Abstract
The aim of this study was to evaluate the differential response to land use changes between native forest and croplands regarding the quantitative soil variables of aggregate weight classes and different carbon pools in extremely kaolinitic soils from the east coast of Brazil. In the soil A horizon, the total (TOC) and dissolved (DOC) organic carbon contents were analyzed. In the 0–0.08 m soil layer, the weight and the organic carbon content (Cag) were determined for six size aggregate classes. The mean differential (Δ) of each property for each area was calculated. Overall, the TOC and DOC were greater in the native forest sites over the counterpart cultivated sites within each area. The ΔDOC of all the five areas were negative. The ΔCag of the 1–2 mm and 0.053–0.105 mm soil aggregate classes of Sooretama were the only ones with mean positive values. The ordination of the five areas by the ΔCag in the six soil aggregate size classes isolated Coruripe as the area with the most negative differentials, because of the forest conservation and management of the cropland. The differentials of organic carbon between forest and agricultural use of the analyzed properties did not reveal a possible effect of soil texture.
1. Introduction
The removal of native vegetation and the improper use of land for agricultural production reduce organic matter content and degrade soil structure [1]. Upon cultivation, soil organic matter (SOM) decreases, and soil aggregate stability is compromised [2,3]. The magnitude of soil structure deterioration, disruption of soil aggregates, losses of total and labile carbon pools as well as soil erosion in deforested areas are governed by crop residue input, original soil carbon content under native vegetation and intensity of cultivation and adopted soil management practices [1,4]. Aggregates are the basic unit of soil structure [5] and SOM improves soil aggregation, which limits the access of the soil organic carbon to decomposers [4,5]. Land use changes strongly affect the maintenance, breakdowns, and neoformation of soil aggregates [6,7], and also affects the organic carbon (OC) associated with these aggregates. The SOM is protected in well structure soils, due to the ability of the clay fraction to encapsulate organic compounds and burial carbon pools within small pores of soil aggregates [4,8,9,10,11].
A reduction in the SOM stored in aggregates is reported for soils cultivated with annual crops over soils under pastures, perennial crops, planted forests, and, especially, under the native forest [12,13,14,15]. This reduction is often related to the destabilization of the soil aggregates because it exposes protected carbon fractions to soil fauna and decomposers, which accelerates SOM decomposition, with a subsequent release of carbon dioxide to the atmosphere and reduction in stored SOM [16,17,18,19].
In cultivated soils, an increase in organic matter decomposition rate contributes to elevate the carbon in the solution [20]. Dissolved organic carbon (DOC) encloses polar, low molecular weight and very reactive organic compounds that play an important role in soil aggregation through the formation of organometallic-clay complexes [20,21]. The DOC content is governed by land use and soil management practices; thus, the conversion of the forest into cropland areas increases soil microbial activity, and consequently, enhances OC in the soil solution [21], as it is normal to observe more DOC in agroforestry systems than in native forest and pasture areas [22], as well as in sugarcane versus eucalyptus plantations and native Cerrado [23]. Thus, the DOC content relies on the stage of soil degradation. The DOC, at least in the short term (temporary flush of carbon in the soil solution), can even be greater in disturbed soils (under agricultural use) over native vegetation, considering that it receives part of the carbon released in a greater rate due to the increased organic matter decomposition in disrupted soil aggregates [19]. As the time evolves, the presence of more stable organic compounds in cultivated soils, mainly in those degraded, decreases the release of DOC to the soil solution [21].
The Brazilian East Coast (from the state of São Paulo northwards) has, at the same time, high rates of population density and removal of primary forest formations (which is part of the Atlantic Forest Biome). Most of these landscapes are formed by sediments from the Tertiary Period, mainly by the Barreiras Formation, and in their less dissected pedoforms (64,235 km2), different land uses gain importance: pastures, planted eucalyptus, sugarcane, manioc (cassava), and fruit trees (mostly under irrigation), among other cropland areas. The largely predominant soils are Ultisols, and, in a lesser proportion, Oxisols [24,25,26]. These soils have a low cation exchange capacity (CEC), low natural soil fertility status (several nutritional plant limitations), and a very uniform mineralogy, with a strong predominance of kaolinite in the clay fraction and quartz in the sand and silt fractions. In addition, they have a wide range of soil texture, varying between texture classes of sand and sandy clay (from 8 to 45 dag kg−1 of clay content). As the content of clay fraction increases, these soils tend to present higher bulk density values, as a reflection of the hard-setting character [27].
The aim of the present study was to evaluate the differential response to land use change from native forests to croplands regarding the quantitative variables of soil aggregate weight classes and soil carbon pools (total, dissolved, and aggregate classes). Surface layers of extremely kaolinitic soils of five areas of the East Coast of Brazil were selected to represent a wide range of soil particle size distribution. The soil carbon pools were compared between two land uses of each area, and the differentials [deltas (Δ) between forest and cropland] of the five areas were compared. The soil sites under native forests of the same areas were already compared to each other by [26], and they observed that the soil texture did not affect the results obtained for soil carbon pools from aggregate size classes, but affected the results obtained for soil aggregate weight classes and DOC. Thus, the study observed that extremely kaolinitic soils have a response differentiated from other low CEC soils, e.g., oxidic soils, which tend to have positive correlations between the clay (or clay and silt) content and diverse soil carbon pools [28,29,30,31,32]. The main hypothesis of this study was that it is possible to identify the main factors regulating the resilience of kaolinitic soils in changing the aggregation stability and carbon contents and pools, when the land use changes from native forest to cropland areas.
2. Materials and Methods
2.1. Study Areas
The study areas are located at the East Coast of Brazil, between the parallels 10° S and 22° S. The following representative areas were selected: Coruripe (C), Umbaúba (U), Nova Viçosa (V), Sooretama (S), and Itaboraí (I) (Figure 1 and Table 1). The degree of conservation of the native forests and the crops cultivated in each area can be observed in Table 2.
Figure 1.
Map of Brazil showing the boundaries of states (a), and study areas (b): Itaboraí (I); Sooretama (S); Nova Viçosa (V); Umbaúba (U); Coruripe (C). Source: adapted from [26].

Table 1.
Characteristics of the study areas.

Table 2.
Land uses and native forests of the studied areas.
Four areas are in landscapes of the Barreiras Formation and one area is in a landscape of the Macacu Formation, both from the Tertiary Period. A common attribute to all soils studied is the cohesiveness, varying in depth and degree of expression, which is significantly affected by soil texture. In Table 3, the studied soils were presented in increasing order of clay contents in the A horizon (S < C < U < I < V). Soils from S had the lowest clay contents (8 and 9 dag kg−1) in the A horizon; soils from V, the greatest (41 and 45 dag kg−1). The clay fraction was predominantly constituted by kaolinite and the sand and silt fractions by quartz. Regarding drainage, V, S, and I areas have well drained soils, and C and U areas enclose moderately drained soils. Subsurface cemented horizons were identified in soils of C and U areas [36,37].

Table 3.
Chemical and physical attributes of the A horizon soil samples; contrasting land use sites from five areas of the Brazilian East Coast (n = 3).
The soils of V area have an influence of granitic gneiss from the Precambrian, which reflects in greater Fe oxides minerals’ contents, more reddish colors, and a greater nutrients reserve than soils of the other areas (Table 3).
All five areas are under a humid tropical climate and sustain a native forest vegetation, though there are contrasts regarding the soil moisture regime. Differences are caused by the amplitude and extent of the dry season (Table 1 and Table 2), the occurrence and depth of cemented horizons at subsoil layers, and soil texture. All the soils studied present a moderately thick A horizon, with medium values of SOM, and are free of redoximorphic features.
2.2. Soil Sampling and Analyses
Locations with two land use conditions (native forest and croplands) were selected in each study area (C, U, V, S, and I). To allow for meaningful comparisons, sites were chosen following two criteria: having soils developed on Tertiary landscapes covering a wide range of texture classes, and having matching areas under native forest and agricultural use. Three soil profiles were sampled under each land use condition (2) of each area (5), totalizing 30 soil sampling sites (five areas × two land uses × three soil profiles).
The total organic carbon (TOC) and the DOC were determined in the soil air-dried fine earth fraction (ADFE) sample from the entire A horizon of each pedon. The TOC was determined in an automatic analyzer using the liquid mode of a TOC machine, with combustions of soil liquid filtrates in a 850 °C combustion chamber and a near infrared light-driven release imidacloprid (NDRI) detector of a Germany elemental analyzer (Elementar, Vario TOC Cube model). The DOC was determined in filtrates of a mixture of 10 g of ADFE and 20 mL of distilled water, which was added to a 35 mL tube, and then this solution was shaken for 1 h at 0.73 g. In sequence, the filtrate samples were centrifuged (15 min at 1814 G) to obtain the extracts for the DOC content analysis [38].
Analysis of the soil aggregate size classes was performed using samples of the uppermost layer (0–0.08 m) of the A horizon of each pedon. These samples were collected by careful removal of the soil from the walls of the soil profile with the assistance of a putty knife in the form of cubes (side edges of the same length in the depth of 0.08 m). The samples were stored, assuring that moisture and structure of soils were preserved. After that, samples were air-dried in the shade for a period of 48 h and then dry sieved, following the procedure adopted by [39], with modifications. The soil aliquots were separated into aggregate size classes (fractions) using a mechanical shaker equipped with six sieves (2.0, 1.0, 0.5, 0.25, 0.105, and 0.053 mm mesh openings), in which the samples were shaken for 15 min at 2.5 cycles per minute (vertical amplitude of 3.5 cm).
After the samples were shaken, the weights of the aggregate size classes were determined and separated into four macroaggregates (MA) classes (>2, 2–1, 1–0.5, and 0.5–0.25 mm) and two microaggregates classes (0.25–0.105 and 0.105–0.053 mm); thus, no aggregates were larger than 4 mm in diameter. In addition, mean weight-diameter of the aggregates (MWD) and mean geometric-diameter of the aggregates (MGD) were determined to assess the distribution of aggregates for each size class, according to Equations (1) and (2):
where wi represents the fraction of weight in each aggregate class (in g) and xi represents the mean diameter of each aggregate class (in mm) [40].
The total organic carbon in all the aggregate size classes (TOCag) was calculated considering the aggregate soil mass of each class, based on the equivalent soil weight basis, as shown in Equation (3):
where TOCag is the TOC of all the aggregates in an equivalent weight basis (g kg−1), wi represents the fraction of aggregate weight (g) in a size class, and Ci is the TOC content (g 100 g−1) in an aggregate size class.
2.3. Differential Carbon Pools of Cultivated and Native Forest Soils (Deltas—Δ)
The differentials were calculated in order to understand the differences of soil OC pools in response to changes in the land use of each studied area. Deltas were calculated by subtracting each soil variable under the cropland from those under the native forest in each area. With this approach, nine deltas (differentials) were generated for each OC compartment (variable) per area (combination of three replicates of the cropland site versus the combination of three replicates of the respective forest site). Only six deltas with the least distance from the mean were used in the statistics analyses.
2.4. Statistical Analyses
Student’s t-test was used to compare the sites under native forest and cropland sites of each area, considering the significant differences between the mean values. When necessary, the data distribution was normalized by the Box-Cox approach.
The values of the differentials (deltas—Δ) used to compare the five areas showed non-parametric distribution; thus, the Kruskal-Wallis test (non-parametric ANOVA of one factor) and the Nemenyi means test (p < 0.05) (non-parametric multiple comparisons test) were applied on them.
In addition to the mean test, the Cag differentials in the six soil aggregate size classes were used as the dataset for a multivariate ordination of non-metric multidimensional scaling (NMS) with Sorensen distances. The data were relativized by the total within each variable, eliminating the effect of the different magnitudes of units. Before relativization, the data were made positive by a mathematical operation (module of the minimum value of Δ found in each aggregate class + 0.1 + Δn), considering that most of the deltas had negative modules. The data were ordered using the program PC-ORD v. 6.0 [41] in the automatic pilot mode, selecting the “slow and thorough” option. The number of dimensions interpreted was chosen based on the stress and stability parameters of the graph solution.
3. Results and Discussion
3.1. Total and Dissolved Organic Carbon
The TOC and DOC soil contents of native forest sites were greater than those under cropland sites in the five areas investigated, regardless of soil texture. The difference was not significant for the DOC mean values in the S area and for the TOC mean values in the V area (Figure 2a,b).
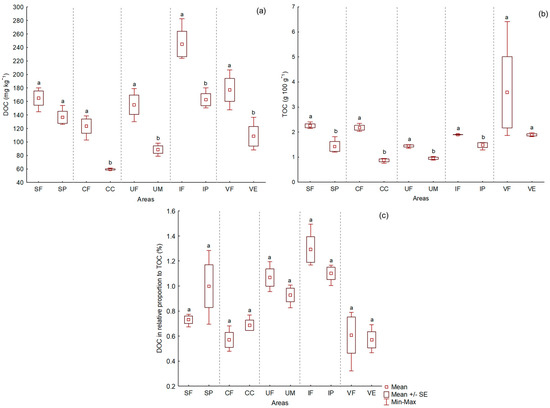
Figure 2.
Dissolved organic carbon (DOC) (a); total organic carbon (TOC) (b); and % DOC of the TOC (c) in A horizon samples of soils. Codes: Areas (first letter) − S = Sooretama, C = Coruripe, U = Umbaúba, I = Itaboraí, and V = Nova Viçosa. Land use (second letter) − F = native forest, P = pasture, C = sugarcane, M = post-cassava fallow, and E = eucalyptus. Average tests were performed between native forest and agricultural use within each area. Mean values followed by different letters differ significantly from each other by the student t test (p < 0.05).
Higher TOC values in soil surface horizons of native forests than in cultivated areas are expected, considering the soil structure, carbon input and slower decomposition rate of SOM in native forests compared to counterparts cultivated [42,43,44,45,46]. Conversion of native forest into cropland is the main reason for the high losses of carbon from soil to air. According to [47], 20–50% of carbon losses are expected in surface soil layers of cultivated soils when compared to native forest soils. The magnitude of these losses may change depending on the native vegetation type and intensity of the cultivation after deforestation. As stated by [48], losses of approximately 5 Mg ha−1 of TOC occurred when native caatinga (scrub) was converted into cropland areas, while a change from caatinga to pasture increased the soil OC value in approximately 6 Mg ha−1. Results that demonstrate a greater accumulation of TOC in cultivated soils over native forest soils are less common, though this may occur. In a Coastal Region of Bahia, [49] obtained similar values of TOC in the soil upon comparing a fragment of the secondary native forest (25.1 g kg−1) to agroforestry sites (27.2 g kg−1) and pasture sites (25.5 g kg−1); however, the values were greater than the site under the traditional agricultural use (18.3 g kg−1).
Overall, native forest surface soils, richer in SOM, tend to have higher DOC values compared to cropland surface soils [50,51,52,53,54].
The mean values of the DOC/TOC ratio did not demonstrate a significant difference in the comparison between native forest and cropland sites in each area (Figure 2c). Reference [55] evaluated soils of the north of Alaska and observed values of the DOC/TOC ratio of 2.6% for acid soils and of 1.8% for non-acidic soils. The results reported here are in line with the results of [55], with the more acid soils of area I (Itaboraí) exhibiting higher values of the DOC/TOC ratio (1.29% in Itaboraí forest sites−IF, and 1.10% in Itaboraí pasture sites−IP), whereas the less acid soils of area V (Nova Viçosa) exhibited a lower ratio (0.61% in Nova Viçosa forest sites−VF, and 0.57% in Nova Viçosa eucalyptus sites−VE). Variations in the DOC/TOC ratio are common, such as those obtained by [56] when evaluating various land uses in the south of Florida. The authors found values of the DOC/TOC ratio ranging from 0.28% (grain crops) to 2.47% (fruit growing), which represent a much broader interval of minimum and maximum values than those obtained here by the mean values of the different combinations of area and land use.
The values of TOC and of the DOC/TOC ratio are not explained by variations in soil silt and clay fraction contents (Table 4); thus, texture is not a key driving force of carbon pools in these soils. The absence of correlation of TOC with soil texture is in line with the results found for extremely kaolinitic soils by [57] and for a subset of the native forest soils studied here, as reported elsewhere [26]. The differences in the response of the extremely kaolinitic soils of the Tertiary age landscapes in relation to the other soils of the low cation exchange capacity in Brazil was extensively discussed in [26]. It is important to report that, although with a low coefficient of correlation, DOC was positively correlated with soil silt and clay fraction contents (p < 0.01, r = 0.47).

Table 4.
Correlation matrix of the sum of silt and clay fraction contents and some attributes of aggregation and soil carbon compartments (n = 30).
3.2. Distribution of Soil Aggregate Size Classes
The area U (Umbaúba) was the only one that demonstrated the native forest sites (UF) with the mean values of the soil aggregate stability indices (MA, MWD, and MGD) statistically greater than the indices of the cropland sites (Umbaúba under post-cassava fallow sites—UM) (Table 5). Despite the current fallow period, the previous cassava harvests, a practice extremely aggressive to the surface structure of the soil, may be determining the soil carbon difference, as reported by [58]. The other areas did not demonstrate differences in comparing the native forest with agricultural use, results that can be considered unexpected. Soil management practices adopted in cultivated sites of areas S, I, and V might not have been extremely aggressive to the soil surface structure, which helps to explain the lower carbon losses verified. In areas S, C, and I, the management systems involving grasses (Sooretama and Itaboraí under Brachiaria decumbens pastures—SP and IP, respectively, and Coruripe under sugarcane—CC) are prone to improve the plant root system proliferation, which may even enhance the soil surface aggregation compared to native forest soils [8,59], compensating for the negative impacts of cropping operations, beyond which they do not involve annual soil turnover. The absence of large negative impacts on soil structure and soil disturbance (aggregates’ stability) when native forest sites were converted into pasture was also reported by [60,61], who evaluated the conversion of native forest into pasture and sugarcane.

Table 5.
Average values of percentage of mass by aggregate size classes of soils and aggregation indices, contrasting land use sites in five areas of the Brazilian East Coast, at 0–0.08 m soil layer (n = 3).
In area V, the absence of difference between the sites of VE and VF is closely related to the low impact management practices of minimum cultivation of eucalyptus, which encompasses a long period without soil disturbance (cycle of seven years) and low traffic of machines. Ref. [62] also did not find significant differences for soil aggregates’ stability between eucalyptus planted and native forest sites.
In addition to the aspects already highlighted for management systems, attention should be paid to the extremely kaolinitic soils in all areas studied, with naturally low aggregates’ stability [63,64], which up to a certain point could make it difficult to differentiate the properties studied between the sites under the native forest vegetation and agricultural use.
The percentage of MA ranged from 71% to 98% (Table 5), values close to indices verified by [65] for soils under native forest vegetation, no-tillage systems, and conventional tillage (intensive use of machine for soil plowing) in the state of Paraná, Brazil (96%, 91%, and 88%, respectively). In the region of Muriaé, Brazil, [66] obtained similar MA values (mean of 60%) among soils under secondary forest, eucalyptus (3–5 years of age), rubber tree plantation (35 years of age), and B. decumbens pasture (50 years after implantation). The MA values obtained by different studies are highly affected by the methodology applied for their determination. The dry sieving method used here was also carried out by [65], however, with a difference in sieving time (15 min here and 20 min there). Conversely, [66] used the wet sieving method, which is more disruptive than the dry sieving procedures, generating lower aggregates’ stability indices.
Soil texture had a striking effect on the aggregation properties (Table 4). The correlation was positive and significant for the percentage of weight of the 2–4 mm aggregate class (p < 0.01, r = 0.88) and for the aggregation indices (p < 0.01, r = 0.68 for MA, r = 0.87 for MWD, and r = 0.80 for MGD, respectively). For the other percentages of the weight of the soil aggregate classes, the values were negative, and were not significant only for the percentage of the weight of aggregates of the 1–2 mm class. Thus, the correlation values confirm that the aggregate stability properties depend on the soil texture and on SOM [67], including the extremely kaolinitic soils of Tertiary age landscapes, such as those studied here.
3.3. Carbon in Soil Aggregate Size Classes
Comparison of the Cag content (OC of the aggregate classes) and of the sum of the classes (TOCag) between native forest and cropland sites showed significant differences for areas, such as C, U, and I (Table 6). There were not significant differences in areas S and V, mainly as a result of the high values of standard deviation (Table S1) in relation to the mean values of Cag and TOCag calculated in these two areas. Even so, in one case only, the mean content of the Cag of an aggregate class was greater in the cropland than in the native forest (Cag of 0.053–0.105 mm class of area S). The destabilization that the management interventions bring about on the aggregates and on the reduction in the OC values [65] explains the results found here. Of all the Cag values, also including the TOCag, only the Cag of the 0.053–0.105 mm class exhibited significant correlation (p < 0.05) with the silt and clay fraction contents (Table 4).

Table 6.
The OC contents of aggregate size classes and sum of aggregate size classes (TOCag—expressed on an equivalent soil mass basis), contrasting land use sites from five areas of the east coast of Brazil, at 0–0.08 m soil layer (n = 3).
3.4. Carbon Pools in Native Forest and Counterpart Cultivated Soils
3.4.1. Differential of DOC (ΔDOC)
All the values of ΔDOC (Table 7) were negative (DOC of croplands < DOC of native forests), which are the expected results, given that surface soils under the native forest are richer in SOM and tend to generate greater values of the DOC compared to cropland soils [50,51].

Table 7.
Mean values of the dissolved differential organic carbon (ΔDOC) at sites from five areas of the Brazilian east coast for A horizon soil samples (n = 6).
The values of ΔDOC were only significantly different between areas S and I, and the other areas had intermediate values. Area S had the lowest absolute value of ΔDOC, being the most conserved forest sites (located at Reserva de Sooretama—SF) among all areas and having a sandy texture in its surface horizon, whereas area I (greatest absolute value of ΔDOC) is the native secondary forest sites situated furthest from an original condition and having a sandy clay soil texture in the entire soil profile. Since the two areas have pasture as the land use, the greatest effect of the difference seems to fall on texture, as the greater clay values (in area I) allow the forest sites to widen the differences in comparison to the cropland sites. A similar tendency was found by [68], who found lower values of DOC under degraded pastures in clayey soils than in sandy soils. However, this is not a generalized tendency, and in area C, the sandy A horizon showed a relatively large ΔDOC, probably affected by burning practices that precede the sugarcane harvest, showing that the effects of management of the cropland sites can sharply change the results of the differentials in relation to the native forests’ sites.
3.4.2. Differential of Organic Carbon of the Aggregate Size Classes (ΔCag) and of the Sum of the Aggregate Size Classes (ΔTOCag)
The differentials ΔCag1–2 mm class, ΔCag0.105–0.25 mm class, and ΔCag0.053–0.105 mm class of area S were the only ones with positive mean values (Table 8). The results followed the expected tendency of higher OC content in the soil aggregates of the sites under native forests over cultivated counterpart sites [69,70,71].

Table 8.
Mean values of the differential OC from aggregate size classes (ΔCag) and sum of the aggregate size classes (ΔTOCag—expressed on an equivalent soil mass basis) at sites from five areas of the Brazilian east coast, at 0–0.08 m of soil layer (n = 6).
The higher values of the differentials (modules of negative values) occurred in area C for all aggregate classes and for the sum of the aggregate size classes. The differentials of areas C and S did not differ from each other only in ΔCag2–4 mm class. The differentials of area C also demonstrated significant differences in relation to those of areas U and V. The use of burning before the sugarcane harvest and a greater machine traffic (although without annual soil turnover) of sugarcane must have accentuated the reduction in Cag and TOCag values of the agricultural sites in area C [72]. In addition, the Coruripe forest sites (CF), along with the SF sites, are better preserved compared to the forest sites of the other areas.
Regarding the reduced changes in the modules of area U, a possible explanation is linked to the limited conservation of the local native forest fragment. The agricultural use of area U, e.g., the cassava cultivation (UM), would constitute the most aggressive management practice in comparison with the other areas, although this effect tends to be minimized by the fallow periods of cassava cropping.
The values of the differentials of the other areas, S, I, and V, were situated between areas C and U. Greater differentials were expected for area S, considering the good conservation status of the forest fragment and the poor condition of the local pasture. However, the input of animal excrement can be a possible explanation for enhanced root growth, consequently, contributing to increase the carbon content and physical protection in soil aggregates [73]. As for area I, the differentials were expected to be narrower, because the local native secondary forest fragment sites represent the most incipient succession stage between the native forest sites studied, and the local pasture was not occupied by cattle for at least two years, and it had considerable leaf biomass. This effect of pasture on Cag corroborates that observed by [74] in the 0–10 cm soil layer of pastures, which obtained an increase from 94% to 162% in the dry matter of roots in the pasture (black oats + ryegrass) according to the increase in the intensity of grazing, in comparison with a non-grazed area. The cropland of area V is the closest to a native forest, as it is a planted forest that undergoes interventions spaced at 7 years, when impacts from harvest machines and soil tillage (subsoiling in the plant row) occur. Overall, the results reported in this study signalize the difficulty to predict which key factors govern the Cag and TOCag losses after the conversion of the native forest into cropland areas.
To understand the differences and similarities of the response of the five areas in relation to the ΔCag, an NMS ordination procedure was performed. As the first axis explained 90% of the variation of dataset, we chose to show only this axis, placing the mean value of clay and silt fraction contents of all the sites from each area on the Y axis (Figure 3). The ΔCag for all aggregate size classes showed high values of negative correlation (p < 0.001) with axis 1, making the most negative differentials remain to the right of the axis. That way, the area C is isolated to the right of the ordination; the area I occupies an intermediate position; and the areas S, U, and V are overlapping. Thus, we have the following increasing gradient of greater (modules) differentials: S < V = U << I << C. A comparison of the mean clay and silt fraction contents of the different areas shows that the response of the differentials does not correlate with this property.
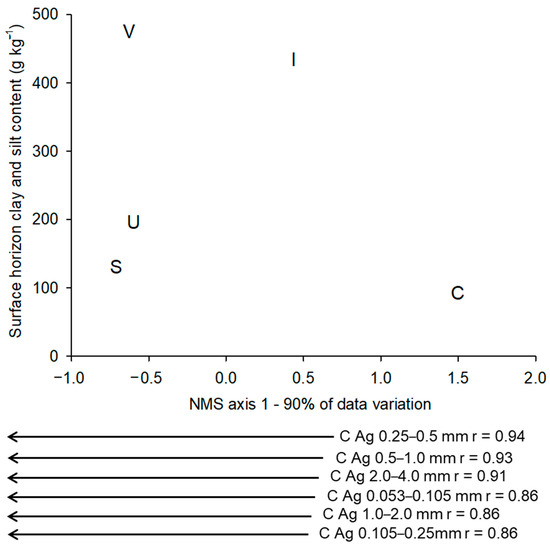
Figure 3.
Average position of the studied areas on axis 1 (90% of the data variation) based upon the ordination obtained by the non-metric multidimensional scaling (NMS) of the differential behavior influenced by the change in land use (n = 6) in the OC contents of the six aggregate size classes, comparing the 0.00–0.08 m soil layer versus the average clay and silt fraction contents of the whole surface horizon of each area. Areas: C = Coruripe; U = Umbaúba; V = Nova Viçosa; S = Sooretama; and I = Itaboraí. Vectors external to the axis 1 of the NMS represent the values of the correlations of each attribute with the axis of the NMS. n = number of differentials with the least distance from the mean used.
4. Conclusions
In general, extremely kaolinitic soils from the Brazilian east coast showed aggregation indices and OC compartments of A horizons higher in soils under native forest than in the cultivated counterpart soils. However, many properties studied did not demonstrate a significant difference, especially the aggregation indices and the OC of the aggregate size classes, both determined in the 0–0.08 m layer. The limited significant differences in the comparison of the soil variables of native forests and cultivated counterparts can be reputed to differential agricultural uses (sugarcane, pasture, post-cassava, and eucalyptus) and managements that served as a term of comparison to the native forest and the variable conservation conditions of native forest fragments in the different areas studied.
The only compartments with a positive OC differential (OC of agricultural use minus OC of the native forest) were those referring to the soil aggregate size classes 1–2, 0.105–0.25, and 0.053–0.105 mm of area Sooretama. These unexpected results, taking into account the good conservation status of the Sooretama native forest, may have been affected by the input of OC from animal excrement in the pastures (cultivated counterparts).
The ordination made for the five areas from the OC differentials in the six soil aggregate size classes isolated Coruripe as the area with the most negative differentials (OC of the aggregate classes in the native forest > OC in agricultural use). This result was attributed to the good conservation condition of the local forest fragment in the area and, especially, the burning practice before the sugarcane harvest.
The differentials of OC between the agricultural use and native forest concerning the properties analyzed did not demonstrate an effect of the soil texture. Probably, such effect was outweighed by the complexity in modeling the losses of soil OC pools considering the cultivated systems’ diversity studied in comparison with the native forests of each area.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15021204/s1, Table S1: Standard deviation (SD) of the OC contents of aggregate size classes and of the sum of aggregate size classes (TOCag—expressed on an equivalent soil mass basis), contrasting land use sites from five areas of the east coast of Brazil, at 0–0.08 m soil layer (n = 3).
Author Contributions
Conceptualization: J.B.V.G. and N.C.; Data curation, formal analysis and investigation: T.M.E., J.B.V.G. and N.C.; Funding acquisition and resources: J.B.V.G.; Writing—original draft preparation: T.M.E. and J.B.V.G.; Writing—review and editing: A.C.V.M., M.M.F., A.V.I., M.M. and N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES), Minas Gerais State Agency for Research and Development (FAPEMIG) and Embrapa.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request.
Acknowledgments
The authors would like to thank the technicians from Embrapa Florestas.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feller, C.; Albrecht, A.; Tessier, D. Aggregation and organic matter storage in kaolinitic and smectitic tropical soils. In Structure and Organic Matter Storage in Agricultural Soils, 1st ed.; Carter, M.R., Stewart, B.A., Eds.; CRC Press: Boca Ranton, FL, USA, 1995; pp. 309–359. [Google Scholar] [CrossRef]
- Chaney, K.; Swift, R.S. The influence of organic matter on aggregate stability in some British soils. Eur. J. Soil Sci. 1984, 35, 223–230. [Google Scholar] [CrossRef]
- Chenu, C.; Le Bissonnais, Y.; Arrouays, D. Organic Matter Influence on Clay Wettability and Soil Aggregate Stability. Soil Sci. Soc. Am. J. 2000, 64, 1479–1486. [Google Scholar] [CrossRef]
- Baldock, J.A.; Nelson, P.N. Soil organic matter. In Handbook of Soil Science; Summer, M.E., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 25–84. [Google Scholar]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. Eur. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9. [Google Scholar] [CrossRef]
- Cheng, X.; Luo, Y.; Xu, X.; Sherry, R.; Zhang, Q. Soil organic matter dynamics in a North America tallgrass prairie after 9 yr of experimental warming. Biogeosciences 2011, 8, 1487–1498. [Google Scholar] [CrossRef]
- Dou, F.; Soriano, J.; Tabien, R.E.; Chen, K. Soil Texture and Cultivar Effects on Rice (Oryza sativa, L.) Grain Yield, Yield Components and Water Productivity in Three Water Regimes. PLoS ONE 2016, 11, e0150549. [Google Scholar] [CrossRef]
- Novelli, L.E.; Caviglia, O.P.; Piñeiro, G. Increased cropping intensity improves crop residue inputs to the soil and aggregate-associated soil organic carbon stocks. Soil Tillage Res. 2017, 165, 128–136. [Google Scholar] [CrossRef]
- Pereira, M.G.; Loss, A.; Batista, I.; de Melo, T.R.; Silva Neto, E.C.; da Silva Rodrigues Pinto, L.A. Biogenic and physicogenic aggregates: Formation pathways, assessment techniques, and influence on soil properties. Rev. Bras. Cienc. Solo. 2021, 45. [Google Scholar] [CrossRef]
- Chi, J.; Fan, Y.; Wang, L.; Putnis, C.V.; Zhang, W. Retention of soil organic matter by occlusion within soil minerals. Rev. Environ. Sci. Bio/Technol. 2022, 21, 727–746. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Yamashita, T.; Flessa, H.; John, B.; Helfrich, M.; Ludwig, B. Organic matter in density fractions of water-stable aggregates in silty soils: Effect of land use. Soil Biol. Biochem. 2006, 38, 3222–3234. [Google Scholar] [CrossRef]
- Powers, J.S.; Corre, M.D.; Twine, T.E.; Veldkamp, E. Geographic bias of field observations of soil carbon stocks with tropical land-use changes precludes spatial extrapolation. Proc. Natl. Acad. Sci. USA 2011, 108, 6318–6322. [Google Scholar] [CrossRef] [PubMed]
- Alkimim, A.; Clarke, K.C. Land use change and the carbon debt for sugarcane ethanol production in Brazil. Land Use Policy 2018, 72, 65–73. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks-a meta-analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Harris, N.L.; Brown, S.; Hagen, S.C.; Saatchi, S.S.; Petrova, S.; Salas, W.; Hansen, M.C.; Potapov, P.V.; Lotsch, A. Baseline Map of Carbon Emissions from Deforestation in Tropical Regions. Science 2012, 336, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Bailey, V.L.; Pries, C.H.; Lajtha, K. What do we know about soil carbon destabilization? Environ. Res. Lett. 2019, 14, e.083004. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; John Wiley and Sons: New York, USA, 1994; p. 512. [Google Scholar]
- Gmach, M.R.; Cherubin, M.R.; Kaiser, K.; Cerri, C.E.P. Processes that influence dissolved organic matter in the soil: A review. Sci. Agricola 2020, 77, e20180164. [Google Scholar] [CrossRef]
- Marques, J.D.D.O.; Luizão, F.J.; Teixeira, W.G.; Ferreira, S.J.F. Variations of dissolved organic carbon and soil physical properties under different land uses in Central Amazonia. Rev. Bras. Ciênc. Solo. 2012, 36, 611–622. [Google Scholar] [CrossRef]
- Da Silva, D.M.L.; Ometto, J.P.H.B.; Lobo, G.D.A.; Lima, W.D.P.; Scaranello, M.A.; Mazzi, E.; Da Rocha, H.R. Can land use changes alter carbon, nitrogen and major ion transport in subtropical brazilian streams? Sci. Agricola 2007, 64, 317–324. [Google Scholar] [CrossRef]
- Silva, F.B.R.; Riché, G.R.; Tonneau, J.P.; Souza Neto, N.C.; Brito, L.T.L.; Correia, R.C.; Cavalcanti, A.C.; Silva, F.H.B.B.; Silva, A.B.; Araújo Filho, J.C.; et al. Zoneamento Agroecológico do Nordeste: Diagnóstico do quadro natural e Agrossocioeconômico; Embrapa-CPATSA: Petrolina, Brazil, 1993. [Google Scholar]
- Jacomine, P.K.T. Evolução do conhecimento sobre solos coesos no Brasil. In Workshop Coesão Em Solos dos Tabuleiros Costeiros; Cintra, L.F.D., Anjos, J.L., Ivo, W.M.P.M., Eds.; Embrapa Tabuleiros Costeiros: Aracaju, Brazil, 2001; pp. 19–46. [Google Scholar]
- Gomes, J.B.V.; Silva, C.A.; Ferreira, T.L.D.A.; Ferreira, M.M.; Inda, A.V.; Curi, N. Carbon Stocks and Pools in Relation to the Texture of Kaolinitic Soils from the Brazilian East Coast. Rev. Bras. Ciênc. Solo. 2018, 42, e0170260. [Google Scholar] [CrossRef]
- Carvalho Filho, A.; Curi, N.; Fonseca, S. Avaliação Informatizada e Validada da Aptidão Silvicultural das Terras dos Tabuleiros Costeiros Brasileiros Para Eucalipto, 1st ed.; Editora UFLA: Lavras, Brazil, 2013; p. 138. [Google Scholar]
- Krull, E.; Baldock, J.; Skjemstad, J. Soil texture effects on decomposition and soil carbon storage. In Net Ecosystem Exchange; Kirschbaum, M.U.F., Mueller, R., Eds.; Cooperative Research Centre for Greenhouse Accounting: Canberra, Australia, 2001; pp. 103–110. [Google Scholar]
- Plante, A.F.; Conant, R.T.; Stewart, C.E.; Paustian, K.; Six, J. Impact of Soil Texture on the Distribution of Soil Organic Matter in Physical and Chemical Fractions. Soil Sci. Soc. Am. J. 2006, 70, 287–296. [Google Scholar] [CrossRef]
- Singh, M.; Sarkar, B.; Sarkar, S.; Churchman, J.; Bolan, N.; Mandal, S.; Menon, M.; Purakayastha, T.J.; Beerling, D.J. Stabilization of Soil Organic Carbon as Influenced by Clay Mineralogy. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2018; Volume 148, pp. 33–84. [Google Scholar]
- Angst, G.; Messinger, J.; Greiner, M.; Häusler, W.; Hertel, D.; Kirfel, K.; Kögel-Knabner, I.; Leuschner, C.; Rethemeyer, J.; Mueller, C.W. Soil organic carbon stocks in topsoil and subsoil controlled by parent material, carbon input in the rhizosphere, and microbial-derived compounds. Soil Biol. Biochem. 2018, 122, 19–30. [Google Scholar] [CrossRef]
- Ge, N.; Wei, X.; Wang, X.; Liu, X.; Shao, M.; Jia, X.; Li, X.; Zhang, Q. Soil texture determines the distribution of aggregate-associated carbon, nitrogen and phosphorous under two contrasting land use types in the Loess Plateau. Catena 2019, 172, 148–157. [Google Scholar] [CrossRef]
- Machado, M.A.B.L.; de Carvalho Chaves, L.D.F.; Neto, J.L.R.; de Lyra-Lemos, R.P. Florística do estrato arbóreo de fragmentos da Mata Atlântica do Nordeste oriental, município de Coruripe, Alagoas, Brasil. Rev Ouricuri. 2012, 2, 55–72. [Google Scholar]
- Magnago, L.F.S.; Edwards, D.P.; Edwards, F.A.; Magrach, A.; Martins, S.V.; Laurance, W.F. Functional attributes change but functional richness is unchanged after fragmentation of Brazilian Atlantic forests. J. Ecol. 2014, 102, 475–485. [Google Scholar] [CrossRef]
- Uhlmann, A.; Bonnet, A.; Curcio, G.R.; Silva, A.D.P.; Gonçalves, F.L.A.; de Resende, A.S. A cobertura vegetal das florestas e pastagens. In Monitoramento da Revegetação do Comperj: Etapa Inicial; Prado, R.B., Fidalgo, E.C.C., Bonnet, A., Eds.; Embrapa: Brasília, Brazil, 2014; pp. 223–244. [Google Scholar]
- IBGE–Instituto Brasileiro de Geografia e Estatística. Manual Técnico de Geomorfologia; IBGE: Rio de Janeiro, Brazil, 1995; p. 113. [Google Scholar]
- Gomes, J.B.V.; Araújo, J.C.; Vidal-Torrado, P.; Cooper, M.; Da Silva, E.A.; Curi, N. Cemented Horizons and Hardpans in the Coastal Tablelands of Northeastern Brazil. Revista Brasileira de Ciência do Solo 2017, 41. [Google Scholar] [CrossRef]
- Scaglia, B.; Adani, F. Biodegradability of soil water soluble organic carbon extracted from seven different soils. J. Environ. Sci. 2009, 21, 641–646. [Google Scholar] [CrossRef]
- Haynes, R.J. Interactions between soil organic matter status, cropping history, method of quantification and sample pretreatment and their effects on measured aggregate stability. Biol. Fertil. Soils 2000, 30, 270–275. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Agregate stability and size distribution. In Methods of Soil Analysis: Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data, Version 6; MjM Software Design: Gleneden Beach, OR, USA, 2011.
- Buringh, P. Organic carbon in soils of the world. In The Role of Terrestrial Vegetation in the Global Carbon Cycle: Measurement by Remote Sensing; Woodwell, G.M., Ed.; SCOPE/ICSU, Wiley & Sons: Chichester, UK, 1984; pp. 91–109. [Google Scholar]
- Yang, Y.; Xie, J.; Sheng, H.; Chen, G.; Li, X.; Yang, Z. The impact of land use/cover change on storage and quality of soil organic carbon in midsubtropical mountainous area of southern China. J. Geogr. Sci. 2009, 19, 49–57. [Google Scholar] [CrossRef]
- Geraei, D.S.; Hojati, S.; Landi, A.; Cano, A.F. Total and labile forms of soil organic carbon as affected by land use change in southwestern Iran. Geoderma Reg. 2016, 7, 29–37. [Google Scholar] [CrossRef]
- Andrade, E.M.; Valbrun, W.; De Almeida, A.M.M.; Rosa, G.; Da Silva, A.G.R. Land-Use Effect on Soil Carbon and Nitrogen Stock in a Seasonally Dry Tropical Forest. Agronomy 2020, 10, 158. [Google Scholar] [CrossRef]
- Dos Santos, O.A.Q.; Silva Neto, E.C.; García, A.C.; Fagundes, H.D.S.; Diniz, Y.V.D.F.G.; Ferreira, R.; Pereira, M.G. Impact of land use on Histosols properties in urban agriculture ecosystems of Rio de Janeiro, Brazil. Rev. Bras. Ciênc. Solo. 2020, 44, e02000041. [Google Scholar] [CrossRef]
- Eswaran, H.; Berg, E.V.D.; Reich, P. Organic Carbon in Soils of the World. Soil Sci. Soc. Am. J. 1993, 57, 192–194. [Google Scholar] [CrossRef]
- Ferreira, A.C.C.; Leite, L.F.C.; de Araújo, A.S.F.; Eisenhauer, N. Land-Use Type Effects on Soil Organic Carbon and Microbial Properties in a Semi-arid Region of Northeast Brazil. Land Degrad. Dev. 2014, 27, 171–178. [Google Scholar] [CrossRef]
- Paes, E.D.C.; Fernandes, I.O.; Dias, F.P.M.; Pereira, E.G.; Santos, D.N.; de Lima, J.M.; Nóbrega, R.S.A.; Nóbrega, J.C.A. Land use, management and physical attributes of dense Ferralsols in tropical northeastern Brazil. Catena 2021, 203, 1–11. [Google Scholar] [CrossRef]
- Khomutova, T.E.; Shirshova, L.T.; Tinz, S.; Rolland, W.; Richter, J. Mobilization of DOC from sandy loamy soils under different land use (Lower Saxony, Germany). Plant Soil 2000, 219, 13–19. [Google Scholar] [CrossRef]
- Aitkenhead-Peterson, J.A.; McDowell, W.H.; Neff, J.C. Sources, Production, and Regulation of Allochthonous Dissolved Organic Matter Inputs to Surface Waters. In Aquatic Ecosystems; Academic Press: Cambridge, MA, USA, 2003; pp. 25–70. [Google Scholar] [CrossRef]
- Chantigny, M.H. Dissolved and water-extractable organic matter in soils: A review on the influence of land use and management practices. Geoderma 2003, 113, 357–380. [Google Scholar] [CrossRef]
- Möller, A.; Kaiser, K.; Guggenberger, G. Dissolved organic carbon and nitrogen in precipitation, throughfall, soil solution, and stream water of the tropical highlands in northern Thailand. J. Plant Nutr. Soil Sci. 2005, 168, 649–659. [Google Scholar] [CrossRef]
- Pesántez, J.; Mosquera, G.M.; Crespo, P.; Breuer, L.; Windhorst, D. Effect of land cover and hydro-meteorological controls on soil water DOC concentrations in a high-elevation tropical environment. Hydrol. Process. 2018, 32, 2624–2635. [Google Scholar] [CrossRef]
- Xu, C.; Guo, L.; Dou, F.; Ping, C.-L. Potential DOC production from size-fractionated Arctic tundra soils. Cold Reg. Sci. Technol. 2009, 55, 141–150. [Google Scholar] [CrossRef]
- Yang, Y.G.; He, Z.L.; Wang, Y.B.; Liu, Y.L.; Liang, Z.B.; Fan, J.H.; Stoffella, P.J. Dissolved Organic Carbon in Association with Water Soluble Nutrients and Metals in Soils from Lake Okeechobee Watershed, South Florida. Water Air Soil Pollut. 2012, 223, 4075–4088. [Google Scholar] [CrossRef]
- Gomes, J.B.V.; Fernandes, M.F.; Barreto, A.C.; Araújo Filho, J.C.D.; Curi, N. Soil attributes under agroecosystems and forest vegetation in the coastal tablelands of northestern Brazil. Ciência Agrotec. 2012, 36, 649–664. [Google Scholar] [CrossRef]
- De Lima, C.A.; Montenegro, A.A.D.A.; Dos Santos, T.E.M.; De Andrade, E.M.; Monteiro, A.L.N. Práticas agrícolas no cultivo da mandioca e suas relações com o escoamento superficial, perdas de solo e água. Rev. Ciênc. Agron. 2015, 46, 697–706. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Miller, R.M.; Lussenhop, J. Contributions of interacting biological mechanisms to soil aggregate stabilization in restored prairie. Soil Biol. Biochem. 1998, 30, 905–916. [Google Scholar] [CrossRef]
- Schwendenmann, L.; Pendall, E. Effects of forest conversion into grassland on soil aggregate structure and carbon storage in Panama: Evidence from soil carbon fractionation and stable isotopes. Plant Soil 2006, 288, 217–232. [Google Scholar] [CrossRef]
- Anaya, C.A.; Huber-Sannwald, E. Long-term soil organic carbon and nitrogen dynamics after conversion of tropical forest to traditional sugarcane agriculture in East Mexico. Soil Tillage Res. 2015, 147, 20–29. [Google Scholar] [CrossRef]
- Ashagrie, Y.; Zech, W.; Guggenberger, G. Transformation of a Podocarpus falcatus dominated natural forest into a monoculture Eucalyptus globulus plantation at Munesa, Ethiopia: Soil organic C, N and S dynamics in primary particle and aggregate-size fractions. Agric. Ecosyst. Environ. 2005, 106, 89–98. [Google Scholar] [CrossRef]
- Ferreira, M.M.; Fernandes, B.; Curi, N. Influência da mineralogia da fração argila nas propriedades físicas de latossolos da região sudeste do Brasil. Rev. Bras. Ciên. Solo. 1999, 23, 515–524. [Google Scholar] [CrossRef]
- Vitorino, A.C.T.; Ferreira, M.M.; Curi, N.; De Lima, J.M.; Silva, M.L.N.; Da Motta, P.E.F. Mineralogia, química e estabilidade de agregados do tamanho de silte de solos da Região Sudeste do Brasil. Pesq. Agropec. Bras. 2003, 38, 133–141. [Google Scholar] [CrossRef]
- Barreto, R.C.; Madari, B.E.; Maddock, J.E.; Machado, P.L.; Torres, E.; Franchini, J.; Costa, A.R. The impact of soil management on aggregation, carbon stabilization and carbon loss as CO2 in the surface layer of a Rhodic Ferralsol in Southern Brazil. Agric. Ecosyst. Environ. 2009, 132, 243–251. [Google Scholar] [CrossRef]
- Vicente, L.C.; Gama-Rodrigues, E.F.; Gama-Rodrigues, A.C.; Marciano, C.R. Organic carbon within soil aggregates under forestry systems and pasture in a southeast region of Brazil. Catena 2019, 182, 104139. [Google Scholar] [CrossRef]
- Carrizo, M.E.; Alesso, C.A.; Cosentino, D.; Imhoff, S. Aggregation agents and structural stability in soils with different texture and organic carbon contents. Sci. Agricola 2015, 72, 75–82. [Google Scholar] [CrossRef]
- Chaplot, V.; Cooper, M. Soil aggregate stability to predict organic carbon outputs from soils. Geoderma 2015, 243-244, 205–213. [Google Scholar] [CrossRef]
- Shrestha, B.M.; Sitaula, B.K.; Singh, B.R.; Bajracharya, R.M. Soil organic carbon stocks in soil aggregates under different land use systems in Nepal. Nutr. Cycl. Agroecosyst. 2004, 70, 201–213. [Google Scholar] [CrossRef]
- Ashagrie, Y.; Zech, W.; Guggenberger, G.; Mamo, T. Soil aggregation, and total and particulate organic matter following conversion of native forests to continuous cultivation in Ethiopia. Soil Tillage Res. 2007, 94, 101–108. [Google Scholar] [CrossRef]
- Laskar, S.Y.; Sileshi, G.W.; Pathak, K.; Debnath, N.; Nath, A.J.; Laskar, K.Y.; Singnar, P.; Das, A.K. Variations in soil organic carbon content with chronosequence, soil depth and aggregate size under shifting cultivation. Sci. Total Environ. 2021, 762, e143114. [Google Scholar] [CrossRef]
- Signor, D.; Zani, C.F.; Paladini, A.A.; Deon, M.D.; Cerri, C.E.P. Estoques de carbono e qualidade da matéria orgânica do solo em áreas cultivadas com cana-de-açúcar. Rev. Bras. Cienc. Solo. 2014, 38, 1402–1410. [Google Scholar] [CrossRef]
- Bayer, C.; Amado, T.J.C.; Tornquist, C.G.; Cerri, C.E.C.; Dieckow, J.; Zanatta, J.A.; Nicoloso, R.S. Estabilização do Carbono no solo e mitigação das emissões de gases de efeito estufa na agricultura conservacionista. Tópicos em Ciência do Solo 2011, 7, 55–118. [Google Scholar]
- De Souza, E.D.; Costa, S.E.V.G.D.A.; De Lima, C.V.S.; Anghinoni, I.; Meurer, E.J.; Carvalho, P.C.D.F. Carbono orgânico e fósforo microbiano em sistema de integração agricultura-pecuária submetido a diferentes intensidades de pastejo em plantio direto. Rev. Bras. Cienc. Solo 2008, 32, 1273–1282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).