Accordingly, many technological interventions, especially those geared towards the use of different compounds based on ecological raw materials, improve urea use efficiency as a main fertilizer of nitrogen and reduce the emissions to the environment [10]. Making nitrogen fertilizer in a controlled- or slow-release form is one of the most prevalent strategies to decrease nitrogen losses, and several methods and materials have been described [10,11]. Thus, the release of nitrogen is controlled so that the amount of excess nitrogen in the soil is reduced in the form of ammonium, which is lost via ammonia to the air, as well as nitrates entering the water, thereby reducing water and air pollution [10,11,12].
To this end, it is important to understand the difference between modified urea nitrogen fertilizer types and the benefits of using them. Therefore, this review aims to focus on conventional urea usage and the technologies that are used to modify urea, the different materials used for urea modification, the differences between the modification processes, and the differences in their mechanisms. This review also outlines the advantages and disadvantages of each process.
2. The Methodology for Preparing This Review
This review extensively covers the establishment of modified urea (slow- and controlled-release) fertilizers. To gather relevant research articles, a literature review was conducted in two steps, as follows:
- i.
- Initially, a search was conducted on Web of Science and Google Scholar using specific keywords like “slow and controlled release fertilizer”, “coated materials”, “coated urea”, “advantages of controlled-release fertilizers”, “disadvantages of slow-release fertilizers”, and “nitrogen use efficiency”. Further inclusion of relevant articles was based on their pertinence to the review’s topic. Figure 1 provides a depiction of the article count from 1970 to 2022.
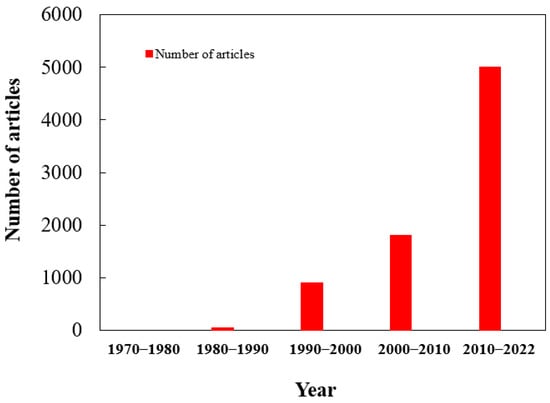 Figure 1. Number of articles published during the period from 1970–2022.
Figure 1. Number of articles published during the period from 1970–2022. - ii.
- Subsequently, the gathered articles were carefully reviewed and classified into distinct sections for the purpose of this review paper. Some articles were cited multiple times to showcase their relevance to different aspects. Overall, 120 articles published between 1970 and 2022 were chosen, and additional references were included to bolster their relevance to specific research topics.
3. Conventional Urea Application
Manufacturing urea fertilizer commercially involves the combination of gaseous CO2 and liquid ammonia under conditions of high compression and temperature. After dehydration, ammonium carbamate is added, and the mixture is passed through a granulator or prilling tower to produce either prills or pellets.
Periodically, farmers require nitrogen fertilizer to supply the precise nutrients necessary for optimal plant growth in their farms and gardens. Urea stands out as the predominant form of solid nitrogen fertilizer, especially in developing regions of the world [13]. Around 80–85% of the production is used as a fertilizer [13,14]. Notably, over 40% of global food production relies on urea fertilization [14]. Around 57% of the world’s agricultural nitrogen usage is currently met by urea addition, and the demand for urea is expected to rise by 1.5% annually shortly [13,15,16].
Furthermore, urea is the most extensively used solid nitrogenous fertilizer in the world, and it has excellent water solubility. In comparison to other nitrogenous fertilizers, urea offers more nitrogen quantity to plants and soil because of its high nitrogen concentration (46%). One of the advantages of using conventional urea is that it holds flexibility in application methods. It can be broadcast over large areas, applied in a band near the plant roots, or incorporated into the soil through tillage. This makes it an excellent choice for both small- and large-scale farming operations. However, there are also some potential drawbacks to using conventional urea.
The transformation processes of N as urea fertilizer to the plant in the soil system are shown in Figure 2. Once urea is applied to soil, urea is hydrolyzed by the urease enzyme, which must be converted to ammonium before it can be used by plants [3,16]. Ammonia (NH3) possesses the highest content of N, nearly 82%, and plants can use it directly [3]. However, before plants can utilize ammonia (NH3) it undergoes conversion from ammonia to ammonium (NH4) and nitrates. As a result, urea, which has an NUE (nitrogen use efficiency) of only 50%, faced hurdles in gaining widespread acceptance as a primary nitrogen fertilizer due to the significant nitrogen loss pathways through the volatilization, fixation, denitrification, and leaching as shown in Figure 2 [17].
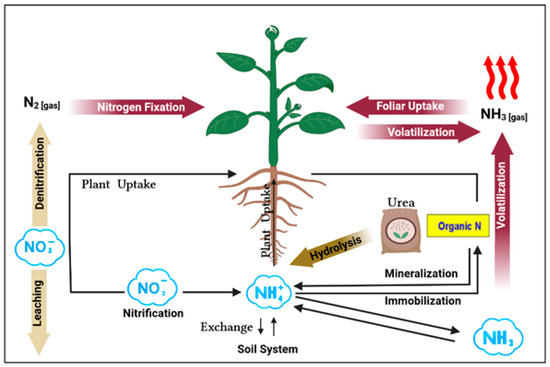
Figure 2.
Urea transformation in the soil system.
This is primarily attributed to urea’s higher susceptibility to N loss through NH3 volatilization, ranging from 2–20% [3,18], reaction in the soil with organic components, which leads to a loss of 15–25%, and leaching into water systems, causing a loss of 2–10% [17]. The hydrolysis process raises the soil pH surrounding the granules, leading to ammonia losses averaging 16% of the applied nitrogen on a global scale, and it can reach 40% or more under warm and humid conditions [3,16]. Although urea and fluids containing urea have the highest potential for volatilization, all surface-applied ammonia and ammonium-based N fertilizers have the capacity to do so. Volatilization alone results in a loss of 20–50% of the nitrogen given to the soil [3]. These losses are contributing to significant environmental concerns [19,20].
4. Urea Modification Approach
Following World War II, the introduction of inorganic nitrogen (N) fertilizers in the 1950s aimed to boost crop productivity and address the growing global population’s food demands. This development paved the way for the “Green Revolution”, which emerged in the 1960s with the introduction of high-yielding crop varieties responsive to fertilizers. Remarkably, over the past five decades, the doubling of crop yields has been closely linked to a seven-fold increase in global N fertilizer usage [21]. From 10.8 million metric tons in 1960, N fertilizer usage surged to 82 million metric tons by the year 2000, with further projections anticipating a rise to 249 million metric tons by 2050. Notably, approximately half of the global food production is estimated to benefit from N fertilizer applications, facilitating rapid crop growth and increased yields [22].
Furthermore, the desire to enhance food security has had a significant impact on global fertilizer consumption during the last few decades. Future demands will most likely be driven by a larger range of issues, such as the desire to limit the environmental implications of nutrient losses. Additionally, the area of accessible arable farmland is shrinking as a result of excessive economic expansion, the conversion of arable land to residential areas, land degradation, and climate change globally [23].
To overcome these issues, careful planning and action are necessary to assess global food shortfalls and future needs. Specific actions, including adjustments in the agriculture sector, are already in place to address these concerns. Some of these issues may be solved by covering urea fertilizer and controlling its release rate. At this stage, using slow- and controlled-release fertilizers would not only increase agricultural output but also minimize environmental damage [24,25]. The growth of slow- and controlled-release fertilizers, as well as stabilized fertilizers, largely fulfills the criteria of an optimal fertilizer. However, it is important to remember that controlled- and slow-release and stabilized fertilizers cannot make up for mistakes in field and crop management. Nonetheless, they can help improve NUE and reduce some environmental impacts. Thus, it is necessary for these unique types of fertilizers to constantly be incorporated into a strong agricultural strategy or best management practices approach [13,26].
5. Modified Urea Fertilizers
Urea modification is a process used to improve the properties of materials by reacting them with urea. Urea is a compound containing nitrogen, carbon, and oxygen that is often used to increase the tolerance of materials to hot and humid climates. This process can also be used to make materials more resistant to corrosion, UV radiation, and other environmental factors. Urea modification is an efficient way to improve the durability and performance of a wide range of materials. Chissoasahi was the first creator of Polyolefin-coated urea (POCU), which is designed to have a sigmoidal release and permits a single, basal application and the simultaneous placement of the fertilizer [26].
The two most common types of modified urea fertilizers are described as “slow-release nitrogen” (SRN) and “controlled-release nitrogen” (CRN) [11,12,27]. Although these terms are often used interchangeably, they have different meanings. Slow-release nitrogen (SRN) and controlled-release nitrogen (CRN) are essentially synonymous and can be collectively referred to as “Enhanced Efficiency Nitrogen.” However, Trenkel (1997) provided a good clarification on the distinction between both [26]. Synthetic-modified urea compounds can be classified into two main categories. The first category is produced by chemical reactions such as urea-formaldehyde to achieve slow release as a by-product.
The second category accomplishes controlled release by employing a coating made of sulfur, wax, or resin around the fertilizer prills [28]. Different factors can affect the release-rate mechanism of nutrients from slow-release fertilizer, such as the soil type and its properties and metrological conditions [26,29]. The mechanism of slow-release fertilizer is completely random. Otherwise, the mechanism of controlled-release nitrogen fertilizer, amount, and rate of release are comprehensively predictable [25,26,29,30].
Even though slow or controlled fertilizers release nitrogen at a rate more gradual than typical urea, their mechanisms are radically different. Furthermore, SRN and CRN sources are separated at the most fundamental level by a single distinguishing feature: whether they are uncoated or coated [17]. SRN doesn’t depend on coatings to deliver extended-release nitrogen, whereas CRN relies entirely on coatings to delay nitrogen release (Figure 3).
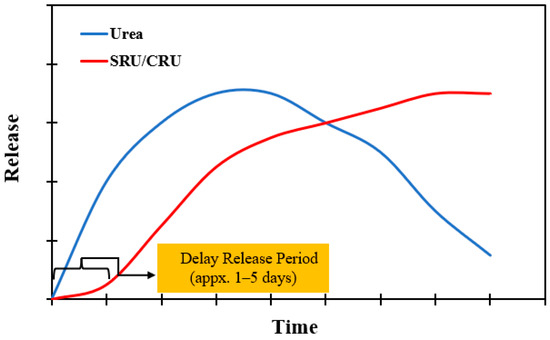
Figure 3.
Differences between the release rate of conventical urea and slow-release (SRU) and controlled-release (CRU) urea.
The major difference between these two forms of nitrogen is based on these coatings. Numerous studies have demonstrated that slow-release or controlled-release fertilizer can enhance nutrient use efficiency, improve crop yields, and reduce environmental harm because it is Eco-friendly and pollutes the environment minimally [19,20,31,32,33,34,35].
5.1. Slow–Release Nitrogen (SRN) Fertilizers and Their Methods
There are many terms identified for slow-release fertilizer (SRF). The Association of American Plant Food Control Officials (AAPFCO) defined slow-release fertilizer as a fertilizer that contains a specific plant nutrient in a form that delays its availability for uptake and utilization by plants after it is applied. This delay in availability can be performed either by retarding the release of the nutrient or by prolonging its availability to the plant for a more extended period compared to a reference fertilizer that provides nutrients rapidly and readily [26,36].
Moreover, SRF has been identified as “a result of interactions with mineral or organic materials” [17,27]. Therefore, according to that definition of (AAPFCO), two major categories of slow-release nitrogen fertilizers may be distinguished [36]. The initial category is composed of natural organic fertilizers such as compost. The term “natural” implies that the nitrogen in these fertilizers comes from animal waste and agricultural processing or any biodegradable sources, rather than being artificially synthesized through industrial processes like urea nitrogen [36]. Synthesized fertilizers are the second type of slow-release N source and include three subsets of chemically reacted slow-release products, physically granulated (crystallization), and mechanosynthesis products [28], as shown in Figure 4.
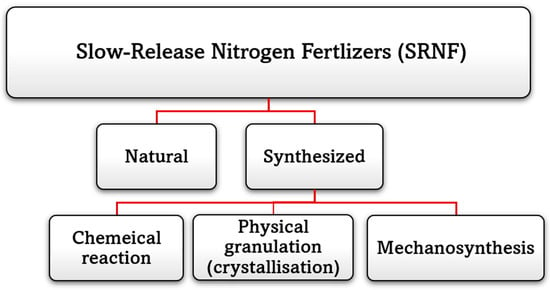
Figure 4.
Slow-release Nitrogen Fertilizers Categories.
The effectiveness of urea over a specific period is influenced by the quantity of nitrogen absorbed by the plants. Slow release of nutrients through fertilizers can be used to counter the swift depletion and wastage seen with conventional urea fertilizers. This gradual release of nutrients is seen as the best way to provide plants with a continuous source of nitrogen to accomplish their requirements [30,37,38]. This has been seen to significantly raise the effectiveness of urea while maintaining adequate crop growth. The slow-release nitrogen fertilizers are produced from urea that has undergone chemical reactions, physical granulation (crystallization), or Mechanosynthesis to delay their release into the soil solution.
The products that have been formulated by the chemical reaction can be grouped into three general categories: urea-formaldehyde (UF) reaction products, isobutylidenediurea (IBDU), and triazone [28]. Urea and formaldehyde are combined to produce slow-release nitrogen fertilizers of the urea-formaldehyde category at various temperatures and reaction durations, resulting in the creation of urea chains and carbon-hydrogen groups. These products are also commonly referred to as “methylene urea” (MUs); however, MUs only represent one step in the UF reaction process. IBDU, on the other hand, is produced through a urea and isobutyraldehyde combination, which releases nitrogen occurring through the hydrolysis process when the reactive product degrades.
Nitrogen release is faster in smaller particles and warmer soil temperatures. Triazones are ammonia-containing cyclic substances that are frequently supplied as a slow-release liquid. While significant attention has been paid to their usage as foliar fertilizers. Triazones are also effective as slow-release nitrogen fertilizers [28]. The other type of slow-release urea is produced by Mechanosynthesis or physical granulated urea fertilizer. Mechanochemical synthesis is a process of chemical synthesis that is accomplished by utilizing mechanical energy.
One of the applications of mechanochemical synthesis is the production of urea plus gypsum as a complex [37,38,39]. This process has become increasingly popular due to its ability to produce high-purity urea with a low environmental impact. Otherwise, physical granulation is a method of producing urea that involves the formation of small pellets or granules. This process involves spraying liquid urea onto a bed of seeds or recycled granules, which are then rotated and heated to form small, spherical particles. Physical granulation typically requires the use of solvents and binders to create the desired shape and size of the urea granules. The final product may contain impurities, such as dust or fines, which can affect the urea’s quality. In terms of the differences between these two methods, the main distinction lies in the process used to produce urea.
Mechanochemical synthesis does not involve the use of solvents or binders, resulting in a high-purity product. Physical granulation, on the other hand, requires the use of solvents and binders, which can introduce impurities into the final product. Additionally, mechanochemical synthesis can be carried out using simple equipment, while physical granulation requires specialized machinery. Mechanochemical synthesis offers a more environmentally friendly and sustainable option for producing high-purity urea. Physical granulation is also a viable option.
5.1.1. Slow-Release Nitrogen (SRN) Materials
Several innovative materials have been used to enhance the effectiveness of urea [26,27]. Three major groups of materials can be employed to provide slow-release nitrogen as shown in Figure 5.
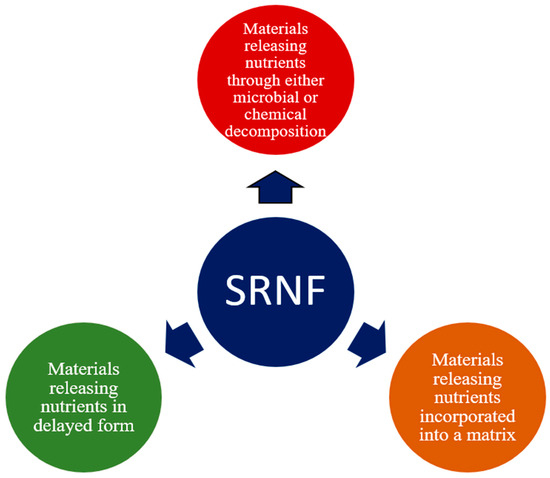
Figure 5.
Classification of materials used to produce slow-release nitrogen fertilizers.
Modifying urea with formaldehyde was the first method to produce a urea blend that enabled nitrogen to be released slowly. Moreover, formaldehyde was used as a material that can release nitrogen through either microbial decomposition [26,28] or compounds that decompose chemically (e.g., isobutyledene diurea—IBDU). This process begins with urea with formaldehyde combining in the catalyst presence, resulting in a white, odor-free solid that contains approximately 38% nitrogen [28,40]. These urea compounds were initially introduced as first-sold fertilizers in 1955. By varying the urea/formaldehyde ratio, temperature, pH, and time of the reaction, different types of UF reaction products can be produced, which can release nitrogen at varying rates [40].
The second type of slow-release fertilizers is renowned water-soluble fertilizers that are equipped with physical barriers produced by incorporating into the matrix using ecologically friendly materials, including cellulose, starch, and lignin, which emerged as substitute materials for SRF performing instead of synthetic polymers [41,42,43]. Starch has gained recognition as a sustainable, affordable, and completely biodegradable polymer that can be combined with synthetic polymers, such as polyvinyl alcohol (PVA), to create starch-based goods that conserve petrochemical resources and reduce the impact on the environment [27,42].
Additional chemical crosslinking techniques can be used to strengthen the blend’s structural integrity [42]. The blend can serve as an effective polymer matrix for the slow release of chemical substances through derivatization and cross-linking. This matrix allows for effective control over fertilizers for extended periods [42]. The matrix component can adopt either a hydrophobic nature, such as polyolefin, rubber, and the like, or a gel-forming polymer that is often denoted as a hydrogel. The hydrogel is endowed with hydrophilic properties, thereby obstructing the dissolution of fertilizer that is dispersed within the hydrogel material due to its capacity to retain considerable volumes of water, leading to swelling [11].
The third category pertained to substances that emit nitrogen over a prolonged period owing to their limited surface area. One of the most prevalent uses of nitrogen-releasing materials with restricted surface areas is gypsum, a naturally occurring mineral that is frequently employed in construction materials. It contains calcium sulfate, which is the critical component utilized in the mechanochemical synthesis of urea [12,37].
The process of producing urea with gypsum involves grinding the gypsum into a fine powder and then adding it to a mixture of ammonia and carbon dioxide [12,44]. The grinding process is crucial in this synthesis method as it helps to break down the gypsum into smaller particles, increasing the surface area for reaction. The ammonia is added to carbon dioxide, they are mixed in a closed chamber, and the reaction is initiated by the mechanical energy provided by the grinding process [44].
The chemical reaction that ensues is the amalgamation of ammonia and carbon dioxide, resulting in the formation of ammonium carbamate. The ammonium carbamate then reacts with the calcium sulfate present in the gypsum to produce urea and calcium carbonate [12]. The urea product is of exceptional purity because the manufacturing procedure does not involve the utilization of any solvents or other substances that could contaminate the outcome. Additionally, the process has a low environmental impact, as it does not produce any harmful by-products.
The mechanochemical synthesis of urea from gypsum has several advantages over traditional methods of urea production. It is a more sustainable and environmentally friendly process that can be carried out using simple equipment. The process can also be easily scaled up to produce large quantities of urea. The mechanochemical synthesis of urea from gypsum is an innovative and sustainable method of urea production. It has significant advantages over traditional methods and offers a more environmentally friendly solution to the growing demand for urea in various industries.
Numerous studies have extensively documented the utilization of diverse substances to generate slow-release nitrogen fertilizers as listed in Table S1. These materials all work by releasing nitrogen slowly over an extended period, which can reduce the amount of fertilizer needed and prevent environmental problems associated with excessive nitrogen runoff. Various techniques have been devised to enhance the dispensation qualities of these substances as shown in Figure 6, guaranteeing the timely and appropriate release of nitrogen for optimal cultivation. Overall, the application of slow-release nitrogen fertilizers is a highly encouraging tactic to advance harvest output whilst mitigating the ecological aftermath of farming.
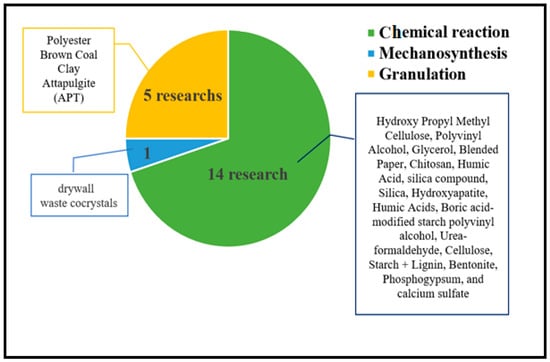
Figure 6.
Various techniques and their substances are used to produce slow-release fertilizers.
5.1.2. Mechanism of Slow-Release Nitrogen (SRN) Fertilizers and the Release Rate
The slow-release nitrogen mechanism mainly depends on the delay of urea blend degradation to save the balance between the plant’s requirements and the fertilizer stock. The postponement of the initial accessibility or the extension of the period of sustained availability can be attributed to diverse mechanisms [45]. These mechanisms may involve the regulated water solubility of the substance by altering the characteristics of the fertilizer in novel chemical configurations, gradually hydrolyzing low molecular weight water-soluble compounds over time, or using alternative, less commonly employed methods. Furthermore, it has been established that the liberation of nitrogen can be attributed to microbial processes and/or chemical hydrolysis [46]. In particular, it has been observed that the length of the reacted chain serves as a key determinant of the temporal profile of nitrogen release from UF products, with longer chains exhibiting a slower rate of nitrogen liberation [28]. The breakdown of these chains into shorter lengths by microorganisms ultimately leads to the release of urea. The necessary release rate of the nutrients for SRN relies on the metabolic needs of the specific crop over a designated period.
The European Standardization Committee (CEN) Task Force has established SRNF certain criteria, specifying that the nutrient release rate must be slower than that of traditional fertilizers, with no more than 15% of nutrients being released within 24 h, and no more than 75% of nutrients being released within 28 days. Moreover, at least 75% of the nutrients must be released within the stated release timeframe [23]. In addition, SRNF must meet certain requirements including cost-effectiveness, environmental friendliness, and sustainability [47,48].
5.2. Controlled Release Nitrogen (CRN) Fertilizers
Controlled-release nitrogen fertilizers (CRNF) are an entirely distinct breed of slow-release nitrogen fertilizers (SRNF), not only in terms of technological advancements but also in the way nitrogen is dispensed. Nevertheless, a few voices in the field have opined that CRF is simply a subset of SRF which belongs to a group of fertilizers that are obstructed by a physical barrier [11,17,49].
Moreover, controlled-release fertilizers (CRF) may be recognized as a type of product that contains a combination of nutrients coated with a polymer or other substance that undergoes gradual degradation in reaction to soil conditions such as moisture and temperature [17,36]. The particles of control-release nitrogen fertilizers are enveloped within either organic or inorganic materials or polymer coatings [11,17,49,50], thereby resulting in the delay of nitrogen release in a controlled-release form. The coatings serve as the key to achieving this effect. Furthermore, controlled urea use (CRU) is an approach that improves nitrogen use efficiency (NUE) and reduces environmental pollution.
Several methods and their materials have been reported in a large number of previous studies [10,11,20,49,50,51,52,53,54]. The physical incorporation of urea pellets into a coating material is one of the most prevalent methods utilized in the manufacture of controlled-release coated urea (CRCU) [11]. This technique involves coating urea granules with appropriate materials that can impede its release into the soil by reducing its solubility in water. This type of fertilizer typically releases nitrogen over several weeks to several months [55].
Polymer-coated urea is like coated urea, but the coating material is a polymer that gradually breaks down over time, releasing nitrogen in a controlled manner. This type of fertilizer can release nitrogen over several months to several years. Sulfur-coated urea is another material used for controlled-release nitrogen production. It is produced by coating urea granules with a sulfur crust that reacts with moisture in the soil to release nitrogen slowly [30,56].
Sulfur-coated urea typically releases nitrogen over 8–10 weeks. The controlled-release coated urea development is an ecological technology that has emerged as a responsible alternative for curtailing nitrogen losses resulting from volatilization and leaching. Additionally, it serves to enhance nitrogen release kinetics and maintain a balance between plants’ needs and nutrient stocks, thus aligning with their metabolic requirements [11,12,32].
5.2.1. Categorization of Coating Materials
The utilization of environmentally friendly natural or semi-natural macromolecule materials for coating has been reported in numerous previous studies [20,57,58]. These materials possess properties that fall between hydrophobicity and hydrophilicity [11,50]. This unique feature allows water to penetrate the coated surface, resulting in the release of nitrogen from the fertilizer. At the same time, the nature of the coating material, which is water-repellent and water-resistant, can reduce the rapid release of nutrients, thus fulfilling the essential nutrient needs for ideal crop growth [11].
The classification of controlled-release fertilizers (CRFs) has been accomplished in a variety of ways in previous research studies, concerning the mechanism and type of coating material employed. By analyzing the previous work of Shaviv [59], Liu [24], Trenkel [26], Azeem [11], Beig [49], Lawrencia [17], and Gutierrez [60], the coating materials could be widely classified into two major categories as illustrated and explained below in Figure 7. The first category is comprised of organic polymer materials, which can be either synthetic polymers (such as polyurethane, polyethylene, and alkyd resin) or natural polymers (such as starch, chitosan, cellulose, and others) [17,26]. The second category comprises inorganic materials such as sulfur and zeolite and other minerals such as clay, bentonite and phosphogypsum [11,38,49].
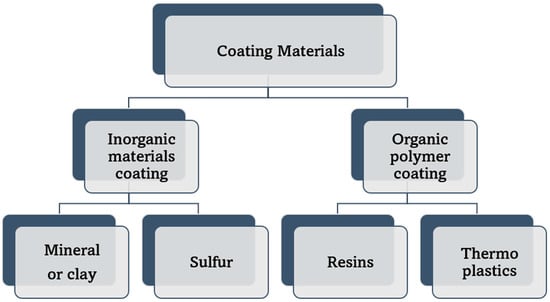
Figure 7.
The classification of coating materials that are used to produce controlled-release nitrogen fertilizers (CRNF).
Furthermore, recent investigations reveal that organic substances like biochar, rosin, and polyphenol are currently in use [20,58,61,62]. Scientists have investigated various mixtures of these materials to assess their impact on the release rate of urea and discover their potential as coatings for controlled-release fertilizers (CRFs). As a result, the scientific community has been very interested in the technology of coating urea fertilizer granules to control the release of nutrients. At first, the most popular urea-coating material was sulfur. Due to their apparent advantages over natural polymers, such as set-to-set consistency, predictable physicochemical properties, and ability to be customized, various synthetic polymers, such as polyethylene, polystyrene, and polyesters, have recently been used.
5.2.2. Constant Diffusion Mechanism
To clarify, the previous studies classified controlled-release fertilizers based on the way they release nutrients (the mechanism), either through the “failure mechanism” and/or the “diffusion mechanism” [11,24,26,49,63]. Figure 8 below shows how water can permeate the protective coating layer of the fertilizer, resulting in the controllable release of nitrogen pellets to the surrounding soil.
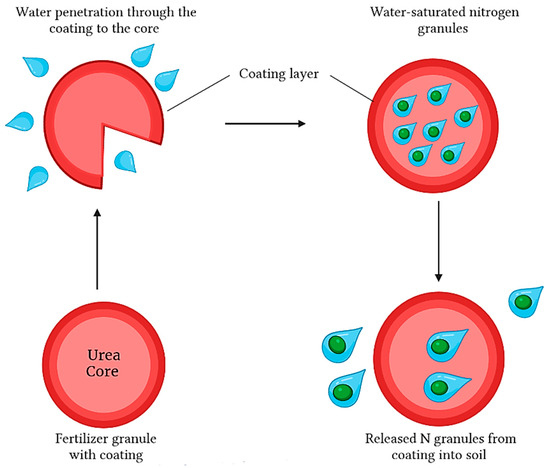
Figure 8.
Constant diffusion mechanism of coated release fertilizers.
The CRF’s effectiveness highly depends on its release mechanism, which is challenging to determine due to several variables such as the properties of coating materials, control release fertilizer type, and the agricultural environment. The scientific literature outlines several different release mechanisms, which are continuously evolving. Among these mechanisms, the multi-stage diffusion model is a commonly suggested approach for coated fertilizers’ release [64].
The nutrients’ release from controlled-release fertilizer occurs through three distinct stages according to the multi-stage diffusion model: the lag stage, the constant release stage, and the decay stage [17,65]. During the lag stage, which is the first stage, the irrigation water breaks through the coating layer and condenses on the core of the solid fertilizer. At this point, one might observe a minuscule fraction of the urea fertilizer dissolving, as depicted in Figure 8. The lag period in this process could be ascribed to two potential variables: the temporal necessity to engorge the internal cavities with an indispensable quantity of water or to establish equilibrium between the influx of water and the outflow of solute [17,64]. During the second stage, as water continues to penetrate the coating, the osmotic pressure inside the granules increases, causing more solid fertilizers to dissolve (Figure 8).
The gradual release of fertilizer from a polymer coating occurs upon the accumulation of a critical volume of the saturated solution. At this point, there are two ways in which the nutrients can be released [11]. The first scenario is observed when osmotic pressure exceeds the resistance of the membrane, resulting in the rupture of the coating, and the entire core is spontaneously released. This mechanism is known as the “failure mechanism” or “catastrophic release”.
The second scenario is in case the membrane withstands the increasing pressure, the fertilizer core is released slowly through diffusion. This release may be driven by a concentration or pressure gradient or a combination of both. This mechanism is defined as the “diffusion mechanism” and is commonly observed in polymer coatings, such as polyolefin. On the other hand, weaker coatings like sulfur or modified sulfur typically exhibit a failure mechanism. Most of the fertilizer has already been dissolved and released during the decay stage, which lowers the concentration gradient and driving force and slows the release rate [17].
6. The Pros and Cons of SRN and CRN Application
The slow-release nitrogen fertilizers usage provides nitrogen to plants over a longer period, while controlled-release nitrogen fertilizers provide nitrogen at a controlled rate. Here, we will discuss the pros and cons of using slow- and controlled-release nitrogen fertilizers. One of the main advantages of slow- or controlled-release nitrogen fertilizers is the long-lasting effect on the soil. The nitrogen is released slowly, and the rate of release can be controlled, which means that the plants receive the nitrogen they need when they need it and can absorb it over a more extended period. This reduces the need for frequent fertilization, costs, time, and energy in terms of economic advantage [66,67,68].
Furthermore, the utilization of slow- or controlled-release nitrogen fertilizers has been shown to improve nitrogen use efficiency (NUE) and diminish nitrogen loss [53,69], mainly by mitigating nitrate leaching, volatilization, and nitrous oxide emissions, resulting in a diminished environmental footprint when compared to conventional nitrogen fertilizers [1]. Additionally, slow- and controlled-release nitrogen fertilizers can increase crop yields by providing a steady supply of nitrogen to the plants. This can help farmers maximize their profits [70,71,72,73].
The controlled- or slow-release nitrogen fertilizers have another advantage; they promote healthy root growth [74,75]. As the nitrogen is released slowly, it allows the roots to grow deeper into the soil in search of nutrients. This helps improve the overall health of the plants and makes them more resilient to environmental stressors such as drought. Furthermore, releasing nutrients in a gradual and regulated manner at an optimal rate can contribute to agronomic safety by mitigating the toxicity levels that plants experience, particularly throughout the initial stages of growth.
This approach is unlike the conventional approach of applying chemical fertilizers which often results in a high local build-up of ions, creating a high concentration that leads to osmotic stress and ultimately harms plants [17,65]. On the other hand, there are disadvantages of controlled- or slow-release nitrogen fertilizers, such that they can be less flexible than traditional nitrogen fertilizers. Once they have been applied, it can be difficult to adjust the release rate if environmental conditions change, such as heavy rainfall [26]. This can lead to a loss of nitrogen from the soil or over-fertilization.
Controlled- or slow-release nitrogen fertilizers are more expensive than conventional nitrogen fertilizers. This can be a significant disadvantage for farmers who are on a tight budget. Controlled- or slow-release nitrogen fertilizers take longer to have an effect on plant growth than traditional nitrogen fertilizers. This means that farmers may have to wait longer to see the results of their fertilization efforts. Controlled-release nitrogen fertilizers can lead to over-fertilization if not used correctly [76]. Therefore, it is important to note that the choice between controlled-release and slow-release nitrogen fertilizers depends on various variables, such as crop type, soil type, and environmental conditions.
For example, slow-release nitrogen fertilizers are more suitable for crops that require a regular supply of nitrogen during the season of growth, while controlled-release nitrogen fertilizers are more suitable for crops that have specific nitrogen requirements during different growth stages. In addition to these advantages and disadvantages, it is worth considering the overall sustainability of using nitrogen fertilizers in the agriculture sector depending on the specific needs of the crop and the farming system. Overuse of nitrogenous fertilizers can have a negative environmental impact, including eutrophication of waterways, greenhouse gas emissions, and soil degradation. Consequently, it is critical to use nitrogen fertilizers judiciously and in combination with other sustainable agriculture practices such as crop rotation, cover cropping, and conservation tillage.
7. Impact of Modified Urea Fertilizers on Crop Yield and NUE
Numerous studies have reported that controlled- or slow-release fertilizers can positively impact nitrogen use efficiency based on several factors including crop type and soil conditions. The increase in crop yield is the main motivation for producers to use controlled- or slow-release fertilizers. Various studies investigated controlled- or slow-release fertilizers to improve crop yield and nitrogen use efficiency as listed in Table 1.
The fertilizer’s use efficiency is crucial in crop production to enhance yields and crop management and decrease the loss of nitrogen. Urea is a common nitrogenous fertilizer that has been utilized in agriculture for over a century. However, the traditional application of urea fertilizer is known to have negative environmental impacts [19,20]. Many studies reported that urea is less effective and its NUE is only around 50%, with 2–20% lost due to ammonia volatilization, approximately 15–25% reacting with soil organic components, and around 2–10% leaching into water systems [17,19,77]. Therefore, there was a necessity to enhance urea nitrogen use efficiency (UNUE) to reduce its negative environmental impacts.

Table 1.
Controlled- and slow–release nitrogen (CRN/SRN) fertilizers and their impact on crop yield and NUE.
Table 1.
Controlled- and slow–release nitrogen (CRN/SRN) fertilizers and their impact on crop yield and NUE.
| Fertilizer Type | Crop Type | Research Findings | Year | Ref. |
|---|---|---|---|---|
| Controlled/slow release | Rice | The NUE increased from 35.7% (Control) to 44.9% (SCRF). The application rate exceeding 90% could have a significant impact on the rice yield. | 2023 | [78] |
| Controlled-release fertilizer | Winter wheat | The applied CRU treatments significantly increased the grain yields by 9.96–15.11% and NUE by 6.71–10.33%. | 2021 | [79] |
| Controlled-release fertilizer | Rice | The results showed that 24% significant increase in N levels in the leaves of plants with CRF. | 2020 | [54] |
| Controlled-release urea | Rice | The CRU treatment reduced the rate of the N application by 20% compared to conventional urea. | 2020 | [80] |
| Controlled-release urea for | Wheat, maize, and rice | The utilization of CRU increased the grain yield by 7.23%, and a substantial upsurge of NUE, NAE, NUR, and NPE by an average of 23.4%, 34.65%, 25.83%, and 15.8%, respectively. | 2020 | [81] |
| Controlled-release urea | Maize | The application of CRU in comparison to urea (the same N rates) increased maize yield by 5.3% and NUE by 24.1%. | 2019 | [82] |
| Controlled-release urea | Wheat-maize | The application of CRF affected the NUE by an increase of 35.7–37.6% for wheat and 13.2–14.3% for maize. | 2017 | [71] |
| Controlled-release urea | Spring Wheat | Grain yields significantly increased from 9.58 to 11.21% and the N level from 19.06 to 23.94%. | 2016 | [83] |
| Controlled-release fertilizer | Rapeseed | An increase in N accumulation and NUE of CRF was 13.66% and 9.74% respectively. | 2016 | [84] |
| Combined application of urea and CRU | The combined application of urea and CRU increased the yield by 11.4% to 12.9% compared to 100% CRU. | 2013 | [85] | |
| Controlled-release fertilizer | Summer maize | The four yield-enhanced CRF treatments exhibited higher N uptake and physiological NUE compared to CCF. | 2013 | [86] |
| Slow-Release | Corn | There were noticeable augmentations in the absorption of nitrogen by plants and nitrogen recovery efficiency with slow-release fertilizers. | 2009 | [87] |
Recently, almost all fertilization research has tended to develop and modify urea compounds that aim to enhance nitrogen utilization and reduce nitrogen losses [66]. Here, we will try to explain the factors that affected the NUE and explore the influences of modified urea compounds on the yields and the agronomic nitrogen use efficiency (ANUE). Controlled- or slow-release nitrogen fertilizers possess the capacity to markedly enhance the efficiency of nitrogen use whilst concurrently preserving crop productivity [88].
Furthermore, controlled- or slow-release nitrogen (CRN/SRN) has the potential to serve as an exceedingly effective nitrogen source for grain crops, thereby augmenting yields. Controlled- or slow-release fertilizers can reduce the recommended rate of the application by 20–30% (or more) compared to conventional fertilizers [26]. Various factors influence nutrient use efficiency (NUE) with a particular emphasis on nitrogen losses. This involves enhancing nutrient uptake by plants, thereby decreasing the amount left in the soil that is vulnerable to environmental loss [26].
The decision to use controlled- or slow-release fertilizers may be influenced by several direct economic factors, including potential nutrient losses and the cost of fertilizer applications [59]. These factors play a vital role in affecting the agronomic use efficiency of controlled- or slow-release fertilizers (CRFs or SRFs). Nutrient loss potentiality is the most important factor, which can occur through various physical, biological, and chemical processes such as surface runoff, leaching, volatilization, denitrification, immobilization, or fixation [89]. The use of SRFs/CRFs and bio-amended ammonium fertilizers has been shown to reduce such losses, leading to better nutrient recovery and crop yield [58,59,90,91].
Another key economic factor is the cost of fertilizer applications [66,69]. Using SRFs or CRFs can result in significant cost savings, as simply one application can provide nutrients for the whole season of growth, reducing the need for multiple applications and spreading costs [66,92]. SRFs or CRFs with a lag in the release can also be applied during less restricted trafficability periods, including fall for spring or winter-planted crops, or before the annual spring rush [51,93].
In addition, SRFs/CRFs may decrease the insistence on short-season and hand labor for top-dressing, which is critical during peak periods such as in rice paddies [20]. Furthermore, applying controlled- or slow-release fertilizers could provide notable benefits in terms of enhancing plant nutrition and physiological functions [26,92]. Conventional soluble fertilizers can lead to an excessive nutrient supply, increasing the concentration of soluble salts in the root area [94,95]. Consequently, this results in several issues, including osmotic stress, particular injuries to plants at different stages of development, and unfavorable developments like lodging; these are just a few issues that can occur [96,97].
In contrast, the application of SRFs or CRFs is accompanied by reduced stress and specific toxicity, as they release nutrients slowly over an extended period, thus reducing the risk of over-fertilization [46,98]. This slow-release mechanism allows plants to absorb the nutrients as needed, avoiding the accumulation of toxic concentrations of nutrients in the soil [24,46]. Research has also shown that the use of SRFs and CRFs is associated with heightened rates of germination and crop productivity and the decreased burning of leaves, stalk breakage, and infestation of disease [71,95,99,100].
In summary, the use of SRFs can improve plant growth, increase yield, and improve crop quality. Additionally, providing the preferred chemical forms of nutrients for plants was one of the factors that affected fertilizer use efficiency [92]. The focus on providing plant nutrients in their preferred chemical forms has received considerable attention over the past two decades, especially in terms of supplying mixed ammonium-nitrate nutrition [34]. Multiple research endeavors have divulged noteworthy advancements in grain production and protein density by employing mixed-N nourishment, by comparison to exclusively nitrate or ammonium consumption alone [66,92,101,102,103,104].
However, it was only in experiments where a proper regulation of the ammonium/nitrate ratio in soil was upheld, that these results were observed. To attain a boost in ammonium nourishment, diverse methods can be employed including the utilization of nitrification inhibitors, controlled or slow fertigation, or heightened local concentrations of ammonium or ammonia [105,106]. Finally, there is assembling evidence to show that different nutrients can work together especially when they are supplied at the same time or placed close to the root surface’s absorption sites or near the root.
For instance, NH4 or K can significantly increase the availability of Fe in calcareous soils, likely as a result of the rhizosphere’s physiological acidification [107]. The utilization of KzSO4 co-granulated with FeSO4 has been demonstrated by researchers as an effective method for correcting Fe deficiency in highly calcareous soils. Conversely, the application of Fe and K sulfates alone resulted in inadequate outcomes. Another example of the efficacy of soil amendments is the ability of NH4 to increase P bioavailability when its nitrification rate is reduced, presumably through rhizosphere acidification. Additionally, the provision of mixed ammonium-nitrate nutrition to plants requires an adequate K supply to achieve increases in yield and protein content. Proper management of the pattern and chemical form of nutrient release from SRFs or CRFs can facilitate the beneficial physiological effects offered by these treatments [108]. However, the effects mentioned above require intensified attention, particularly on the field level, to assess their actual contribution, besides simply reducing nutrient losses [92].
8. Conclusions
In recent times, the utilization of urea-modified fertilizers has emerged as a crucial factor in improving the availability of mineral nitrogen in the soil. These fertilizers not only enhance nitrogen levels but also can decrease the nitrogen release rate, resulting in higher efficiency of fertilizers in crop cultivation. Moreover, numerous studies have highlighted the beneficial impact of urea-controlled or slow-release compounds on crop productivity. However, despite these advancements, there is still a requirement for more extensive investigations to better understand the effects of these compounds. By conducting further research, scientists aim to refine the formulations of urea-modified fertilizers and integrate them with effective agronomic practices, ultimately leading to reduced nitrogen losses and improved nitrogen use efficiency in agriculture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su16010188/s1. References [27,37,38,40,42,52,57,108,109,110,111,112,113,114,115,116,117,118,119,120] are cited in the Supplementary Materials.
Author Contributions
R.M. and J.B. conceived the study. S.S. collected and analyzed the works of literature, visualized the data, and wrote the original draft of the manuscript. K.B. and D.D. revised the manuscript. All the authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Engineering for Agricultural Production Systems program grant no. 2020-67022-31144 from the USDA National Institute of Food and Agriculture.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used in the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bacon, P. Nitrogen Fertilization in the Environment, 13th ed.; Marcel Dekker: New York, NY, USA, 1995. [Google Scholar]
- Rosmarina, A.K.; Khanif, Y.M.; Hanafi, M.M.; Aminuddin, H.; Abdul Rahim, K.B. Nitrogen Loss Pathways in Anaerobic Soils and Mitigation Approaches Through Inhibitors—A Review. Am. Eurasian J. Agric. Environ. Sci. 2016, 16, 641–651. [Google Scholar]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen Losses from the Soil/Plant System: A Review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Terman, G.L. Volatilization Losses of Nitrogen as Ammonia from Surface-Applied Fertilizers, Organic Amendments, and Crop Residues. Adv. Agron. 1980, 31, 189–223. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.L.; Li, Q.; Zeng, X.P.; Liu, Y.; Li, Y.R. Fate of Nitrogen in Agriculture and Environment: Agronomic, Eco-Physiological and Molecular Approaches to Improve Nitrogen Use Efficiency. Biol. Res. 2020, 53, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Cao, W.; Fang, J.; Cai, L. Nitrogen and Phosphorus Losses from Agricultural Systems in China: A Meta-Analysis. Mar. Pollut. Bull. 2014, 85, 727–732. [Google Scholar] [CrossRef]
- Li, S.Q.; Ji, C.R.; Fang, Y.N.; Chen, X.L.; Li, S.X. Advances in Nitrogen Loss Leached by Precipitation from Plant Canopy. Agric. Sci. China 2008, 7, 480–486. [Google Scholar] [CrossRef]
- Fenn, L.B.; Miyamoto, S. Ammonia Loss and Associated Reactions of Urea in Calcareous Soils. Soil Sci. Soc. Am. J. 1981, 45, 537–540. [Google Scholar] [CrossRef]
- Mateo-Sagasta, J.; Zadeh, S.M.; Turral, H.; Burke, J. More People, More Food, Worse Water? A Global Review of Water Pollution from Agriculture, Executive Summary; FAO: Rome, Italy, 2017. [Google Scholar]
- Azeem, B.; KuShaari, K.; Man, Z. Effect of Coating Thickness on Release Characteristics of Controlled Release Urea Produced in Fluidized Bed Using Waterborne Starch Biopolymer as Coating Material. Procedia Eng. 2016, 148, 282–289. [Google Scholar] [CrossRef]
- Azeem, B.; Kushaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on Materials & Methods to Produce Controlled Release Coated Urea Fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [CrossRef]
- Barčauskaite, K.; Braziene, Z.; Avižienyte, D.; Silva, M.; Drapanauskaite, D.; Honer, K.; Gvildiene, K.; Slinksiene, R.; Jancaitiene, K.; Mazeika, R.; et al. Mechanochemically Synthesized Gypsum and Gypsum Drywall Waste Cocrystals with Urea for Enhanced Environmental Sustainability Fertilizers. J. Environ. Chem. Eng. 2020, 8, 103965. [Google Scholar] [CrossRef]
- Heffer, P.; Prud’homme, M. Global Nitrogen Fertilizer Demand and Supply: Trend, Current Level and Outlook. In Proceedings of the International Nitrogen Initiative Conference, “Solutions to Improve Nitrogen Use Efficiency for the World”, Melbourne, Australia, 4–8 December 2016. [Google Scholar]
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision; FAO: Rome, Italy, 2012. [Google Scholar]
- IFA. Short-Term fertilizer Outlook 2017–2018 Report; IFA International Fertilizer Association, Services PITaA: Paris, France, 2017. [Google Scholar]
- Cantarella, H.; Otto, R.; Soares, J.R.; de Brito Silva, A.G. Agronomic Efficiency of NBPT as a Urease Inhibitor: A Review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Sanabria, J.; Austin, E.R.; Agyin-Birikorang, S. Nitrogen Transformation, Ammonia Volatilization Loss, and Nitrate Leaching in Organically Enhanced Nitrogen Fertilizers Relative to Urea. Soil Sci. Soc. Am. J. 2012, 76, 1842–1854. [Google Scholar] [CrossRef]
- Savci, S. Investigation of Effect of Chemical Fertilizers on Environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Gil-Ortiz, R.; Naranjo, M.Á.; Ruiz-Navarro, A.; Caballero-Molada, M.; Atares, S.; García, C.; Vicente, O. New Eco-Friendly Polymeric-Coated Urea Fertilizers Enhanced Crop Yield in Wheat. Agronomy 2020, 10, 438. [Google Scholar] [CrossRef]
- Han, M.; Wong, J.; Su, T.; Beatty, P.H.; Good, A.G. Identification of Nitrogen Use Efficiency Genes in Barley: Searching for QTLs Controlling Complex Physiological Traits. Front. Plant Sci. 2016, 7, 1587. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Jie, C.; Jing-zhang, C.; Man-zhi, T.; Zi-tong, G. Soil Degradation: A Global Problem Endangering Sustainable Development. J. Geogr. Sci. 2002, 12, 243–252. [Google Scholar] [CrossRef]
- Liu, L.S.; Kost, J.; Fishman, M.L.; Hicks, K.B. A Review: Controlled Release Systems for Agricultural and Food Applications. ACS Symp. Ser. 2008, 992, 265–281. [Google Scholar] [CrossRef]
- Beig, B.; Bilal Khan Niazi, M.; Jahan, Z.; Hussain, A.; Hussain Zia, M.; Taqi Mehran, M.; Taqi, M. Coating Materials for Slow Release of Nitrogen from Urea Fertilizer: A Review. J. Plant Nutr. 2020, 43, 1510–1533. [Google Scholar] [CrossRef]
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture, 2nd ed.; International Fertilizer Industry Association: Paris, France, 2010. [Google Scholar]
- Kareem, S.A.; Dere, I.; Gungula, D.T.; Andrew, F.P.; Saddiq, A.M.; Adebayo, E.F.; Tame, V.T.; Kefas, H.M.; Joseph, J.; Patrick, D.O. Synthesis and Characterization of Slow-Release Fertilizer Hydrogel Based on Hydroxy Propyl Methyl Cellulose, Polyvinyl Alcohol, Glycerol and Blended Paper. Gels 2021, 7, 262. [Google Scholar] [CrossRef] [PubMed]
- Guertal, E.A. Slow-Release Nitrogen Fertilizers in Vegetable Production: A Review. Horttechnology 2009, 19, 16–19. [Google Scholar] [CrossRef]
- Shaviv, A. Advances in Controlled-Release Fertilizers; Elsevier: Amsterdam, The Netherlands, 2001; Volume 71, pp. 1–49. [Google Scholar] [CrossRef]
- Blouin, G.M.; Rindt, D.W.; Moore, O.E. Sulfur-Coated Fertilizers for Controlled Release: Pilot Plant Production. J. Agric. Food Chem. 1971, 19, 801–808. [Google Scholar] [CrossRef]
- Roshanravan, B.; Soltani, S.M.; Rashid, S.A.; Mahdavi, F.; Yusop, M.K. Enhancement of Nitrogen Release Properties of Urea–Kaolinite Fertilizer with Chitosan Binder. Chem. Speciat. Bioavailab. 2015, 27, 44–51. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for Nano-Biotechnology Enabled Protection and Nutrition of Plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhou, X.; Ding, Z.; Liu, Q.; Xie, G.; Peng, J.; Rong, X.; Zhang, Y.; Yang, Y.; Eissa, M.A. Controlled-Release N Fertilizer to Mitigate Ammonia Volatilization from Double-Cropping Rice. Nutr. Cycl. Agroecosyst. 2021, 119, 123–137. [Google Scholar] [CrossRef]
- Shoji, S. Innovative Use of Controlled Availability Fertilizers with High Performance for Intensive Agriculture and Environmental Conservation. Sci. China Ser. C Life Sci./Chin. Acad. Sci. 2005, 48, 912–920. [Google Scholar] [CrossRef]
- Chen, J.; Wei, X. Controlled-Release Fertilizers as a Means to Reduce Nitrogen Leaching and Runoff in Container-Grown Plant Production. In Nitrogen in Agriculture—Updates; IntechOpen: London, UK, 2018; pp. 34–52. [Google Scholar] [CrossRef]
- AAPFCO. Association of American Plant Food Control Officials (AAPFCO): Official Publication No. 57; Association of American Plant Food Control Officials Inc.: West Lafayette, IN, USA, 1997. [Google Scholar]
- Malinowski, P.; Biskupski, A.; Gtowiłski, J. Preparation Methods of Calcium Sulphate and Urea Adduct. Pol. J. Chem. Technol. 2007, 9, 111–114. [Google Scholar] [CrossRef]
- Borowik, M.; Malinowski, P.; Biskupski, A.; Dawidowicz, M.; Schab, S.; Rusek, P.; Igras, J. Production Technology of Nitrogen-Sulphur-Calcium Fertilizers on the Base of Urea and Phosphogypsum. Chemik 2012, 66, 525–534. [Google Scholar]
- Villiers, J.P.R.D.E.; Boeyens, J.C.A. Crystal Structure of a Calcium Sulfate-Urea Complex. J. Cryst. Mol. Struct. 1975, 5, 215–226. [Google Scholar] [CrossRef]
- Yamamoto, C.F.; Pereira, E.I.; Mattoso, L.H.C.; Matsunaka, T.; Ribeiro, C. Slow Release Fertilizers Based on Urea/Urea–Formaldehyde Polymer Nanocomposites. Chem. Eng. J. 2016, 287, 390–397. [Google Scholar] [CrossRef]
- Mulder, W.J.; Gosselink, R.J.A.; Vingerhoeds, M.H.; Harmsen, P.F.H.; Eastham, D. Lignin Based Controlled Release Coatings. Ind. Crops Prod. 2011, 34, 915–920. [Google Scholar] [CrossRef]
- Lum, Y.H.; Shaaban, A.; Mohamad, N.; Dimin, F.; Yatim, N.M. Boric Acid Modified Starch Polyvinyl Alcohol Matrix for Slow Release Fertilizer. E-Polymers 2016, 16, 151–158. [Google Scholar] [CrossRef]
- Özen, İ.; Okyay, G.; Ulaş, A. Coating of Nonwovens with Potassium Nitrate Containing Carboxymethyl Cellulose for Efficient Water and Fertilizer Management. Cellulose 2018, 25, 1527–1538. [Google Scholar] [CrossRef]
- Alshamaileh, E.; Al-Rawajfeh, A.E.; Alrbaihat, M. Mechanochemical Synthesis of Slow-Release Fertilizers: A Review. Open Agric. J. 2018, 12, 11–19. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Sharma, M.; Sharma, P.; Tomar, R. Synthesis and Surfactant Modification of Clinoptilolite and Montmorillonite for the Removal of Nitrate and Preparation of Slow Release Nitrogen Fertilizer. J. Hazard Mater. 2012, 227–228, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.T.; Cushman, K.E.; Sato, S. Release Mechanisms for Slow- and Controlledrelease Fertilizers and Strategies for Their Use in Vegetable Production. Horttechnology 2009, 19, 10–12. [Google Scholar] [CrossRef]
- Ashitha, A.; Rakhimol, K.R.J.M. Chapter 2—Fate of the Conventional Fertilizers in Environment; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Naz, M.Y.; Sulaiman, S.A. Muhammad Yasin Naz, Shaharin Anwar Sulaiman, Slow release coating remedy for nitrogen loss from conventional urea: A review. J. Control. Release 2016, 225, 109–120, ISSN 0168-3659. [Google Scholar] [CrossRef]
- Chen, J.; Lü, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally Friendly Fertilizers: A Review of Materials Used and Their Effects on the Environment. Sci. Total Environ. 2018, 613–614, 829–839. [Google Scholar] [CrossRef]
- Jariwala, H.; Santos, R.M.; Lauzon, J.D.; Dutta, A.; Wai Chiang, Y. Controlled Release Fertilizers (CRFs) for Climate-Smart Agriculture Practices: A Comprehensive Review on Release Mechanism, Materials, Methods of Preparation, and Effect on Environmental Parameters. Environ. Sci. Pollut. Res. 2022, 29, 53967–53995. [Google Scholar] [CrossRef] [PubMed]
- Rizki Pradana, A.; Royani, A.; Zulfikri, K.; Tuffahati, N.; Zulfa Azzahra, R.; Qori Amini, T.; Bayu Dani Nandiyanto Setiabudi No, A.; City, B.; Java, W. Review: Synthesis of Urea in Several Methods. Mediterr. J. Chem. 2021, 2021, 146–161. [Google Scholar] [CrossRef]
- Swify, S.; Avizienyte, D.; Mazeika, R.; Braziene, Z. Influence of Modified Urea Compounds to Improve Nitrogen Use Efficiency under Corn Growth System. Sustainability 2022, 14, 14166. [Google Scholar] [CrossRef]
- Gil-Ortiz, R.; Naranjo, M.Á.; Ruiz-Navarro, A.; Atares, S.; García, C.; Zotarelli, L.; Bautista, A.S.; Vicente, O. Enhanced Agronomic Efficiency Using a New Controlled-Released, Polymeric-Coated Nitrogen Fertilizer in Rice. Plants 2020, 9, 1183. [Google Scholar] [CrossRef] [PubMed]
- Cahill, S.; Osmond, D.; Israel, D. Nitrogen Release from Coated Urea Fertilizers in Different Soils. Commun. Soil Sci. Plant Anal. 2010, 41, 1245–1256. [Google Scholar] [CrossRef]
- Zaman, A.; Khyber, K.; Ali, B.; Afzal, M.; Wahab, S.; Zaman Khan, A. Effects of Sulfur and Urease Coated Controlled Release Urea on Dry Matter Yield, N Uptake and Grain Quality of Rice. J. Anim. Plant Sci. 2015, 25, 679–685. [Google Scholar]
- Nilwala Kottegoda*, I.M.; Nadeesh, M.; Veranja, K. A Green Slow-Release Fertilizer Composition Based on Urea-Modified Hydroxyapatite Nanoparticles Encapsulated Wood. Curr. Sci. 2011, 101, 73–78. [Google Scholar]
- Mukerabigwi, J.F.; Wang, Q.; Ma, X.; Liu, M.; Lei, S.; Wei, H.; Huang, X.; Cao, Y. Urea Fertilizer Coated with Biodegradable Polymers and Diatomite for Slow Release and Water Retention. J. Coat Technol. Res. 2015, 12, 1085–1094. [Google Scholar] [CrossRef]
- Shaviv, A.; Mikkelsen, R.L. Controlled-Release Fertilizers to Increase Efficiency of Nutrient Use and Minimize Environmental Degradation—A Review. Fertil. Res. 1993, 35, 1–12. [Google Scholar] [CrossRef]
- Gutiérrez, C.A.; Ledezma-Delgadillo, A.; Juárez-Luna, G.; Neri-Torres, E.E.; Ibanez, J.G.; Quevedo, I.R. Production, Mechanisms, and Performance of Controlled-Release Fertilizers Encapsulated with Biodegradable-Based Coatings. ACS Agric. Sci. Technol. 2022, 2, 1101–1125. [Google Scholar] [CrossRef]
- Shi, W.; Ju, Y.; Bian, R.; Li, L.; Joseph, S.; Mitchell, D.R.G.; Munroe, P.; Taherymoosavi, S.; Pan, G. Biochar Bound Urea Boosts Plant Growth and Reduces Nitrogen Leaching. Sci. Total Environ. 2020, 701, 134424. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, I.; Majeed, Z.; Ajab, Z.; Ahmad, B.; Khurshid, K.; Mubashir, M. Optimized Tuning of Rosin Adduct with Maleic Anhydride for Smart Applications in Controlled and Targeted Delivery of Urea for Higher Plant’s Uptake and Growth Efficiency. Ind. Crops Prod. 2019, 133, 395–408. [Google Scholar] [CrossRef]
- Shaviv, A. Controlled Release Fertilizers. In Enhanced-Efficiency Fertilizers; IFA International Workshop on Enhanced-Efficiency Fertilizers: Frankfurt, Germany, 2005. [Google Scholar]
- Shaviv, A.; Raban, S.; Zaidel, E. Modeling Controlled Nutrient Release from Polymer Coated Fertilizers: Diffusion Release from Single Granules. Environ. Sci. Technol. 2003, 37, 2251–2256. [Google Scholar] [CrossRef]
- Sempeho, S.I.; Kim, H.T.; Mubofu, E.; Hilonga, A. Meticulous Overview on the Controlled Release Fertilizers. Adv. Chem. 2014, 2014, 363071. [Google Scholar] [CrossRef]
- Buresh, R.J.; Baanante, C.A. Potential Economic Benefits of Modifications to Urea That Increase Yield through Reduction in Nitrogen Losses. Agron. J. 1993, 85, 947–954. [Google Scholar] [CrossRef]
- Mi, W.; Yang, X.; Wu, L.; Ma, Q.; Liu, Y.; Zhang, X. Evaluation of Nitrogen Fertilizer and Cultivation Methods for Agronomic Performance of Rice. Agron. J. 2016, 108, 1907–1916. [Google Scholar] [CrossRef]
- Hassan, W.Z. Preparation and Properties of Urea Slow Release Coated with Potassium Humate, Bentonite and Polyacrylamide as Compositely Fertilizer Which Reflected on the Productivity of Wheat Crop. J. Soil Sci. Agric. Eng. Mansoura Univ. 2018, 9, 627–635. [Google Scholar] [CrossRef]
- Xu, X.; He, P.; Wei, J.; Cui, R.; Sun, J.; Qiu, S.; Zhao, S. Use of Controlled-Release Urea to Improve Yield, Nitrogen Utilization, and Economic Return and Reduce Nitrogen Loss in Wheat-Maize Crop Rotations. Agronomy 2021, 11, 723. [Google Scholar] [CrossRef]
- Raun, W.R.; Johnson, G.V. Improving Nitrogen Use Efficiency for Cereal Production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Z.; Zhang, M.; Shi, Y.; Zhu, Q.; Sun, Y.; Zhou, H.; Li, C.; Yang, Y.; Geng, J. Improving Crop Yields, Nitrogen Use Efficiencies, and Profits by Using Mixtures of Coated Controlled-Released and Uncoated Urea in a Wheat-Maize System. Field Crops Res. 2017, 205, 106–115. [Google Scholar] [CrossRef]
- Shivay, Y.S.; Prasad, R.; Pal, M. Effect of Nitrogen Levels and Coated Urea on Growth, Yields and Nitrogen Use Efficiency in Aromatic Rice. J. Plant Nutr. 2016, 39, 875–882. [Google Scholar] [CrossRef]
- Aghdam, S.M.; Yeganehpoor, F.; Kahrariyan, B.; Shabani, E. Effect of Different Urea Levels on Yield and Yield Components of Corn 704. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 300–305. [Google Scholar]
- Zhang, M.; Nyborg, M.; Malhi, S.S.; Solberg, E.D. Localized Root Growth in Soil Induced by Controlled-Release Urea Granule and Barley Nitrogen Uptake. J. Plant Nutr. 2000, 23, 413–422. [Google Scholar] [CrossRef]
- Tang, L.; Sun, H.; Sun, R.; Niu, Y.; Song, J.; Li, S.; Shen, Y. Optimized Nitrogen Application Increases Soil Water Extraction by Changing In-Season Maize Root Morphology and Distribution in Rainfed Farmland. Agronomy 2020, 10, 1606. [Google Scholar] [CrossRef]
- Lammel, J. Cost of the Different Options Available to the Farmers: Current Situation and Prospects; IFA International Workshop on Enhanced-Efficiency Fertilizers: Frankfurt, Germany, 2005. [Google Scholar]
- Achat, D.L.; Augusto, L.; Gallet-Budynek, A.; Loustau, D. Future Challenges in Coupled C–N–P Cycle Models for Terrestrial Ecosystems under Global Change: A Review. Biogeochemistry 2016, 131, 173–202. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Li, G.; Jiang, Y.; Hou, P.; Xue, L.; Yang, L.; Ding, Y. Lower Dose of Controlled/Slow Release Fertilizer with Higher Rice Yield and N Utilization in Paddies: Evidence from a Meta-Analysis. Field Crops Res. 2023, 294, 108879. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, M.; Zheng, G.; Yao, Y.; Tao, R.; Zhu, M.; Ding, J.; Li, C.; Guo, W.; Zhu, X. Twice-Split Application of Controlled-Release Nitrogen Fertilizer Met the Nitrogen Demand of Winter Wheat. Field Crops Res. 2021, 267, 108163. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q.; Ma, J.; Zou, P.; Jiang, L. Impact of Controlled-Release Urea on Rice Yield, Nitrogen Use Efficiency and Soil Fertility in a Single Rice Cropping System. Sci. Rep. 2020, 10, 10432. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, L.; Xu, Y.; Yang, Y.; Shi, R. Application of Controlled Release Urea Improved Grain Yield and Nitrogen Use Efficiency: A Meta-Analysis. PLoS ONE 2020, 15, e0241481. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Z.; He, X.; Wang, X.; Shi, X.; Zou, C.; Chen, X. The Effects of Controlled Release Urea on Maize Productivity and Reactive Nitrogen Losses: A Meta-Analysis. Environ. Pollut. 2019, 246, 559–565. [Google Scholar] [CrossRef]
- Shivay, Y.S.; Pooniya, V.; Prasad, R. Sulphur-Coated Urea as a Source of Sulphur and an Enhanced Efficiency of Nitrogen Fertilizer for Spring Wheat. Cereal Res. Commun. 2016, 44, 513–523. [Google Scholar] [CrossRef]
- Tian, C.; Zhou, X.; Liu, Q.; Peng, J.W.; Wang, W.M.; Zhang, Z.H.; Yang, Y.; Song, H.X.; Guan, C.Y. Effects of a Controlled-Release Fertilizer on Yield, Nutrient Uptake, and Fertilizer Usage Efficiency in Early Ripening Rapeseed (Brassica napus L.). J. Zhejiang Univ. Sci. B 2016, 17, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Su-ping, W.; Li, X.; Jian-wei, L.; Hui, L.; Bo, L.; Qing-feng, W.; Hang, W.; Guo-bin, X.; Xin-xin, X.; Zheng-wei, X. Effects of Combined Application of Urea and Controlled-Release Urea on Yield, Profits of Rapeseed and Soil Inorganic Nitrogen. Chin. J. Oil Crop Sci. 2013, 35, 295. [Google Scholar] [CrossRef]
- Zhao, B.; Dong, S.; Zhang, J.; Liu, P. Effects of Controlled-Release Fertiliser on Nitrogen Use Efficiency in Summer Maize. PLoS ONE 2013, 8, e70569. [Google Scholar] [CrossRef] [PubMed]
- Noellsch, A.J.; Motavalli, P.P.; Nelson, K.A.; Kitchen, N.R. Corn Response to Conventional and Slow-Release Nitrogen Fertilizers across a Claypan Landscape. Agron. J. 2009, 101, 607–614. [Google Scholar] [CrossRef]
- Blaylock, A.D.; Kaufmann, J.; Dowbenko, R.D. Nitrogen Fertilizer Technologies. West. Nutr. Manag. 2005, 6, 8–13. [Google Scholar]
- Marchetti, R.; Castelli, F. Mineral Nitrogen Dynamics in Soil during Sugar Beet and Winter Wheat Crop Growth. Eur. J. Agron. 2011, 35, 13–21. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Medina, J.; González, A.; Diez, M.C.; Cartes, P.; Monreal, C.; Navia, R. Evaluation of Biodegradable Polymers as Encapsulating Agents for the Development of a Urea Controlled-Release Fertilizer Using Biochar as Support Material. Sci. Total Environ. 2015, 505, 446–453. [Google Scholar] [CrossRef]
- Geng, J.; Sun, Y.; Zhang, M.; Li, C.; Yang, Y.; Liu, Z.; Li, S. Long-Term Effects of Controlled Release Urea Applica-tion on Crop Yields and Soil Fertility under Rice-Oilseed Rape Rotation System. Field Crops Res. 2015, 184, 65–73. [Google Scholar] [CrossRef]
- Christianson, C.B. Factors Affecting N Release of Urea from Reactive Layer Coated Urea. Fert. Res. 1988, 16, 273–284. [Google Scholar] [CrossRef]
- Jackson, L.E.; Burger, M.; Cavagnaro, T.R. Roots, Nitrogen Transformations, and Ecosystem Services. Annu. Rev. Plant Biol. 2008, 59, 341–363. [Google Scholar] [CrossRef]
- Vazquez-Carrasquer, V.; Laperche, A.; Bissuel-Bélaygue, C.; Chelle, M.; Richard-Molard, C. Nitrogen Uptake Efficiency, Mediated by Fine Root Growth, Early Determines Temporal and Genotypic Variations in Nitrogen Use Efficiency of Winter Oilseed Rape. Front. Plant Sci. 2021, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Hauck, R.D. Slow-Release and Bioinhibitor-Amended Nitrogen Fertilizers. In Fertilizer Technology and Use; Soil Science Society of America, Inc.: Madison, WI, USA, 1985; pp. 293–322. [Google Scholar] [CrossRef]
- Mikkelsen, R.L.; Williams, H.M.; Behel, A.D. Nitrogen Leaching and Plant Uptake from Controlled-Release Fertilizers. Fertil. Res. 1994, 37, 43–50. [Google Scholar] [CrossRef]
- Oertli, J.J. Controlled-Release Fertilizer. Fertil. Res. 1980, 1, 103–123. [Google Scholar] [CrossRef]
- Bashir, N.; Malik, S.A.; Mahmood, S. Influence of Urea Application on Growth, Yield and Mineral Uptake in Two Corn (Zea mays L.) Cultivars. Afr. J. Biotechnol. 2012, 11, 10494–10503. [Google Scholar] [CrossRef]
- Ghafoor, I.; Habib-ur-Rahman, M.; Ali, M.; Afzal, M.; Ahmed, W.; Gaiser, T.; Ghaffar, A. Slow-Release Nitrogen Fertilizers Enhance Growth, Yield, NUE in Wheat Crop and Reduce Nitrogen Losses under an Arid Environment. Environ. Sci. Pollut. Res. 2021, 28, 43528–43543. [Google Scholar] [CrossRef] [PubMed]
- Yerokun, O.A. Response of Maize to Ammonium Nitrate, Urea and Cogranulated Urea-Urea Phosphate. South Afr. J. Plant Soil 1997, 14, 63–66. [Google Scholar] [CrossRef]
- Uknowledge, U.; Murdock, L.W. Comparative Effectiveness of Urea, Ammonium Nitrate, and Urea Comparative Effectiveness of Urea, Ammonium Nitrate, and Urea Ammonium Polyphosphate on Fescue Production Ammonium Polyphosphate on Fescue Production. Agron. Notes 1982, 15, 86. [Google Scholar]
- Ostrom, A.K. Comparing the Effect of Controlled-Release, Slow-Release, and Water-Soluble Fertilizers on Plant Growth and Nutrient Leaching. Master’s thesis, The Ohio State University, Columbus, OH, USA, 2011. [Google Scholar]
- Li, Y.; Sun, Y.; Liao, S.; Zou, G.; Zhao, T.; Chen, Y.; Yang, J.; Zhang, L. Effects of Two Slow-Release Nitrogen Fertilizers and Irrigation on Yield, Quality, and Water-Fertilizer Productivity of Greenhouse Tomato. Agric. Water Manag. 2017, 186, 139–146. [Google Scholar] [CrossRef]
- Wang, H.; Köbke, S.; Dittert, K. Use of Urease and Nitrification Inhibitors to Reduce Gaseous Nitrogen Emissions from Fertilizers Containing Ammonium Nitrate and Urea. Glob. Ecol. Conserv. 2020, 22, e00933. [Google Scholar] [CrossRef]
- Shoji, S.; Delgado, J.; Mosier, A.; Miura, Y. Use of Controlled Release Fertilizers and Nitrification Inhibitors to Increase Nitrogen Use Efficiency and to Conserve Air Andwater Quality. Commun. Soil Sci. Plant Anal. 2001, 32, 1051–1070. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants: Third Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Patricia, M.; Maud, L.; Frederique, B.; Jean-Pierre, R.; Xavier, B.; Christian, M.; Daniel-Vedele, F. Physiological and Transcriptomic Aspects of Urea Uptake and Assimilation in Arabidopsis Plants. Plant Physiol. 2008, 147, 1225–1238. [Google Scholar] [CrossRef]
- Lu, L.; Wang, Y.; Li, T.; Wang, S.; Yang, S.; Qing, Y.; Li, X.; Wu, Y.; Liu, M. Calcium Carbonate Modified Urea–Formaldehyde Resin Adhesive for Strength Enhanced Medium Density Fiberboard Production. RSC Adv. 2021, 11, 25010–25017. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Ye, X.; Ma, C.; Wang, Q.; Tu, R.; Zhang, L.; Gao, H. Greenhouse Gas Emissions from Synthetic Nitrogen Manufacture and Fertilization for Main Upland Crops in China. Carbon Balance Manag. 2019, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.M.; Li, H.F.; Wang, C.S.; Yang, J.; Huang, G.; Meng, X.; Zhou, Q. Degradable Polyester/Urea Inclusion Complex Applied as a Facile and Environment-Friendly Strategy for Slow-Release Fertilizer: Performance and Mechanism. Chem. Eng. J. 2020, 381, 122704. [Google Scholar] [CrossRef]
- de Silva, M.; Siriwardena, D.P.; Sandaruwan, C.; Priyadarshana, G.; Karunaratne, V.; Kottegoda, N. Urea-Silica Nanohybrids with Potential Applications for Slow and Precise Release of Nitrogen. Mater. Lett. 2020, 272, 127839. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Madushanka, D.; Arachchige, B.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; et al. Urea-Hydroxyapatite Nanohybrids for Slow Release of Nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef]
- Chen, X.; Kou, M.; Tang, Z.; Zhang, A.; Li, H. The Use of Humic Acid Urea Fertilizer for Increasing Yield and Utilization of Nitrogen in Sweet Potato. Plant Soil Environ. 2017, 63, 201–206. [Google Scholar] [CrossRef]
- Saha, B.K.; Rose, M.T.; Wong, V.; Cavagnaro, T.R.; Patti, A.F. Hybrid Brown Coal-Urea Fertiliser Reduces Nitrogen Loss Compared to Urea Alone. Sci. Total Environ. 2017, 601–602, 1496–1504. [Google Scholar] [CrossRef]
- Rose, M.T.; Perkins, E.L.; Saha, B.K.; Tang, E.C.W.; Cavagnaro, T.R.; Jackson, W.R.; Hapgood, K.P.; Hoadley, A.F.A.; Patti, A.F. A Slow Release Nitrogen Fertiliser Produced by Simultaneous Granulation and Superheated Steam Drying of Urea with Brown Coal. Chem. Biol. Technol. Agric. 2016, 3, 1–14. [Google Scholar] [CrossRef]
- Golbashy, M.; Sabahi, H.; Allahdadi, I.; Nazokdast, H.; Hosseini, M. Synthesis of Highly Intercalated Urea-Clay Nanocomposite via Domestic Montmorillonite as Eco-Friendly Slow-Release Fertilizer. Arch. Agron. Soil Sci. 2017, 63, 84–95. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Su, Y.; Yue, Q.; Gao, B.; Su, Y. A Novel Wheat Straw Cellulose-Based Semi-IPNs Superabsorbent with Integration of Water-Retaining and Controlled-Release Fertilizers. J. Taiwan Inst. Chem. Eng. 2015, 55, 170–179. [Google Scholar] [CrossRef]
- Ariyanti, S.; Zakaria, M.; Azmi, B.M. Improvement of Hydrophobicity of Urea Modified Tapioca Starch Film with Lignin for Slow Release Fertilizer. Adv. Mat. Res. 2013, 626, 350–354. [Google Scholar] [CrossRef]
- Xiaoyu, N.; Yuejin, W.; Zhengyan, W.; Lin, W.; Guannan, Q.; Lixiang, Y. A Novel Slow-Release Urea Fertiliser: Physical and Chemical Analysis of Its Structure and Study of Its Release Mechanism. Biosyst. Eng. 2013, 115, 274–282. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lü, S.; Xie, L.; Wang, Y. Environmentally Friendly Slow-Release Nitrogen Fertilizer. J. Agric. Food Chem. 2011, 59, 10169–10175. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).